Submitted:
12 October 2023
Posted:
17 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Agriculture parameters for antimicrobial inputs estimates
2.3. Antimicrobial Analysis of top soil improvers and irrigation waters
2.4. Risk assessment for the onset of Antimicrobial Resistance
3. Results
4. Discussion
4.1. Analysis
4.2. Antimicrobials profiling in top soil improvers and irrigation waters
4.3. Antimicrobial Inputs in lettuce cultivation cycle
Acknowledgments
Declaration of Interest
List of abbreviations
| AMR | Anti-Microbial Resistance |
| cWTTPs | Civil wastewater treatment Plants |
| HI | Hazard Index |
| LOD | Limit of Detection |
| PNEC | Predicted No Effect Concentration |
| SM | Supplementary Materials |
| TSI | Top Soil Improvers |
| ww | wet weight |
References
- Anderson N, Snaith R, Madzharova G, Bonfait J, Doyle L, Godley A, Lam M, Day G, 2021. From: Ricardo. Energy and Environment Sewage sludge and the circular economy. Version: 7 Date: 17/05/2021 EEA activity: 1.5.2. Consulted at: https://forum.eionet.europa.eu/nrc-eionet-freshwater/library/urban-waste-water-treatment/sewage-sludge-and-circular-economy/download/en/1/Sewage%20Sludge%20and%20the%20Circular%20Economy%20-%20Final%20Report.pdf.
- Albero B, Tadeo J, del Mar Delgado M, Miguel E, and Pérez RA, 2019. Analysis of Multiclass Antibiotics in Lettuce by Liquid Chromatography–Tandem Mass Spectrometry to Monitor Their Plant Uptake. Molecules 24:4066. [CrossRef]
- Bengtsson-Palme J, Joakim Larsson DG, 2016. Concentrations of antibiotics predicted to select for resistant bacteria: Proposed limits for environmental regulation. Environ Int. 86: 140–149.
- Bhalsod GD, Chuang YH, Jeon S, Gui W, Li H, Ryser ET, Guber AK, Zhang W, 2018. Uptake and Accumulation of Pharmaceuticals in Overhead- and Surface-Irrigated Greenhouse Lettuce. J Agric Food Chem. 66:822-830.
- Binsker U, Käsbohrer A, Hammerl JA, 2022. Global colistin use: a review of the emergence of resistant Enterobacterales and the impact on their genetic basis, FEMS Microbiology Reviews, 46:fuab049. [CrossRef]
- Commission Implementing Decision (EU) 2022/1307 of 22 July 2022 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council 26.7.2022 EN Official Journal of the European Union L 197 p. 117.
- Commission Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. D. Of. Unión Eur. p. 1–114 .
- Commission Regulation (EU) 1258/2011 of 2 December 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for nitrates in foodstuffs OJ L 320 ,3.12.2011, p. 15.
- Congilosi JL, Aga DS, 2021. Review on the fate of antimicrobials, antimicrobial resistance genes, and other micropollutants in manure during enhanced anaerobic digestion and composting. J Hazard Mater. 405:123634. [CrossRef]
- Cycon´ M, Mrozik A and Piotrowska-Seget Z, 2019. Antibiotics in the Soil Environment—Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 10:338. [CrossRef]
- ECDC, EMA, EFSA and OECD, 2022 Antimicrobial Resistance in the EU/EEA - A One Health response. Available at: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-eueea-one-health-response.
- EEA (European Environmental Agency), 2020a. Report 04/2020 Bio-waste in Europe — turning challenges into opportunities ISBN 978-92-9480-223-1. ISSN 1977-8449. 1977. [CrossRef]
- EEA (European Environmental Agency), 2020b. Report 17/2020 Water and agriculture: towards sustainable solutions ISBN 978-92-9480-359-7. [CrossRef]
- EFSA (European Food Safety Authority), Alvarez F, Arena M, Auteri D, Borroto J, Brancato A, Carrasco Cabrera L, Castoldi AF, Chiusolo A, Colagiorgi A, Colas M, et al., 2021. Updated peer review of the pesticide risk assessment of the active substance asulam (variant evaluated asulam-sodium). EFSA Journal 19:6921, 31 pp. [CrossRef]
- European Commission Notice, 2022. Guidelines to support the application of Regulation 2020/741 on minimum requirements for water reuse (2022/C 298/01). Consulted at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52022XC0805(01)&from=EN.
- European Union Council Directive 91/676/EEC of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources (OJ L 375, 31.12.1991, p.1.
- FAO and WHO. 2023. Foodborne antimicrobial resistance – Compendium of Codex standards. First revision. Codex Alimentarius Commission. Rome. [CrossRef]
- Ferreira PFA, Xavier JF, Nunes JF, Fonseca IP, de Mattos de Oliveira Coelho S, Soares de Souza MM, da Silva Coelho I, 2023. Bacteria and antimicrobial resistance profile during the composting process of wastes from animal production. Braz J Microbiol. 54:1157-1167. [CrossRef]
- Gigliucci F, Brambilla G, Tozzoli R, Michelacci V, Morabito S, 2017. Comparative analysis of metagenomes of Italian top soil improvers. Environ Res. 155:108-115. doi: 10.1016/j.envres.2017.02.004. Grenni P., Ancona V, Barra Caracciolo A. Ecological effects of antibiotics on natural ecosystems: A review Microchemical Journal 136 (2018) 25–39 https://doi.org/10.1016/j.microc.2017.02.006.
- Holden, NM, White EP, Lange MC, and Olfield TL, 2018. Review of the sustainability of food systems and transition using the Internet of Food. npj Sci Food 2: 18. [CrossRef]
- ISPRA 2011. Guidelines for composts sampling (in Italian). Available at: (https://www.isprambiente.gov.it/files/pubblicazioni/manuali-lineeguida/man-3-2001-compost/1-10-manuale_3_2011_compost-2.pdf-1.
- Kumirska J, 2020. Pharmaceutical Residues in the Environment. Molecules. 25:2941. [CrossRef]
- Lasaridi KE, Manios T, Stamatiadis S, Chroni C, Kyriacou A, 2018. The Evaluation of Hazards to Man and the Environment during the Composting of Sewage Sludge. Sustainability 10:2618. [CrossRef]
- Matamoros V, Escolà Casas M, Mansilla S, Tadić Đ, Cañameras N, Carazo N, Portugal J, Piña B, Díez S, Bayona JM, 2022. Occurrence of antibiotics in Lettuce (Lactuca sativa L.) and Radish (Raphanus sativus L.) following organic soil fertilisation under plot-scale conditions: Crop and human health implications, J Haz Mat. 436:129044. [CrossRef]
- Mathews A, Naushad S, Duceppe MO, Kang M, Wang LR, Huang H, 2022. Complete Genome Sequence of a Listeria monocytogenes Strain with Antimicrobial Resistance Genes Isolated from Lettuce in Canada. Microbiol Resour Announc. 11:e0029822. [CrossRef]
- Mecella G. (2001). Analytical methods of waters intended for irrigation and animal farming (Metodi di analisi delle acque per uso agricolo e zootecnico). Franco Angeli, Milano (Italy) ISBN: 9788846429247.
- Moretti S, Cruciani G, Romanelli S, Rossi R, Saluti G, Galarini R, 2016. Multiclass method for the determination of 62 antibiotics in milk. J Mass Spectrom. 51:792-804. [CrossRef]
- Palumbo MT, Russo S, Polesello S, Guzzella L, Roscioli C, Marziali L, Valsecchi L, Cappelli F, Pascariello S, Tasselli S, et al., 2022. Integrated Exposure and Algal Ecotoxicological Assessments of Effluents from Secondary and Advanced-Tertiary Wastewater-Treatment Plants. Environ Toxicol Chem. 41: 2404-2419. [CrossRef]
- Patyra E, Nebot C, Gavilán RE, Kwiatek K, and Cepeda, A, 2023. Prevalence of veterinary antibiotics in natural and organic fertilizers from animal food production and assessment of their potential ecological risk. J Sci Food Agric. 103:3638–3644. doi 10.1002 jsfa/12435.
- Regulation (EU) 2019/4 of the European Parliament and of the Council of 11 December 2018 on the manufacture, placing on the market and use of medicated feed, amending Regulation (EC) No 183/2005 of the European Parliament and of the Council and repealing Council Directive 90/167/EEC. Official Journal of the European Union L 4/7.1.2019, p.1.
- Regulation 2020/741 of the European Parliament and of the Council of 25 May 2020 on minimum requirements for water reuse. Official Journal of the European Union L 177, 5.6.2020, p 32.
- Sagaseta de Ilurdoz M, Sadhwani JJ, Vaswani Reboso J, 2022. Antibiotic removal processes from water & wastewater for the protection of the aquatic environment - a review. J. Water Process Eng. 45:102474. [CrossRef]
- Saluti G, Diamanti I, Giusepponi D, Pucciarini L, Rossi R, Moretti S, Sardella R, Galarini R, 2018. Simultaneous determination of aminoglycosides and colistins in food. Food Chem 266:9-16. [CrossRef]
- Sargenti M, Bartolacci S, Luciani A, Di Biagio K, Baldini M, Galarini R, Giusepponi D, Capuccella M, 2020. Investigation of the Correlation between the Use of Antibiotics in Aquaculture Systems and Their Detection in Aquatic Environments: A Case Study of the Nera River Aquafarms in Italy. Sustainability 12:5176. [CrossRef]
- Seyoum MM, Lichtenberg R, Orlofsky E, Bernstein N, Gillor O, 2022. Antibiotic resistance in soil and tomato crop irrigated with freshwater and two types of treated wastewater. Environ Res. 211:113021. [CrossRef]
- Slobodiuk S, Niven C, Arthur G, Thakur S, Ercumen A, 2021. Does Irrigation with Treated and Untreated Wastewater Increase Antimicrobial Resistance in Soil and Water: A Systematic Review. Int J Environ Res Public Health 18:11046. [CrossRef]
- Solaiman S, Handy E, Brinks T, Goon K, Bollinger C, Sapkota AR, Sharma M, Micallef SA, 2022. Extended Spectrum β-Lactamase Activity and Cephalosporin Resistance in Escherichia coli from U.S. Mid-Atlantic Surface and Reclaimed Water. Appl Environ Microbiol. 88:e0083722. [CrossRef]
- Wang J, Xu S, Zhao K, Song G, Zhao S, Liu R, 2023. Risk control of antibiotics, antibiotic resistance genes (ARGs) and antibiotic resistant bacteria (ARB) during sewage sludge treatment and disposal: A review. Sci Total Environ. 877:162772. [CrossRef]

| Sulfonamides | Tetracyclines | Fluoroquinolones | |||||||||||||||||
| ID | top soil improver | GUA | ANI | DIA | MERA | META | MMTX | DMTX | TRMP | Tetra* | Oxy* | Doxy | Nor | Cipro | Flum | Enro | Marbo | TYLA | LIN |
| 1.0 | mixed compost | <LOD | 1832 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 4.0 | mixed compost | <LOD | 1710 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 5.0 | mixed compost | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 5.2 | mixed compost | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 6.0 | mixed compost | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 11 | mixed compost | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 14 | mixed compost | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 19 | mixed compost | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 2.0 | Bovine manure and meat and bone meals | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 27 | 57 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 111 | <LOD | <LOD | <LOD |
| 3.0 | Bovine + equine manure | <LOD | <LOD | <LOD | <LOD | 20 | <LOD | 51 | 178 | <LOD | <LOD | 95 | <LOD | <LOD | <LOD | 95 | <LOD | <LOD | <LOD |
| 7.0 | Bovine manure from organic farm | 37 | <LOD | 41 | <LOD | 33 | <LOD | 80 | 274 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 179 | <LOD | <LOD | <LOD |
| 8.0 | Pig Slurry | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 190 |
| 10 | Pig Slurry | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 76 | 54 | <LOD | <LOD | <LOD | <LOD | 14 | <LOD | 959 |
| 13 | Poultry Litter | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 15 | Bovine manure | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 26 | Bovine manure | <LOD | <LOD | 36 | 14 | 14 | 20 | <LOD | <LOD | <LOD | 58 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 30 | Poultry Litter | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 4487 | <LOD |
| 9.0 | Digestate | <LOD | <LOD | 12 | <LOD | 11 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 24 | Digestate | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 13 | <LOD | <LOD |
| 25 | Digestate | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 28 | Digestate | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 29 | Digestate | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 16 | WWTP biosolid | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 202 | <LOD | <LOD | <LOD | <LOD | <LOD |
| 17 | Cheese plant biosolid | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 18 | WWTP biosolid | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 22 | <LOD | 63 | <LOD | 353 | <LOD | <LOD | <LOD | <LOD | <LOD |
| 20 | WWTP biosolid | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 34 | 29 | 885 | <LOD | <LOD | <LOD | <LOD | <LOD |
| 21 | WWTP biosolid | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 41 | 36 | 632 | <LOD | <LOD | <LOD | <LOD | <LOD |
| 22 | WWPT biosolid + lime | <LOD | <LOD | 58 | <LOD | <LOD | <LOD | <LOD | <LOD | 81 | <LOD | 470 | <LOD | 406 | 22 | <LOD | <LOD | <LOD | <LOD |
| 23 | WWPT biosolid + lime | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 572 | 58 | <LOD | <LOD | <LOD | <LOD |
| 27 | WWPT biosolid + lime | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 307 | <LOD | <LOD | <LOD | <LOD | <LOD |
| Origin | Sampling date | ID | GUA | DIA | PYRI | ANI | MTX | TRMP | CIPRO | SPIRA | DOXI | TIAM | FLUM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dd/mm/yy | |||||||||||||
| Reused waters | 23/05/2022 | 1297 | 22 | D | 26 | <LOD | 136 | 50 | 12 | 30 | 61 | 11 | 10 |
| River freshwater | 23/05/2022 | 1298 | <LOD | <LOD | <LOD | <LOD | D | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Irrigation channel | 23/05/2022 | 1299 | <LOD | <LOD | <LOD | <LOD | D | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Irrigation channel | 23/05/2022 | 1301 | 14 | D | 13 | <LOD | 77 | 18 | <LOD | <LOD | <LOD | D | D |
| Reused waters | 20/06/2022 | 1551 | 25 | 11 | 22 | 11 | 181 | 35 | <LOD | <LOD | <LOD | <LOD | <LOD |
| Irrigation channel | 20/06/2022 | 1552 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| River freshwater | 20/06/2022 | 1553 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Irrigation channel | 20/06/2022 | 1554 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Reused waters | 30/08/2022 | 2211 | 28 | <LOD | 17 | <LOD | 221 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Irrigation channel | 30/08/2022 | 2212 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Irrigation channel | 30/08/2022 | 2213 | 16 | <LOD | <LOD | <LOD | 15 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Inputs | Sulfonamides | Tetracyclines | Macrolides | Fluoroquinolones | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GUA | ANI | DIA | MET | SDM | MTX | PYR | TRP | DOX | OXY* | TYL | SPIR | LYN | ENR | NOR | CIPR | MARB | TIA | |||
| TSI | ID | tons | ||||||||||||||||||
| manure | 7 | 0.30 | 11.1 | ND | 12.3 | 9.9 | 24 | ND | ND | 82.2 | ND | ND | ND | ND | ND | 53.7 | ND | ND | ND | ND |
| Mixed Compost | 1 | 0.15 | ND | 275 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Sludge compost | 15 | 0.15 | ND | ND | ND | ND | ND | ND | ND | ND | 2.04 | ND | ND | ND | ND | ND | 1.74 | 53.1 | ND | ND |
| Poultry Litter | 30 | 0.02 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 89.7 | ND | ND | ND | ND | ND | ND | ND |
| Pig Slurry | 10 | 0.02 | ND | ND | ND | ND | ND | ND | ND | ND | 1.08 | 1.52 | ND | ND | 19.2 | ND | ND | ND | 0.28 | ND |
| Waters | tons | |||||||||||||||||||
| Reused (N=3) | 350 | 8.75 | 1.30 | 1.30 | ND | ND | 62.7 | 7.70 | 9.80 | 7.00 | ND | ND | 3.50 | ND | ND | ND | 1.40 | ND | 1.30 | |
| Rivers (N=2) | 350 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Channels (N=6) | 350 | 1.75 | ND | 0.63 | ND | ND | 5.25 | 0.77 | 1.05 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Worst case | ||||||||||||||||||||
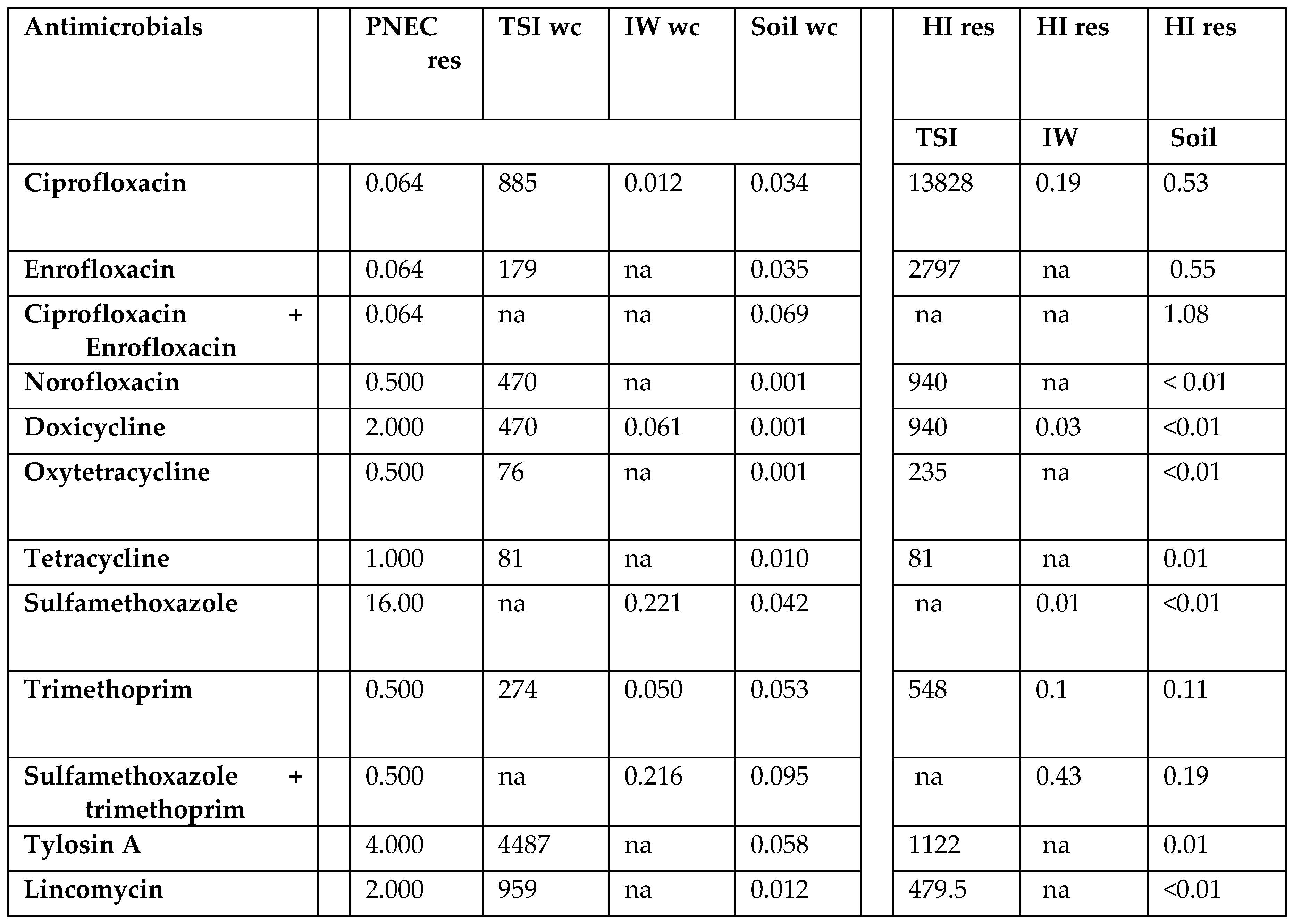
| Top soil + water | mg | 12.9 | 276 | 12.3 | 9.90 | 24.0 | 62.7 | 7.70 | 82.2 | 2.04 | 1.52 | 89.6 | 3.50 | 19.8 | 53.7 | 1.74 | 53.1 | 0.28 | 1.30 | |
| Dilution factor | 1542 | tons | ||||||||||||||||||
| final input in soil | ng/g | 0.008 | 0.179 | 0.008 | 0.006 | 0.016 | 0.042 | 0.005 | 0.053 | 0.001 | 0.001 | 0.058 | 0.002 | 0.012 | 0.035 | 0.001 | 0.034 | 0.001 | 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





