Submitted:
12 October 2023
Posted:
16 October 2023
You are already at the latest version
Abstract
Keywords:
Figure 1.
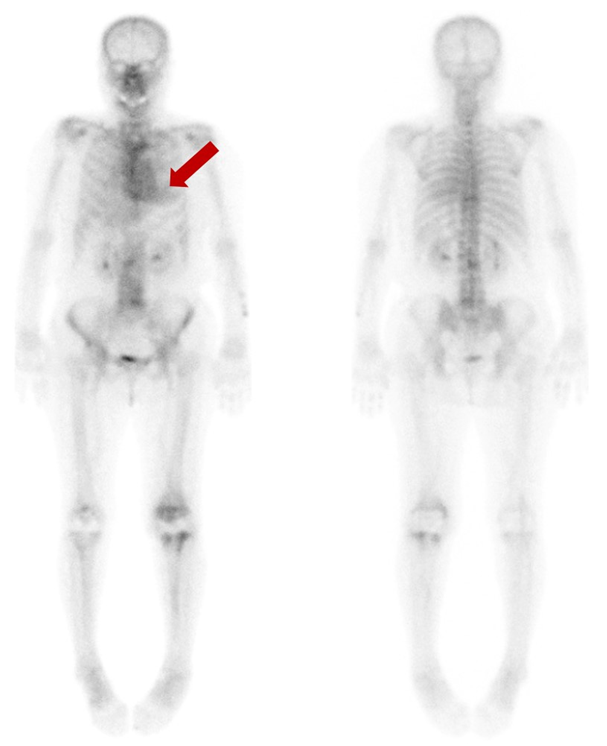
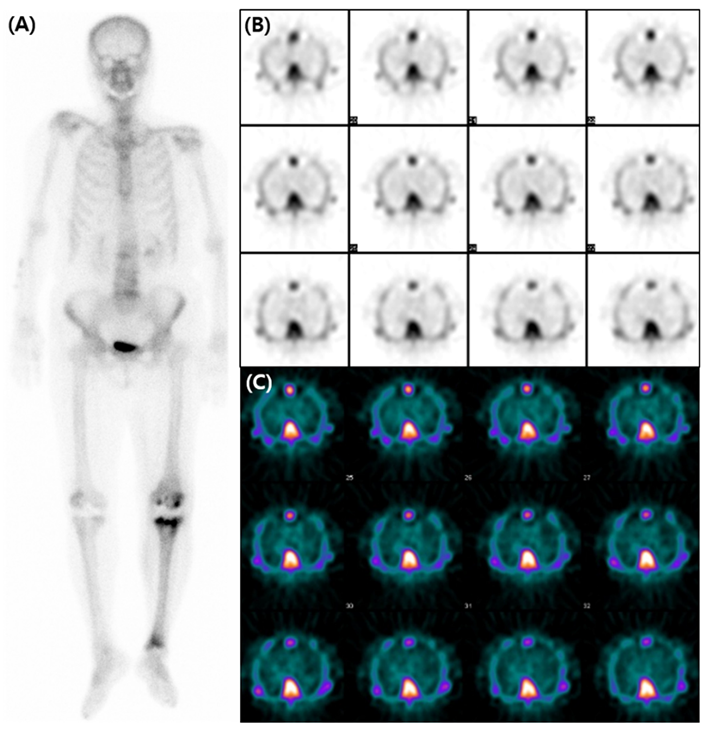
Figure 2.
References
- Perugini, E.; Guidalotti, P.L.; Salvi, F.; Cooke, R.M.T.; Pettinato, C.; Riva, L.; Leone, O.; Farsad, M.; Ciliberti, P.; Bacchi-Reggiani, L.; et al. Noninvasive Etiologic Diagnosis of Cardiac Amyloidosis Using 99mTc-3,3-Diphosphono-1,2-Propanodicarboxylic Acid Scintigraphy. Journal of the American College of Cardiology 2005, 46, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Glaudemans, A.; Bertagna, F.; Hazenberg, B.P.C.; Erba, P.A.; Giubbini, R.; Ceriani, L.; Prior, J.O.; Giovanella, L.; Slart, R. Diagnostic accuracy of bone scintigraphy in the assessment of cardiac transthyretin-related amyloidosis: a bivariate meta-analysis. Eur J Nucl Med Mol Imaging 2018, 45, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Brownrigg, J.; Lorenzini, M.; Lumley, M.; Elliott, P. Diagnostic performance of imaging investigations in detecting and differentiating cardiac amyloidosis: a systematic review and meta-analysis. ESC Heart Fail 2019, 6, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- de Haro-del Moral, F.J.; Sánchez-Lajusticia, A.; Gómez-Bueno, M.; García-Pavía, P.; Salas-Antón, C.; Segovia-Cubero, J. Role of cardiac scintigraphy with ⁹⁹mTc-DPD in the differentiation of cardiac amyloidosis subtype. Rev Esp Cardiol (Engl Ed) 2012, 65, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, S.; Castaño, A.; Pozniakoff, T.; Deslisle, S.; Latif, F.; Maurer, M.S. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging 2013, 6, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Quarta, C.C.; Guidalotti, P.L.; Pettinato, C.; Fanti, S.; Leone, O.; Ferlini, A.; Longhi, S.; Lorenzini, M.; Reggiani, L.B.; et al. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging 2011, 4, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.A.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.; et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI Expert Consensus Recommendations for Multimodality Imaging in Cardiac Amyloidosis: Part 2 of 2-Diagnostic Criteria and Appropriate Utilization. J Card Fail 2019, 25, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Oh, M.; Sung, C.; Kim, K.H.; Ryu, J.S. Altered Biodistribution of (99m)Tc-DPD on Bone Scan After Intravenous Iron Supplement. Nucl Med Mol Imaging 2017, 51, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Choy, D.; Murray, I.P.; Hoschl, R. The effect of iron on the biodistribution of bone scanning agents in humans. Radiology 1981, 140, 197–202. [Google Scholar] [CrossRef] [PubMed]
- VanAntwerp, J.; Hall, J.; OMara, R.; Hilts, S. Bone scan abnormality produced by interaction of Tc-99m diphosphonate with iron dextran (Imferon). JOURNAL OF NUCLEAR MEDICINE: SOC NUCLEAR MEDICINE INC 1850 SAMUEL MORSE DR, RESTON, VA 20190-5316; 1975:577-577.
- Parker, J.A.; Jones, A.G.; Davis, M.A.; Mcilmoyle, G.; Tow, D.E. Reduced uptake of bone-seeking radiopharmaceuticals related to iron excess. Clinical Nuclear Medicine 1976, 1, 267–268. [Google Scholar] [CrossRef]
- Forauer, A.R.; Grossman, S.J.; Joyce, J.M. Altered biodistribution of Tc-99m HMDP on bone scintigraphy from recent intravenous iron therapy. Clin Nucl Med 1994, 19, 817–818. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




