1. Introduction
Mitral valve replacement (MVR) in patients with small mitral annulus (<25 mm diameter) is technically difficult and carries an increased risk of poor prognosis, which results in very limited options for those [
1,
2,
3,
4,
5]. Obviously, surgery is very difficult in patients with small mitral valve redo-MVR and involves a substantial risk of mitral annular disruption, ventricular dysfunction, or perivalvular leak [
6,
7,
8,
9,
10].
The chimney technology was first reported to be used to solve patient-prosthesis mismatch (PPM) in pediatrics undergoing MVR [
11]. The technique was subsequently reported to be used to solve PPM in adults undergoing Bentall procedure [
12] and to avoid complete mitral annular calcification (MAC) debridement in adults with severe mitral stenosis and massive MAC [
13,
14]. The main challenges in small mitral valve redo-MVR are the difficulty of intraoperative removal of diseased tissue from the annulus and postoperative PPM, and there is no feasible treatment option that can effectively address these challenges. In our center, 77 patients with small mitral valve successfully performed redo-MVR with the chimney technology, with excellent clinical results.
2. Materials and Methods
A retrospective analysis of all patients who underwent an redo-MVR using the chimney technology between 2019 and 2020 with access to follow-up echocardiography was carried out after institutional review board approval.
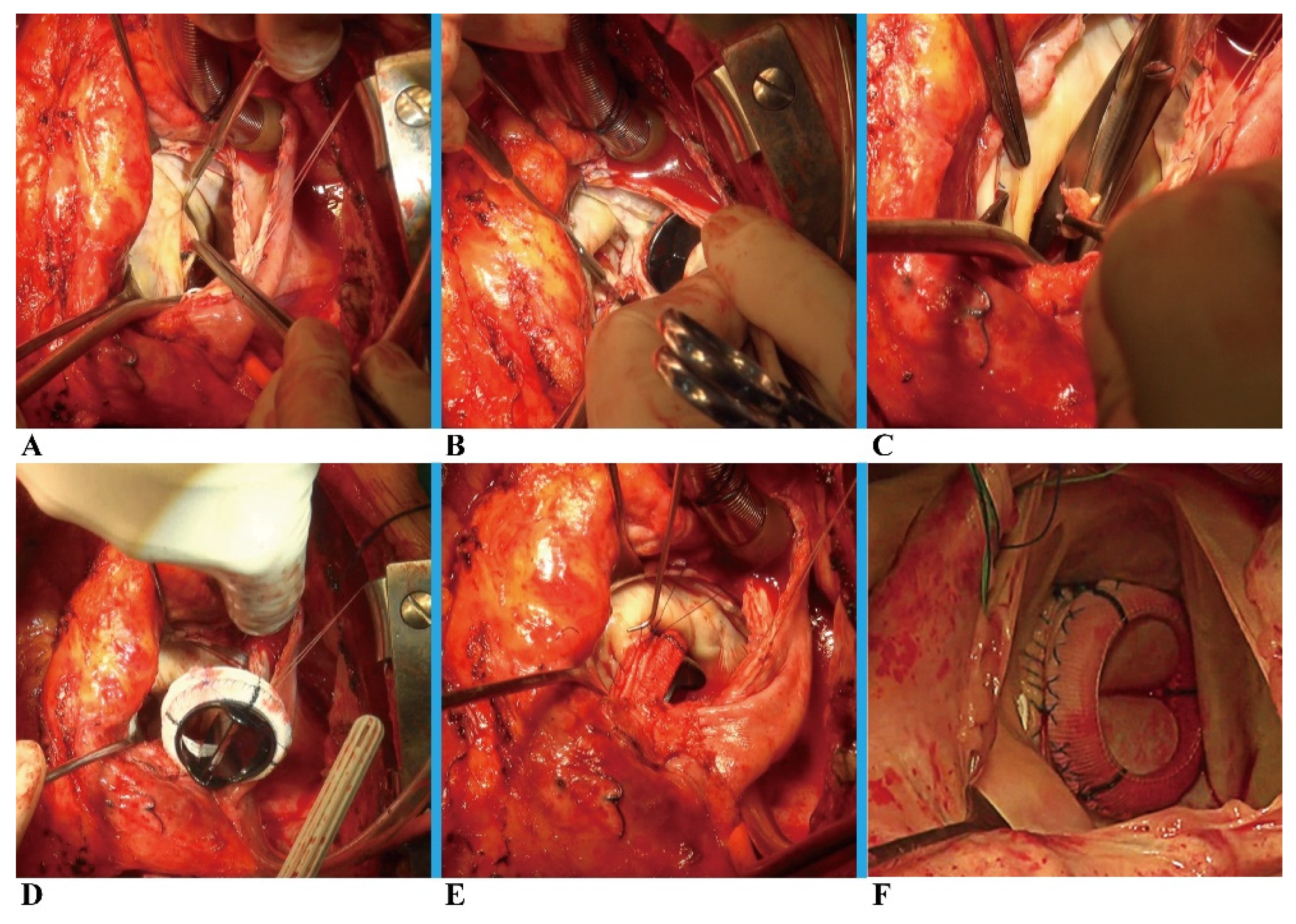
After a routine re-sternotomy, the patient is administered systemically Heparin (300-400 Units/Kg). After bicaval cannulation with anterograde and retrograde cardioplegic arrest, the mitral valve was accessed through an incision of the right atrium and atrial septum. (
Figure 1A). The prior mitral prosthesis was removed (
Figure 1B), and the annulus underwent significant debridement (
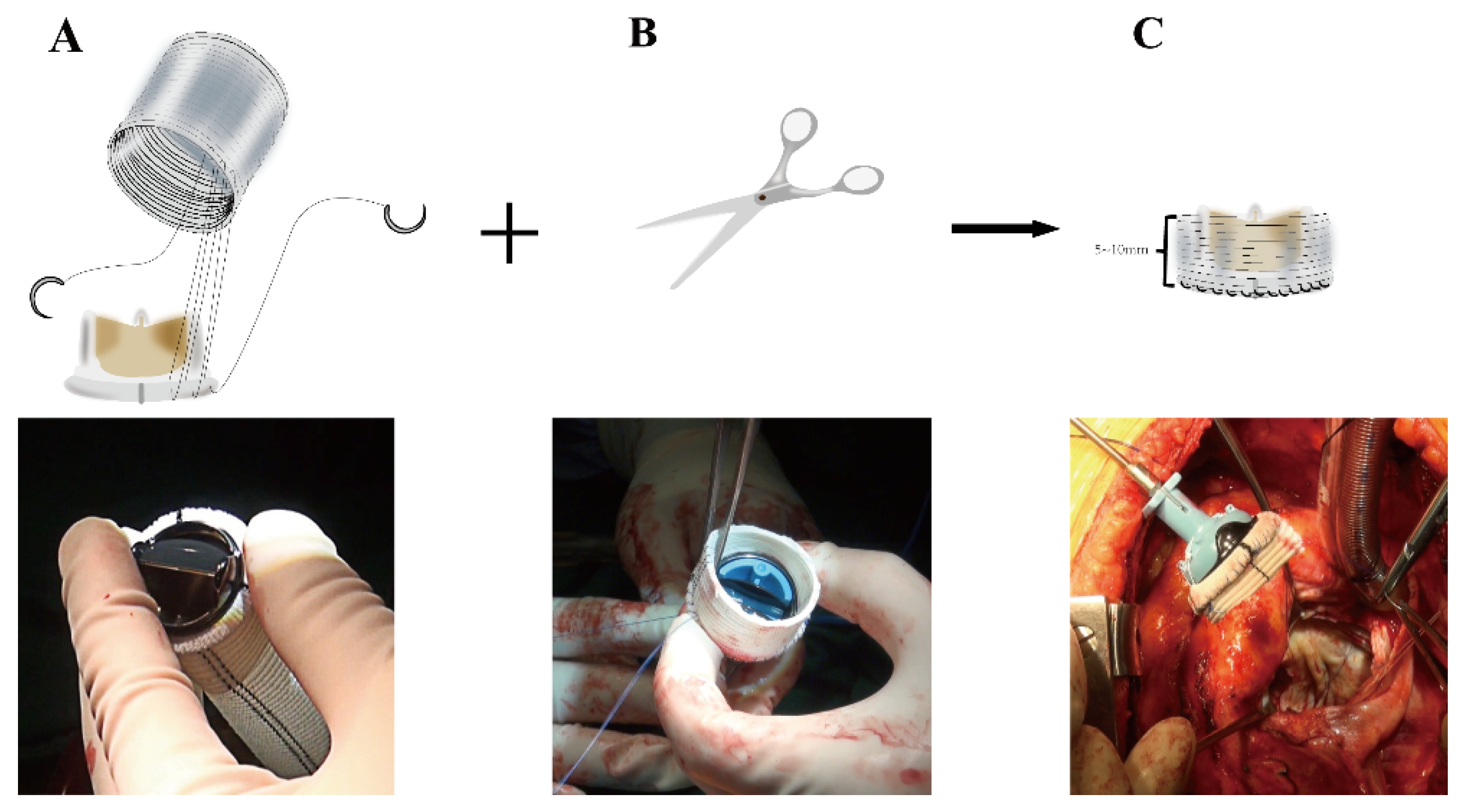
Figure 1C), resulting in a complete or at least partial loss of annular tissue. The next critical step is to assemble the new mitral valvular conduit. A woven polyester vascular graft (InterGard; Maquet Cardiovascular, La Ciotat, France) and any valve prosthesis including the bileaflet St Jude (St Jude Medical, Inc, St Paul, MN) prosthetic valve and Medtronic Aortic AP (Medtronic Inc, Minneapolis, MN) could be used to assemble the new valvular conduit. Artificial blood vessels are at least 3 sizes larger than mechanical prostheses and 5 sizes larger than bio-prostheses. The valve is fixed within the vascular graft with running 4-0 polypropylene sutures (
Figure 2A). The self-assembled valvular conduit leaves an approximately 5 to 10 mm free margin above the valve. According to the need for surgery, the margin above the valve's length is chosen (
Figure 2B-2C). The valvular conduit was sutured to the annulus with a continuous stitch of 4/0Prolene (
Figure 1D), and the suture is passed from the atrial side through the left atrial outflow tract endocardium and the "chimney" of valved conduit without suturing to the prosthetic textile suturing ring (
Figure 1E-1F). Suture should pay attention to the posterior annulus suture and exposure, usually, the mitral valve disease is accompanied by left atrial enlargement. In most patients, the left atrium is enlarged with a septal incision, and a bi-atrial incision can be considered in patients with a small left atrium or an atrium that is not large in the first place. The cardiopulmonary bypass was eventually weaned, and the atrial septum and right atrium were closed (Supplemental Video S1). Temporary epicardial pacing wires are routinely placed. The incision is typically closed in layers after chest tubes are inserted and hemostasis is obtained. After transfer to the ICU, anticoagulation is routinely administered as soon as possible to maintain the INR between 1.8 to 2.5.
Statistical Methods
Data analysis was performed with R software (version 4.2.1; http://www.Rproject.org). All
categorical data were expressed as proportions and continuous variables were expressed as mean ± SD or median with date range. Estimates of survival were measured from the date of surgery to the date of death or the date of the last known time of survival (censored observations). Survival rates at 1-year and 4-year intervals were estimated using the Kaplan-Meier method. The log-log transformation method was used to calculate 95% confidence intervals (CIs) for survival. The reported statistical significance levels were all two-sided, with statistical significance set at .05.
3. Results
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3.1. Patients’ Characteristics
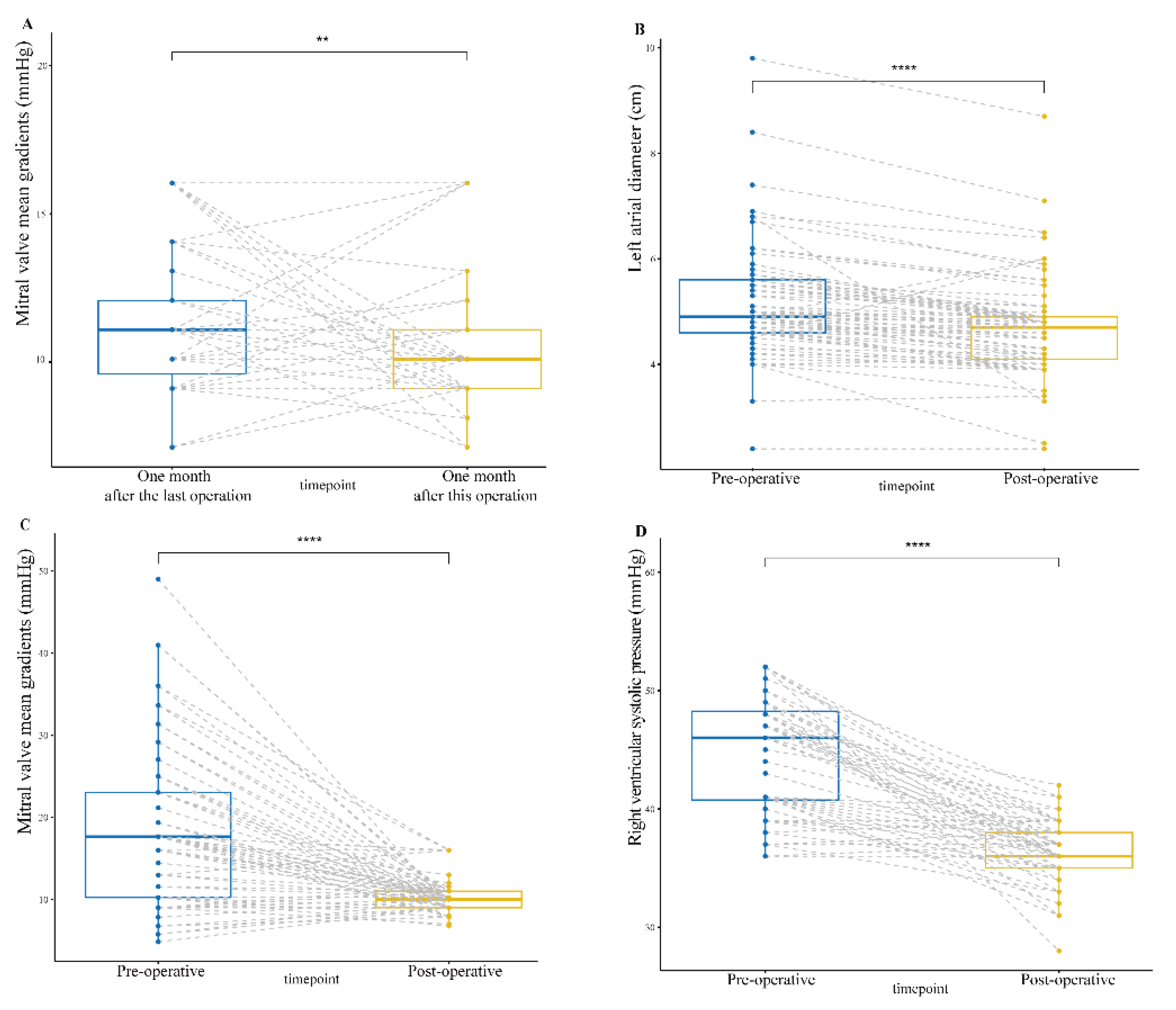
The mean age of the patients was 56.7±15.98 years, with 48 females and 58 patients with atrial fibrillation. All patients underwent mitral valve surgery of which 62 were mitral valve replacements, 7 mitral valve repairs, and 8 double valve replacements. 52 patients (67.5%) with 2 to 5 years since the last valve surgery. The mitral valve mean gradient (MG) one month after the last surgery in all patients was 11.15 ± 2.26 mmHg. Preoperative left atrial diameter (LAD) and right ventricular systolic pressure (RVSP) were 5.14 ± 1.06 cm and 47.14 ± 9.92 mmHg, respectively. Most patients (96.1%) had underlying preoperative mitral stenosis, but 29.9% also had at least moderate mitral regurgitation preoperatively. The patient's preoperative mitral valve mean gradient and peak gradient were 18.07 ± 9.40 mmHg and 22.43 ± 4.69 mmHg, respectively. Mean preoperative mitral valve diameter and EOAI were 24.5 ± 0.42 mm and 0.95 ± 0.27 cm
2/m
2, respectively. No patient had periprosthetic leakage and left ventricular outflow tract obstruction (LVOTO) (
Table 1).
3.2. Procedural and In-Hospital Outcomes
3.2.1. Procedural Data
Other concurrent procedures were performed in 42 of 77 patients (54.5%), including aortic valve replacement (n=12), coronary bypass (n=9), tricuspid repair (n=11), Modified Morrow procedure (n=4) and Cox Maze IV (n=12). The total cardiopulmonary bypass and aortic cross-clamp times were 179.40 ± 68.56 min and 112.90 ± 42.70 min, respectively. There were no intraoperative deaths (
Table 2).
3.2.2. Periprocedural Outcomes
The incidence of atrial fibrillation postoperatively was 23.7% (n=21). Other events and respective incidences were tracheostomy: 5.2% (n=4), prolonged ventilation (>24 h): 17% (n=5), reoperations due to bleeding: 3% (n=1), stroke: 1.3% (n=1), and temporary neurological impairment: 6.5% (n=5). Mechanical circulatory support including an intra-aortic balloon was required in 1 (1.3%) patient. Two perioperative deaths occurred, both because of respiratory failure and malignant arrhythmia. The total intensive care unit (ICU) and hospital stay lengths were 6 ± 2 and 21 ± 5 days, respectively (
Table 3).
Postoperative transthoracic echocardiograms showed that the size of the mitral valve prosthesis was 28.51 ± 1.22 mm and also demonstrated a great performance of the composite valved conduit through the chimney technique (Supplemental
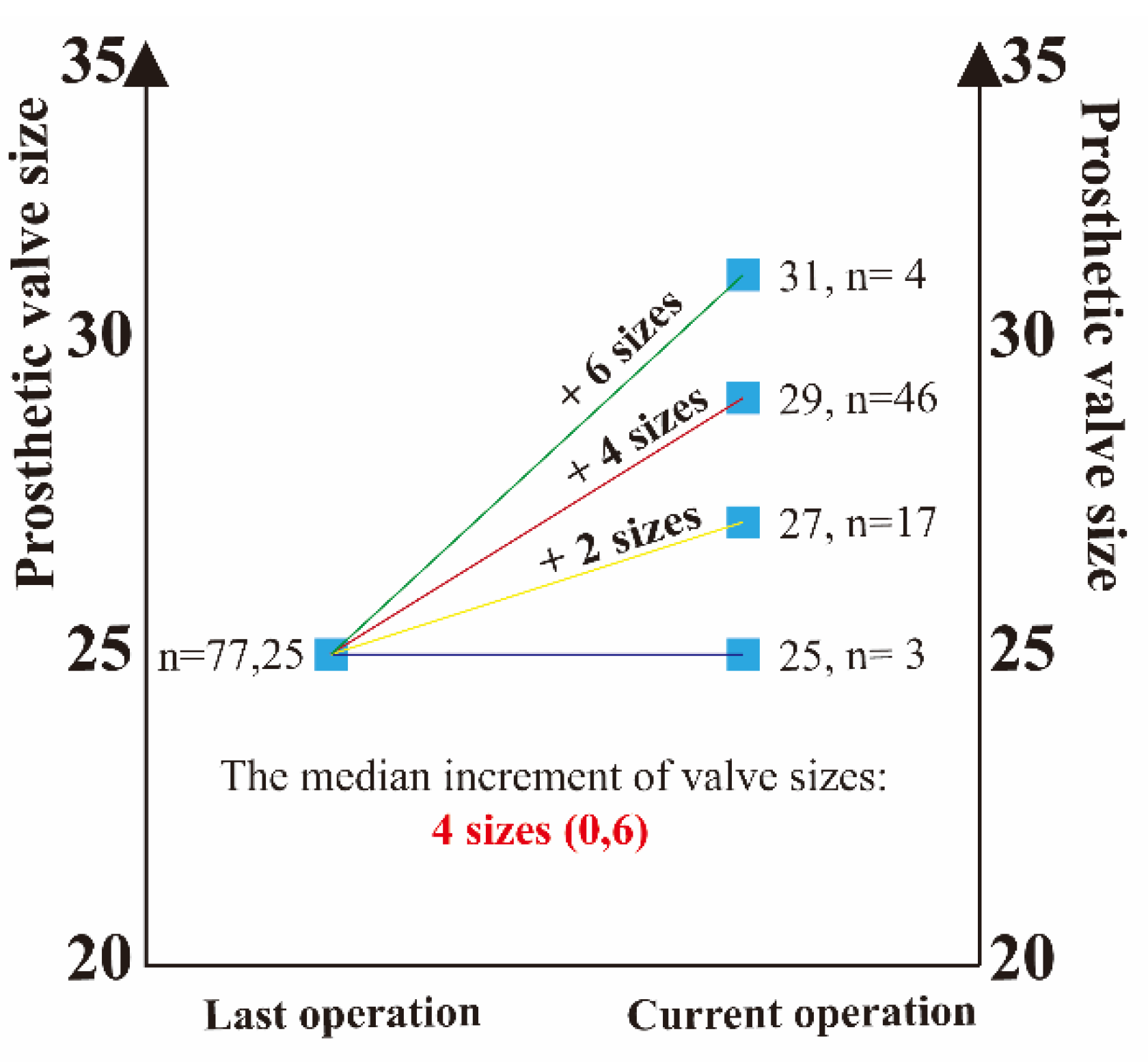
Figure S1). The median increment of annulus enlargement was 4 (0, 6) valve sizes (
Figure 3). The peak gradient (PG) and MG of the mitral prosthesis were 11.73 ± 3.42 mmHg and 10.34 ± 2.12 mmHg, respectively, which were significantly statistically decreased (p0.001) from preoperative data (
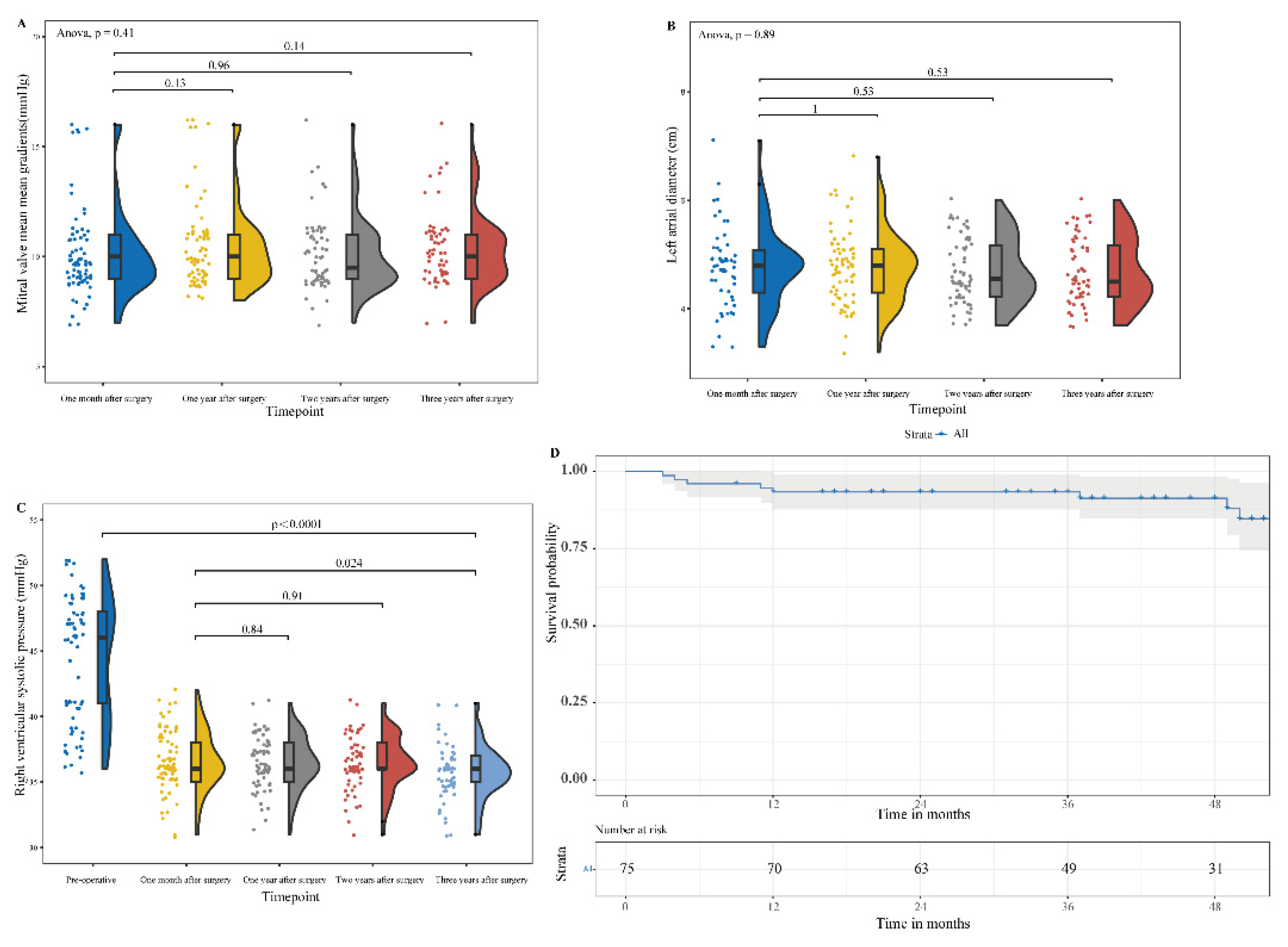
Figure 4). Postoperative LAD and RVSP were 4.68 ± 0.92 cm and 36.30 ± 2.42 mmHg, respectively, which were significantly statistically decreased (p0.001) from preoperative data (
Figure 4). The cohort had 77.9% of patients with normal left ventricle (LV) function, 16.9% with mild dysfunction, 3.9% with moderate dysfunction, and 1.3% with severe LV dysfunction (
Table 3).
3.3. Follow-Up Outcomes
3.3.1. Clinical Outcomes
The mean follow-up period was 1160 ± 420 days (30 patients follow-up beyond 4 years). Four-year patient survival was 89.3% (
Figure 5). Causes of death were respiratory failure in 4 patients with COVID-19, sudden death in 1 patient, 1 patient with terminal stomach cancer, 1 patient with end-stage heart failure, and failure to thrive in a 77-year-old patient. At a median patient follow-up of 43 months (range: 3 to 54 months), no patient required reoperation and no patient had periprosthetic leakage, LVOTO, and thrombosis. Median clinical heart failure symptoms at follow-up were New York Heart Association (NYHA) class I, which statistically significantly improved from preoperative symptoms of class II (p0.001). At 4-year follow-up, living patients were NYHA class I in 25 of 30 patients (83%), class II in 3 of 30 patients (10%, with 1 of 3 having significant bronchiectasis), and class III in 1 patient with severe premature ventricular contraction.
3.3.2. Echocardiographic Follow-Up
Follow-up echocardiography showed that the MG of the mitral prosthesis at one, two, and three years postoperatively fluctuated slightly but was not statistically significant (p > 0.001) compared to it at one month postoperatively. Likewise, LAD and RVSP at one, two, and three years postoperatively fluctuated slightly but were not statistically significant (p > 0.001) compared to that at one month postoperatively. By 3-year follow-up echocardiogram, RVSP had fallen from 47.14 ± 9.92 mm Hg to 35.76 ± 2.03 mm Hg (p0.0001) (
Figure 5). During the 1-month follow-up period, there was no LV dysfunction in 75% of the patients, while 21% had mild, 3% had moderate LV dysfunction, and 1 patient had severe LV dysfunction. During the 3-year follow-up period, there was no LV dysfunction in 91% of the patients, while 7% had mild, and 1 patient had severe LV dysfunction (
Table 4).
4. Discussion
To our knowledge, this is the largest series of redo-mitral valve replacement with the use of the chimney technology for a small mitral valve annulus (<25 mm). The chimney technology is currently described in case reports for the resolution of PPM in pediatric patients undergoing MVR [
11] and adult patients undergoing the Bentall procedure [
12] and for the resolution of MVR in severe MAC patients [
13,
14]. The small mitral annulus may be the result of extensive calcification of the mitral annulus to the leaflets, small patient size, or previous mitral repair with a small annular ring5. Surgical options including extensive annular debridement and reconstruction, placement of prosthesis in a supra-annular intra-atrial position, or placement of a transcatheter aortic prosthesis in the mitral position are limited and technically challenging for redo-MVR patients with a small mitral annulus [
3,
5,
7]. All of these techniques are associated with an increased risk of disruption of the ventriculoatrial and mitral annulus, ventricular dysfunction, perivalvular leakage, or LVOTO. In our series, early and mid-term outcomes were excellent with relatively few complications in this high-risk group of patients for redo-MVR.
In our series, the preoperative mitral valve size was 25# and 96% of the patients had mitral stenosis with a mitral EOAI of 0.95 ± 0.27 cm2/m2, all of which suggested that all patients had severe PPM. The median increment of mitral size enlargement after MVR with the chimney technology was 4 (0, 6) valve sizes, which can effectively avoid postoperative PPM. It was the enlargement of the implanted prosthesis size that significantly reduced the patient's postoperative mitral valve MG compared to the preoperative period. Moreover, the patient's mitral valve MG one month after this operation was 10.11 ± 1.99 mmHg, which was significantly statistically decreased from data one month after the last operation. Thus, the chimney technique allows patients with small mitral annulus to obtain ideal valve size at redo-MVR, effectively avoiding postoperative PPM. All patients in our series had prior mitral surgery and required redo sternotomy. Additionally, the majority of patients had complex cardiac and no-cardiac comorbidities. Thus, this group of patients is a high-risk surgical group but with a relatively low postoperative complication rate.
The MG of the mitral valve after operation remained at 10.34 ± 2.12 mmHg. Additionally, follow-up echocardiographic results at one, two, and three years postoperatively showed mild stenosis of the mitral valve prosthesis (MG > 10 mmHg). This may be because the composite graft valve is not flush with the native annulus. The anterior annulus and the left atrial wall make up one side of the valve. The atrial side of the composite graft is higher than the annulus side because they are not on the same horizontal surface, which causes the valve to lean toward the annulus. The blood flowing through the valve may impact the atrial wall under the composite graft and create a turbulent flow that generates a high-pressure gradient. The postoperative high-pressure gradient of mitral valve is also partly due to the use of cardiotonic drugs. LAD and RVSP at one, two, and three years postoperatively fluctuated slightly but were not statistically significant (p > 0.001) compared to that at one month postoperatively. LAD and RVSP Follow-up results suggest that mitral stenosis is artifactual and that the mitral high-pressure gradient does not reverse conduction. The composite graft valve alters the local hemodynamics of the mitral valve including the generation of local vortices, which may lead to the distant complication of thrombosis and pannus ingrowth in the mitral position. But, our mid-term follow-up results did not show any of these complications. In our series, the previous prostheses for patients were removed, and extensive debridement of the annulus was performed, which led to a total (or at least partial) removal of annular tissue. The skirt end of this valved conduit was sutured to the endocardium of the posterior wall of the left atrium, from the lateral to the medial fibrous trigones. The patient’s endocardial tissue is quite fragile, this suturing technique may lead to perivalvular leakage or even avulsion of the valved conduit. Nevertheless, periprosthetic leakage was not detected in our group at one, two, and three years postoperatively.
The intraoperative operation of the chimney technique requires attention as follows. Firstly, there is a possibility of the valved conduit graft kinking in the left atrium due to the graft remaining free in the left atrium. The mechanical valved conduit graft height should be tailored to 5 mm, which should be sufficient to allow free movement of leaflets and to avoid kinking. But, the Bioprosthetic valved conduit graft height should be tailored to 6 to 10 mm with the one side left slightly longer because the bioprosthetic valve has the valved stent (Supplemental
Figure S2). The 6 to 10 mm sleeve of the prosthetic valve rarely bends due to using a rigid Dacron graft. Secondly, there is a possibility that vortexing occurs locally in the mitral valve, which can lead to changes in local hemodynamics. By inclining the composite graft during trimming, so that the anterior side is higher than the posterior side, we believe that this problem can be avoided [
13].
The limitation of this study is that it is a small series of highly selected patients for whom the use of the chimney technique in redo-MVR was considered to be a more feasible treatment option than alternatives such as annular enlargement and extensive annular debridement. Although there has been short to medium-term follow-up, there is no long-term follow-up of these patients, and exercise test results are not available for these patients. However, the improvement in clinical symptoms in the short and medium term suggests that these patients were able to benefit from this treatment in the short and medium term.
5. Conclusions
These results suggest that use of the chimney technology in redo-MVR in the small mitral annulus could be an additional option for replacement of small mitral valves or annular enlargement. While avoiding the technical difficulties and risks associated with other alternative approaches, this approach has produced excellent short and medium term results in this limited series. Further experience with multiple institutions and large cohorts of patients should show if there are any relative contraindications to this approach or long-term complications.
6. Limitations
The limitation of this study is that it is a small series of highly selected patients for whom the use of the chimney technique in redo-MVR was considered to be a more feasible treatment option than alternatives such as annular enlargement and extensive annular debridement. Although there has been short to medium-term follow-up, there is no long-term follow-up of these patients, and exercise test results are not available for these patients. However, the improvement in clinical symptoms in the short and medium term suggests that these patients were able to benefit from this treatment in the short and medium term.
Supplementary Materials
Supplemental Video S1; Supplemental Figure S1; Supplemental Figure S2.
Author Contributions
MY conceived and designed the study. MY and LC performed statistical design and analysis. LC and YX acquired the data. MY drafted the manuscript. LT handled funding and supervision. LT, LC, MY and YX made critical revision of the manuscript for key intellectual content. All authors contributed to the article and approved the submitted version.
Funding
There is no separate funding support in this study.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
Throughout the writing of this dissertation I have received a great deal of support and assistance. I would first like to thank my supervisor, Liang Tao, whose expertise was invaluable in formulating the research questions and methodology. Your insightful feedback pushed me to sharpen my thinking and brought my work to a higher level. I would particularly like to acknowledge my team members for their wonderful collaboration and patient support. I would also like to thank study units, Wuhan Asian Heart Hospital.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Urena, M.; Vahanian, A.; Brochet, E.; Ducrocq, G.; Iung, B.; Himbert, D. Current Indications for Transcatheter Mitral Valve Replacement Using Transcatheter Aortic Valves: Valve-in-Valve, Valve-in-Ring, and Valve-in-Mitral Annulus Calcification. Circulation 2021, 143, 178–196. [Google Scholar] [CrossRef]
- Joury, A.; Duran, A.; Stewart, M.; Gilliland, Y.E.; Spindel, S.M.; Qamruddin, S. Prosthesis-patient mismatch following aortic and mitral valves replacement – A comprehensive review. Prog. Cardiovasc. Dis. 2022, 72, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Redondo, A.; Lopez-Menendez, J.; Varela, L.; Muñoz, R.; Rodriguez-Roda, J. Overcoming a Surgical Challenge: Inverted Aortic Prosthetic Valves in Small Mitral Annulus. Ann. Thorac. Surg. 2018, 106, 637–638. [Google Scholar] [CrossRef] [PubMed]
- Cammack, P.L.; Edie, R.N.; Edmunds, L.H. Bar calcification of the mitral anulus. A risk factor in mitral valve operations. J Thorac Cardiovasc Surg 1987, 94, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Barac, Y.D.; Zwischenberger, B.; Schroder, J.N.; Daneshmand, M.A.; Haney, J.C.; Gaca, J.G.; Wang, A.; Milano, C.A.; Glower, D.D. Using a Regent Aortic Valve in a Small Annulus Mitral Position Is a Viable Option. Ann. Thorac. Surg. 2018, 105, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- David, T.E.; Feindel, C.M.; Armstrong, S.; Sun, Z. Reconstruction of the mitral anulus: A ten-year experience. J. Thorac. Cardiovasc. Surg. 1995, 110, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Atoui, R.; Lash, V.; Mohammadi, S.; Cecere, R. Intra-atrial implantation of a mitral valve prosthesis in a heavily calcified mitral annulus☆. Eur. J. Cardio-Thoracic Surg. 2009, 36, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Polomsky, M.; Koulogiannis, K.P.; Kipperman, R.M.; Cohen, B.M.; Magovern, C.J.; Slater, J.P.; Xydas, S.; Marcoff, L.; Brown, J.M. Mitral Valve Replacement With Sapien 3 Transcatheter Valve in Severe Mitral Annular Calcification. Ann. Thorac. Surg. 2017, 103, e57–e59. [Google Scholar] [CrossRef]
- Roberts, W.C.; Morrow, A.G. Causes of early postoperative death following cardiac valve replacement. J. Thorac. Cardiovasc. Surg. 1967, 54, 422–437. [Google Scholar] [CrossRef]
- Abramowitz, Y.; Jilaihawi, H.; Chakravarty, T.; Mack, M.J.; Makkar, R.R. Mitral Annulus Calcification. J Am Coll Cardiol. 2015, 66, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- González Rocafort, Á.; Aroca, Á.; Polo, L.; Rey, J.; Villagrá, F. Chimney technique for mitral valve replacement in children. Ann Thorac Surg. 2013, 96, 1885–1887. [Google Scholar] [CrossRef]
- Song, L.; He, G.; Imin, E.; Tao, C.; Xu, M.; Wang, B.; Li, X.; Tao, L. ''Chimney'' Bentall Procedure in the Small Aortic Root After Prior Aortic Valve Operations. Ann Thorac Surg. 2020, 110, e241–e243. [Google Scholar] [CrossRef]
- Go, S.; Furukawa, T.; Yamada, K.; Hiraoka, T.; Mochizuki, S. A case of supra-annular mitral valve replacement using chimney technique for severe mitral stenosis with extensive mitral annular calcification. Gen. Thorac. Cardiovasc. Surg. 2019, 68, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Tan, T.; Li, X.; Li, J.; Zhang, Z.; Yuan, H. Mitral Valve Replacement Adopting Chimney Technique in Mitral Insufficiency And Extensive Mitral Annular Calcification: A Case Report. Hear. Surg. Forum 2022, 25, E718–E720. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).