1. Introduction
Polyethylene terephthalate (PET) is a linear polymer formed from the condensation of terephthalic acid and ethylene glycol, which has the advantages of high cost performance, creep resistance, friction resistance, fatigue resistance and other advantages [
1]. The global consumption of polyester still maintains a growth rate of about 5%, and China has become the world's largest consumer of plastics and plastic products, plastics industry is an important pillar of the national economy. Most PET packaging materials are disposable and are discarded as garbage after one use [
2,
3,
4]. The good thermal stability of PET makes it difficult to be degraded by microorganisms, resulting in more and more serious white pollution. Therefore, the recycling and utilization of PET polyester has become one of the hot issues to solve environmental pollution and the recycling and utilization of polymer materials [
5,
6,
7,
8].
In general, in addition to being directly buried in landfills, PET waste can be recycled through direct combustion, physical recovery, chemical recovery and other ways [
9,
10]. Direct combustion is simple and easy, low cost, is the most extensive recovery method at present, but PET will emit a lot of greenhouse gas CO2 while recycling energy by incineration, which is not in line with the current dual-reduction policy, and indirectly pollute the environment. Physical recycling refers to the separation, cleaning, crushing and other pretreatment of waste PET to remove labels, dust and other pollutants, and then heating and melting to create particles, and finally processing into new PET products. The recycled particles recovered by physical method are not suitable for manufacturing high-grade plastic products, and the mechanical properties and thermal stability of recycled plastics are greatly reduced compared with raw materials, and the application range of recycled plastics is narrow, so it is subject to certain restrictions [
11,
12]. At present, the chemical recovery methods of PET include hydrolysis method, glycol alcoholysis method, methanol alcoholysis method, ammonia hydrolysis method, etc [
13,
14]. Among them, hydrolysis is a very widely used method to recover PET [
15,
16]. Using the hydrolysis of the ester group, PET can be hydrolyzed and depolymerized under alkaline, acidic or neutral conditions to produce terephthalate or acid (TPA) and ethylene glycol (EG), TPA and EG are just the common raw materials for the production of primary PET, so as to achieve the chemical recycling of PET [
17,
18,
19]. However, the defects of chemical monomer recovery at present are harsh reaction conditions, low product yield, and difficult separation and purification of the product.
In this paper, waste PET was used as raw material, after swelling with ethanol, hydrolysis reaction was carried out with acetic acid at 80 oC for 3 h, and the degradation product was used as a hydrophobic modification agent to modify the stainless steel mesh, and the structure and oil-water separation performance of the modified stainless steel mesh were tested. At the same time, the hydrolysis reactions carried out in this paper do not add any catalyst, the purpose is not only to protect the environment and save costs, but also to reduce the reaction conditions and reduce the energy consumption of the whole process. The degradation products as hydrophobic modifiers enhance the recovery value of PET.
2. Experiment
2.1. Materials
Ethanol (C2H5OH, analytically pure), purchased from Chengdu Colon Chemical Co., LTD., China; Acetic acid (C2H4O2, analytically pure), produced in Shanghai Titan Technology Co., LTD. Stainless Steel mesh (SSM) was purchased from Zilianzhong (Guangzhou) Stainless Steel Co., LTD. PET waste (Coca-Cola Company). Deionized water (DI) self-made. PET waste is Coca-Cola waste bottles.
2.2. PET pretreatment
The PET waste was washed with detergent, hot water and ethanol first, and then put it in the drying box to dry, and then cut it into pieces after drying, and crush it into 2.5*2.5 mm pieces with a grinder.
2.3. PET swelling
4 g PET particles were weighed and put into a 100 mL three-mouth flask, then 30 mL anhydrous ethanol was added, after that, the three-mouth flask was placed in a magnetic stirrer and swelled at 70 oC for 5 h. After the reaction is completed, a suction filter bottle was used for filtration. The weight of S-PET after swelling is 8.6 g, and the swelling rate is 115%.
2.4. PET degradation
The swollen S-PET tablets and acetic acid were added to a 50 mL three-mouth flask and reacted in a magnetic stirrer. The reaction temperature is set at 80 oC and the reaction time is 3 h. After the reaction is complete, the reaction mixture is transparent and the reaction vessel is cooled to room temperature.
2.5. Pre-processing of SSM
SSM was put into 4 mol/L aqueous solution of HNO3 to remove the surface oxide, and then heated in a water bath at 60 oC for 4 h. Then wash with anhydrous ethanol for 3 min, dry and set aside.
2.6. SSM modification
The modified SSM was obtained by immersing the pre-treated SSM in PET degradation solution, ultrasonic treatment for 20 min, natural drying, and then drying in 60 oC oven for 24 h.
2.7. Characterization of modified SSM
The surface morphology of the sample was observed and analyzed by SEM of Hitachi su8020, the acceleration voltage was 15 kV, and the components of the sample surface were analyzed by X-ray energy dispersion spectrometer (EDX). Before observation, the sample was fixed on the sample table with conductive adhesive and treated with gold spraying. Contact Angle (CA) was measured by JC2000D2H contact Angle tester manufactured by Shanghai Zhongchen Technical Equipment Co., LTD. The surface roughness of the sample was determined by LEXT OLS4100 laser scanning confocal microscope of Shanghai Fulai Optical Technology Co., LTD. The resin was placed on the sample table at 25 oC and the contact Angle of water and chloroform was determined separately. The amounts of water and chloroform were both 3 μL, and the average values of 3 different monitoring points on the surface of the sample were obtained.
3. Results and analysis
3.1. Surface morphology analysis of modified SSM
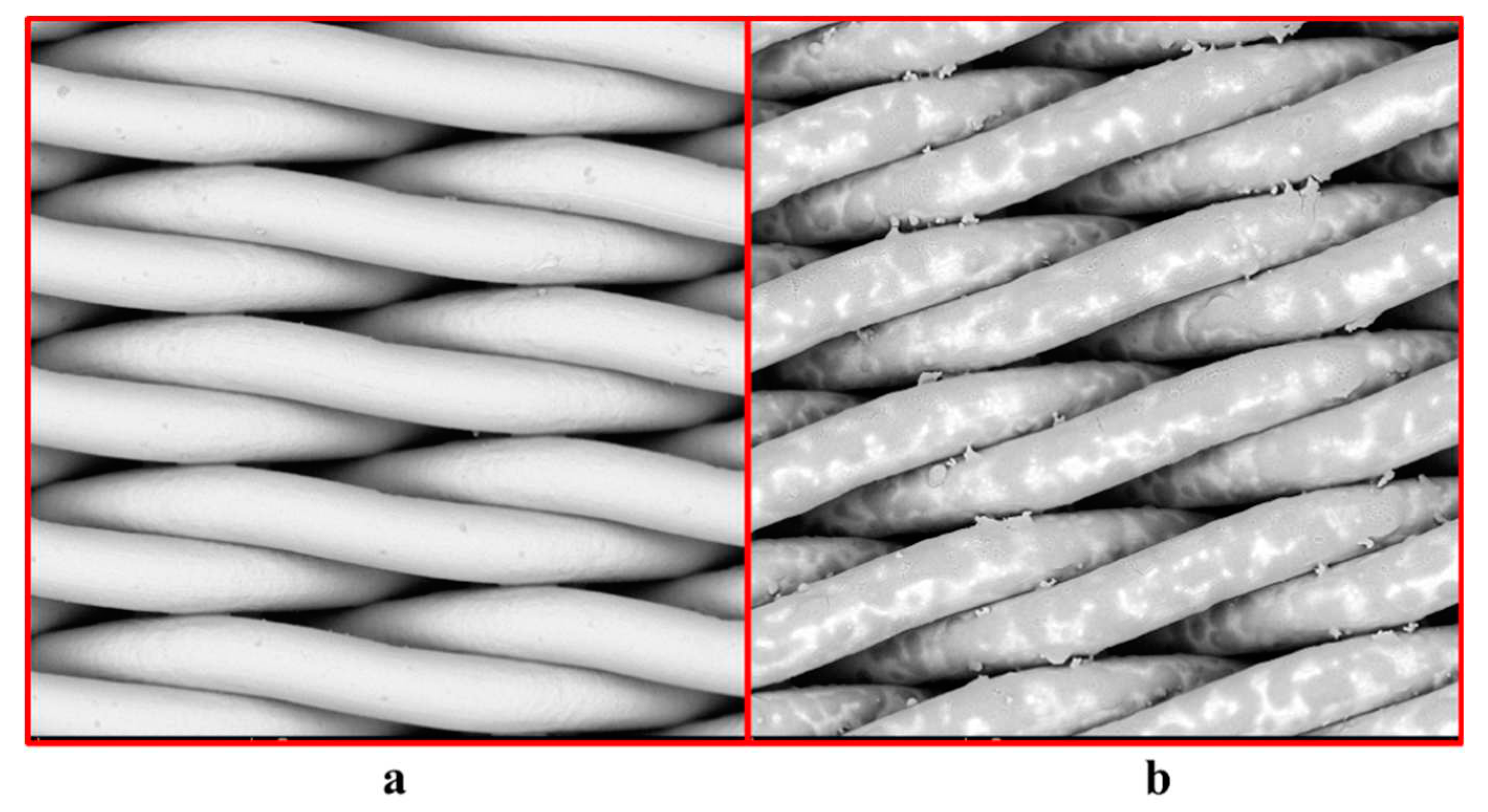
The effect of product modification on SSM surface morphology was observed by SEM, and the experimental results were shown in
Figure 1. The surface of the unmodified SSM is smooth and the pore size is about 0.8 μm. After soaking in the solution, the product adheres to the screen of SSM, making the surface from smooth to rough, and the product "grows" along the stainless steel mesh.
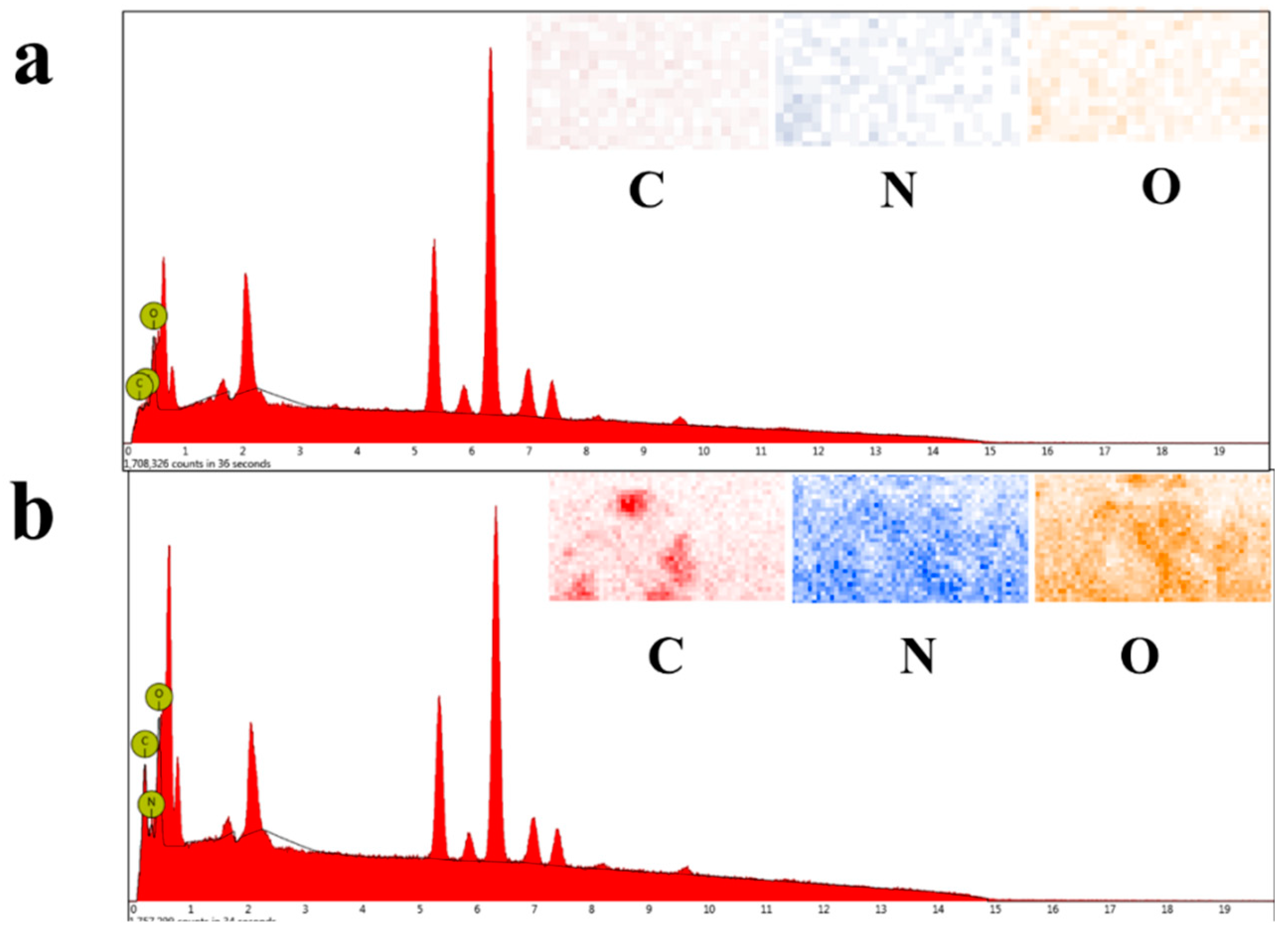
In order to test the load and distribution of the product on the surface of SSM, EDX was used to test the element content on the surface of the sample. The mapping color of C, N and O in the original SSM was very light. Due to the existence of a very small amount of organic impurities on the surface of SSM, the content of C and N elements were 2.07% and 8.90% respectively. When SSM was soaked in the product, the color of C, N and O was deepened due to the load of the product. The EDX results showed that the content of C, N and O elements on the modified SSM surface increased, and the content of C and N elements on the modified SSM surface increased to 31.95% and 15.17%, respectively.
Figure 2.
The EDX and mapping of (a) SSM and (b) modified SSM.
Figure 2.
The EDX and mapping of (a) SSM and (b) modified SSM.
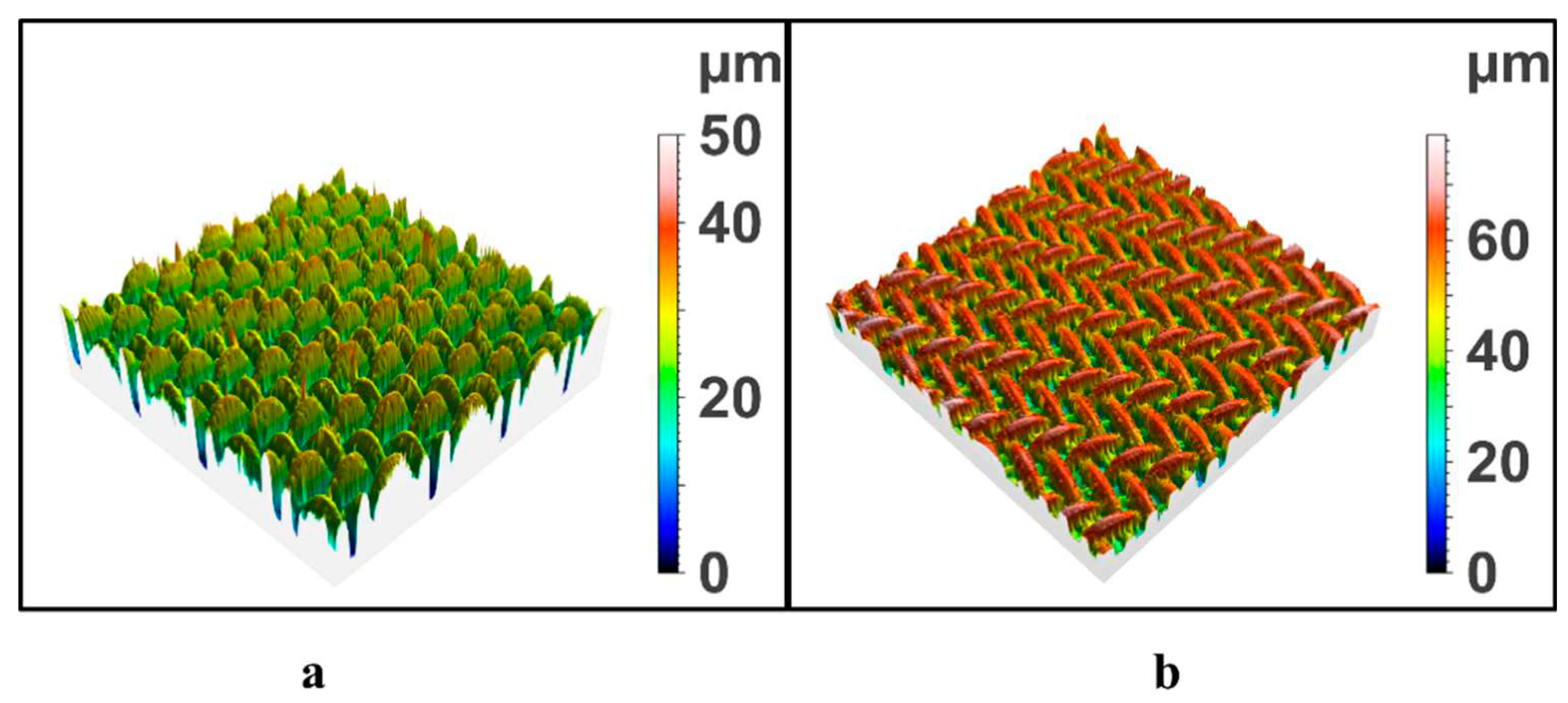
The surface roughness of modified SSM was analyzed and measured by confocal laser scanning microscope, and the influence of the load of degradation products on the surface roughness of SSM was studied. The results are shown in
Figure 3. The surface of the initial SSM was smooth with a roughness of 19.09 μm, which was consistent with the SEM observation results. After the modification of low concentration DEP, the coarse excess is slightly increased. After modified by PET degradation products, the roughness of SSM increased significantly to 62.33 μm.
3.2. Surface wettability analysis of modified SSM
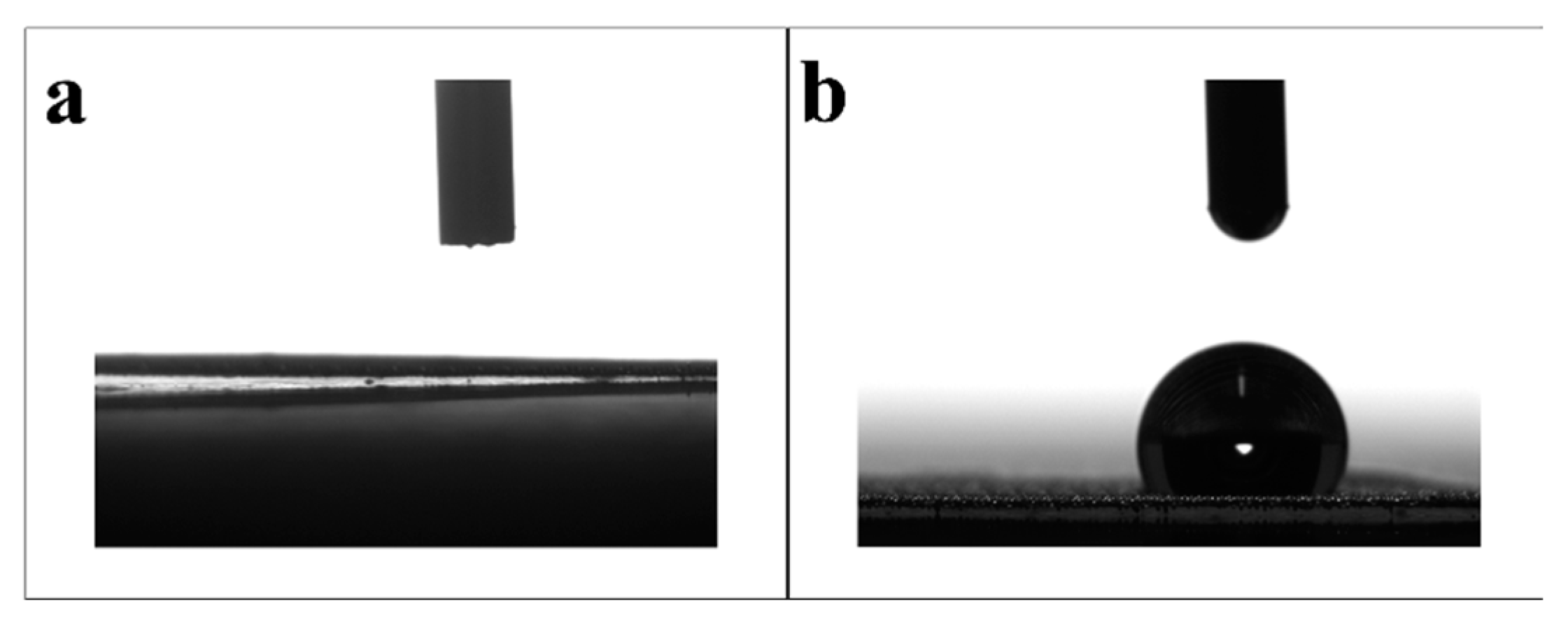
Wettability is an important index of oil-water separation materials, which can affect the oil-water separation effect of materials. Therefore, the water contact Angle (WCA) of PET degradation products before and after SSM modification was measured, and the experimental results are shown in
Figure 4. After pretreatment, the surface of SSM is hydrophilic, and when the degradation products are loaded, SSM becomes hydrophobic, and the water contact angle is 123
o. This is because the degradation products contain a large number of hydrophobic groups.
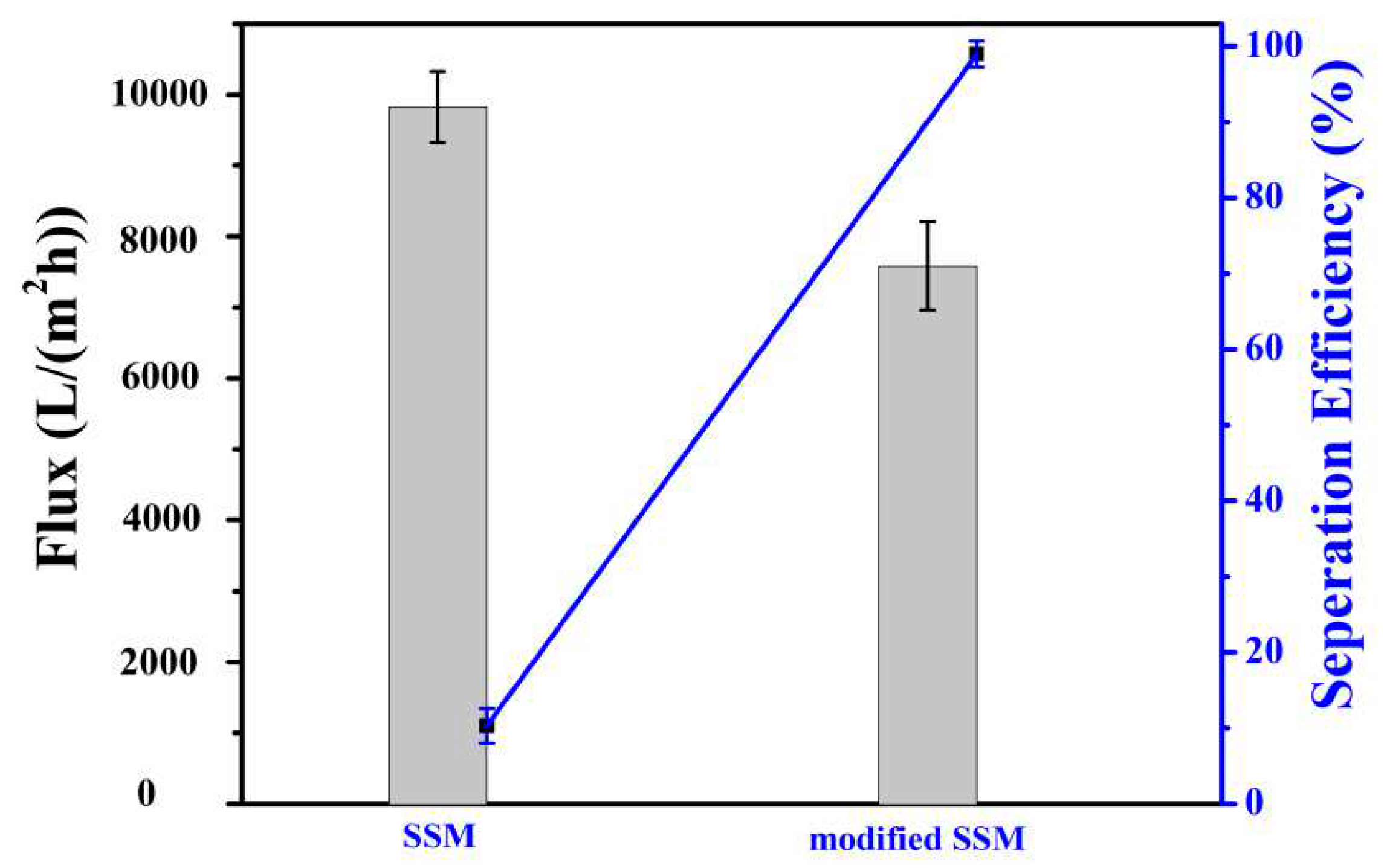
3.3. Analysis of oil-water separation efficiency of modified SSM
The experimental results of using modified materials to test oil-water separation performance are shown in
Figure 5. The separation efficiency of the original SSM was only 10.3%. Under the action of its own gravity, the separation efficiency of the modified SSM reached 98.99% and the flux reached 9825 L/(m
2·h). SSM was modified by PET degradation products to achieve a good separation effect of oil and water, and at the same time, waste PET was degraded and recycled with high value.
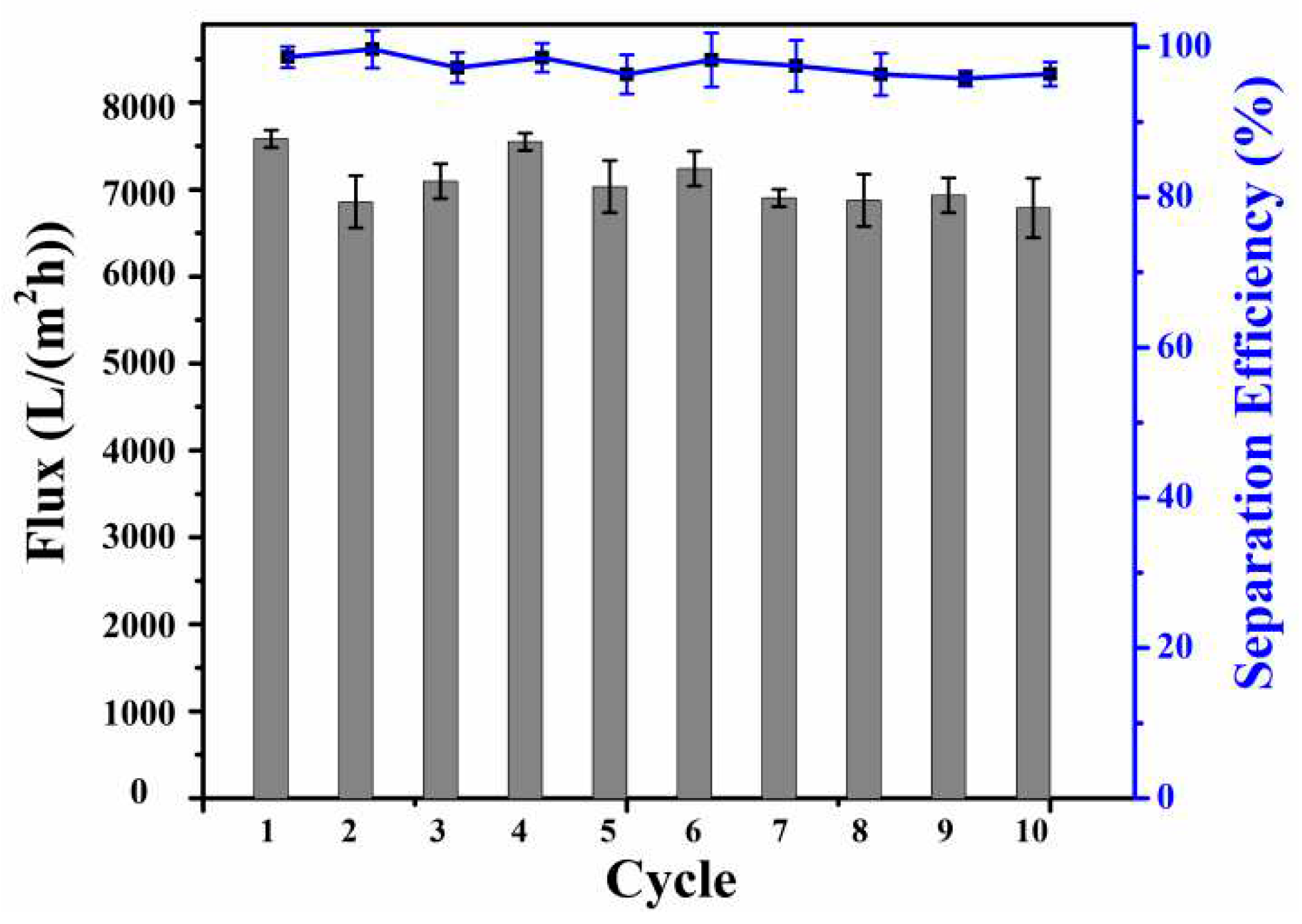
In order to verify the reusability of the modified SSM, the oil-water separation cycle experiment was carried out on the modified SSM, and the experimental results were shown in
Figure 6. After 10 cycles, the separation efficiency of modified SSM for emulsion remained between 95.7%-99.6%, and the flux remained above 7000 L/(m
2·h). The test results show that the modified SSM has good recycling performance.
4. Conclusion
In this experiment, PET was pretreated with ethanol for swelling, and then the waste plastic was completely degraded in acetic acid solution at 80 oC for 3 h. Hydrophobicity modification of porous substrates by degradation products was used to prepare oil-water separation materials. After the product was loaded on SSM, the surface roughness and water contact Angle were increased, the separation flux of oil-water mixture reached 9825 L/(m2·h), and the separation efficiency reached 98.99%, and the separation efficiency did not decrease significantly after 10 times of repeated use. It not only solves the problem of waste thermosetting plastics, but also provides new raw materials for the preparation of oil-water separation materials and saves petrochemical resources.
Acknowledgments
We gratefully acknowledge the financial support by 2023 Jiangsu Degree and Postgraduate Education Teaching Reform project (JGKT23_C085), 2023 Jiangsu Graduate Research and Practice Innovation plan (KYCX23_3469), the Funding for School-level Research projects of Yancheng Institute of Technology (No. xjr2019007 and No. xjr2023031), 2023 Excellent Graduation Project (Thesis) Cultivation project of Yancheng Institute of Technology and also thank the testing service provided by Analysis and Testing Center in Yancheng Institute of Technology.
References
- Kratish, Y.; Li, J.; Liu, S.; Gao, Y.; Marks, T.J. Polyethylene terephthalate deconstruction catalyzed by a carbon-supported single-site nolybdenum-dioxo complex. Angew. Chem. Int. Ed. 2020, 59, 19857–19861. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, R.; Chen, Y.; Wang, J.; Song, H. New effective catalysts for glycolysis of polyethylene terephthalate waste: Tropine and tropine-zinc acetate complex. J. Mol. Liq. 2021, 334, 116419. [Google Scholar] [CrossRef]
- Scé, F.; Cano, I.; Martin, C.; Beobide, G.; Castillo. ; de Pedro, I. Comparing conventional and microwave-assisted heating in PET degradation mediated by imidazolium-based halometallate complexes. New J. Chem. 2019, 43, 3476–3485. [Google Scholar] [CrossRef]
- Eshaq, G.; ElMetwally, A. (Mg–Zn)–Al layered double hydroxide as a regenerable catalyst for the catalytic glycolysis of polyethylene terephthalate. J. Mol. Liq. 2015, 214, 1–6. [Google Scholar] [CrossRef]
- Zhang, F.; Zeng, M.; Yappert, R.D.; Sun, J.; Lee, Y.-H.; LaPointe, A.M.; Peters, B.; Abu-Omar, M.M.; Scott, S.L. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 2020, 370, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Lindqvist, K.; de la Motte, H. An efficient recycling process of glycolysis of PET in the presence of a sustainable nanocatalyst. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Tawfik, M.E.; Eskander, S.B. Chemical recycling of poly(ethylene terephthalate) waste using ethanolamine. Sorting of the end products. Polym. Degrad. Stab. 2010, 95, 187–194. [Google Scholar] [CrossRef]
- Bäckström, E.; Odelius, K.; Hakkarainen, M. Ultrafast microwave assisted recycling of PET to a family of functional precursors and materials. Eur. Polym. J. 2021, 151. [Google Scholar] [CrossRef]
- Wang, L.; Xiang, D.; Zhu, W.; Zhao, C.; Li, Y.; Han, H.; Wang, P.; Wang, J. Flexible piezoresistive strain sensor with high sensitivity based on carbonised waste thermosetting resin. Plast. Rubber Compos. 2020, 49, 300–306. [Google Scholar] [CrossRef]
- Jiao, X.; Zheng, K.; Hu, Z.; Zhu, S.; Sun, Y.; Xie, Y. Conversion of Waste Plastics into Value-Added Carbonaceous Fuels under Mild Conditions. Adv. Mater. 2021, 33, 2005192. [Google Scholar] [CrossRef] [PubMed]
- Pingale, N.; Shukla, S. Microwave assisted ecofriendly recycling of poly (ethylene terephthalate) bottle waste. Eur. Polym. J. 2008, 44, 4151–4156. [Google Scholar] [CrossRef]
- Park, R.; Sridhar, V.; Park, H. Taguchi method for optimization of reaction conditions in microwave glycolysis of waste PET. J. Mater. Cycles Waste Manag. 2019, 22, 664–672. [Google Scholar] [CrossRef]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.L.; Texier, H.; Gavalda, S.; Cot, M.; Guémard, E.; Dalibey, M.; Nomme, J.; Cioci, G.; Barbe, S.; Chateau, M.; André, I.; Duquesne, S.; Marty, A. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Breite, D.; Song, C.; Gräsing, D.; Ploss, T.; Hille, P.; Schwerdtfeger, R.; Matysik, J.; Schulze, A.; Zimmermann, W. Biocatalytic Degradation Efficiency of Postconsumer Polyethylene Terephthalate Packaging Determined by Their Polymer Microstructures. Adv. Sci. 2019, 6, 1900491. [Google Scholar] [CrossRef] [PubMed]
- Al Mahmood, A.; Hossain, R.; Bhattacharyya, S.; Sahajwalla, V. Recycling of polymer laminated aluminum packaging (PLAP) materials into carbonaceous metallic microparticles. J. Clean. Prod. 2020, 269, 122157. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Duque-Ingunza, I.; de Rivas, B.; Flores-Giraldo, L.; Gutiérrez-Ortiz, J.I. Kinetics of catalytic glycolysis of PET wastes with sodium carbonate. Chem. Eng. J. 2011, 168, 312–320. [Google Scholar] [CrossRef]
- Martinez, P.L.; Zapata, T.T.; Sanchez, N.M. Directing depolymerization of PET with subcritical and supercritical ethanol to different monomers through changes in operation conditions. ACS Sustain. Chem. Eng. 2021, 9, 9846–9853. [Google Scholar] [CrossRef]
- Merkel, D.R.; Kuang, W.; Malhotra, D.; Petrossian, G.; Zhong, L.; Simmons, K.L.; Zhang, J.; Cosimbescu, L. Waste PET Chemical Processing to Terephthalic Amides and Their Effect on Asphalt Performance. ACS Sustain. Chem. Eng. 2020, 8, 5615–5625. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Kirlikovali, K.O.; Gong, X.; Atilgan, A.; Ma, K.; Schweitzer, N.M.; Gianneschi, N.C.; Li, Z.; Zhang, X.; et al. Catalytic Degradation of Polyethylene Terephthalate Using a Phase-Transitional Zirconium-Based Metal–Organic Framework. Angew. Chem. Int. Ed. 2022, 61, e202117528. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).