Submitted:
13 October 2023
Posted:
17 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Molecular basis of cardiometabolic calpain isozymes and calpastatin
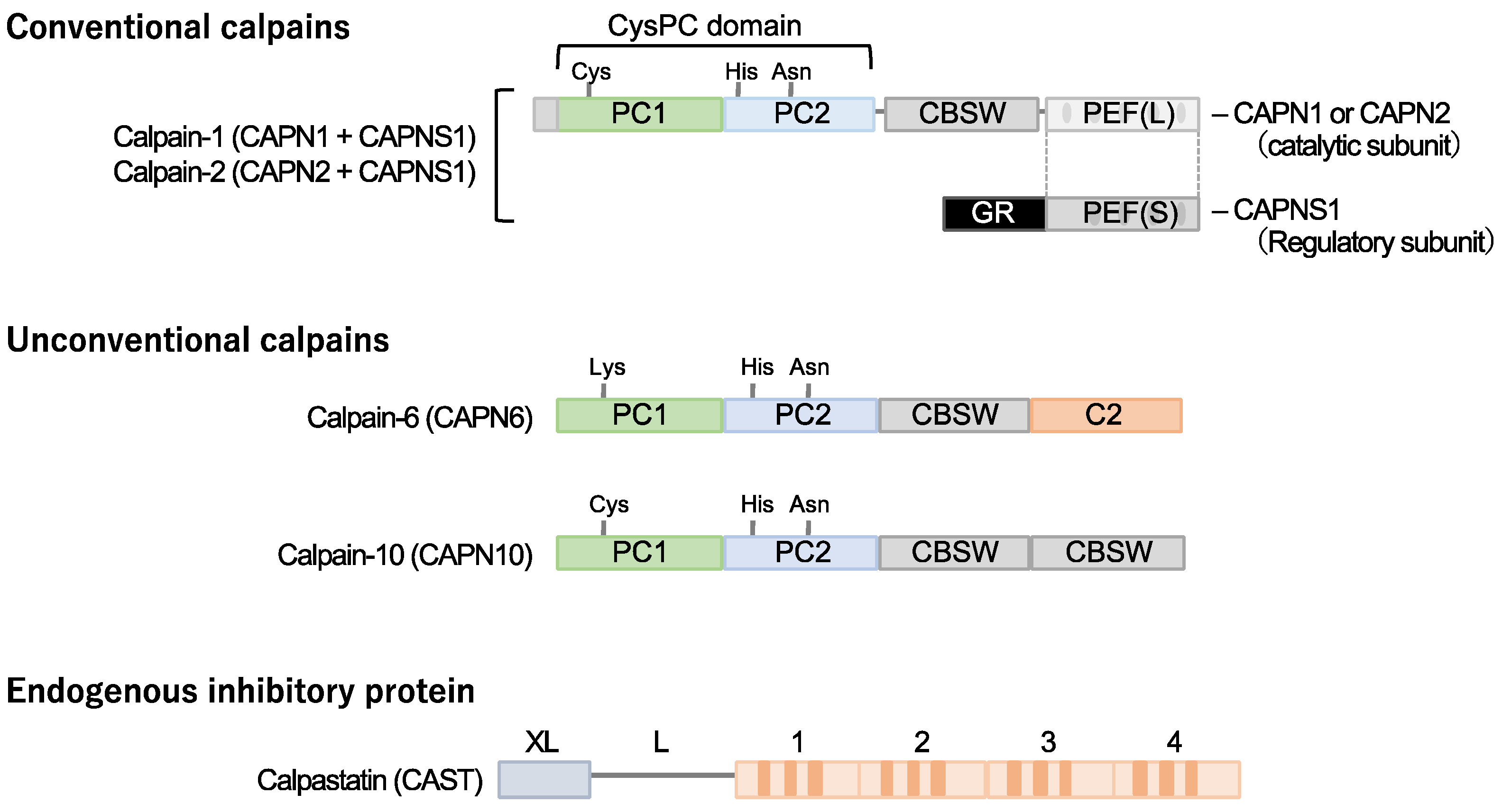
2.1. Conventional calpains and calpastatin
2.2. Calpain-10
2.3. Calpain-6
3. Calpain and atherosclerosis
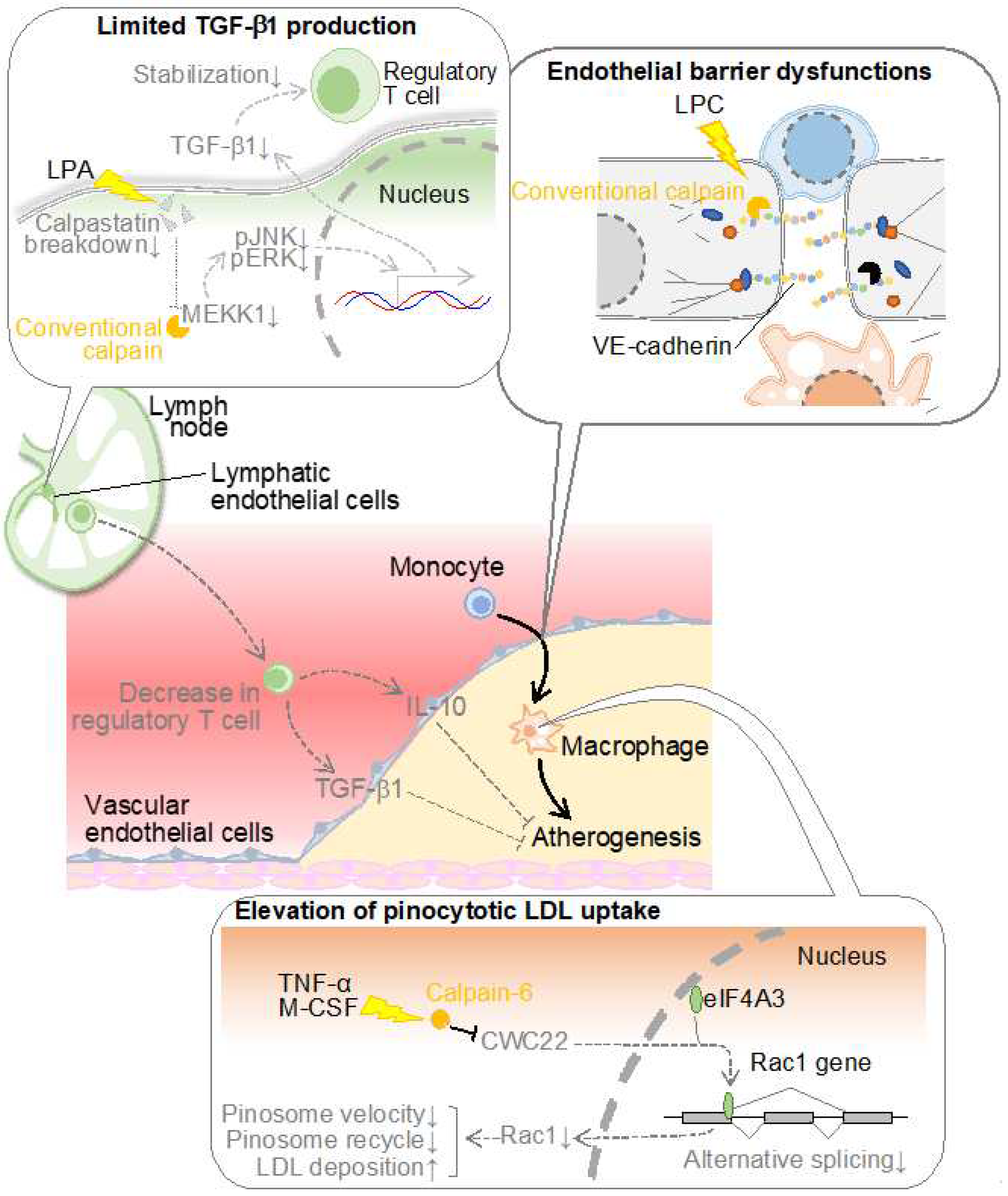
3.1. conventional calpain in vascular endothelial cells
3.2. conventional calpain in lymphatic endothelial cells
3.3. Calpain-6 in macrophages
4. Calpain and diabetes
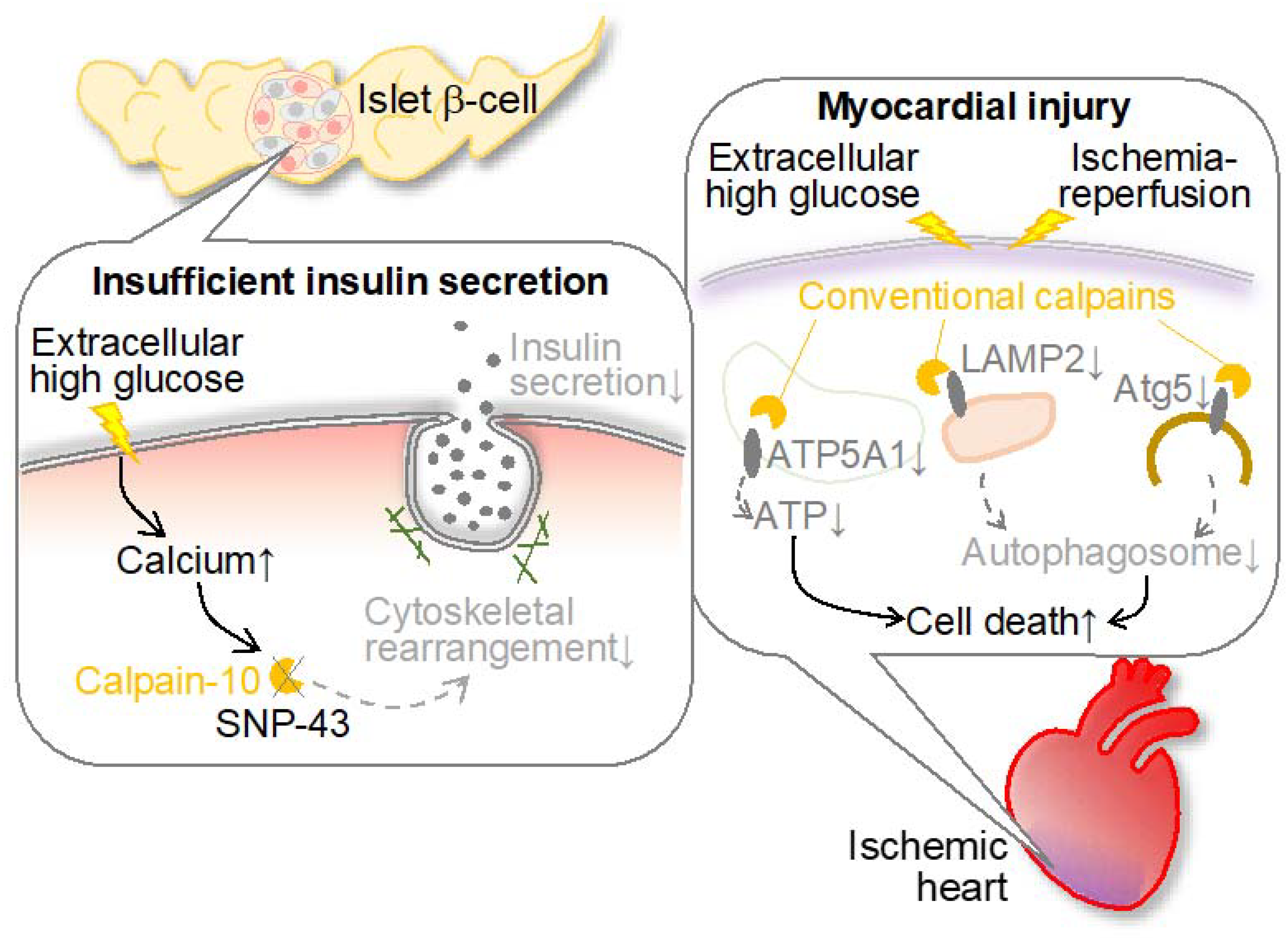
4.1. Calpain-10 and diabetes
4.2. Calpain and diabetic cardiomyopathy
5. Calpain and liver disease
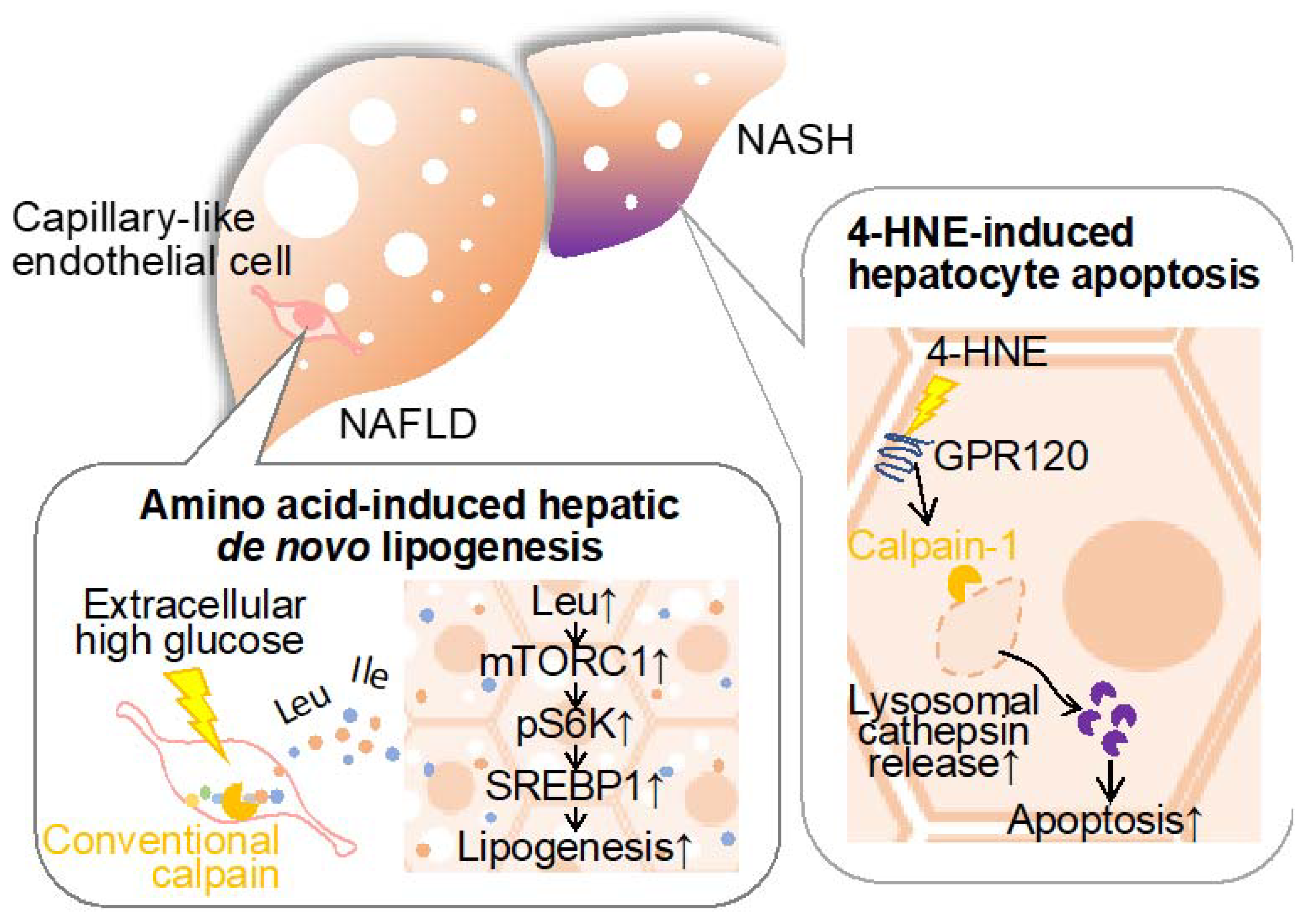
5.1. Conventional calpains and amino acid metabolism in the liver
5.2. Conventional calpains and hepatocellular insults
5.3. Conventional calpains and hepatic ischemic insults
6. Calpain and obesity
7. Conclusions and future perspective
Author Contributions
Funding
References
- Sattar, N.; Gill, J.M.R.; Alazawi, W. Improving prevention strategies for cardiometabolic disease. Nat. Med. 2020, 26, 320–325. [Google Scholar] [CrossRef]
- O’sullivan, J.W.; A Ashley, E.; Elliott, P.M. Polygenic risk scores for the prediction of cardiometabolic disease. Eur. Hear. J. 2022, 44, 89–99. [Google Scholar] [CrossRef]
- Ogata, H.; Takeshima, A.; Ito, H. An update on phosphate binders for the treatment of hyperphosphatemia in chronic kidney disease patients on dialysis: a review of safety profiles. Expert Opin. Drug Saf. 2022, 21, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Amann, K.; Bangalore, S.; Cavalcante, J.L.; Charytan, D.M.; Craig, J.C.; Gill, J.S.; Hlatky, M.A.; Jardine, A.G.; Landmesser, U.; Newby, L.K.; Herzog, C.A.; Cheung, M.; Wheeler, D.C.; Winkelmayer, W.C.; Marwick, T.H. ; Conference Participants. Chronic kidney disease and coronary artery disease: jacc state-of-the-art review. J. Am. Coll. Cardiol. 2019, 74, 1823–1838. [Google Scholar] [PubMed]
- Muzurović, E.; Peng, C.C.-H.; Belanger, M.J.; Sanoudou, D.; Mikhailidis, D.P.; Mantzoros, C.S. Nonalcoholic Fatty Liver Disease and Cardiovascular Disease: a Review of Shared Cardiometabolic Risk Factors. Hypertension 2022, 79, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Gastaldelli, A.; Foschi, F.G. Fatty liver, cardiometabolic disease, and mortality. Curr. Opin. Lipidol. 2021, 31, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.O.; Budoff, M. Effect of statins on atherosclerotic plaque. Trends Cardiovasc. Med. 2019, 29, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K.; P, P.; Y, L.; S, M.; C, H.; S, K.; V, T.; S, K.; et al. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]
- Targher, G.; Mantovani, A.; Byrne, C.D. Mechanisms and possible hepatoprotective effects of glucagon-like peptide-1 receptor agonists and other incretin receptor agonists in non-alcoholic fatty liver disease. Lancet Gastroenterol. Hepatol. 2023, 8, 179–191. [Google Scholar] [CrossRef]
- Brunner, K.T.; Henneberg, C.J.; Wilechansky, R.M.; Long, M.T. Nonalcoholic Fatty Liver Disease and Obesity Treatment. Curr. Obes. Rep. 2019, 8, 220–228. [Google Scholar] [CrossRef]
- Marton, A.; Kaneko, T.; Kovalik, J.-P.; Yasui, A.; Nishiyama, A.; Kitada, K.; Titze, J. Organ protection by SGLT2 inhibitors: role of metabolic energy and water conservation. Nat. Rev. Nephrol. 2020, 17, 65–77. [Google Scholar] [CrossRef]
- Brown, E.; Heerspink, H.J.L.; Cuthbertson, D.J.; Wilding, J.P.H. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet 2021, 398, 262–276. [Google Scholar] [CrossRef]
- Miyazaki, T.; Miyazaki, A. Emerging roles of calpain proteolytic systems in macrophage cholesterol handling. Cell. Mol. Life Sci. 2017, 74, 3011–3021. [Google Scholar] [CrossRef]
- Miyazaki, T.; Akasu, R.; Miyazaki, A. Calpain-Associated Proteolytic Regulation of the Stromal Microenvironment in Cancer. Curr. Pharm. Des. 2021, 27, 3128–3138. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Miyazaki, A. Impact of Dysfunctional Protein Catabolism on Macrophage Cholesterol Handling. Curr. Med. Chem. 2019, 26, 1631–1643. [Google Scholar] [CrossRef]
- Miyazaki, T.; Miyazaki, A. Defective Protein Catabolism in Atherosclerotic Vascular Inflammation. Front. Cardiovasc. Med. 2017, 4, 79. [Google Scholar] [CrossRef]
- Miyazaki, T.; Akasu, R.; Miyazaki, A. Calpain proteolytic systems counteract endothelial cell adaptation to inflammatory environments. Inflamm. Regen. 2020, 40, 1–8. [Google Scholar] [CrossRef]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The calpain system. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Sorimachi, H. Calpains: an elaborate proteolytic system. Biochim. Biophys. Acta. 2012, 1824, 224–236. [Google Scholar] [CrossRef]
- Ono, Y.; Saido, T.C.; Sorimachi, H. Calpain research for drug discovery: challenges and potential. Nat. Rev. Drug Discov. 2016, 15, 854–876. [Google Scholar] [CrossRef]
- Miyazaki, T.; Miyazaki, A. Dysregulation of Calpain Proteolytic Systems Underlies Degenerative Vascular Disorders. J. Atheroscler. Thromb. 2018, 25, 1–15. [Google Scholar] [CrossRef]
- Shinkai-Ouchi, F.; Koyama, S.; Ono, Y.; Hata, S.; Ojima, K.; Shindo, M.; Duverle, D.; Ueno, M.; Kitamura, F.; Doi, N.; et al. Predictions of Cleavability of Calpain Proteolysis by Quantitative Structure-Activity Relationship Analysis Using Newly Determined Cleavage Sites and Catalytic Efficiencies of an Oligopeptide Array. Mol. Cell. Proteom. 2016, 15, 1262–1280. [Google Scholar] [CrossRef]
- Miyazaki, T.; Taketomi, Y.; Saito, Y.; Hosono, T.; Lei, X.-F.; Kim-Kaneyama, J.-R.; Arata, S.; Takahashi, H.; Murakami, M.; Miyazaki, A. Calpastatin Counteracts Pathological Angiogenesis by Inhibiting Suppressor of Cytokine Signaling 3 Degradation in Vascular Endothelial Cells. Circ. Res. 2015, 116, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Barefield, D.Y.; McNamara, J.W. ; Lynch, TL; Kuster, D. W.D.; Govindan, S.; Haar L; Wang Y; Taylor EN; Lorenz JN; Nieman ML; Zhu G; Luther PK; Varró A; Dobrev D; Ai X; Janssen PML; Kass DA; Jones WK; Gilbert RJ; Sadayappan S. Ablation of the calpain-targeted site in cardiac myosin binding protein-c is cardioprotective during ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2019, 129, 236–246. [Google Scholar]
- Maki, M.; Takano, E.; Mori, H.; Sato, A.; Murachi, T.; Hatanaka, M. All four internally repetitive domains of pig calpastatin possess inhibitory activities against calpains I and II. FEBS Lett. 1987, 223, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Takano, E.; Murachi, T. Purification and Some Properties of Human Erythrocyte Calpastatin1. J. Biochem. 1982, 92, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Morales-Corraliza, J.; Berger, J.D.; Mazzella, M.J. ; Veeranna; Neubert, T. A.; Ghiso, J.; Rao, M.V.; Staufenbiel, M.; Nixon, R.A.; Mathews, P.M. Calpastatin modulates APP processing in the brains of β-amyloid depositing but not wild-type mice. Neurobiol. Aging. 2012, 33, 1125.e9–18. [Google Scholar]
- Sato, K.; Minegishi, S.; Takano, J.; Plattner, F.; Saito, T.; Asada, A.; Kawahara, H.; Iwata, N.; Saido, T.C.; Hisanaga, S.-I. Calpastatin, an endogenous calpain-inhibitor protein, regulates the cleavage of the Cdk5 activator p35 to p25. J. Neurochem. 2011, 117, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Iwata, N.; Matsuba, Y.; Takano, J.; Suemoto, T.; Maeda, J.; Ji, B.; Ono, M.; Staufenbiel, M.; Suhara, T.; et al. Mechanistic involvement of the calpain-calpastatin system in Alzheimer neuropathology. FASEB J. 2011, 26, 1204–1217. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.J.; Kloock, S.J.; Nagel, M.; Ortiz-Rios, M.M.; Hofmann, J.; Riess, O.; Nguyen, H.P. Calpastatin ablation aggravates the molecular phenotype in cell and animal models of Huntington disease. Neuropharmacology 2018, 133, 94–106. [Google Scholar] [CrossRef]
- Ling, C.; Groop, L.; Del Guerra, S.; Lupi, R. Calpain-10 Expression Is Elevated in Pancreatic Islets from Patients with Type 2 Diabetes. PLOS ONE 2009, 4, e6558. [Google Scholar] [CrossRef]
- Horikawa, Y.; Oda, N.; Cox, N.J.; Li, X.; Orho-Melander, M.; Hara, M.; Hinokio, Y.; Lindner, T.H.; Mashima, H.; Schwarz, P.E.; et al. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat. Genet. 2000, 26, 163–175. [Google Scholar] [CrossRef]
- Bayramci, N.S.; Açik, L.; Kalkan. ; Yetkin, I. Investigation of glucocorticoid receptor and calpain-10 gene polymorphisms in Turkish patients with type 2 diabetes mellitus. Turk. J. Med Sci. 2017, 47, 1568–1575. [Google Scholar] [CrossRef]
- Karambataki, M.; Malousi, A.; Tzimagiorgis, G.; Haitoglou, C.; Fragou, A.; Georgiou, E.; Papadopoulou, F.; Krassas, G.E.; Kouidou, S. Association of two synonymous splicing-associated CpG single nucleotide polymorphisms in calpain 10 and solute carrier family 2 member 2 with type 2 diabetes. Biomed. Rep. 2016, 6, 146–158. [Google Scholar] [CrossRef]
- Tonami, K.; Hata, S.; Ojima, K.; Ono, Y.; Kurihara, Y.; Amano, T.; Sato, T.; Kawamura, Y.; Kurihara, H.; Sorimachi, H. Calpain-6 Deficiency Promotes Skeletal Muscle Development and Regeneration. PLOS Genet. 2013, 9, e1003668. [Google Scholar] [CrossRef]
- Dear, N.; Matena, K.; Vingron, M.; Boehm, T. A New Subfamily of Vertebrate Calpains Lacking a Calmodulin-Like Domain: Implications for Calpain Regulation and Evolution. Genomics 1997, 45, 175–184. [Google Scholar] [CrossRef]
- Björkegren, J.L.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis is an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Yurdagul, A. Jr.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Malik, A.B. Signaling Mechanisms Regulating Endothelial Permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef]
- Bobryshev, Y.V.; Cherian, S.M.; Inder, S.J.; Lord, R.S. Neovascular expression of VE-cadherin in human atherosclerotic arteries and its relation to intimal inflammation. Cardiovasc. Res. 1999, 43, 1003–1017. [Google Scholar] [CrossRef]
- Foteinos, G.; Hu, Y.; Xiao, Q.; Metzler, B.; Xu, Q.; L, Z.; S, I.B.; T, C.; B, Z.; Q, X.; et al. Rapid Endothelial Turnover in Atherosclerosis-Prone Areas Coincides With Stem Cell Repair in Apolipoprotein E–Deficient Mice. Circ. 2008, 117, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Rios-Doria, J.; Day, K.C.; Kuefer, R.; Rashid, M.G.; Chinnaiyan, A.M.; Rubin, M.A.; Day, M.L. The Role of Calpain in the Proteolytic Cleavage of E-cadherin in Prostate and Mammary Epithelial Cells. J. Biol. Chem. 2003, 278, 1372–1379. [Google Scholar] [CrossRef]
- Jang, Y.-N.; Jung, Y.-S.; Lee, S.H.; Moon, C.-H.; Kim, C.-H.; Baik, E.J. Calpain-Mediated N-Cadherin Proteolytic Processing in Brain Injury. J. Neurosci. 2009, 29, 5974–5984. [Google Scholar] [CrossRef]
- Miyazaki, T.; Taketomi, Y.; Takimoto, M.; Lei, X.-F.; Arita, S.; Kim-Kaneyama, J.-R.; Arata, S.; Ohata, H.; Ota, H.; Murakami, M.; Miyazaki, A. m-calpain induction in vascular endothelial cells on human and mouse atheromas and its roles in VE-cadherin disorganization and atherosclerosis. Circulation. 2011, 124, 2522–2532. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Kowalczyk, A.P. The VE-cadherin cytoplasmic domain undergoes proteolytic processing during endocytosis. Mol. Biol. Cell 2017, 28, 76–84. [Google Scholar] [CrossRef]
- Friedrich, E.E.; Hong, Z.; Xiong, S.; Zhong, M.; Di, A.; Rehman, J.; Komarova, Y.A.; Malik, A.B. Endothelial cell Piezo1 mediates pressure-induced lung vascular hyperpermeability via disruption of adherens junctions. Proc. Natl. Acad. Sci. 2019, 116, 12980–12985. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-W.; Zhang, H.; Huang, J.-Q.; Wang, S.-N.; Lu, Y.; Cheng, B.; Dong, S.-H.; Wang, Y.-Y.; Li, F.-S.; Li, Y.-W. PIEZO1 Ion Channel Mediates Ionizing Radiation-Induced Pulmonary Endothelial Cell Ferroptosis via Ca2+/Calpain/VE-Cadherin Signaling. Front. Mol. Biosci. 2021, 8. [Google Scholar] [CrossRef]
- Sun, X.; Sun, Y.; Jiang, P.; Qi, G.; Chen, X. Crosstalk between endothelial cell-specific calpain inhibition and the endothelial-mesenchymal transition via the HSP90/Akt signaling pathway. Biomed. Pharmacother. 2020, 124, 109822. [Google Scholar] [CrossRef]
- Sato, H.; Taketomi, Y.; Murakami, M. Metabolic regulation by secreted phospholipase A2. Inflamm. Regen. 2016, 36, 7. [Google Scholar] [CrossRef]
- Chisolm, G.M.; Steinberg, D. The oxidative modification hypothesis of atherogenesis: an overview. Free. Radic. Biol. Med. 2000, 28, 1815–1826. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kobayashi, T.; Kamata, K. Role of Lysophosphatidylcholine (LPC) in Atherosclerosis. Curr. Med. Chem. 2007, 14, 3209–3220. [Google Scholar] [CrossRef]
- Mueller, P.; Ye, S.; Morris, A.; Smyth, S.S. Lysophospholipid mediators in the vasculature. Exp. Cell Res. 2015, 333, 190–194. [Google Scholar] [CrossRef]
- Engelbrecht, E.; MacRae, C.A.; Hla, T. Lysolipids in Vascular Development, Biology, and Disease. Arter. Thromb. Vasc. Biol. 2020, 41, 564–584. [Google Scholar] [CrossRef]
- Bandaru, S.; Ala, C.; Salimi, R.; Akula, M.K.; Ekstrand, M.; Devarakonda, S.; Karlsson, J.; Van den Eynden, J.; Bergström, G.; Larsson, E.; Levin, M.; Borén, J.; Bergo, M.O.; Akyürek, L.M. Targeting filamin a reduces macrophage activity and atherosclerosis. Circulation. 2019, 140, 67–79. [Google Scholar] [CrossRef]
- Yu, L.; Yin, M.; Yang, X.; Lu, M.; Tang, F.; Wang, H. Calpain inhibitor I attenuates atherosclerosis and inflammation in atherosclerotic rats through eNOS/NO/NF-κB pathway. Can J Physiol Pharmacol. 2018, 96, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yin, M.; Yu, L.; Lu, M.; Wang, H.; Tang, F.; Zhang, Y. Simvastatin inhibited oxLDL-induced proatherogenic effects through the calpain-1-PPARγ-CD36 pathway. Can. J. Physiol. Pharmacol. 2016, 94, 1336–1343. [Google Scholar] [CrossRef]
- Yin, M.; Liu, Q.; Yu, L.; Yang, Y.; Lu, M.; Wang, H.; Luo, D.; Rong, X.; Tang, F.; Guo, J. Downregulations of CD36 and Calpain-1, Inflammation, and Atherosclerosis by Simvastatin in Apolipoprotein E Knockout Mice. J. Vasc. Res. 2017, 54, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Miyazaki, A. Hypercholesterolemia and lymphatic defects: the chicken or the egg? Front. Cardiovasc. Med. 2021, 8, 701229. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Thiam, C.H.; Yeo, K.P.; Bisoendial, R.; Hii, C.S.; McGrath, K.C.; Tan, K.W.; Heather, A.; Alexander, J.S.J.; Angeli, V. Lymphatic Vessels Are Essential for the Removal of Cholesterol from Peripheral Tissues by SR-BI-Mediated Transport of HDL. Cell Metab. 2013, 17, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Kutkut, I.; Meens, M.J.; McKee, T.A.; Bochaton-Piallat, M.; Kwak, B.R. Lymphatic vessels: an emerging actor in atherosclerotic plaque development. Eur. J. Clin. Investig. 2014, 45, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Martel, C.; Li, W.; Fulp, B.; Platt, A.M.; Gautier, E.L.; Westerterp, M.; Bittman, R.; Tall, A.R.; Chen, S.-H.; Thomas, M.J.; et al. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J. Clin. Investig. 2013, 123, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Rademakers, T.; van der Vorst, E.P.C.; Daissormont, I.T.M.N.; Otten, J.J.T.; Theodorou, K.; Theelen, T.L.; Gijbels, M.; Anisimov, A.; Nurmi, H.; Lindeman, J.H.N.; et al. Adventitial lymphatic capillary expansion impacts on plaque T cell accumulation in atherosclerosis. Sci. Rep. 2017, 7, srep45263. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Taketomi, Y.; Higashi, T.; Ohtaki, H.; Takaki, T.; Ohnishi, K.; Hosonuma, M.; Kono, N.; Akasu, R.; Haraguchi, S.; et al. Hypercholesterolemic Dysregulation of Calpain in Lymphatic Endothelial Cells Interferes With Regulatory T-Cell Stability and Trafficking. Arter. Thromb. Vasc. Biol. 2023, 43, E66–E82. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.M.; Teitelbaum, S.L.; Kim, T.-H.; Ross, F.P.; Kim, S.-Y.; Kim, H.-J. Calpain-6, a target molecule of glucocorticoids, regulates osteoclastic bone resorption via cytoskeletal organization and microtubule acetylation. J. Bone Miner. Res. 2010, 26, 657–665. [Google Scholar] [CrossRef]
- Andrique, C.; Morardet, L.; Linares, L.K.; Cissé, M.Y.; Merle, C.; Chibon, F.; Provot, S.; Haÿ, E.; Ea, H.-K.; Cohen-Solal, M.; et al. Calpain-6 controls the fate of sarcoma stem cells by promoting autophagy and preventing senescence. J. Clin. Investig. 2018, 3. [Google Scholar] [CrossRef]
- Miyazaki, T.; Tonami, K.; Hata, S.; Aiuchi, T.; Ohnishi, K.; Lei, X.F.; Kim-Kaneyama, J.R.; Takeya, M.; Itabe, H.; Sorimachi, H.; Kurihara, H.; Miyazaki, A. Calpain-6 confers atherogenicity to macrophages by dysregulating pre-mRNA splicing. J. Clin. Invest. 2016, 126, 3417–3432. [Google Scholar] [CrossRef]
- Tonami, K.; Kurihara, Y.; Arima, S.; Nishiyama, K.; Uchijima, Y.; Asano, T.; Sorimachi, H.; Kurihara, H. Calpain-6, a microtubule-stabilizing protein, regulates Rac1 activity and cell motility through interaction with GEF-H1. J. Cell Sci. 2011, 124, 1214–1223. [Google Scholar] [CrossRef]
- Steckelberg, A.-L.; Altmueller, J.; Dieterich, C.; Gehring, N.H. CWC22-dependent pre-mRNA splicing and eIF4A3 binding enables global deposition of exon junction complexes. Nucleic Acids Res. 2015, 43, 4687–4700. [Google Scholar] [CrossRef]
- Woodward, L.A.; Mabin, J.W.; Gangras, P.; Singh, G. The exon junction complex: a lifelong guardian of mRNA fate. Wiley Interdiscip. Rev. RNA 2016, 8. [Google Scholar] [CrossRef]
- Steckelberg, A.-L.; Boehm, V.; Gromadzka, A.M.; Gehring, N.H. CWC22 connects pre-mRNA splicing and exon junction complex assembly. Cell Rep. 2012, 2, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Kruth, H.S. Receptor-independent fluid-phase pinocytosis mechanisms for induction of foam cell formation with native low-density lipoprotein particles. Curr. Opin. Infect. Dis. 2011, 22, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Kruth, H.S.; Jones, N.L.; Huang, W.; Zhao, B.; Ishii, I.; Chang, J.; Combs, C.A.; Malide, D.; Zhang, W.-Y. Macropinocytosis Is the Endocytic Pathway That Mediates Macrophage Foam Cell Formation with Native Low Density Lipoprotein. J. Biol. Chem. 2005, 280, 2352–2360. [Google Scholar] [CrossRef] [PubMed]
- Seachrist, J.L.; Ferguson, S.S. Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases. Life Sci. 2003, 74, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Buono, C.; Anzinger, J.J.; Amar, M.; Kruth, H.S. Fluorescent pegylated nanoparticles demonstrate fluid-phase pinocytosis by macrophages in mouse atherosclerotic lesions. J. Clin. Investig. 2009, 119, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Kruth, H.S. Fluid-Phase Pinocytosis of LDL by Macrophages: A Novel Target to Reduce Macrophage Cholesterol Accumulation in Atherosclerotic Lesions. Curr. Pharm. Des. 2013, 19, 5865–5872. [Google Scholar] [CrossRef]
- Steinbrecher, U.P.; Lougheed, M.; J, D.; S, A.; K, B.; C, O.; Y, W.; C, P.; K, B.; T, C.; et al. Scavenger receptor-independent stimulation of cholesterol esterification in macrophages by low density lipoprotein extracted from human aortic intima. Arter. Thromb. A J. Vasc. Biol. 1992, 12, 608–625. [Google Scholar] [CrossRef]
- Paul, D.S.; Harmon, A.W.; Winston, C.P.; Patel, Y.M. Calpain facilitates GLUT4 vesicle translocation during insulin-stimulated glucose uptake in adipocytes. Biochem. J. 2003, 376, 625–632. [Google Scholar] [CrossRef]
- Brown, A.E.; Yeaman, S.J.; Walker, M. Targeted suppression of calpain-10 expression impairs insulin-stimulated glucose uptake in cultured primary human skeletal muscle cells. Mol. Genet. Metab. 2007, 91, 318–324. [Google Scholar] [CrossRef]
- Turner, M.D.; Fulcher, F.K.; Jones, C.V.; Smith, B.T.; Aganna, E.; Partridge, C.J.; Hitman, G.A.; Clark, A.; Patel, Y.M. Calpain facilitates actin reorganization during glucose-stimulated insulin secretion. Biochem. Biophys. Res. Commun. 2007, 352, 650–655. [Google Scholar] [CrossRef]
- Sreenan, S.K.; Zhou, Y.-P.; Otani, K.; Hansen, P.A.; Currie, K.P.; Pan, C.-Y.; Lee, J.-P.; Ostrega, D.M.; Pugh, W.; Horikawa, Y.; et al. Calpains Play a Role in Insulin Secretion and Action. Diabetes 2001, 50, 2013–2020. [Google Scholar] [CrossRef]
- Zhou, Y.-P.; Sreenan, S.; Pan, C.-Y.; Currie, K.P.; Bindokas, V.P.; Horikawa, Y.; Lee, J.-P.; Ostrega, D.; Ahmed, N.; Baldwin, A.C.; et al. A 48-hour exposure of pancreatic islets to calpain inhibitors impairs mitochondrial fuel metabolism and the exocytosis of insulin. Metabolism 2003, 52, 528–534. [Google Scholar] [CrossRef]
- Hatta, T.; Iemura, S.-I.; Ohishi, T.; Nakayama, H.; Seimiya, H.; Yasuda, T.; Iizuka, K.; Fukuda, M.; Takeda, J.; Natsume, T.; et al. Calpain-10 regulates actin dynamics by proteolysis of microtubule-associated protein 1B. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Teng, X.; Ji, C.; Zhong, H.; Zheng, D.; Ni, R.; Hill, D.J.; Xiong, S.; Fan, G.-C.; Greer, P.A.; Shen, Z.; et al. Selective deletion of endothelial cell calpain in mice reduces diabetic cardiomyopathy by improving angiogenesis. Diabetologia 2019, 62, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Yu, Z.; Che, Z.; Zhang, H.; Yu, Y.; Yang, D.; Qian, D.; Chen, R.; Yu, M. Experimental diabetes exacerbates autophagic flux impairment during myocardial I/R injury through calpain-mediated cleavage of Atg5/LAMP2. J. Cell. Mol. Med. 2023, 27, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Cao, T.; Zhang, L.-L.; Fan, G.-C.; Qiu, J.; Peng, T. Targeted inhibition of mitochondrial calpain alleviates oxidative stress-induced myocardial injury. Acta Pharmacol. Sin. 2021, 42, 909–920. [Google Scholar] [CrossRef]
- White, P.J.; Newgard, C.B. Branched-chain amino acids in disease. Science 2019, 363, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Le Couteur, D.G.; Solon-Biet, S.M.; Cogger, V.C.; Ribeiro, R.; de Cabo, R.; Raubenheimer, D.; Cooney, G.J.; Simpson, S.J. Branched chain amino acids, aging and age-related health. Ageing Res. Rev. 2020, 64, 101198. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Metabolomics and metabolic diseases: where do we stand? Cell Metab. 2017, 25, 43–56. [Google Scholar] [CrossRef]
- Fiehn, O.; Garvey, W.T.; Newman, J.W.; Lok, K.H.; Hoppel, C.L.; Adams, S.H. Plasma Metabolomic Profiles Reflective of Glucose Homeostasis in Non-Diabetic and Type 2 Diabetic Obese African-American Women. PLOS ONE 2010, 5, e15234. [Google Scholar] [CrossRef] [PubMed]
- Uno, K.; Yamada, T.; Ishigaki, Y.; Imai, J.; Hasegawa, Y.; Sawada, S.; Kaneko, K.; Ono, H.; Asano, T.; Oka, Y.; et al. A hepatic amino acid/mTOR/S6K-dependent signalling pathway modulates systemic lipid metabolism via neuronal signals. Nat. Commun. 2015, 6, 7940. [Google Scholar] [CrossRef] [PubMed]
- Onodera, J.; Ohsumi, Y. Autophagy Is Required for Maintenance of Amino Acid Levels and Protein Synthesis under Nitrogen Starvation. J. Biol. Chem. 2005, 280, 31582–31586. [Google Scholar] [CrossRef]
- Suraweera, A.; Münch, C.; Hanssum, A.; Bertolotti, A. Failure of Amino Acid Homeostasis Causes Cell Death following Proteasome Inhibition. Mol. Cell 2012, 48, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T.; Huibregtse, J.M.; Matouschek, A. A masked initiation region in retinoblastoma protein regulates its proteasomal degradation. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Akasu, R.; Miyazaki, T.; Elhussiny, M.Z.; Sugiura, Y.; Tomitsuka, Y.; Haraguchi, S.; Otsu, K.; Chowdhury, V.S.; Miyazaki, A. Calpain-mediated proteolytic production of free amino acids in vascular endothelial cells augments obesity-induced hepatic steatosis. J. Biol. Chem. 2022, 298, 101953. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L.; Xu, D.; Deng, W.; Yang, W.; Tang, F.; Da, M. Knockout of calpain-1 protects against high-fat diet-induced liver dysfunction in mouse through inhibiting oxidative stress and inflammation. Food Sci. Nutr. 2020, 9, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Haraguchi, S.; Kim-Kaneyama, J.-R.; Miyazaki, A. Endothelial calpain systems orchestrate myofibroblast differentiation during wound healing. FASEB J. 2018, 33, 2037–2046. [Google Scholar] [CrossRef]
- Sato, T.; Head, K. Z.; Li, J.; Dolin, C. E.; Wilkey, D.; Skirtich, N.; Smith, K.; McCreary, D. D.; Liu, S.; Beier, J. I.; Singhi, A. D.; McEnaney, R. M.; Merchant, M. L.; Arteel, G. E. Fibrosis resolution in the mouse liver: role of Mmp12 and potential role of calpain 1/2. Matrix Biol. Plus. 2022, 17, 100127. [Google Scholar] [CrossRef]
- Seike, T.; Boontem, P.; Yanagi, M.; Li, S.; Kido, H.; Yamamiya, D.; Nakagawa, H.; Okada, H.; Yamashita, T.; Harada, K.; et al. Hydroxynonenal Causes Hepatocyte Death by Disrupting Lysosomal Integrity in Nonalcoholic Steatohepatitis. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 925–944. [Google Scholar] [CrossRef]
- Flores-Toro, J.; Chun, S.-K.; Shin, J.-K.; Campbell, J.; Lichtenberger, M.; Chapman, W.; Zendejas, I.; Behrns, K.; Leeuwenburgh, C.; Kim, J.-S. Critical Roles of Calpastatin in Ischemia/Reperfusion Injury in Aged Livers. Cells 2021, 10, 1863. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, S.; Tang, H.; Yang, H.; Zhang, J.; Shi, X.; Li, J.; Guo, W.; Zhang, S. miR-140-5p alleviates mouse liver ischemia/reperfusion injury by targeting CAPN1. Mol. Med. Rep. 2021, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, B.; Gustafson, B. Adipose tissue, inflammation, and atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 332–341. [Google Scholar]
- Gutiérrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, J. Pathophysiological Molecular Mechanisms of Obesity: A Link between MAFLD and NASH with Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 11629. [Google Scholar] [CrossRef] [PubMed]
- Muniappan, L.; Javidan, A.; Jiang, W.; Mohammadmoradi, S.; Moorleghen, J.J.; Katz, W.S.; Balakrishnan, A.; Howatt, D.A.; Subramanian, V. Calpain Inhibition Attenuates Adipose Tissue Inflammation and Fibrosis in Diet-induced Obese Mice. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




