1. Introduction
The genus Baccharis is part of the Asteraceae family, one of the largest families in the Plantae kingdom. It is estimated that there are around 500 species of the genus Baccharis from North to South America, of which 210 inhabit the southern cone (Abstract et al., 2019). It is possible to find members of Baccharis in climatic zones including temperate, tropical, and desert climates, either cool or warm and at any elevation. The species of Baccharis genus share many anatomical and histologic characteristics, however few studies extensively describe the genus. The species of the genus Baccharis are usually branched leafy shrubs that can measure from 0.5 to 4 meters in height. Some species, such as Baccharis concava Pers. (syn. B. macraei) can be found at elevations close to sea level. B. concava is abundant in the central coastline of Chile and considered a pioneer specie with ability to grow in poor soils, including sands of the first line of the coast. Another example is Baccharis obovata, found in Chile and Argentina, closely related to other Baccharis, such as B. concava and B. magellanica (Molares et al., 2009).
Figure 1.
Details of B. concava small branches and leaves used for the study. Branches and leaves were collected from a female plant located in a private garden around 400 m from San Sebastian beach and 80 m from the Cartagena estuary, during February (middle summer) 2017. Precise location (33°31'46.8"S 71°35'59.3"W) is shown at the right panel, obtained from Google Earth App. By the end of summer (late February and onwards) intense blooming, as seen at the left, massively attract bees and bumblebees (left panel).
Figure 1.
Details of B. concava small branches and leaves used for the study. Branches and leaves were collected from a female plant located in a private garden around 400 m from San Sebastian beach and 80 m from the Cartagena estuary, during February (middle summer) 2017. Precise location (33°31'46.8"S 71°35'59.3"W) is shown at the right panel, obtained from Google Earth App. By the end of summer (late February and onwards) intense blooming, as seen at the left, massively attract bees and bumblebees (left panel).
Table 1.
Qualitative determination of secondary metabolites in hydro-alcoholic extracts of B. concava.
Table 1.
Qualitative determination of secondary metabolites in hydro-alcoholic extracts of B. concava.
| Assay |
Compounds tested |
Positive results |
Result |
| Dragendorff |
Alkaloids |
red precipitated |
+ |
| Borntrager |
Free Anthraquinones |
red color in aqueos fase |
- |
| Flurescence under UV |
Cumarines |
blue color under UV light |
- |
| Libermann-Burchard |
Steroids y terpenes |
green-blue or purple red |
+ |
| Aluminum chloride |
Flavonoids |
yellow green fluorescence under UV light |
+ |
| Keller-Killiani |
Cardiac glycosides |
greenish blue color |
- |
| Foam formation |
Saponins |
stable foam production (stands 10 min) |
- |
| Ferric chloride |
Tannins and phenolic compounds |
green or dark blue color |
+ |
While systematic studies provide support for popular and ancestral uses of several species of the genus Baccharis from Brazil, Argentina, and Uruguay, few studies describe medicinal properties and the phytochemical characteristics of B. concava and other species of Baccharis in Chile. In 1985, Houghton and Manby, found from a list of 136 species with medicinal properties use by the native Mapuche people in Chile, three species of the Baccharis genus: B. concava used as vermifuge; B. rosmarinifolia, whose resins has been used for treating rheumatism and respiratory and genitourinary infections; and B. sagitallis for the treatment of fractured limbs (Houghton and Manby, 1985). Other species of the Baccharis genus such as B. articulata, B. crispa and B. dracunculifolia have been used for the treatment of ulcers, wounds, alleviation of gastrointestinal discomfort and infections, usually used as a decoct of leaves applied on skin or for drinking (Abad and Bermejo, 2007; Desmarchelier, 2015). In 1986, preparing ethanolic extracts of B. linearis, B. rhomboidalis, and B. solieri , Labbé et al.described , after partition with solvents of higher polarity, the presence of terpenes, coumarins and fatty esters of different nature.(Labbe et al., 1986).
Preparations of B. concava, has been used in pre- and post-Columbian traditional medicine to treat wounds, preventing infections, as diuretic and as a tonic beverage. Up-to-day, there are few scientific studies describing medicinal properties and the phytochemical characteristics of B. concava. In 1989, preparing dichloromethane extracts of aereal parts of B. concava, Gambaro et al. found derivatives of the clerodane diterpenes such as hardwickiic acid, hautriwaic acid, and derivatives of bacchasmacranone (Gambaro et al., 1986). Also using the aerial parts of B. concava, but performing a hydromethanolic followed by partitions with petroleum ether, ethyl ether and ethyl acetate, the flavonoids salvigenin, cirsimaritin and pectolinarigenin were purified (Zamorano et al., 1987). In 2012, antimicrobial activity of B. concava agains Staphylococcus aureus (Gram-positive) but not for Escherichia coli (Gram-negative) was described in essential oils. From a total of 102 identified compound; limonene, miricene, α-pineno, murolene espatulene, δ-cadineno and lachnophyllum ester were the most abundant compounds, with different proportion, depending in the gender of plants (Santander Meyer, 2012).
Antibacterial effects have been reported for species of the Baccharis genus, including B. concava, the wound healing process is promoted by B. concava (according to traditional medicine) and it could be due to antimicrobial effects. Because B. concava have not been extensively described in the scientific literature, we sought in this study describing the possible antibacterial effects and phytochemically describing the hydroalcoholic extracts of B. concava.
2. Material and Methods
Plant Material.
Leaves and small branches of B. concava were collected in San Sebastian Beach, V region, Chile (33°31'46.8"S 71°35'59.3"W) from a female plant which material was validated by MRD and deposited at the herbarium of Facultad de Ciencias Quimicas y Farmaceuticas, Universidad de Chile under code number SQF #22.889. Leaves and small branches of B. concava were dried for 14 days at 21°C and crushed in a porcelain mortar. 100g of dry plant powder were macerated with 1000 mL of ethanol (70% in water) for 3 days at 50 rpm and 30°C. after 3 days the extract was filtered and rotovaped from 30 to 70°C before further drying with a lyophilizer at -50°C, until the solvents are eliminated. Finally, 42.51 g of dry extract was obtained. The extract was stored in an amber bottle, at a temperature of 4°C, according to methodologies reported and standardized in the literature for this type of study (Tiwari et al., 2011).
Phytochemical characterization
Standard reactions were performed to qualitatively identify the main families of compounds present in the hydroalcoholic extract of B. concava. The performed tests include: Dragendorff for alkaloids, Borntrager for anthraquinones, fluorescence for coumarins, Libermann-Burchard for steroids and terpenes, aluminum chloride for flavonoids, Keller-Killiani for cardiac glycosides, foam formation for saponins and Ferric chloride for tannins and phenolic compounds. Details in how to perform these tests have extensively been described in the literature (María et al., 2018; Pandey and Tripathi, 2014).
Antimicrobial activity assays by an agar-diffusion test.
90mm Petri-like plates were filled with 20 mL of Miller-Hinton agar. Once Miller-Hinton agar was jellified, the agar was seeded to forma a lawn of bacteria, by zigzag streaking a cotton swab soaked in diluted bacteria (McFarland 0,5 in saline serum). Right after seeding the lawn, each plate was 6mm punched to form 4 holes, 3 peripheric to test 100 μL of the B. concava extract (333.33 mg/mL dried extract dissolved in 70% EtOH) and one at the center to test for 100 μL of 70% EtOH vehicle. After incubating 16 h, the diameter was measured through 3 different sections for each inhibition halo. Independent experiments were performed at least 3 times. To test susceptibility of yeast such as Candida albicans and Cryptococcus neoformans, the assay was as described, but Miller-Hinton agar was replaced with Potato-Dextrose agar.
Minimum inhibitory concentration (MIC).
Starting with a solution of 166,67 mg/mL of lyophilized extract, base two serial dilutions were prepared in LB-broth (for bacteria) or Potato-Dextrose broth (for yeasts). 150 μL of each dilution were loaded through the rows of a flat-bottom 96-well plate to finally seed with 50 µL of diluted bacteria or yeast (adjusted to McFarland 0.5 in saline serum). After 16 h incubation, turbidity of cultures was measured at a longitude of 600 nm to calculate IC50, which was consider as the MIC. As a control, bacteria were treated with same amount of vehicle to look for possible effects in growth.
Minimum biocidal concentration (MBC).
From the same set of experiments intended to determine the MIC (or IC50), after 16 h incubation, aliquots of 5 μL from each dilution were seeded on top of a LB agar or Potato-Dextrose agar, incubated for 16 h to finally count the colony forming units (CFU). MBC was estimated as the concentration capable of eliminate 99.9% of a microorganisms compared to the control without treatment.
Thin layer chromatography (TLC).
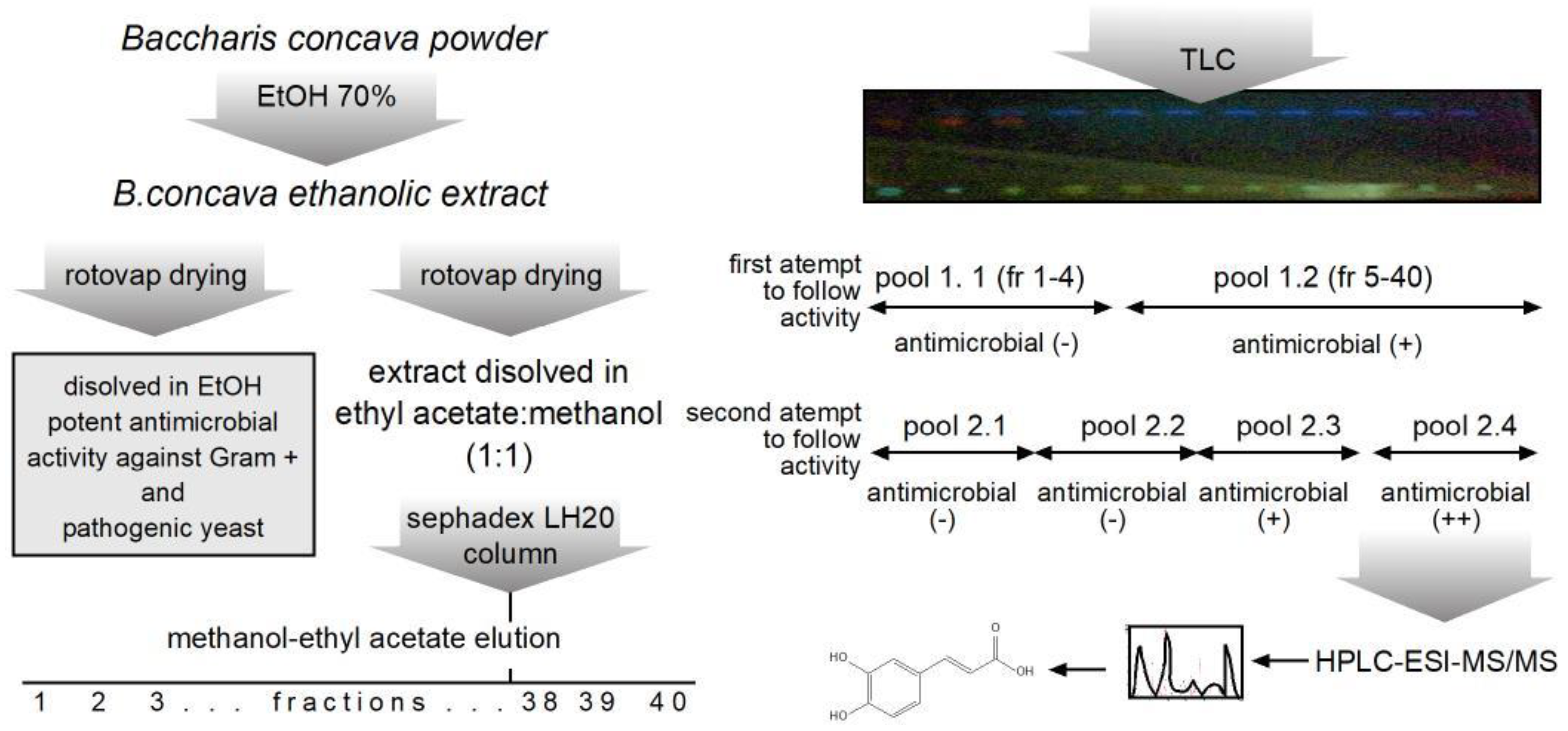
Characterization of extracts and eluted fractions were characterized by thin layer chromatography using aluminum oxide (AL2O3) as the stationary phase and methanol/ethyl acetate (1:1) as the mobile phase. UV (254 nm y 365 nm) and visible longitudes were used to visualize characteristic bands in crude and purified extracts.
Sephadex preparatory column.
A 290 mm long x 21 mm
wide LH-20 column was selected as the stationary phase to separate 300 mg of dried
B. concava extract dissolved in 3 mL of methanol/ethyl acetate (1:1). To elute the column methanol/ethyl acetate (1:1) was used as mobile phase. A total of 40 fractions of approximately 5 mL each were collected, resolved by TLC and pooled by their patterns as shown in
Figure 2. Finally, antimicrobial activity was determined to continue with further chemical characterization.
LC-MS analysis.
The Baccharis extract was examined on an LC-MS system consisting of the HPLC HP 1100 (Agilent Technologies Inc., CA-USA) coupled to an electrospray ion-trap mass spectrometer Esquire 4000 ESI-IT (Bruker Daltonik GmbH, Germany). For the HPLC separation, a Kromasil 100-5C18 250×4.6 mm, 5 µm, 100 A column (Eka Chemicals AB, Sweden) was used, the column outlet was connected to a split that divided the flow to the UV detector and the mass spectrometer. The analysis was performed at room temperature by the injection of 20 µL of extract, at a flow rate of 1.0 mL/min. The mobile phase components were formic acid 0.1% v/v (component A) and methanol (component B) according to the following elution gradient: 0-5 min, 5% B; 5-7.5 min, 5-20% B; 7.5-20 min, 20-30% B; 20-40 min, 30-40% B; 40-45 min, 40-60% B; 45-50 min, 60-80% B; 50-55 min, 80% B; 55-57.5 min, 80-5% B; and 57.5-60 min, 5% B. The UV detection was performed at 254 nm. The ionization process (nebulization) by electrospray was performed at 3,000 V assisted by nitrogen as nebulizer gas at a pressure of 50 psi and flow rate of 10 L/min and assisted by nitrogen as drying gas at a temperature of 365ºC. Chromatograms and mass spectra were acquired in positive and negative polarity. The trap parameters were set in ion charge control (ICC) using manufacturer default parameters, and maximum accumulation time of 200 ms. Collision induced dissociation (CID) was performed by collisions with the helium background gas present in the trap. Fragmentation was controlled by SmartFrag. All data obtained was analyzed using DataAnalysis 3.2 (Bruker Daltonik GmbH, Germany). The identification of compounds was carried out by precursor and fragmentation pattern comparison with a library from the Unidad de Espectrometría de Masas at Universidad de Chile. The identification of compounds was carried out by comparison of their precursors and corresponding fragmentation patterns with a library developed at the Mass Spectrometry Unit of the Universidad de Chile.
3. Results
Alcaloids, steroids and therpenes, flavonoids and phenolic compounds are present the hydroalcoholic extract of B. concave.
Before testing biological activity in the B. concava, the phytochemical composition was qualitatively studied. The extract obtained in 70% EtOH, resulted positive for Dragendorff, Libermann-Burchard, aluminum chloride, and Ferric chloride tests. Indicating presence of detectable amount of alcaloids, steroids and therpenes, flavonoids, and phenolic compounds.
Column fractionation of B. concava extract.
Sephadex LH-20 was used to separate 300 mg of dried extract dissolved in 3 mL of methanol/ethyl acetate (1:1). The same solvent was used to elute the column. A total of 40 fractions of approximately 50 mL each were collected, resolved by TLC as described in material and methods and fractions pooled according to presence of similar patterns under UV light. As seen in
Figure 2, in a first attempt to follow antimicrobial activity present in the pooled fractions, fractions 1 through 4 and 5 through 40 were pooled, to rescue antimicrobial in the bigger pool. Next, in a second attempt, fractions were pooled in lots of 10 consecutive fractions, this time the fourth pool (pool 2.4 in
Figure 2) was the one with the higher antimicrobial activity measured against
S. aureus. Therefore, pool 2.4 was selected for further chemical composition analysis.
HPLC/mass spectrometry identification of phenolic compounds.
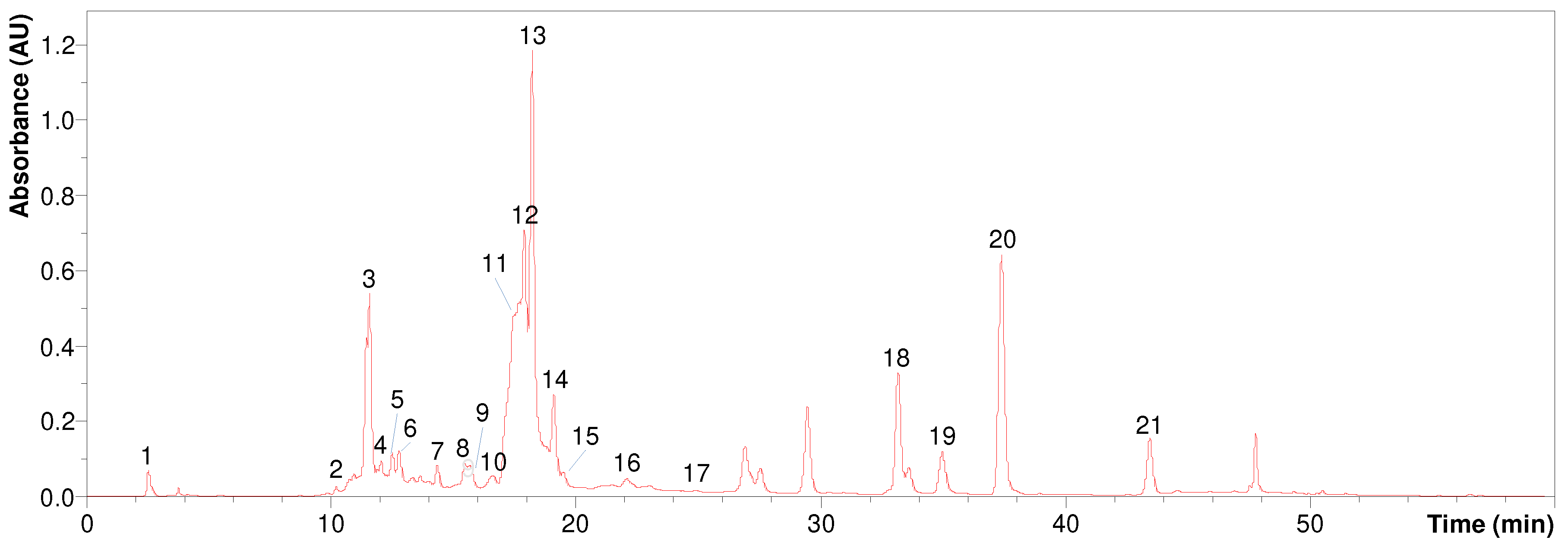
Figure 3 shows the UV chromatogram at 254 nm obtained for the
B. concava extract, pooled fraction 2.4. The identification of the labeled peaks is detailed in
Table 4 which contains the precursors observed in negative polarity, as well as their corresponding fragmentations. The fragmentations are arranged by decreasing intensity from left (base peak) to right.
As seen from the table 4, three isomers of caffeoylquinic acid (m/z 353, [M-H]-) were observed at tR 11.6 min (peak 3), tR 12.2 min (peak 4) and tR 13.3 min (peak 6). Peaks 10-15 were identified as isomers of dicaffeoylquinic acid as indicated by the observation of signal m/z 515 in negative polarity ([M-H]-) and m/z 499 in positive polarity ([M-H2O+H]+). Peak 17 (tR 24.9 min) would correspond to caffeoylquinic acid-O-hexoside as suggested by the observation of signal m/z 677 and its fragment m/z 515. Among the flavones, apigenin was identified at tR 33.1 min (peak 18) through signal m/z 269 in negative polarity ([M-H]-) and m/z 271 in positive polarity ([M+H]+); and apigenin-di-C-hexoside (probably vicenin 2) at tR 11.6 min (peak 3) on the basis of signal m/z 593 ([M-H]-) and its corresponding fragmentation. Among the flavonols, several quercetin derivatives were identified as: peak 5 (tR 12.8 min) identified as rutin-O-pentoside based on the signal m/z 741 ([M-H]-) and its fragmentation; peak 7 (tR 14.3 min) which was identified as quercetin-O-rhamnosyl hexoside based on the signal m/z 609 ([M-H]-) and m/z 611 ([M+H]+) together with its fragmentations; peak 8 (tR 15. 4 min) identified as quercetin-O-hexoside according to the observation of signal m/z 463 ([M-H]-) and m/z 465 ([M+H]+); and peak 9 (tR 15.6 min) which was identified as quercetin-O-glucuronide based on the signals m/z 477 ([M-H]-) and m/z 479 ([M+H]+) and their corresponding fragmentations. Several kaempferol derivatives were also identified: peak 19 (tR 34.9 min) identified as kaempferol methoxy methyl ether based on the signals m/z 329 ([M-H]-) and m/z 331 ([M+H]+); peak 20 (tR 37. 3 min) which according to the signal m/z 300 ([M-H]-) and its fragmentation would correspond to kaempferol methyl ether (probably kaempferide); and peak 21 (tR 43.4 min) identified as dimethoxy kaempferol according to the signal m/z 313 ([M-H]-) and its corresponding fragmentation. Other compounds identified were quinic acid (peak 1), coumarylhexaric acid (peak 2) and caffeoyl feruloylquinic acid (peak 16).
4. Discussion
This work describes potent antimicrobial activity, of a hydroalcoholic extract of B. concava against Gram-positive bacterium, C. albicans, and C. neoformans. Previously, antimicrobial activity was reported against S. aureus in essential oil prepared from B. concava. In the same line with our results, the essential oil derived from B. concave was inactive against the Gram -negative bacteria E. coli. it is possible that the active molecules present in the B. concava are inactive against Gram-positive. However, some Baccharis have presented discrete activity against Gram-negatives, such is the case of Baccharis revoluta, although its activity against Gram-positive is higher (Rodríguez A et al., 2016). Whether the extracts of B. concava are inactive against Gram-negative or Gram-negative are just highly resistance to B. concava extracts was assessed by using S. Typhimurium mutants. Increased permeability turned S. Typhimurium susceptible to B. concava extracts. Therefore, we speculate that active molecules in the tested extract poorly penetrates the bacterial envelope of Gram-negatives with an intact LPS. Moreover, new evidence indicates that this kind of mutants accumulate oxidative species, that in turn may facilitate killing by antimicrobial agents (Seregina et al., 2022).
Few reports exist about antimycotic effects within the Baccharis genus, and none are published for B. concava, in this study we found potent activity against pathogenic yeast C. albicans and C. neoformns. Previous report indicates some discrete to poor antifungal activity in some Baccharis species, however some efforts were made to evaluate synergistic effects of different Baccharis extracts in combination with terbinafine against Trichophyton rubrum. Some promising synergistic and additive effect were reported in regard of chemical composition of extracts (Rodriguez et al., 2013). Further studies, in regard of antimycotic activity, will be important in the light of emergent yeast and fungi with multiresistant phenotypes (Arendrup and Patterson, 2017).
This study describes the phytochemical composition of a polar extract of B. concava. Fifteen compounds were identified and among them fourteen are described for the first time in this specie. These compounds were diterpenoids, flavonoids, and chlorogenic acid derivatives, which are natural products frequently found in Baccharis species. The antimicrobial effects of polar compounds such as phenolic acids, flavonoids and heteroside derivatives have been reported. An example is chlorogenic acid, a compound found in extracts of B. concava and from which its antimicrobial activity has been reported, probably as a disruptor of bacterial membranes, therefore, disrupting cellular homeostasis (Lou et al., 2011). Other molecules described in the ethanolic extract of B. concava are various caffeic acid-derivative molecules. Because of their abundance, pure commercial caffeic acid was tested for antimicrobial activity, against S. aureus, using 6mm filter disks impregnated with 0.3 or 0.5 mg. The effect of pure caffeic acid was discrete with haloes ranging from 8 to 9 mm, compared with 6mm in the DMSO control (data not shown). It is plausible that the blend of caffeic acid-derivatives and other molecules account for the potent antimicrobial activity observed in ethanolic B. concava extract.
B. concava has been used in traditional medicine for wound healing, as diuretic and as a tonic in beverages. It is possible that its antimicrobial effects accounts for its curative effect on wounds. The antimicrobial effects, found in the ethanolic extract, are exclusive to Gram-Positive bacteria and pathogenic yeast, with no effect over Gram-negative bacteria. It would be interesting to continue learning about the molecules responsible for the spectrum in the ethanolic extract of B. concava, which in the future might allow developing more specific therapies with less unwanted effects on the Gram-negative microbiota.
Acknowlegments
We thank Dr. Juan Fuentes for providing with strains S. Typhimurium ΔrfaD and S. Typhimurium ΔrfaE. Parts of this work have been executed and/or presented as the qualifying exams for FEP, PMM, JIC and CFM. The authors gratefully appreciate funding from FONDECYT/ANID Grant 11150588, FONDECYT/ANID Grant 1231676, and UNAB Regular grant DI-15-19/R.
References
- Abad:, M. : Bermejo, P., 2007. Baccharis (compositae): A review update. Arkivoc 2007, 76–96.
- Abstract, Zuloaga, F., C, A., Zanotti, C., 2019. An update of the Catalogue of the Vascular Plants of the Southern Cone 7, 208–278. [CrossRef]
- Arendrup, M.C. , Patterson, T.F., 2017. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J Infect Dis 216, S445–S451. [CrossRef]
- Desmarchelier, C. , 2015. Plantas Medicinales Autóctonas de la Argentina - Bases Científicas para su Aplicación en Atención Primaria de la Salud.
- Gambaro, V. , Chamy, M.C., Garbarino, J.A., San-Martin, A., Castillo, M., 1986. Neo-clerodane diterpenoids from Baccharis macraei. Phytochemistry 25, 2175–2177. [CrossRef]
- Houghton, P.J. , Manby, J., 1985. Medicinal plants of the Mapuche. Journal of Ethnopharmacology 13, 89–103. [CrossRef]
- Labbe, C. , Rovirosa, J., Faini, F., Mahu, M., San-Martin, A., Castillo, M., 1986. Secondary Metabolites from Chilean Baccharis species. J. Nat. Prod. 49, 517–518. [CrossRef]
- Lou, Z. , Wang, H., Zhu, S., Ma, C., Wang, Z., 2011. Antibacterial activity and mechanism of action of chlorogenic acid. J Food Sci 76, M398-403. [CrossRef]
- María, R. , Shirley, M., Xavier, C., Jaime, S., David, V., Rosa, S., Jodie, D., 2018. Preliminary phytochemical screening, total phenolic content and antibacterial activity of thirteen native species from Guayas province Ecuador. Journal of King Saud University - Science 30, 500–505. [CrossRef]
- Molares, S. , González, S.B., Ladio, A., Agueda Castro, M., 2009. Etnobotánica, anatomía y caracterización físico-química del aceite esencial de Baccharis obovata Hook. et Arn. (Asteraceae: Astereae). Acta Bot. Bras. 23, 578–589. [CrossRef]
- Pandey, A. , Tripathi, S., 2014. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacogn Phytochem 2, 115–119.
- Rodríguez A, Ó.E. , Roa A, V.P., Palacios O, É.A., 2016. Actividad antibacteriana y antioxidante de Baccharis revoluta Kunth. Nova 14, 57–65.
- Rodriguez, M.V. , Sortino, M.A., Ivancovich, J.J., Pellegrino, J.M., Favier, L.S., Raimondi, M.P., Gattuso, M.A., Zacchino, S.A., 2013. Detection of synergistic combinations of Baccharis extracts with Terbinafine against Trichophyton rubrum with high throughput screening synergy assay (HTSS) followed by 3D graphs. Behavior of some of their components. Phytomedicine 20, 1230–1239. [CrossRef]
- Santander Meyer, R. del P., 2012. Análisis de los componentes químicos y actividad antibacteriana de los aceites esenciales de dos plantas endémicas: Baccharis concava y Haplopappus foliosus. Tesis Universidad de Santiago de Chile, Santiago.
- Seregina, T.A. , Petrushanko, I.Y., Shakulov, R.S., Zaripov, P.I., Makarov, A.A., Mitkevich, V.A., Mironov, A.S., 2022. The Inactivation of LPS Biosynthesis Genes in E. coli Cells Leads to Oxidative Stress. Cells 11, 2667. [CrossRef]
- Tiwari, P. , Kumar, B., Kaur, M., Kaur, G., Kaur, H., 2011. Phytochemical screening and Extraction: A Review. Internationale Pharmaceutica Sciencia 1, 98–106.
- Zamorano, R. , Aguirre, M.E., Peña, A.M.D.L., Cordano, G., Medina, J., Timmermann, B., 1987. Flavonoids from baccharis concava pers. Boletin De La Sociedad Chilena De Quimica.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).