Introduction
Endometrial cancer is the most common gynecologic malignancy in the United States, with more than 65 000 new cases per year. In a landmark study published in 1983, Bokhman et al described two distinct types of endometrial cancer, based on histologic and molecular characteristics [
1]. Endometrioid type (Type I), comprises 80–90% of all sporadic endometrial cancers, while non-endometrioid (Type II), encompasses the remaining 10–20%. While the incidence of Type II tumors is low compared to Type I, excess mortality is associated with Type II EC. In an analysis of Surveillance, Epidemiology, and End Results (SEER) data, Hamilton et al. reported that while 11% of ECs were Type II, 47% of deaths occurred in this subtype and stage-adjusted 5-year overall survival rates for Type II tumors are significantly worse compared to Type I tumors [
2].
Endometrial cancer is surgically staged with hysterectomy, bilateral salpingo-oophorectomy, and lymph node evaluation. Laparoscopy has become the standard surgical approach for patients with early-stage uterine carcinoma due to the results of studies such as LAP2, which demonstrated no negative effect of the MIS approach on oncologic outcomes [
3]. Another randomized trial (Laparoscopic Approach to Cancer of the Endometrium, LACE) showed no difference in overall and recurrence-free survival between the two surgical approaches [
4]. Both studies established the superiority of the MIS approach regarding perioperative outcomes. However, the results of these studies may not apply to patients with aggressive histology types. Type II tumors were poorly represented in LAP2 accounting only for 20% of the enrolled patients, while LACE included only patients with endometrioid adenocarcinoma.
In 2018, the Laparoscopic Approach to Cervical Cancer (LACC) trial reported worse oncologic outcomes in patients undergoing minimally invasive surgery for cervical cancer [
5]. Multiple factors have been implicated in this unexpected finding, including the aggressive biology of cervical cancer and its propensity to overcome the patient’s immune system and grow primarily through VEGF activation and angiogenesis. Type II endometrial cancer has more aggressive behavior and a higher metastatic potential compared to Type I, with a significant risk for intraperitoneal and lymphatic recurrences. The findings of the LACC trial have raised concerns about the oncologic safety of MIS in patients with Type II endometrial cancers. Furthermore, an association of worse disease-free survival in patients with intermediate-risk endometrial cancer who underwent robotic-assisted laparoscopy as opposed to open surgery was recently reported by Song et al [
6]. In the widest single institution retrospective study comparing MIS with open surgery in Type II endometrial cancer published to date, Monterossi et al found no difference in survival outcomes in Stages I and II and a trend for worse overall survival with MIS in stage III [
7]. In a systematic review of the literature that included nine retrospective studies, MIS appears to offer better perioperative and postoperative outcomes and comparable oncological outcomes [
8]. The low incidence of Type II endometrial tumors makes this disease difficult to study in a prospective randomized manner and a large epidemiologic study based on a national cancer registry would be the best next alternative.
We designed a study to evaluate survival and surgical outcomes using a large cohort of patients from the National Cancer Database [
10]. The primary objective was to compare overall survival between patients with Type II endometrial cancer who underwent minimally invasive to those who underwent an open hysterectomy. A secondary objective was to compare surgical outcomes such as length of stay, readmission rates, and postoperative mortality.
Materials and Methods
Data were obtained from the National Cancer Database, which includes data on patients who received some element of their cancer care (treatment or diagnosis) at a cancer program accredited by the Commission on Cancer-Accredited Centers [
11]. Data covers more than 70% of newly diagnosed cases collected in approximately 1500 facilities. We identified a cohort of women who underwent hysterectomy as the primary treatment for Type II endometrial cancer (serous, clear cell, carcinosarcoma) between January 2010 and December 2014. We excluded patients who did not have a hysterectomy as primary treatment, those whose primary treatment was unknown, those with stage IV disease, and those for whom there was a lack of pathological confirmation of cancer. The surgical approach documented included open or minimally invasive (laparoscopic or robotic assisted). Our analysis was based on the intention-to-treat model in which we included any cases initiated as minimally invasive surgery even if later converted to open surgery. We compared the two groups in terms of age, race, co-morbidities, stage, histology type, primary tumor size, and adjuvant therapy. Comorbid conditions were analyzed using the Charlson/Deyo Score provided by the National Cancer Database. The Charlson/Deyo value is a weighted score derived from the sum of the scores for each of the comorbid conditions listed in the Charlson Comorbidity Score; A score of 0 indicates "no comorbid conditions recorded." Histology in the NCDB PUF dictionary was reported as ICD-O-3 codes reported by SEER registries.
The primary outcome was to compare overall survival between patients undergoing minimally invasive (MIS) versus open surgical management. Secondary endpoints included length of hospital stay, readmission within 30 days of discharge, and 30 and 90-day mortality.
Statistical Analysis
Patient demographics, clinical scores, treatment, and tumor characteristics were analyzed using Pearson’s chi-square or Fisher’s exact test for categorical data, and Mann-Whitney U for continuous data.
A propensity score was calculated to control for confounding using multivariable logistic regression with nine potential confounding variables (Year of diagnosis, Age, Race, Histology, Analytic Stage Group, Chemotherapy, Radiation Therapy, Lymph nodes dissected, and Tumor Size). We then applied inverse propensity weighting to create a pseudo-population to balance the measured confounding variables. To reduce variability in the weighted sample, we applied stabilized weights. Where traditional Inverse Propensity Score Weighting applies a weight of 1/ (propensity score) where group = MIS and 1/(1-propensity score) where group = Open, the stabilized weights are applied as follows: Where group = MIS; (probability of receiving MIS)/(propensity score). Where group = Open; (1-probability of receiving MIS)/(1-propensity score).
For the Inverse probability of treatment weighted (IPTW) sample, we compared groups using weighted binary logistic regression for all patient characteristics, clinical scores, tumor characteristics, treatment, and outcomes.
Rates of 30-day mortality, 30-day readmit, and 90-day mortality were plotted over time, both using raw percentages and IPTW percentages.
Median survival was estimated using the product-limit method, plotted with a Kaplan-Meier curve, and compared with a log-rank test. This analysis was done for both raw and IPTW samples.
Finally, multivariable Cox proportional hazards models were created to estimate the effect of MIS on the hazards function, while controlling for relevant covariates. A weighted Cox model was used to estimate the same, for the IPTW sample. These results were compared as a sensitivity analysis to demonstrate the robustness of the findings.
A two-tailed p-value of less than 0.05 was considered statistically significant. Analyses were performed using SPSS Version 28 (IBM Corp, Armonk NY).
Results
3.1. Patient characteristics
Between January 2010 and December 2014, we identified 12905 patients that underwent hysterectomy for Type II uterine cancer. 7123 of these women (55%) underwent minimally invasive surgery. The rate of MIS increased from 39% in 2010 to 64% in 2014. Women who underwent minimally invasive hysterectomy were more likely to be white, privately insured, and have higher income. The mean age for both groups was relatively similar (67 versus 68). Carcinosarcoma histology was more common in the open group (30.7% vs 23.4%). In the open group, patients were more likely to have stage III disease (38.4% vs 27.4) and larger primary tumors. Postoperative radiation was more common in the MIS group (40.1% vs 37%) while chemotherapy was more common in the open group (37.6% vs 33.9%) (Table-1).
Table 1.
Patient demographics and disease characteristics.
Table 1.
Patient demographics and disease characteristics.
| |
|
Unweighted |
|
|
Weighted |
|
| |
|
Surgical type |
|
|
Surgical type |
|
| Variables |
|
|
|
|
|
|
| |
Open (n=5782) |
MIS (n=7123) |
P-value |
Open (n=5578) |
MIS (n=6867) |
P-value |
| Age |
67 (61- 74) |
68 (62 - 74) |
<0.001 |
68 (62 - 74) |
67 (62 - 74) |
0.965 |
| Race, n (%) |
|
|
|
|
|
|
| White |
3967 (68.1) |
5450 (75.7) |
|
4037 (72.4) |
4970 (72.4) |
0.985 |
| Black |
1559 (26.8) |
1378 (19.1) |
|
1257 (22.5) |
1546 (22.5) |
| Asian/Pacific Islander |
178 (3.1) |
218 (3) |
<0.001 |
168 (3) |
207 (3) |
| Other |
63 (1.1) |
78 (1.1) |
|
61 (1.1) |
74 (1.1) |
| Unknown |
60 (1) |
73 (1) |
|
56 (1) |
69 (1) |
| Ethnicity, n (%) |
|
|
|
|
|
|
| Non-Hispanic |
5277 (90.6) |
6574 (91.3) |
|
5056 (90.6) |
6276 (91.4) |
0.13 |
| Hispanic |
367 (6.3) |
444 (6.2) |
0.072 |
358 (6.4) |
417 (6.1) |
| Unknown |
183 (3.1) |
179 (2.5) |
|
164 (2.9) |
174 (2.5) |
| Insurance, n (%) |
|
|
|
|
|
|
| No |
219 (3.8) |
141 (2) |
<0.001 |
194 (3.5) |
147 (2.1) |
0.001 |
| Private |
1968 (33.8) |
2541 (35.3) |
1836 (32.9) |
2478 (36.1) |
| Medicaid/Medicare/Other Public |
3541 (60.8) |
4456 (61.9) |
3463 (62.1) |
4187 (61) |
| Unknown |
99 (1.7) |
59 (0.8) |
85 (1.5) |
55 (0.8) |
| Median Income Quartiles, n (%) |
|
|
|
|
|
|
| <$30,000 |
885 (17) |
840 (13.1) |
<0.001 |
794 (16) |
860 (14.1) |
<0.001 |
|
$30,000 - $34,999 |
934 (17.9) |
1007 (15.7) |
860 (17.3) |
967 (15.8) |
|
$35,000 - $45,999 |
1381 (26.5) |
1721 (26.9) |
1330 (26.7) |
1642 (26.9) |
|
$46,000 |
2018 (38.7) |
2826 (44.2) |
1992 (40) |
2647 (43.3) |
| Charlson Comorbidity Index, n (%) |
|
|
|
|
|
|
| 0 |
4117 (70.7) |
5200 (72.3%) |
0.164 |
3949 (70.8) |
4935 (71.9) |
0.162 |
| 1 |
1336 (22.9) |
1584 (22%) |
1269 (22.7) |
1522 (22.2) |
| 2 |
293 (5) |
317 (4.4%) |
281 (5) |
317 (4.6) |
| 3 |
81 (1.4) |
96 (1.3%) |
79 (1.4) |
93 (1.4) |
| Year of diagnosis, n (%) |
|
|
|
|
|
|
| 2010 |
1267 (21.7) |
823 (11.4%) |
<0.001 |
1217 (21.8) |
790 (11.5) |
<0.001 |
| 2011 |
1228 (21.1) |
1163 (16.2%) |
1179 (21.1) |
1119 (16.3) |
| 2012 |
1140 (19.6) |
1383 (19.2%) |
1088 (19.5) |
1317 (19.2) |
| 2013 |
1064 (18.3) |
1811 (25.2%) |
1023 (18.3) |
1724 (25.1) |
| 2014 |
1128 (19.4) |
2017 (28%) |
1070 (19.2) |
1917 (27.9) |
| Histology, n (%) |
|
|
|
|
|
|
| Serous Carcinoma |
3288 (52.9) |
4539 (63.7%) |
<0.001 |
3395 (60.9) |
4182 (60.9) |
0.958 |
| Clear Cell Carcinoma |
708 (12.2) |
901 (12.6%) |
694 (12.4) |
855 (12.5) |
| Carcinosarcoma |
1786 (30.9) |
1683 (23.6%) |
1489 (26.7) |
1830 (26.6) |
| Lymph Nodes Dissection |
4937 (85.4%) |
6380 (89.6%) |
<0.001 |
4767 (85.5) |
6139 (89.4) |
<0.001 |
| Tumor Size (mm) |
59 (32 - 120) |
45 (27 - 92) |
<0.001 |
55 (30 - 115) |
48 (29 - 92) |
0.99 |
| FIGO stage, n (%) |
|
|
|
|
|
|
| I |
2988 (51.7%) |
4646 (65.2%) |
<0.001 |
3318 (59.5) |
4085 (59.5) |
0.991 |
| II |
571 (9.9%) |
525 (7.4%) |
473 (8.5) |
581 (8.5) |
| III |
2223 (38.4%) |
1952 (27.4%) |
1786 (32) |
2201 (32) |
| Chemotherapy, n (%) |
2156 (37.6%) |
2407 (33.9%) |
<0.001 |
2004 (35.9) |
2461 (35.8) |
0.912 |
| Days from surgery to chemotherapy |
41 (30-56) |
38 (29-51) |
<0.001 |
41 (30-56) |
38 (29-51) |
<0.001 |
| Radiation therapy, n (%) |
2101 (37%) |
2813 (40.1%) |
<0.001 |
2191 (39.3) |
2697 (39.3) |
0.992 |
| Length of Hospital Stay |
4 (3-5) |
1 (1-2) |
<0.001 |
4 (3 - 5) |
1 (1 - 2) |
<0.001 |
| Months from Surgery to Last visit |
53.55 (21.4 - 77.48) |
58.63 (29.21 - 75.55) |
<0.001 |
55.94 (23.57 - 78.94) |
57.22 (26.07 - 74.63) |
0.805 |
| Death, n (%) |
2839 (48.7%) |
2902 (40.3%) |
<0.001 |
2607 (46.7) |
2932 (42.7) |
<0.001 |
| 30 Day Readmission, n (%) |
289 (5.0%) |
177 (2.5%) |
|
266 (4.8) |
175 (2.6) |
<0.001 |
| 30 Day Mortality, n (%) |
76 (1.3%) |
37 (0.5%) |
<0.001 |
64 (1.2) |
37 (0.5) |
<0.001 |
| 90 Day Mortality, n (%) |
207 (3.6%) |
109 (1.5%) |
<0.001 |
165 (3) |
112 (1.6) |
<0.001 |
3.2. Survival analysis
The median follow-up was 54 months in the open group and 59 months in the MIS group. In multivariate analysis, demographic factors associated with worse OS include older age (HR 1.035, p<0.001) and African American race (HR 1.28, P<0.001). Pathologic factors associated with increased mortality were stage, histology type, and size of the primary tumor. Stage II and III were associated with worse OS (HR 1.8 and 3 respectively, p<0.001). Serous and carcinosarcoma histologies were associated with worse OS compared to clear cell (HR 1.2 and 1.7 respectively, P<0.001). Larger size of the primary tumor was also associated with decreased OS. Evaluation of adjuvant therapy modalities showed that both chemotherapy and radiation were associated with improved survival (HR 0.8 for both, p<0.001).
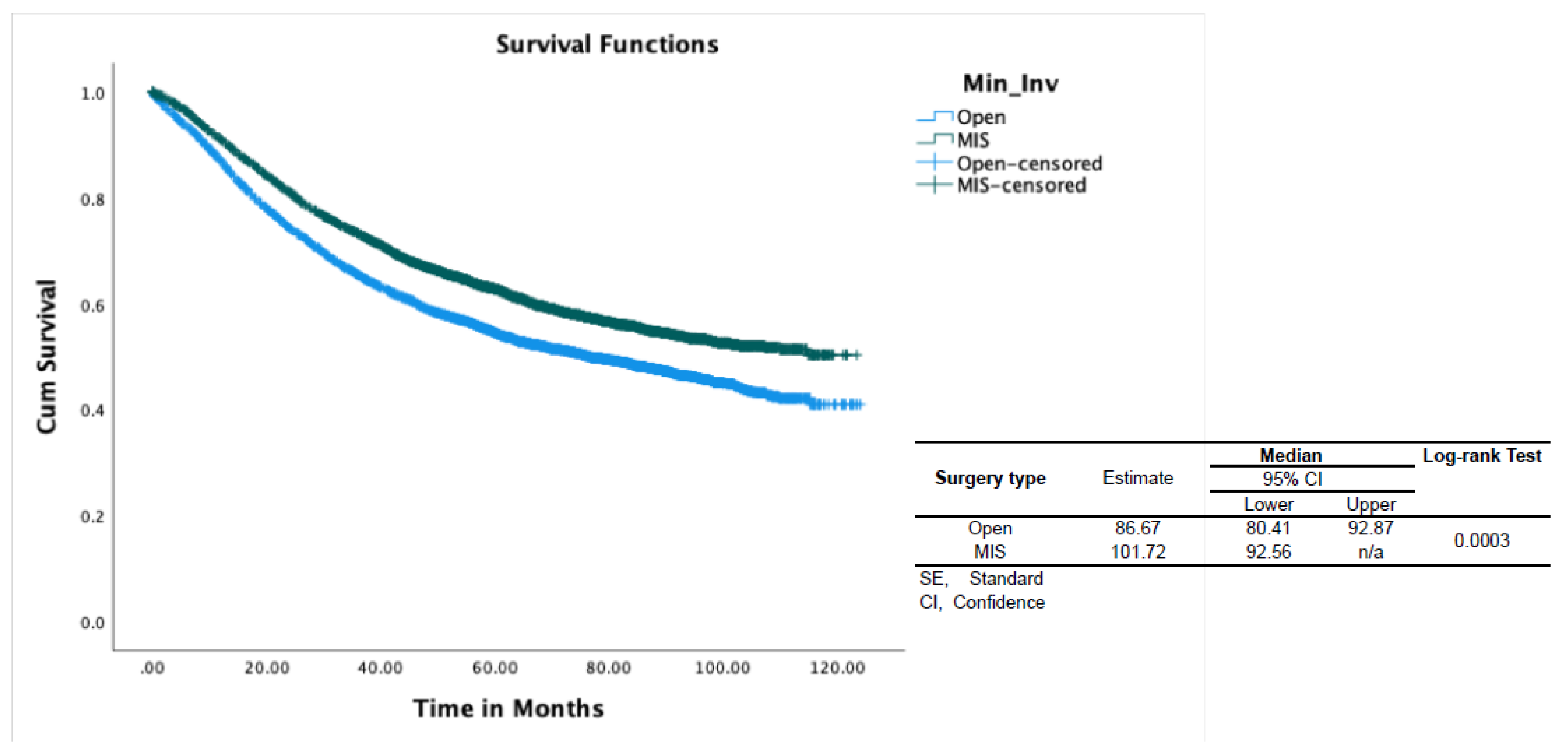
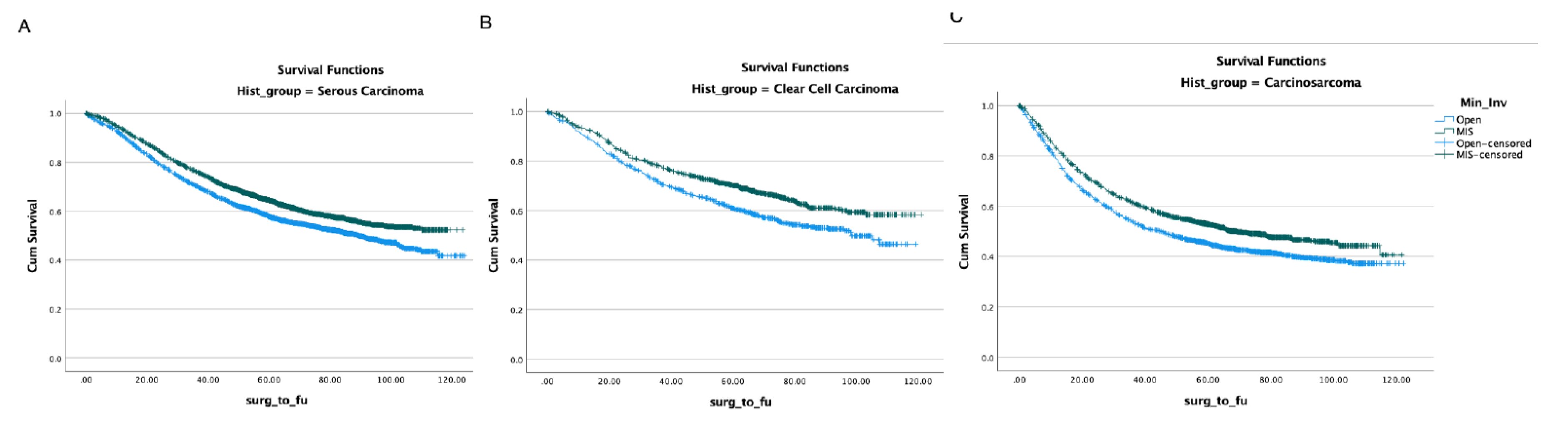
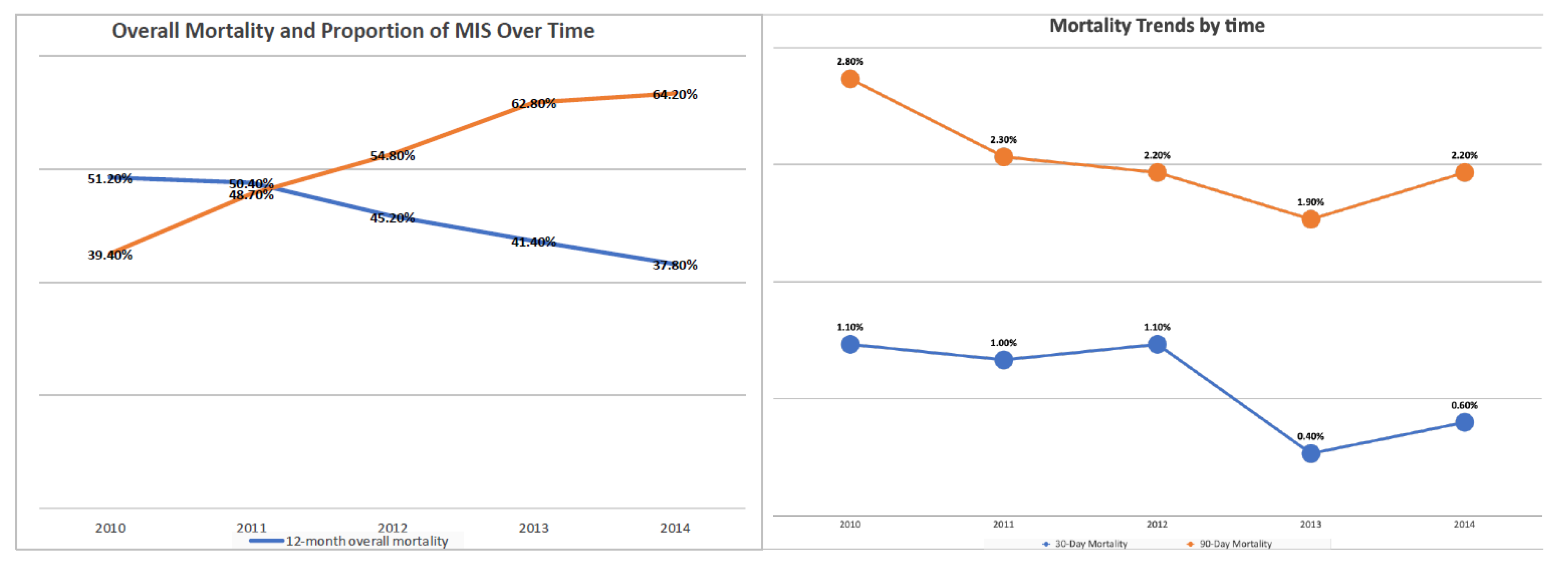
With regards to the surgical approach, MIS was associated with better OS (HR 0.9, p<0.001) (Table-2). In propensity-score–weighted analyses, MIS was associated with superior overall survival (102 vs 87 months, P=0.003 by the long-rank test) which corresponds to a 10% lower risk of death from any cause (HR 0.9; CI 0.857-0.954, p=0.0002). Weighted survival functions for the MIS group and the open group are plotted in Figure-1. MIS was associated with better survival outcomes across all three histology types and stages (Figure-2). The increased adoption of MIS from 2010 to 2014 corresponds to a decrease in the overall mortality (51% to 38% at 12 months, r= -0.95; p=0.006) (Figure-3).
Table 2.
Multivariable overall survival analysis of patients with Type II EC.
Table 2.
Multivariable overall survival analysis of patients with Type II EC.
| |
HR |
95% CI |
|
Sig. |
| Variables |
|
Lower |
Upper |
|
| Age |
1.032 |
1.029 |
1.035 |
<.001 |
| Race |
|
|
|
|
| White |
ref. |
ref. |
ref. |
ref. |
| Black |
1.261 |
1.184 |
1.343 |
<.001 |
| Asian/Pacific Islander |
0.871 |
0.73 |
1.038 |
0.123 |
| Other |
1.067 |
0.819 |
1.39 |
0.63 |
| Unknown |
1.126 |
0.861 |
1.474 |
0.386 |
| Histology |
|
|
|
|
| Serous Carcinoma |
ref. |
ref. |
ref. |
ref. |
| Clear Cell Carcinoma |
0.886 |
0.81 |
0.969 |
0.008 |
| Carcinosarcoma |
1.53 |
1.442 |
1.624 |
<.001 |
| FIGO stage |
|
|
|
|
| I |
ref. |
ref. |
ref. |
ref. |
| II |
1.775 |
1.609 |
1.957 |
<.001 |
| III |
3.107 |
2.933 |
3.291 |
<.001 |
| MIS |
0.905 |
0.857 |
0.956 |
<.001 |
| Chemotherapy |
0.77 |
0.725 |
0.817 |
<.001 |
| Radiation therapy |
0.801 |
0.756 |
0.848 |
<.001 |
| Lymph node dissection |
0.586 |
0.545 |
0.631 |
<.001 |
| Tumor Size (10mm increment) |
0.998 |
0.998 |
0.999 |
<.001 |
Figure 1.
Overall survival of patients with Type II endometrial cancer by surgical approach. CI, confidence interval; SE, standard error; OS, overall survival.
Figure 1.
Overall survival of patients with Type II endometrial cancer by surgical approach. CI, confidence interval; SE, standard error; OS, overall survival.
Figure 2.
Overall survival by surgical approach in different histology types. (A) serous carcinoma, (B) clear cell carcinoma, (C) carcinosarcoma.
Figure 2.
Overall survival by surgical approach in different histology types. (A) serous carcinoma, (B) clear cell carcinoma, (C) carcinosarcoma.
Figure 3.
The increased adoption of MIS from 2010 to 2014 corresponds to a decrease in the postoperative mortality and overall mortality.
Figure 3.
The increased adoption of MIS from 2010 to 2014 corresponds to a decrease in the postoperative mortality and overall mortality.
3.3. Perioperative outcomes
MIS was associated with superior perioperative outcomes. The mean length of stay was shorter in the MIS group (1 vs. 4 days with p-value <0.001). The 30-day readmission rate was higher in the open group (5% vs 2.5% with p-value<0.001). Furthermore, MIS was associated with lower 30 and 90-day postoperative mortality (0.5% vs 1.3% and 1.5% vs 3.6%, respectively) (p-value<0.001) (Table-1). The increase in the use of MIS between 2010 and 2014 corresponds to a decrease in postoperative death rates (Figure 3). The time interval from surgery to chemotherapy was shorter for the MIS group (38 vs 41 days, p=0.013) (Table-1).
Discussion
Our findings suggest that minimally invasive surgery is associated with better overall survival compared to open hysterectomy in patients with Type II uterine cancer. MIS was also associated with improved perioperative outcomes such as a shorter length of hospital stay, decreased readmission, and 30 and 90-day mortality rates. Prior studies have demonstrated superior perioperative outcomes with MIS without detrimental effect on the oncologic outcomes. However, this is the first study that demonstrates the survival benefit of MIS in Type II uterine cancer.
LAP-2 is a landmark study that established the oncologic safety of MIS in patients with endometrial cancer. In a posthoc analysis of LAP2 patients with uterine serous, clear cell, carcinosarcoma, and Grade III endometrial adenocarcinoma, there was no difference in progression-free and overall survival between MIS and open surgical approach [
9]. In the largest systematic review published to date, MIS is associated with improved perioperative outcomes and similar oncologic outcomes compared to open surgery [
10].
To our knowledge, the present study offers the largest analysis to date on the use of MIS in Type II uterine cancer. Likely, the larger number of patients that was included in this study, compared to prior studies provided sufficient power for the detection of the survival benefit offered by MIS. Furthermore, we assessed several potential confounding factors between the study groups, such as histology type, stage, tumor size, and adjuvant therapy. According to our data, MIS is an independent prognostic factor for improved survival in patients with Type II uterine cancer.
Several factors may be implicated in our study's superior survival outcomes with MIS. First, MIS is associated with decreased postoperative mortality rates at 30 and 90 days, affecting the overall survival of the study cohort. Second, the distinct biology of Type II uterine cancer may make this disease suitable for an MIS approach. Etiologic factors that have been implicated in the worse oncologic outcomes with MIS in cervical cancer include the increased propensity for tumor spillage due to the uterine manipulator [5, 10] or an effect of CO2 on tumor cell spread. Microscopic peritoneal spread at the time of surgery can lead to subsequent growth since most cervical cancer patients do not receive adjuvant therapy after a radical hysterectomy. However, most patients with Type II endometrial cancer receive adjuvant therapy, and chemotherapy has a role in managing all stages of the disease. Adjuvant therapy targets microscopic disease in the immediate postoperative setting, and treatment delays have been associated with worse outcomes in various malignancies [
12,
13]. A possible explanation for the superior survival outcomes with MIS in the present study is that the superior perioperative outcomes with MIS allow faster recovery and earlier initiation of adjuvant therapy leading to superior survival outcomes. Interestingly, the time interval from surgery to chemotherapy was shorter for the MIS cohort in our study. Furthermore, an association between surgical stress and immunosuppression has been well described in the literature. The use of MIS could likely decrease the surgical stress and the negative impact of surgery on the antitumor immune response [
14,
15,
16,
17].
We acknowledge several important limitations in our study. Although the NCDB captures women from a large number of hospitals, these data may not be representative of the entire population. Another significant limitation of this study is the absence of information about recurrence, subsequent treatment at the time of recurrence, and cause of death in the National Cancer Database. Furthermore, there is no information on factors affecting the selection between the open and MIS approach that could lead to potential selection bias. It may be suggested that high-risk patients (based on preoperative pathology or imaging) were selected to undergo open surgery. To eliminate the effect of confounding factors a propensity-score–weighted analysis was performed and demonstrated that MIS is independently associated with improved survival. Finally, operative morbidity is a significant factor that affects a surgical approach's choice, and our data lack information on that.
The histologic classification of endometrial cancer used in this study is prone to misdiagnosis, with studies indicating approximately 30% uncertainty among pathologists [18, 19, 20]. Molecular classification has become a valuable tool for prognosis assessment and treatment guidance in recent years. Endometrial cancer has been categorized into four molecular subgroups (POLE ultramutated, MSI-H, copy number high, and copy number low), each with distinct prognosis and response to treatment. Investigating the connection between tumor molecular classification and surgical approach could be a potential topic for future studies [
21].
In conclusion, our study includes a large cohort of patients with Type II uterine cancer derived from a nationwide, multicentered database. Based on our multivariate propensity score weighted analysis, MIS is associated with improved overall survival compared to open hysterectomy while offering improved postoperative outcomes. Based on this large data set, MIS, when feasible, should be the recommended surgical approach for patients with Type II uterine cancer.
Conflicts of Interest Statement
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Author Contributions Statement
The authors confirm contribution to the paper as follows: Study conception and design: Ioannis Alagkiozidis MD, Qi Zhang, MD; Data collection: Qi Zhang, MD. Analysis and interpretation of results: Michael Silver, MS, Qi Zhang, MD, Yi-Ju Amy Chen, MD, Jennifer Wolf, MD, Judy Hayek, MD, Ioannis Alagkiozidis MD. Draft manuscript preparation: Qi Zhang, MD, Ioannis Alagkiozidis MD. All authors reviewed the results and approved the final version of the manuscript.
References
- Bockman, J.V. Two pathogenic types of endometrial carcinoma. Gynecol Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.A.; Kapp DS Chan, J.K. Clinical aspects of uterine papillary serous carcinoma. Curr Opin Obstet Gynecol. 2008, 20, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Piedmonte, M.R.; Spirtos, N.M.; et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009, 27, 5331–5336. [Google Scholar] [CrossRef]
- Janda, M.; Gebski, V.; Davies, L.C.; Forder, P.; Brand, A.; Hogg, R.; Jobling, T.W.; Land, R.; Manolitsas, T.; Nascimento, M.; Neesham, D. Effect of Total Laparoscopic Hysterectomy vs Total Abdominal Hysterectomy on Disease-Free Survival Among Women with Stage I Endometrial CancerA Randomized Clinical Trial. JAMA 2017, 317, 1224–1233. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, M.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Le, T.; Hopkins, L.; Fung-Kee-Fung, M.; Lupe, K.; Gaudet, M.; Choan, E.; Samant, R. A comparison of disease recurrence between robotic versus laparotomy approach in patients with intermediate-risk endometrial cancer. Int. J. Gynecol. Cancer 2019, 30, 160–166. [Google Scholar] [CrossRef]
- Monterossi, G.; Ghezzi, F.; Vizza, E.; Zannoni, G.F.; Uccella, S.; Corrado, G.; Restaino, S.; Quagliozzi, L.; Casarin, J.; Dinoi, G.; Scambia, G. Minimally Invasive Approach in Type II Endometrial Cancer. JMIG 2017, 24, 438–445. [Google Scholar]
- Scaletta, G.; Dinoi, G.; Capozzi, V.; Cianci, S.; Pelligra, S.; Ergasti, R.; Fagotti, A.; Scambia, G.; Fanfani, F. Comparison of minimally invasive surgery with laparotomic approach in the treatment of high risk endometrial cancer: A systematic review. Eur. J. Surg. Oncol. (EJSO) 2019, 46, 782–788. [Google Scholar] [CrossRef]
- Impact of histology and surgical approach on survival among women with early-stage, high-grade uterine cancer: An NRG Oncology/Gynecologic Oncology Group ancillary analysis. Gynecol Oncol. 2016, 143, 460–465. [CrossRef]
- Chiva, L.; Zanagnolo, V.; Querleu, D.; Martin-Calvo, N.; Ar, J.; Capîlna, M.E.; et al. , SUCCOR study: an international European cohort observational study comparing minimally invasive surgery versus open abdominal radical hysterectomy in patients with stage IB1 cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 1269–1277. [Google Scholar] [CrossRef]
- Melamed, A.; Margul, D.J.; Chen, L.; Keating, N.L.; del Carmen, M.G.; Yang, J.; Seagle, B.-L.L.; Alexander, A.; Barber, E.L.; Rice, L.W.; et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. New Engl. J. Med. 2018, 379, 1905–1914. [Google Scholar] [CrossRef]
- Tewari, K.S.; Java, J.J.; Eskander, R.N.; et al. Early initiation of chemotherapy following complete resection of advanced ovarian cancer associated with improved survival: NRG Oncology/Gynecologic Oncology Group study. Annals of Oncology 2016, 27, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.; Clark, R.M.; Dizon, D.S.; Lee, M.S.; Goodman, A.; Boruta, D.; Schorge, J.O.; del Carmen, M.G.; Growdon, W.B. Delay in chemotherapy administration impacts survival in elderly patients with epithelial ovarian cancer. Gynecol. Oncol. 2015, 137, 401–405. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, P.; Xu, Y.; Yan, J.; Liu, Z.; Lau, W.B.; Lau, B.; Li, Y.; Zhao, X.; Wei, Y.; Zhou, S. Surgical stress and cancer progression: the twisted tango. Molecular Cancer 2019, 18, 132. [Google Scholar] [CrossRef] [PubMed]
- Bakos, O.; Lawson, C.; Rouleau, S.; Tai, L.-H. Combining surgery and immunotherapy: turning an immunosuppressive effect into a therapeutic opportunity. J. Immunother. Cancer 2018, 6, 86. [Google Scholar] [CrossRef]
- Krall, J.A.; Reinhardt, F.; Mercury, O.A.; Pattabiraman, D.R.; Brooks, M.W.; Dougan, M.; Lambert, A.W.; Bierie, B.; Ploegh, H.L.; Dougan, S.K.; et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Coffey, J.C.; Wang, J.H.; Smith, M.J.; Bouchier-Hayes, D.; Cotter, T.G.; Redmond, H.P. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 2003, 4, 760–768. [Google Scholar] [CrossRef]
- Alexa, M.; Hasenburg, A.; Battista, M.J. The TCGA molecular classification of endometrial cancer and its possible impact on adjuvant treatment decisions. Cancers 2021, 13, 1478. [Google Scholar] [CrossRef]
- Gilks, C.B.; Oliva, E.; Soslow, R.A. Poor Interobserver Reproducibility in the Diagnosis of High-grade Endometrial Carcinoma. Am. J. Surg. Pathol. 2013, 37, 874–881. [Google Scholar] [CrossRef]
- Han, G.; Sidhu, D.; Duggan, M.A.; Arseneau, J.; Cesari, M.; Clement, P.B.; Ewanowich, C.A.; Kalloger, S.E.; Köbel, M. Reproducibility of Histological Cell Type in High-Grade Endometrial Carcinoma. Mod. Pathol. 2013, 26, 1594–1604. [Google Scholar] [CrossRef]
- Levine, D; The Cancer Genome Atlas Research Network. A. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).