1. Introduction
Syringa pubescens Turcz. (Oleaceae), which is a deciduous shrub, is widely distributed in mountainous areas of Henan, Shanxi, Shaanxi, Hebei, Shandong Provinces and Beijing City [
1]. The dried flower of
Syringa pubescens (Syringae Pubescentem Flos, SPF) is often used as a Chinese folk medicine and flower tea for healthcare in Funiu Mountains in Henan Province. To date, modern scientific investigations on SPF have indicated that it has diverse biological and pharmacological properties including antioxidant [
2,
3], hepatoprotective [
4], anti-inflammatory and antibacterial [
5] activities. Furthermore, our recent study has demonstrated that SPF possessed strong inhibitory activity against α-glucosidase [
1]. These biological properties are due to the presence of chemical constituents in SPF. The main compounds in SPF are phenylethanoid glycosides and iridoids [
1,
2,
3,
6,
7].
Drying method of medicinal plants is one of key steps affecting their the compounds and biological activities [
8,
9,
10]. Among the various drying methods, shade-drying (SHD), sun-drying (SD) and oven drying (OD) are commonly used to process medical plants at industrial scales [
11,
12]. In recent years with studies conducted, novel drying methods including the microwave drying (MD) and infrared drying (IRD) have been developed for the plant materials drying [
13,
14]. Different drying method is suitable for different medicinal herbal because the drying process can lead to the loss of some compounds or can prevent the loss of some compounds under different treatment conditions. However, the drying method of SPF and the differences in main bioactive compounds during the different drying methods have not been studied. The correlation between SPF bioactivity and the drying method is lacking. Therefore, the aim of this present work was to explore the influences and changes of drying methods on the bioactive compound contents, the antioxidant capacities, anti-inflammatory properties and enzyme inhibition effects of SPF, to determine the suitable drying method.
2. Results and Discussion
2.1. The Contents of Bioactive Compound
The contents of bioactive compounds of SPF prepared using five drying methods were shown in
Table 1. The data revealed that echinacoisde, forsythoside B, verbascoside, and oleuropein were the major compounds of SPF extract, which was agreement with previous study [
1]. Meanwhile, it could be found that the contents of active ingredient exhibited significant differences under five drying methods (
p<0.05). The content of salidroside was the lowest content in SD method. The maximal ayringin was observed in SD (0.317±0.040 mg/g) > OD and SHD (0.161±0.005, 0.149±0.015 mg/g) > MD (0.028±0.004 mg/g) and minimal from IRD (0.018±0.007 mg/g). As for echinacoside, the contents from different drying methods were significant different (
p<0.05). The highest content of echinacoside was found in SD and the minimal in IRD. The contents of forsythoside B and verbascoside showed similar trend, and the concentrations in OD was higher than those in other drying methods. The oleuropein content was MD > SD > OD and SHD > IRD. It could be concluded that the drying of SPF should not be done using IRD, this was possible due to the fact that IRD could cause degradation in active compounds of SPF [
15]. Based on the contents of bioactive compound, MD, SD, OD and SHD could be used to treat SPF. Meanwhile, MD was the fastest method among these drying processes. However, SD, OD and SHD have been considered to be the most prevalent drying methods for industrial production [
16]. In consideration of energy savings, SD and SHD are the appropriate drying methods for SPF.
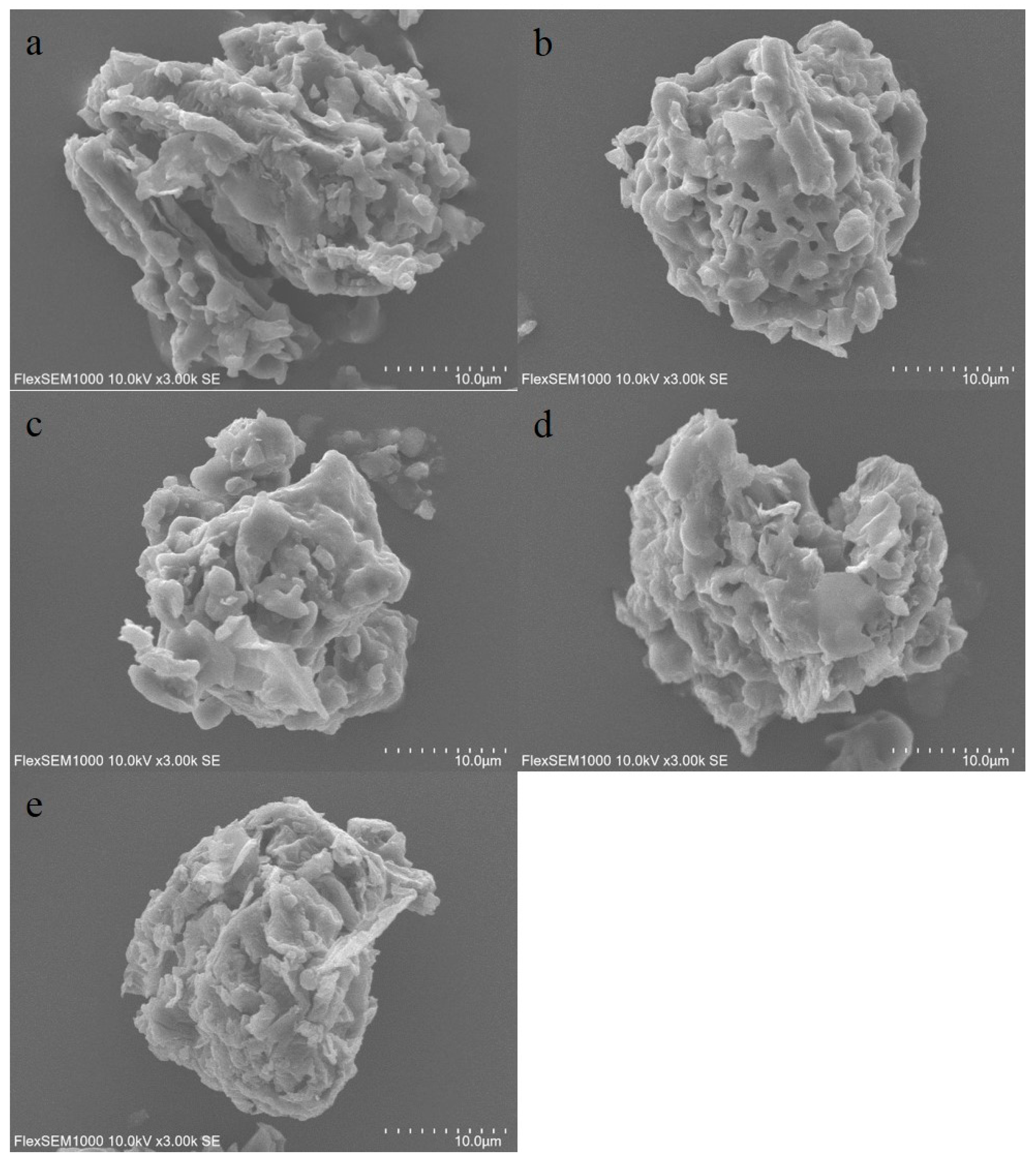
2.2. SEM Analysis
The powder characterization of SPF was used to assess the difference of samples treated by five drying methods.
Figure 1a–e showed the micro-morphology of different samples. It could be seen that powder features exhibited a rough and irregular surface. Meanwhile, it was evident that micro-morphological characters of samples treated by different drying methods possessed significantly difference. This reason was possibly attributed to the fact that five drying methods resulted in physical and chemical changes of SPF materials [
17].
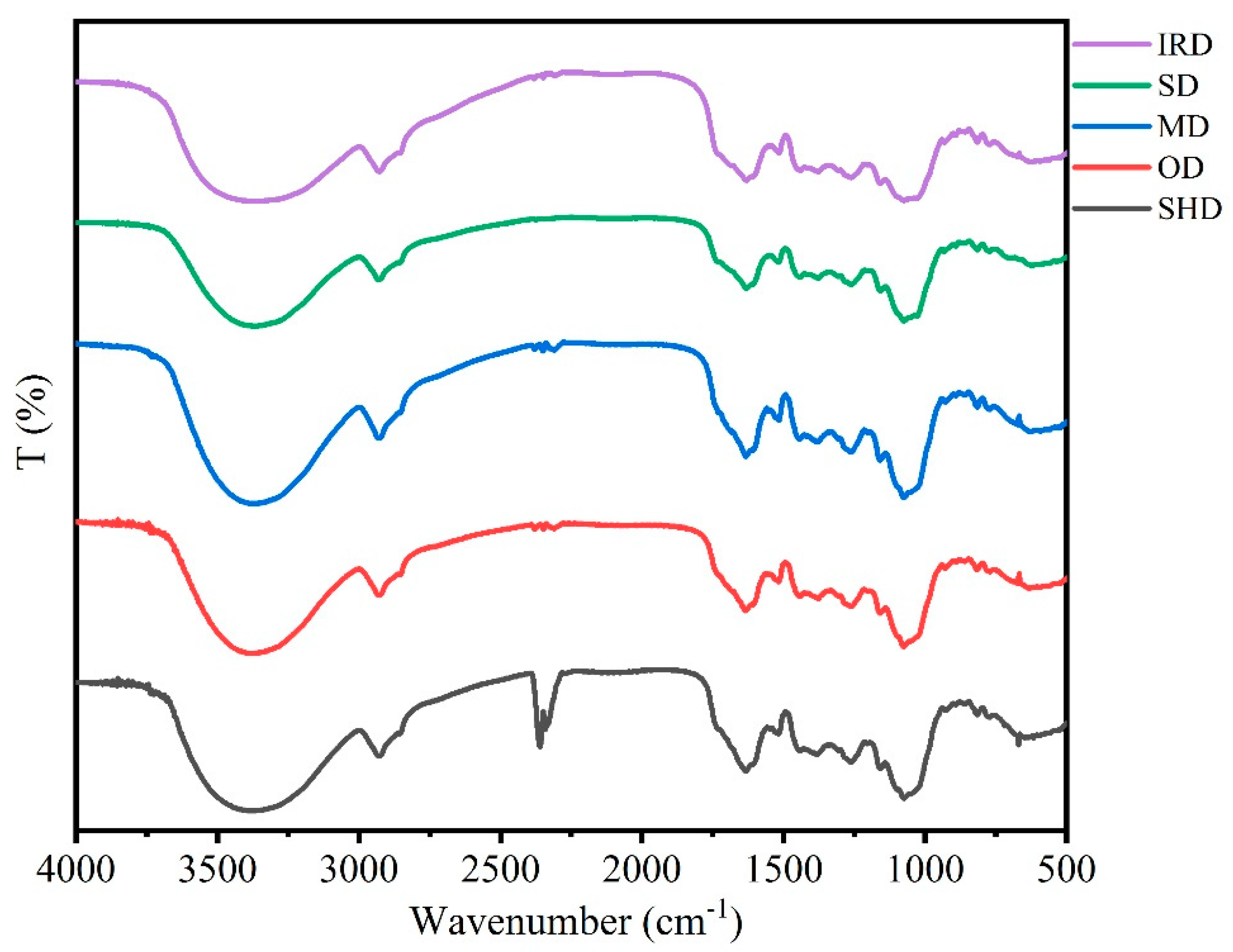
2.3. FT-IR Spectra Analysis
The intensity, shape and position of FT-IR spectra peaks from samples prepared by five drying methods were used to compare the differences and similarities (
Figure 2). It could be observed that the main chemical compounds of SPF samples dried by different methods were similar owing to the similarity of FT-IR spectra. The characteristic absorption peaks of glycosides including echinacoisde, forsythoside B, verbascoside and oleuropein could be found in the FT-IR spectra. On the other hand, differences of the positions and intensities in the FT-IR spectra were found in the 1700-850 cm
−1 wavenumber regions. The changes of position and intensity of the absorption peaks revealed the differences of component contents of samples prepared by five drying methods. Compared with other drying methods, the absorption bands of sample treated by IRD at 1634–1632 cm
-1, 1517-1512 cm
-1, 1382-1378 cm
-1, 1263-1256 cm
-1, 1157-1152 cm
-1, 1074-1066 cm
-1, 927 cm
-1 and 860-858 cm
-1 were low, suggesting that the active compounds might be degraded after the IRD process.
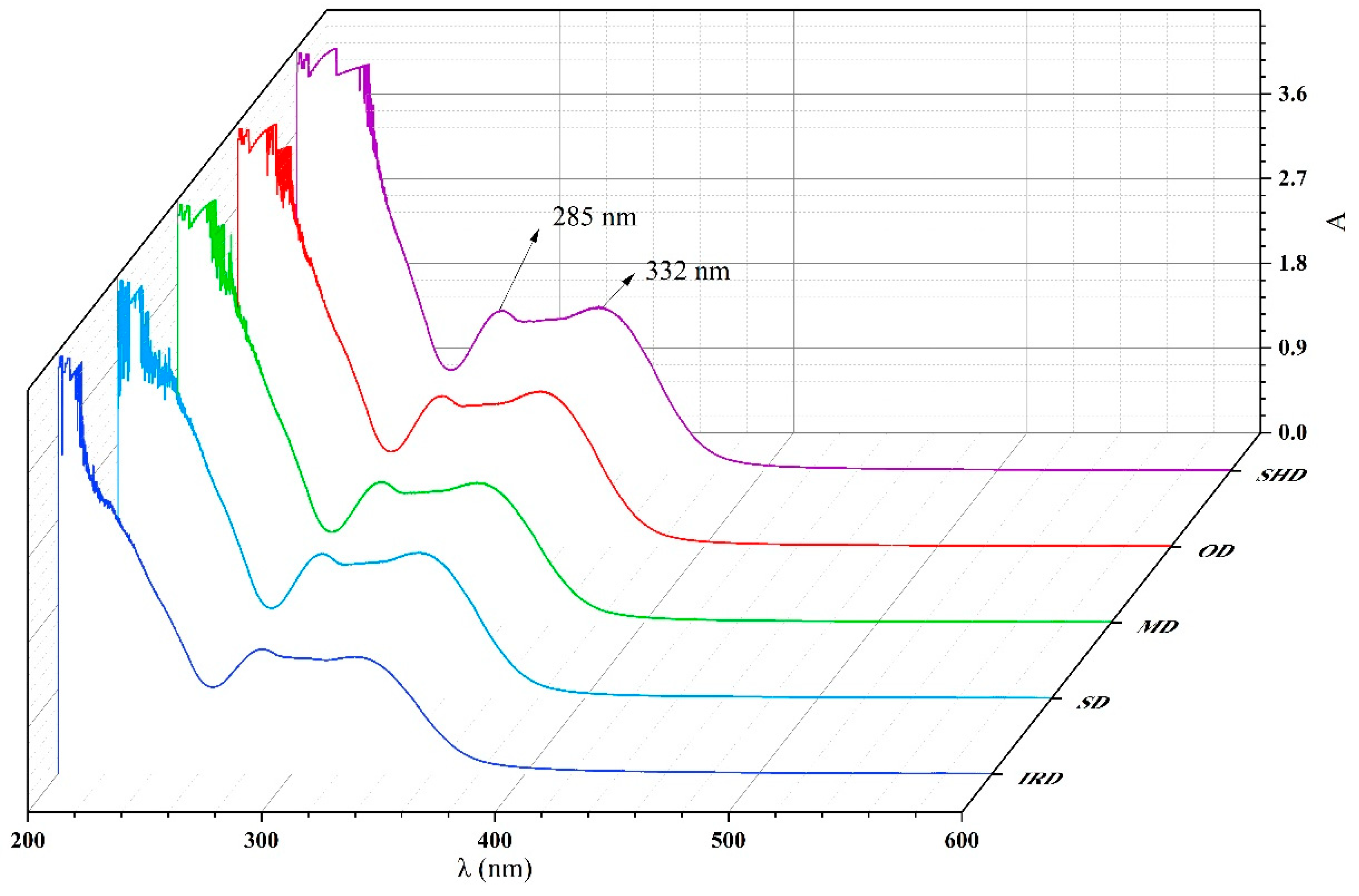
2.4. UV Spectroscopy Analysis
The UV-Vis spectra of the ethanol extract of five samples were illustrated in
Figure 3. The peaks at 285 and 332 nm were identified as oleuropein and phenylethanoid glycosides, respectively [
18,
19]. It could be observed that the major compounds of SPF extract after different drying methods were similar. However, there were some variation on absorbance values of samples prepared by the different drying methods.
2.5. Antioxidant Activities In Vitro
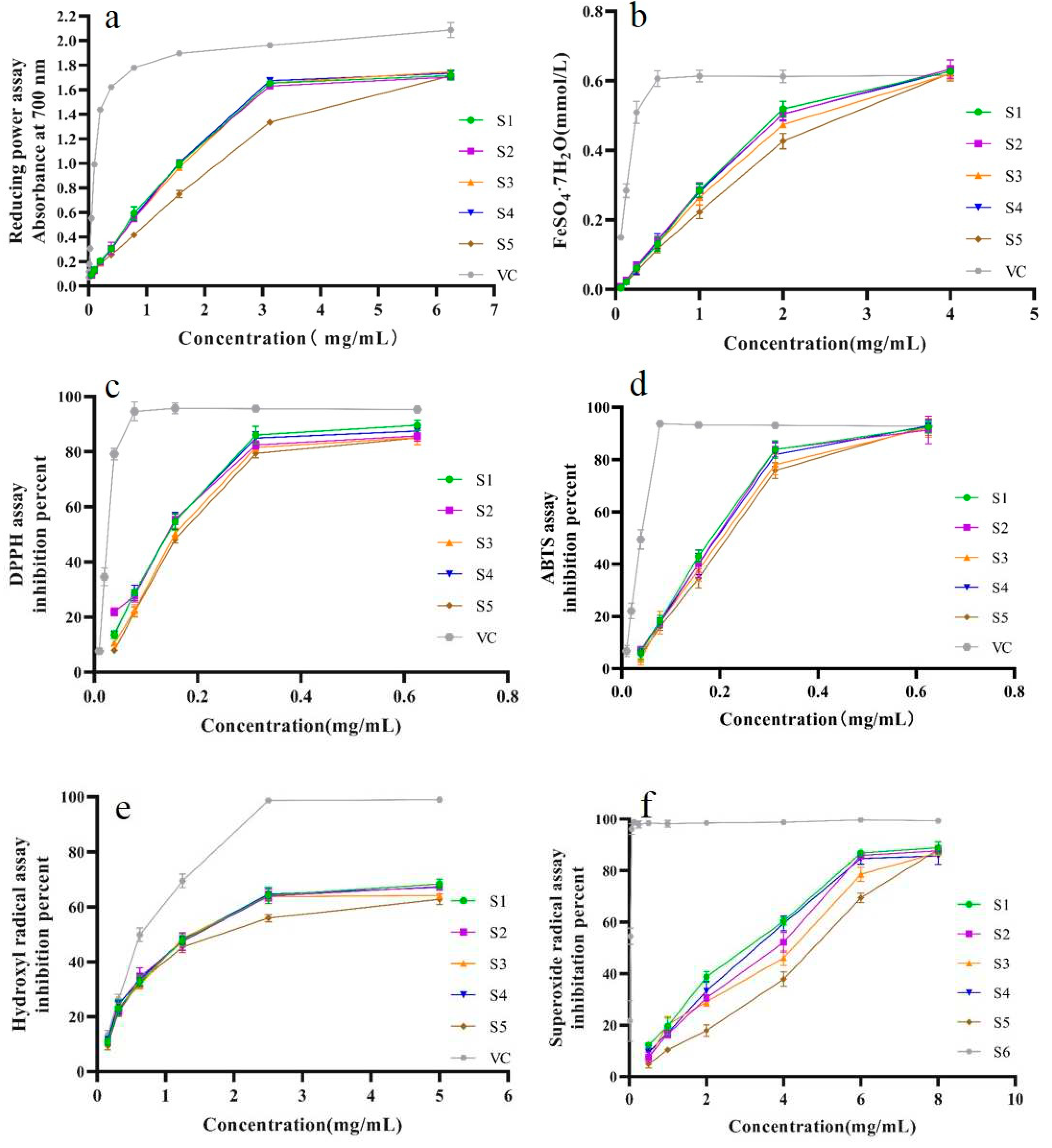
2.5.1. Influence of Five Drying Methods on Reducing Power
The reducing power of SPF sample was showed in
Figure 4a,b.
Figure 4a showed that reducing power of SPF samples from five drying methods lower than that of the VC in the testing concentration range. Meanwhile, compared with other drying methods, reducing capacity of sample after IRD was weaker. And the reducing capacities of the samples prepared by SD, SHD, MD and OD exhibited similarly trend and was linear within the concentration from 0 to 3.25 mg/mL. When the concentration was more than 3.25 mg/mL, the reducing power did not increase. Likewise, the results of FRAP confirmed SPF samples prepared by five drying methods possessed different antioxidant properties (
Figure 4b); meanwhile, sample after SHD had the highest antioxidant effect. However, SPF sample exhibited stronger antioxidant properties compared to the Fe(III) reducing power method. Furthermore, the antioxidant capacity of SPF sample was closer to that of the VC at the high concentration (4 mg/mL).
2.5.2. Influence of Different Drying Methods against DPPH, ABTS+ and •OH Scavenging Ability
In the present work, three free radicals including DPPH, ABTS
+ and •OH were used to investigate scavenging capacity of SPF sample.
Figure 4c showed DPPH·free radical scavenging effects of different SPF samples. It was evident that DPPH radical scavenging capacity of VC was significantly stronger than that of SPF sample within the test concentration (
p<0.05). The IC
50 values of samples prepared by SHD, OD, MD, SD and IRD were 0.111, 0.118, 0.144, 0.123 and 0.142 mg/mL, respective, and the sample after SHD possessed the strongest DPPH scavenging effect. The ABTS
+ radical scavenging effects of SPF sample were similar trends to that of DPPH radical scavenging effect (
Figure 4d). However, IC
50 values of ABTS
+ radical scavenging effect were higher than those of DPPH radical scavenging effect. The •OH scavenging effect of SPF samples was similar to those found for ABTS
+ and DPPH (
Figure 4e). However, the IC
50 values of •OH scavenging ability were higher compared to ABTS
+ and DPPH free radicals. Among five drying methods, the samples after SHD had the highest •OH scavenging effect with relative low IC
50 value of 1.521 mg/mL.
2.5.3. Influence of Different Drying Methods on Superoxide Radical Scavenging Effect
The SPF sample against superoxide radical scavenging activity was showed in
Figure 4f. In the test concentration range of 0.25-6.0 mg/mL, the superoxide radical scavenging abilities of SPF prepared by five drying methods were linear. Compared to the other SPF samples, the SHD sample possessed stronger superoxide radical scavenging capacity. Nevertheless, the activity of SPF sample was weaker than that of the VC.
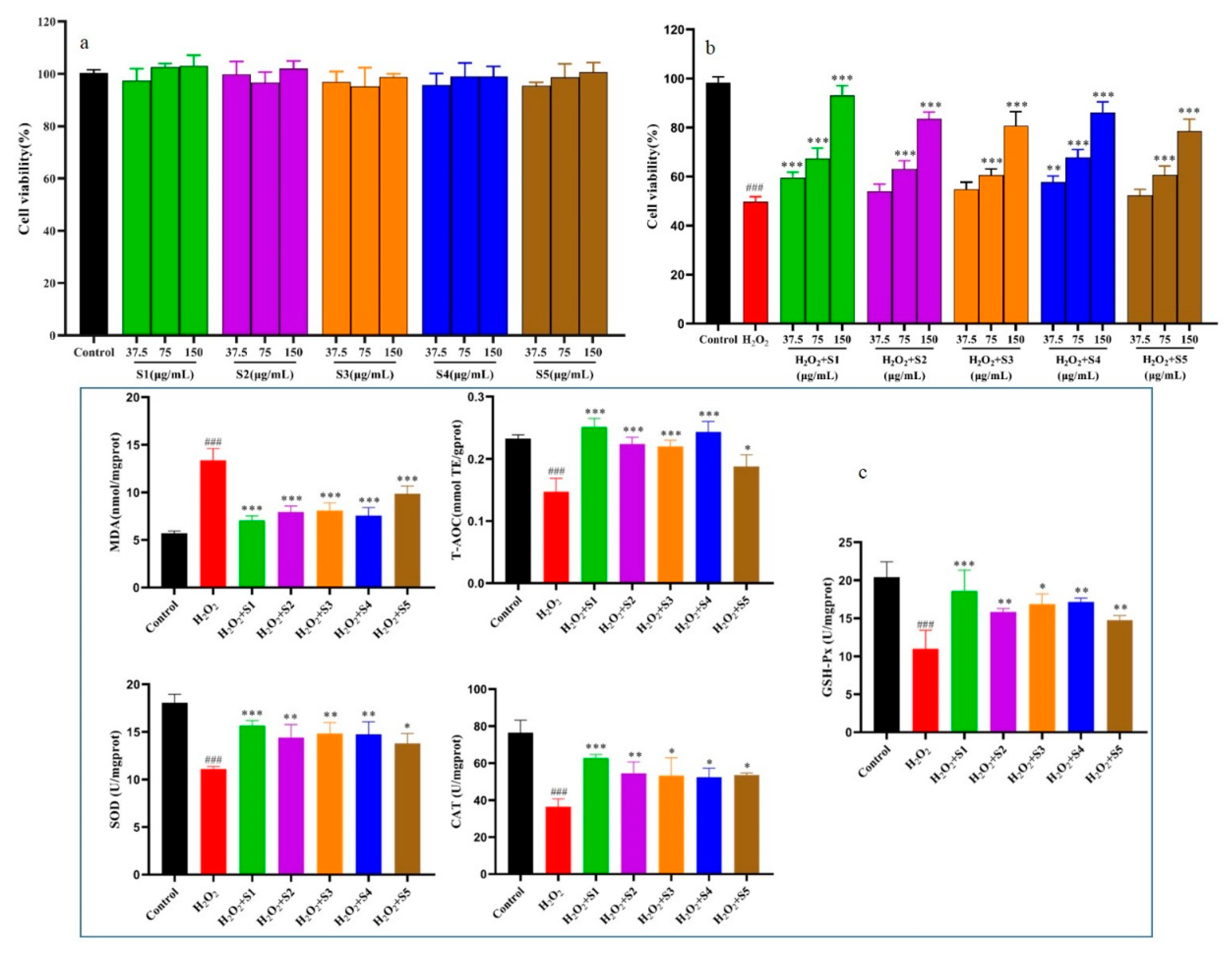
2.6. Protective Effect of SPF Extract on H2O2-Induced Oxidative Injury in L02 Cells
2.6.1. SPF of Cytotoxicity on L02 Cells
The cytotoxicity of SPF extract was showed
Figure 5a. It could be found that the cell viabilities treated by SPF extract were more than 95 % at the tested concentration (37.5 μg/mL, 75 μg/mL and 150 μg/mL), indicating that the SPF extract was non-cytotoxicity on L02 cells [
20]. Therefore, the SPF extract concentrations of 37.5 μg/mL, 75 μg/mL and 150 μg/mL were used in the following investigation.
2.6.2. Protective Effect of SPF Extract against Oxidative Damage by H2O2-Induced and Determination of Cells Biochemical Indexes
Protective effect of SPF extract on oxidative damage by H
2O
2-induced was displayed in
Figure 5b. Compared to control group, the cell survival rate induced by H
2O
2 significantly decreased at 150 μmol/L (
p<0.01). The cell viability notably increased after treatment with SPF extract (75 μg/mL and 150 μg/mL) compared with the H
2O
2 group. Meanwhile, the cell viability of SHD and SD samples exhibited an obvious increase at the concentration of 37.5 μg/mL (
p<0.01).
The results of SPF on levels of MDA, T-AOC, SOD, CAT and GSH-PX in the cells were showed
Figure 5c. It could be found that MDA level in H
2O
2 group significantly increased compared with the control group (
p<0.01), and obviously decreased in SPF groups (150 μg/mL) compared with H
2O
2 group (
p<0.01). Furthermore, MDA level treated by SPF prepared by SHD was lower than that of the other SPF groups. The antioxidant enzymes activities including T-AOC, SOD, CAT and GSH-PX in H
2O
2 group significantly decreased compared with control group (
p<0.01). The antioxidant enzymes activities in SPF groups (150 μg/mL) considerably increased compared with H
2O
2 group (
p<0.01). Meanwhile, the increase trends antioxidant enzymes activities were similar. As for T-AOC, SHD group had relatively strong enzyme activity, followed by SD group, OD group, MD group and IRD group. The findings obtained from this study were consistent with previous studies that the cells exposed to excessive hydrogen peroxide, the enzymatic activities were remarkably decreased together with an increase in the MDA level [
21]. These results demonstrated that SPF could ameliorate oxidative damage by H
2O
2-induced in L02 cells
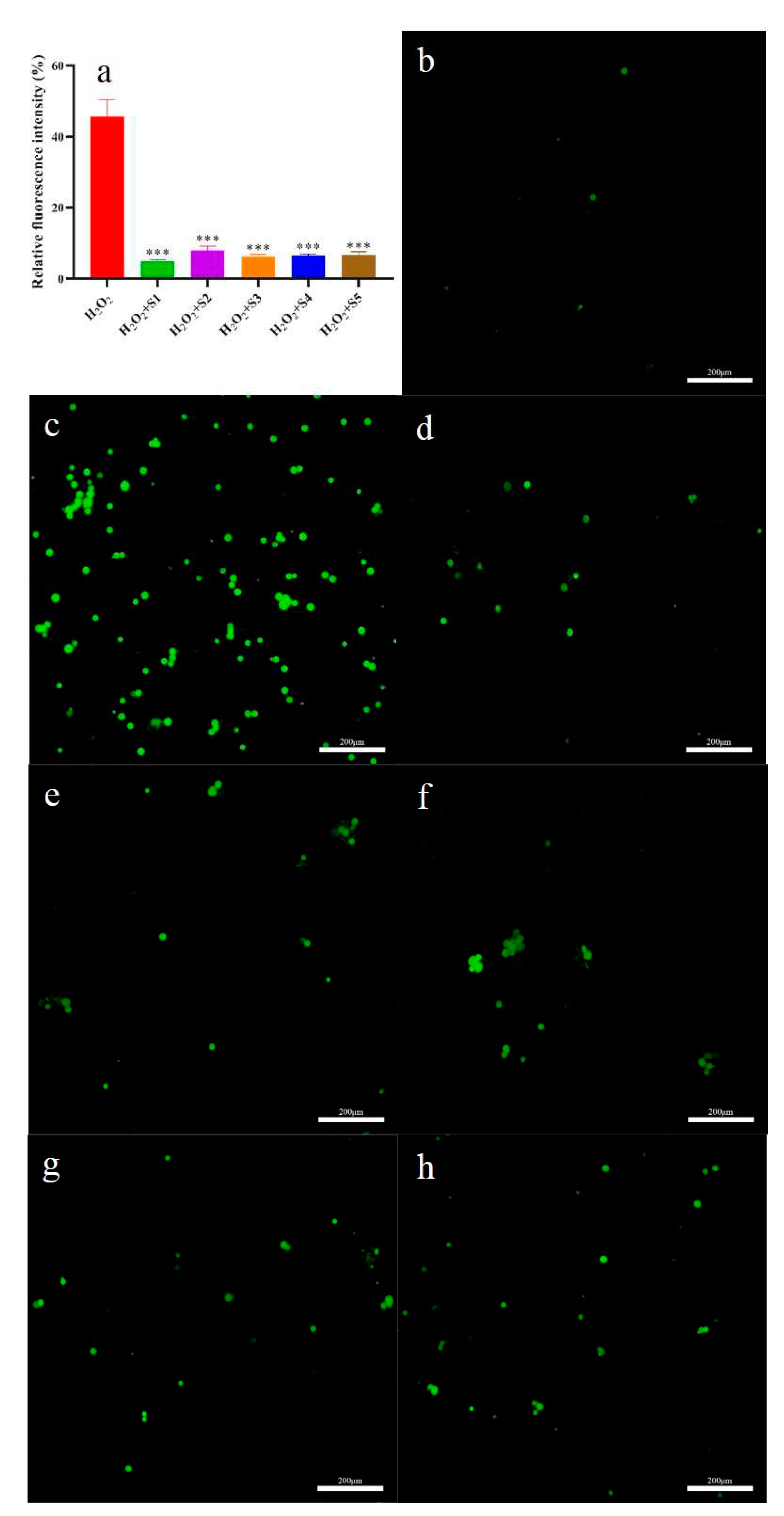
2.6.3. Intracellular ROS Evaluation
The level of intracellular ROS and ROS scavenging ability of SPF were given
Figure 6a–h. The highest intracellular ROS level was found in the H
2O
2 group among the test groups (
Figure 6c). Compared to the H
2O
2 group, the intracellular ROS level in the SPF groups significantly decreased (
p<0.05). Meanwhile, intracellular ROS level in the SHD group was lowest (
Figure 6a, 6d). These results indicated that SPF could scavenge the intracellular ROS. Similar results have been obtained by Shen, et al. [
22], who confirmed that phenylethanoid glycosides from
Incarvillea compacta possessed intracellular ROS scavenging capacity.
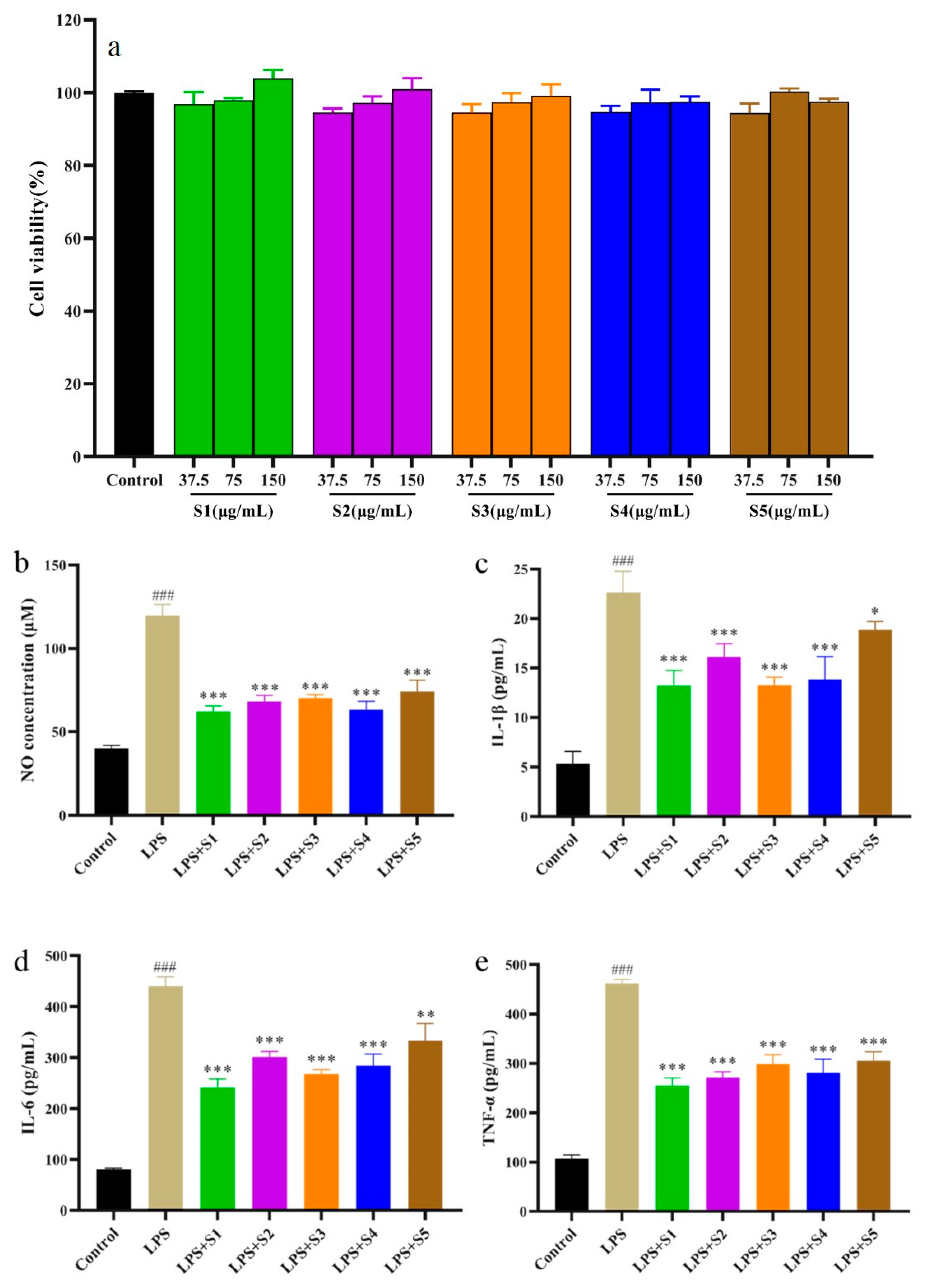
2.7. Anti-Inflammatory Activity of SPF
2.7.1. Cytotoxicity of SPF
The cytotoxicity of SPF extract on RAW264.7 cells was showed
Figure 7a. Compared with the control group, the viability of RAW264.7 cells exceeded 90%, suggesting SPF extract (37.5, 75, 150 μg/mL) showed non-cytotoxicity to RAW264.7 cells.
2.7.2. Determination of NO, IL-6, IL-1β, and TNF-α of RAW.264.7Cells
The NO is an inflammatory mediator and plays a vital role the pathogenesis of inflammation [
23]. The results were showed in
Figure 7b. Compared with control group, the NO production significantly increased (
p<0.01). However, NO production in SPF groups were remarkably suppressed compared with LPS group. Meanwhile, SPF samples prepared by SHD and SD strongly possessed inhibition on the NO production.
The inflammation process could lead to overexpression of inflammatory cytokines including IL-6, IL-1β, and TNF-α [
24,
25]. These inflammatory factors can promote the development of degree of inflammation. It could be found from
Figure 7c-e that the levels of IL-6, IL-1β, and TNF-α in RAW264.7 cells simulated by LPS notably increased compared with control group (
p<0.01). However, the levels of inflammatory factors in SPF groups significantly reduced compared with the LPS group. Furthermore, the SPF sample prepared by SHD could evidently inhibit the expression of IL-6, IL-1β, and TNF-α.
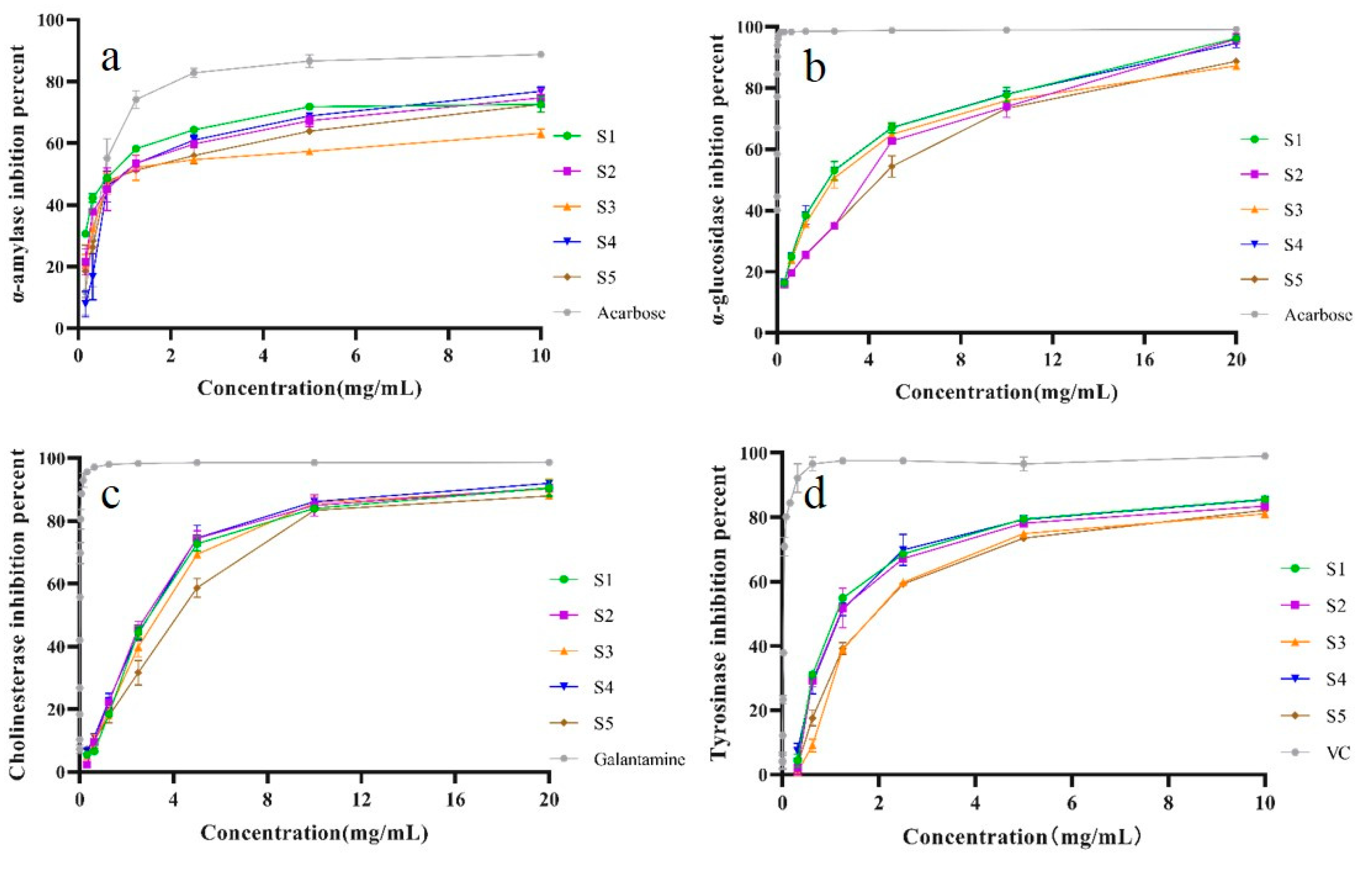
2.8. Enzyme Inhibitory Activity
The α-amylase and α-glucosidase are the key enzymes hydrolyzing carbohydrates into glucose [
26], which causes increase of postprandial blood glucose. The inhibition activities on α-amylase and α-glucosidase of SPF extracts were presented
Figure 8a-b. It could be observed that SPF extract exhibited enzyme properties in a concentration dependent manner. Meanwhile, the IC
50 value of sample prepared by SHD was lower than that of other samples. However, IC
50 values of all tested SPF samples were higher than that of positive reference (Acarbose). These results were compatible with our previous work that different original sample possessed α-glucosidase inhibition activities [
1].
As ROS have been confirmed to be strongly correlated with neurodegenerative diseases such as Alzheimer’s disease and Parkinson disease [
27,
28]. Previous studies have revealed that inflammation is involved in Alzheimer’s disease and Parkinson disease [
29,
30]. The SPF extract possessed strongly antioxidant and anti-inflammatory activity [
6,
7]. The inhibition of AChE has been considered as the main approach for treatment of Alzheimer’s disease [
31]. Moreover, studies have indicated that the inhibition of tyrosinase is a vital target in developing new drug for Parkinson’s disease [
32]. In this study, enzymes inhibitory activities of SPF samples prepared by five drying methods were investigated. The results were presented in
Figure 8c-d. It was evident that SPF extracts possessed enzymes inhibitory activities in a. As for AChE inhibitory activity, the IC
50 value of sample after SD was lower than that of other samples. Compared with all tested samples, the IC
50 value of control was lower. Meanwhile, we also found a dose-response effect of tryosimase inhibitory activity of SPF sample (
Figure 8). It could be observed that IC
50 values (1.717±0.188, 1.723±0.058, Supplementary
Figure 3) of samples after SHD and SD were lower that of other samples. The IC
50 value of control (VC) was 0.033±0.003, which was significantly lower than that of all tested SPF samples. The findings obtained from this work showed that SPF had strong AChE and tyrosimase inhibition degree and the sample prepared SHD and SD possessed higher enzyme inhibitory capacity.
3. Materials and Methods
3.1. Plant Materials and Reagents
The flowers of Syringa pubescens Turcz. were obatined from Xin’an County, Henan Province, China and were identified by Professor Yanfang Wu. Standard reference compounds, namely, salidroside, syringin, echinacoisde, forsythoside B, verbascoside, isoacteoside and oleuropein were purchased from Chengdu Biopurify Phytochemicals Ltd. (Chengdu, China), and the purity was more than 98 %. 2,2′-Azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,4,6-tris (2-pyridyl)-S-triazine(TPTZ), α-glucosidase (from baker’s yeast), p-nitrophenyl-α-Dglucopyranoside (pNPG) were provided by Aladdin Chemical Co. (Shanghai, China). The α-amylase, cholinesterase and tyrosinase were obtained from Macklin (Shanghai, China). All other chemicals and solvents used were of analytical grade.
3.2. Drying Methods
The equal amounts of Syringa pubescens flowers were processed using five different drying methods. For the SD method, the fresh flower samples were exposed to directly to the sunlight at well ventilated place. For the SHD method, the fresh flower samples were placed under the shade in a laboratory. The temperature of this laboratory was 25 oC with appropriate ventilation. In regards to the OD method, the process was conducted in a BPG-9106A drying oven (Yiheng Scientific Instrument Co., Shanghai, China) at 45 oC. For the MD method, the process was performed at 700W in an PM20A0 microwave oven (Midea Group Co.,Ltd., Guangdong, China). For the IRD method, the drying process was carried out in an infrared drying oven (Hangzhou Qiwei Instrument Co., LTD,. Hangzhou, China). All samples were processed by different drying methods to constant weight, and then were powdered using a mill and sieved through a 60-mesh sieve. The sample powder prepared by five drying methods were kept at 4 oC until use.
3.3. Preparation of the Standard Solution
A mixed stock solution, which contains 90.00 μg/mL of salidroside, 63.64 μg/mL of syringing, 86.36 μg/mL of echinacoisde, 86.36 μg/mL of forsythoside B, 94.55 μg/mL of verbascoside, 90.91 μg/mL of isoacteoside and 70.00 μg/mL of oleuropein, was prepared according to previous study [
1]. The solution was serially diluted in methanol to plot calibration curves.
3.4. Extraction of Bioactive Compounds of the SPF Samples
The extraction was conducted according to previous study [
1]. Briefly, 2 g sample powder was accurately weighed and mixed with 20 mL of 70%. The extraction was conducted using a Ymnl-2008D ultrasonic probe extraction device (Nanjing Immanuel Instrument Equipment Co. Ltd., Nanjing, China) for 40 min at 40
oC. After extraction, the crude extract was subjected to centrifuge at 12,000 rpm for 15 min. The supernatant was transferred into a volumetric flask of 25 mL and diluted to volume using 70% ethanol.
3.5. Chromatographic Methods
HPLC analysis was performed on an Eclassical 3200 HPLC system (Dalian Elite Analytical Instrument Co., Ltd., Dalian, China) equipped with an ultraviolet detector, an autosampler, a binary pump, and a column oven. The separation of active compounds was conducted with an Elite Supersil ODS2 column (4.6mm × 250 mm, 5 μm, Dalian Elite Analytical Instrument Co., Ltd., Dalian, China). The chromatographic conditions were the same as those described by Wang, Zhang, Wang, Zhang, Yang, Zhang, Ma, Wu, Ma and Fan [
1].
3.6. Scanning Electron Microscopy (SEM)
After drying treatments, the morphological feature of sample was analyzed with an SEM (FlexSEM 1000, Hitachi, Tokyo, Japan). The sample powers were placed on conductive adhesive tape and sprayed with a very thin layer of gold for SEM analysis.
3.7. FT-IR Analysis
An IRTracer-100 spectrometer (Shimadzu, Japan) was employed to record the diffuse reflectance spectra of the SPF powder. Briefly, the KBr powder was added into each sample, and then the mixture was ground again, and pressed into a tablet. The FTIR spectra were recorded in the range of 4,000-400 cm-1 at resolution of 4 cm-1 with 16 scans for each spectrum.
3.8. UV Spectroscopy
A UV-2700 spectrophotometer (Shimadzu, Japan) was empolyed to measure the absorbance of the ethanol extract within the wavelength range of 600–200 nm. The characteristic peaks were identified and the peak values were recorded.
3.9. Assay of Antioxidant Activities
3.9.1. Determination of Reducing Capacity
The measure method of reducing power was performed by our previously published work [
33]. The absorbance at 700 nm was determined as the reducing capacity. In all the antioxidant activity experiments, the Vitamin C (VC) was used as a positive reference.
3.9.2. Assay of the Ferric Reducing Antioxidant Power (FRAP)
The reducing power was also evaluated using the FRAP method according to the study reported by Yang, Wang, Zhang, Li, Wang, Zhang, Liu, Wu, Shen and Ma [
2]. In this study, the FeSO
4 solution was employed to plot linear regression equation as follows: Y = 5.8853x + 0.1395 (R
2 = 0.999).
3.9.3. DPPH and ABTS Radical Scavenging Assay
The DPPH and ABTS radical scavenging capacities of SPF extracts were performed as the method described by Wu, et al. [
34].
3.9.4. Hydroxyl Radical (•OH) Scavenging Assay
The •OH scavenging ability was measured using the orthophenanthroline method [
35] with a slight adjustment. Briefly, the mixed solution containing 1mL sample, 1 mL phosphate buffer solution (0.2 M, pH 7.4), 1 mL orthophenanthroline (2.5 mM), 1 mL ferrous sulfate solution (2.5 mM) and 1 mL H
2O
2 was incubated for 60 min at 37
oC. Then, the absorbance value was recorded as
Ai at 532 nm. The absorbance value was recorded as
A0 without tested sample. The ·OH scavenging ability was obtained as following equation (1).
3.9.5. Superoxide Radical (O2•-) Scavenging Assay
The ability of SPF extract to scavenge O
2•- was evaluated with a pyrogallol auto-oxidation system [
36] with a few adjustments. Briefly, the mixed solution containing 0.5 mL Tris–HCl buffer (pH 8.2, 50 mM) and 0.4mL sample solution was kept for 10 min at 25
oC. Then 150 μL pyrogallol solution (3mM) was added and incubated for 5 min at 25
oC. The mixed reaction system was ended after added 150 μL HCl. The absorbance value of mixed solution at 325 nm was recorded as
Ai. The absorbance value was recorded as
A0 without tested sample. The superoxide radicals scavenging ability was calculated using equation (1).
3.9.6. Protective Effect of SPF Extract on H2O2-Induced Oxidative Injury in L02 Cells
3.9.6.1. SPF of Cytotoxicity on L02
Hepatic L02 cells were cultured as described by Wang, et al. [
37]. Briefly, the DMEM medium containing 10% FBS, penicillin (100 U/mL) and streptomycin (100 μg/mL) were used to culture L02 cells. And then, L02 cells were placed in a humidified incubator at 37
oC under 5% CO
2 with continuous growth for 48 h. The obtained L02 cells were seeded at a density of 1.0 × 10
5 cells/mL with 100 μL/well. The L02 cells were continuously incubated to adherence, the initial culture medium was removed. The L02 cells were pretreated using different concentrations of SPF extract dissolved in DMSO, and DMEM and DMSO were used as references. The L02 cells were continued to be treated for an additional 24h, the supernatants were removed, and PBS was used to wash cells for 3 times. The cell viability was measured using MTT assay.
3.9.6.2. Protective Effect of SPF Extract against Oxidative Damage by H2O2-Induced and Determination of Cells Biochemical Indexes
The protective effect of five drying method SPF extract against H
2O
2-induced oxidative injury in hepatic L02 was measured as the method reported by Chen, et al. [
38]. Briefly, after the L02 cells were incubated in 96-well at 37.0
oC for 24 h, different concentrations of SPF extract were added to 96-well and treated for 12 h. And then 100 μL of H
2O
2 (300μmol/L) was transferred into 96-well and incubated for 6 h to induce oxidative damage. MTT was added and the absorbance value was read at 492 nm. Meanwhile, the cell culture media induced by H
2O
2 were collected to determine the biochemical indexes. The parameters including SOD, GSH-PX, MDA, CAT and T-AOC were measured using detection kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) based on the manufacturers’ instructions.
3.9.6.3. Intracellular ROS Evaluation
The assay of intracellular ROS was conducted based on the previous study [
38]. Briefly, the oxidative damage was generated according to the above mentioned method. Each well was added with DCFH-DA (10 μM). After incubation for 1 h, the intracellular ROS levels were measured using fluorescent microscope (Olympus IX73, Olympus Corporation, Tokyo, Japan). The cells induced-H
2O
2 were used as negative reference.
3.10. Anti-Inflammatory Property of SPF in LPS-Activated RAW264. 7 Cells
3.10.1. Cell Culture and Cytotoxic Effect of SPF
RAW 264.7 cells were provided by Procell Life Science & Technology Co., Ltd. (Wuhan, China). The DMEM containing 10% FBS and 1% streptomycin/penicillin was employed to culture RAW 264.7 cells under 37
oC and 5% CO
2 conditions. The cytotoxicity of SPF extract was assessed by the method reported by Zhen, et al. [
39]. Briefly, RAW 264.7 cells were incubated in 96-well plates (1×10
5 cells/well). After 24 h, different concentrations SPF extract (37.5, 75, 150 μg/mL) were added to 96-well for another 24 h. Then, the MTT (20 μl) was added to each well and cell culture was kept for 4 h. The absorbance value of cell culture was read at 570 nm to evaluate cytotoxicity of SPF extract.
3.10.2. Determination of NO, IL-6, IL-1β, and TNF-α of RAW.264.7Cells
The RAW.264.7 cells were transferred into a 6-well plate and treated for 24 h. And then, different concentrations of SPF extract were added to test well and cultured for 2 h. Finally, LPS solution was added to induce inflammation models and kept for additional 24 h. The cell culture media was obtained by centrifugation. The NO, IL-6, IL-10, and TNF-α were measured with ELISA kits based on the kit instructions.
3.11. Enzyme Inhibitory Activity
3.11.1. α-Amylase Inhibitory Property Assay
The α-amylase inhibitory activity was determined using starch as a substrate as reported previously [
40] with slight adjustments. The testing sample solution (1 mL) and 0.5 mL of α-amylase solution dissolved in phosphate buffer were mixed and kept for 5 min at 37
oC. And then the reaction system was activated by adding the starch solution (0.5mL) and incubated 5 min at 37
oC. The reaction was terminated by the addition of HCl (1mL, 1 M). Finally, the I
2-KI solution (0.5 mL) was added to the reaction mixtures. The absorbance value was read as
Ai at 630 nm. The absorbance value was read as
A0 without tested sample. The acarbose was sued as a positive reference. The α-amylase inhibition activity was calculated with the equation (2).
3.11.2. Inhibitory Activity on α-Glucosidase
The inhibitory activity on α-glucosidase was investigated based on our previous study [
1]. Briefly, the reaction system containing 100 μL testing sample, 100 μL glutathione, 100 μL α-glucosidase solution dissolved in phosphate buffer (pH 6.8) and PNPG (50 μL) was treated for 15 min at 37
oC. After pretreatment, the mixed solution was terminated by addition 100 μL sodium carbonate (0.2 M). The absorbance value was read as
Ai at 405 nm. The absorbance value was read as
A0 without α-glucosidase. The α-glucosidase inhibition activity was calculated with the equation (2).
3.11.3. Inhibitory Activity on Tyrosinase Assay
The inhibitory property on tyrosinase was measured as previously published paper [
41]. Briefly, the reaction system containing L-DOPA or L-tyrosine aqueous solution (50μL, 2 mM) and SPF solution was treated at 37
oC for 10 min. And then, the tyrosinase (100 μg/mL) was mixed to the solution to activate the reaction. After 10 min, the absorbance value with sample was read as
Ai at 475 nm. The absorbance value without sample was read as
A1. The absorbance value without tyrosinase was read as
A0. The tyrosinase inhibitory activity was calculated with the equation (3).
3.11.4. Cholinesterase Inhibitory Activity Assay
The inhibitory activity ChE was assessed using previously reported method [
42]. The reaction system containing different concentration SPF extract (50 μL), DTNB solution (125 μL), AChE or BChE solution dissolved in Tris-HCl buffer (pH 8.0) (25 μL) was incubated for 15 min at 25
oC. The absorbance value with sample was read as
Ai at 405 nm, and without enzyme was read as
A0. The ChE inhibitory activity was calculated with the equation (2).
4. Conclusions
In this work, the influences of five drying methods on the active ingredient contents, antioxidant property, anti-inflammatory activity and enzyme inhibition activity of SPF were investigated for the first time. The results obtained from this study demonstrated that drying methods could considerably influence active compounds and biological activities. On the whole, SHD and SD were the suitable to dry the SPF because of high bioactive constituent contents, strong antioxidant activity, high anti-inflammatory property and enzyme inhibitory activity.
Author Contributions
Investigation, Data curation, Writing-Original draft preparation, W.-D. X.; J.-M. Z.; Z.-C. Z.; Provided analysis samples, Writing-draft revised, Project administration Y.-F. W.; Writing-review and editing, Funding acquisition, Project administration X.-S. W.; Writing-review, Project administration J.-Y. M. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All data is included in the manuscript in the form of figures and tables.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. U1804175), the Joint Fund of Provincial Science and Technology Research and Development plan of Henan Province (232103810055, 232301420100), the College Students’ innovation and entrepreneurship training program (2023176, 2023363).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Wang, X.; Zhang, J.; Wang, P.; Zhang, Y.; Yang, Q.; Zhang, Z.; Ma, Z.; Wu, Y.; Ma, J.; Fan, E. , Evaluation of alpha-glucosidase inhibition activity and glycosides in the Syringa pubescens Turcz from different geographical origin. Scientia Horticulturae 2023, 320, 112198. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Zhang, Y.; Li, M.; Wang, P.; Zhang, J.; Liu, K.; Wu, Y.; Shen, G.; Ma, Z. , Simultaneous Determination of Phenylethanoid Glycosides and Antioxidant Activity of Syringa pubescens Turcz. from Different Geographical Origin in China. J. Chromatogr. Sci. 2022. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Q.; Wang, D.; Deng, R.; Yu, M.; Wang, X. J. P. J. o. P. S. , A comparative study of echinacoside, oleuropein content and antioxidant properties of different solvent extracts from Syringa pubescens Turcz. Pak. J. Pharm. Sci. 2022, 35, 35–40. [Google Scholar]
- Zhang, H. , Protective effects of Syringa pubescens extract on liver injury induced by cadmium in mice. Clinical Research and Practice 2018, 23, 154–156. [Google Scholar]
- He, X. Y.; Shi, J. J.; Gong, R. C.; Miao, T. T. , Anti-inflammatory effect of Syringa pubescens Turcz. Journal Of Tonghua Normal University 2013, 34, 55–57. [Google Scholar]
- Wang, X.; Wu, Y.; Li, J.; Wang, A.; Li, G.; Ren, X.; Yin, W. , Ultrasound-assisted deep eutectic solvent extraction of echinacoside and oleuropein from Syringa pubescens Turcz. Industrial Crops and Products 2020, 151, 112442. [Google Scholar] [CrossRef]
- Wu, Y.; Chu, Y.; Yang, Q.; Li, M.; Yu, M.; Deng, R.; Liu, K.; Wang, X.; Fan, E. , Response surface methodology optimised solvothermal system enables an efficient extraction of echinacoside and oleuropein from Syringa pubescens Turcz. Phytochem. Anal. 2021, 32, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Ke, Z.; Chai, D.; Miao, Y.; Luo, K.; Li, W. , Lycopene, polyphenols and antioxidant activities of three characteristic tomato cultivars subjected to two drying methods. Food Chem. 2021, 338, 128062. [Google Scholar] [CrossRef] [PubMed]
- Ng, Z. X.; Than, M. J. Y.; Yong, P. H. , Peperomia pellucida (L.) Kunth herbal tea: Effect of fermentation and drying methods on the consumer acceptance, antioxidant and anti-inflammatory activities. Food Chem. 2021, 344, 128738. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Z.; Qin, L.; Shen, J.; He, Z.; Shao, Q.; Lin, D. , Drying methods affect bioactive compound contents and antioxidant capacity of Bletilla striata (Thunb.) Reichb.f. flower. Industrial Crops and Products 2021, 164, 113388. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Stankevičius, M.; Drevinskas, T.; Akuneca, I.; Kaškonas, P.; Bimbiraitė-Survilienė, K.; Maruška, A.; Ragažinskienė, O.; Kornyšova, O.; Briedis, V.; Ugenskienė, R. , Evaluation of phytochemical composition of fresh and dried raw material of introduced Chamerion angustifolium L. using chromatographic, spectrophotometric and chemometric techniques. Phytochemistry 2015, 115, 184–193. [Google Scholar] [CrossRef]
- Silpa, S. G.; Smitha, G. R.; Ranjitha, K. , Drying and packaging methods impact the bacoside profile and microbiological quality of Brahmi herb (Bacopa monnieri L.) during storage. Industrial Crops and Products 2021, 159, 113064. [Google Scholar] [CrossRef]
- Su, X.; Wu, Y.; Li, Y.; Huang, Y.; Liu, Y.; Luo, P.; Zhang, Z. , Effect of Different Post-Harvest Processing Methods on the Chemical Constituents of Notopterygium franchetii by an UHPLC-QTOF-MS-MS Metabolomics Approach. Molecules 2019, 24, 3188. [Google Scholar] [CrossRef]
- Qu, F.; Zhu, X.; Ai, Z.; Ai, Y.; Qiu, F.; Ni, D. , Effect of different drying methods on the sensory quality and chemical components of black tea. LWT 2019, 99, 112–118. [Google Scholar] [CrossRef]
- Li, M.; Ai, Z.; Xiao, H.; Mowafy, S.; Pei, Y.; Liu, Y. , Improvement of drying efficiency and physicochemical quality of kiwifruit slices using infrared-assisted tilted tray air impingement drying. Drying Technol. 2023, 41, 1159–1170. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Begum, N.; Xia, P.; Liu, J.; Liang, Z. , Effects of Different Processing Methods Based on Different Drying Conditions on the Active Ingredients of Salvia miltiorrhiza Bunge. Molecules 2022, 27, 4860. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhao, Y.; Huang, J.; Zeng, H.; Zheng, B. , Effects of different drying methods on the product quality and volatile compounds of whole shiitake mushrooms. Food Chem. 2016, 197, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-t.; Mei, X.-g. , Improvement of phenylethanoid glycosides production by a fungal elicitor in cell suspension culture of Cistanche deserticola. Biotechnol. Lett. 2003, 25, 1437–1439. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A. C. P.; Paiva, J. P.; Diniz, R. R.; dos Anjos, V. M.; Silva, A. B. S. M.; Pinto, A. V.; dos Santos, E. P.; Leitão, A. C.; Cabral, L. M.; Rodrigues, C. R.; de Pádula, M.; Santos, B. A. M. C. , Photoprotection assessment of olive (Olea europaea L.) leaves extract standardized to oleuropein: In vitro and in silico approach for improved sunscreens. J. Photochem. Photobiol. B: Biol. 2019, 193, 162–171. [Google Scholar] [CrossRef]
- Wang, C.; Gao, X.; Santhanam, R. K.; Chen, Z.; Chen, Y.; Xu, L.; Wang, C.; Ferri, N.; Chen, H. , Effects of polysaccharides from Inonotus obliquus and its chromium (III) complex on advanced glycation end-products formation, α-amylase, α-glucosidase activity and H2O2-induced oxidative damage in hepatic L02 cells. Food Chem. Toxicol. 2018, 116, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, M.; Kang, M.-C.; Lee, H. H. L.; Cho, C. H.; Choi, I.; Park, Y.; Lee, S.-H. , Antioxidant Effects of Turmeric Leaf Extract against Hydrogen Peroxide-Induced Oxidative Stress In Vitro in Vero Cells and In Vivo in Zebrafish. 2021, 10, 112.
- Shen, T.; Li, X.; Hu, W.; Zhang, L.; Xu, X.; Wu, H.; Ji, L. , Hepatoprotective effect of phenylethanoid glycosides from Incarvillea compacta against CCl4-induced cytotoxicity in HepG2 cells. Journal of the Korean Society for Applied Biological Chemistry 2015, 58, 617–625. [Google Scholar] [CrossRef]
- Sharma, J. N.; Al-Omran, A.; Pavathy, S. S. , Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Carlson, N. G.; Wieggel, W. A.; Chen, J.; Bacchi, A.; Rogers, S. W.; Gahring, L. C. , Inflammatory Cytokines IL-1α, IL-1β, IL-6, and TNF-α Impart Neuroprotection to an Excitotoxin Through Distinct Pathways1. The Journal of Immunology 1999, 163, 3963–3968. [Google Scholar] [CrossRef]
- Cavalcanti, M. R. M.; Passos, F. R. S.; Monteiro, B. S.; Gandhi, S. R.; Heimfarth, L.; Lima, B. S.; Nascimento, Y. M.; Duarte, M. C.; Araujo, A. A. S.; Menezes, I. R. A.; Coutinho, H. D. M.; Zengin, G.; Ceylan, R.; Aktumsek, A.; Quintans-Júnior, L. J.; Quintans, J. S. S. , HPLC-DAD-UV analysis, anti-inflammatory and anti-neuropathic effects of methanolic extract of Sideritis bilgeriana (lamiaceae) by NF-κB, TNF-α, IL-1β and IL-6 involvement. J. Ethnopharmacol. 2021, 265, 113338. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, J.; Yang, W.; Chen, S.; Liu, D.; Fang, H.; Zhang, H.; Ye, X. , Inhibition mechanism of ferulic acid against α-amylase and α-glucosidase. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef] [PubMed]
- Altun, M. L.; Yılmaz, B. S.; Orhan, I. E.; Citoglu, G. S. , Assessment of cholinesterase and tyrosinase inhibitory and antioxidant effects of Hypericum perforatum L. (St. John’s wort). Industrial Crops and Products 2013, 43, 87–92. [Google Scholar] [CrossRef]
- Jia, X.; Yang, Y.; Wang, Q.; Tian, Y.; Hong, Y.; Tian, M.; Tang, D. , Phytochemical composition, antioxidant, anti-tyrosinase, anti-cholinesterase, and anti-inflammatory activities of Rhynchanthus beesianus rhizome extracts. Arabian Journal of Chemistry 2023, 16, 104952. [Google Scholar] [CrossRef]
- Saeedi, M.; Khezri, K.; Seyed Zakaryaei, A.; Mohammadamini, H. , A comprehensive review of the therapeutic potential of α-arbutin. Phytother. Res. 2021, 35, 4136–4154. [Google Scholar] [CrossRef] [PubMed]
- Khattabi, L.; Boudiar, T.; Bouhenna, M. M.; Chettoum, A.; Chebrouk, F.; Chader, H.; Lozano-Sánchez, J.; Segura-Carretero, A.; Nieto, G.; Akkal, S. , RP-HPLC-ESI-QTOF-MS Qualitative Profiling, Antioxidant, Anti-Enzymatic, Anti-Inflammatory, and Non-Cytotoxic Properties of Ephedra alata Monjauzeana. Foods 2022, 11, 145. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, V.; Anand, P.; Kumar, V.; Ranjan Dwivedi, A.; Kumar, V. , Advancements in the development of multi-target directed ligands for the treatment of Alzheimer’s disease. Biorg. Med. Chem. 2022, 61, 116742. [Google Scholar] [CrossRef]
- Li, Q.; Mo, J.; Xiong, B.; Liao, Q.; Chen, Y.; Wang, Y.; Xing, S.; He, S.; Lyu, W.; Zhang, N.; Sun, H. , Discovery of Resorcinol-Based Polycyclic Structures as Tyrosinase Inhibitors for Treatment of Parkinson’s Disease. ACS Chem. Neurosci. 2022, 13, 81–96. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Wu, Y.; Chen, G.; Yue, W.; Liang, Q. , Response Surface Optimized Ultrasonic-Assisted Extraction of Flavonoids from Sparganii Rhizoma and Evaluation of Their in Vitro Antioxidant Activities. Molecules 2012, 17, 6769–6783. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Xue, J.; Fan, E. , Plant Phenolics Extraction from Flos Chrysanthemi: Response Surface Methodology Based Optimization and the Correlation Between Extracts and Free Radical Scavenging Activity. J. Food Sci. 2017, 82, 2726–2733. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xu, Z.; Wang, F.; Liu, L.; Wei, Y.; Li, J.; Zhang, L.; Zheng, K.; Wu, L.; Men, X.; Zhang, H. , Extraction, characterization, and biological activities of exopolysaccharides from plant root soil fungus Fusarium merismoides A6. Braz. J. Microbiol. 2023, 54, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Ban, X.; He, J.; Zeng, H.; Zhang, P.; Wang, Y. , Hepatoprotective and antioxidant effects of the methanolic extract from Halenia elliptica. J. Ethnopharmacol. 2010, 131, 276–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Q.; Zhuang, S.; Wen, Y.; Cheng, W.; Zeng, Z.; Jiang, T.; Tang, C. , Effect of Anoectochilus roxburghii flavonoids extract on H2O2 - Induced oxidative stress in LO2 cells and D-gal induced aging mice model. J. Ethnopharmacol. 2020, 254, 112670. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Xu, L.; Jia, Y.; Xue, Z.; Zhang, M.; Phisalaphong, M.; Chen, H. , Ultrasound-assisted modified pectin from unripe fruit pomace of raspberry (Rubus chingii Hu): Structural characterization and antioxidant activities. LWT 2020, 134, 110007. [Google Scholar] [CrossRef]
- Zhen, D.; Xuan, T.-q.; Hu, B.; Bai, X.; Fu, D.-n.; Wang, Y.; Wu, Y.; Yang, J.; Ma, Q. , Pteryxin attenuates LPS-induced inflammatory responses and inhibits NLRP3 inflammasome activation in RAW264.7 cells. J. Ethnopharmacol. 2022, 284, 114753. [Google Scholar] [CrossRef]
- Uysal, S. , A comparative study of three drying methods on the phenolic profile and biological activities of Salvia absconditiflora. Journal of Food Measurement and Characterization 2019, 13, 162–168. [Google Scholar] [CrossRef]
- Moonrungsee, N.; Shimamura, T.; Kashiwagi, T.; Jakmunee, J.; Higuchi, K.; Ukeda, H. , Sequential injection spectrophotometric system for evaluation of mushroom tyrosinase-inhibitory activity. Talanta 2012, 101, 233–239. [Google Scholar] [CrossRef]
- Zengin, G. , A study on in vitro enzyme inhibitory properties of Asphodeline anatolica: New sources of natural inhibitors for public health problems. Industrial Crops and Products 2016, 83, 39–43. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).