1. Introduction
The patella and achilles tendons are subject to a heavy load during high-impact activity and are prone to overuse injury. Most commonly, this occurs in competitive and recreational sports such as running, basketball, and volleyball. It has been estimated that more than half of elite runners will develop achilles tendinopathy during their lifetime[
1], with similar estimates for patellar tendinopathy in volleyball and basketball athletes[
2]. Given these high numbers, it is crucial to be able to recognize tendinopathy in its early stages and to correct factors that may contribute to structural and mechanical changes.
Historically, standard brightness-mode (B-mode) ultrasound imaging has enabled researchers and clinicians to understand these reactive changes of tendons and fascia with respect to this increased load. The appropriately trained clinician can determine normal from abnormal tissue and correlate it with the stage of tendon pathology[
3]. Yet, delineating normal from abnormal tendons does not encompass the entirety of tendinopathy, and thus, B-mode imaging has its limitations. B-mode imaging is unable to quantify tissue stiffness, which is integral to understanding the pathophysiology of tendinopathy, and potentially contributes to injury prevention[
4].
Recently, ultrasound shear wave elastography (SWE) has emerged within musculoskeletal imaging to address this gap. SWE works through compressive acoustic waves emitted by the ultrasound transducer. These acoustic waves create shear waves that ultimately yield quantitative measurements of tissue stiffness by means of Young’s modulus[
5]. Prior studies have demonstrated that shear wave values can be used in the evaluation of tendon pathology, citing the difference between tendinopathic, normal, and ruptured tissue[
6,
7,
8,
9,
10]. Even further, SWE has been used to demonstrate a response to treatment with potential prognostic implications[
11].
The difficulty with ultrasound SWE is that the results are a function of the frequency of the shear wave, and consequently, values are machine-specific [
12]. Normative values for the achilles and patellar tendon need to be established to compare to pathological tissue. The primary goal of this study, then, was to investigate tendon stiffness in normal subjects on a single machine and establish a protocol for future clinical use.
2. Materials and Methods
This was a cross-sectional study performed at a tertiary referral center for sports medicine. IRB approval was obtained prior to the initiation of the study. Participants were included if they were healthy adults aged 18-65 without active lower extremity injury or systemic, metabolic, or endocrine disorders. Participants were excluded if they had lower extremity surgery or previous trauma; orthopedic knee injuries such as tendinopathy, bursitis, ligament, and meniscus injuries; neurological or cardiopulmonary diseases; rheumatic disease such as Gout, Rheumatoid Arthritis, Systemic Lupus; active pain in the achilles or Patellar Tendon; inability to consent; not met the age requirement; pregnant; prisoners. The demographic data collected included sex, age, BMI, dominant leg, and TAS score.
2.1. Shear Wave Elastography
Imaging Procedures: A Samsung RS85 Prestige Ultrasound machine with a 14L-2 MHz transducer was used throughout the study. The elasticity range was set to 200 kPa for the patellar tendon and 300 kPa for the achilles tendon in HQ mode with a persistence of 50%. All examinations were performed by an experienced sports medicine-trained clinician with a registered musculoskeletal sonography (RMSK) certification.
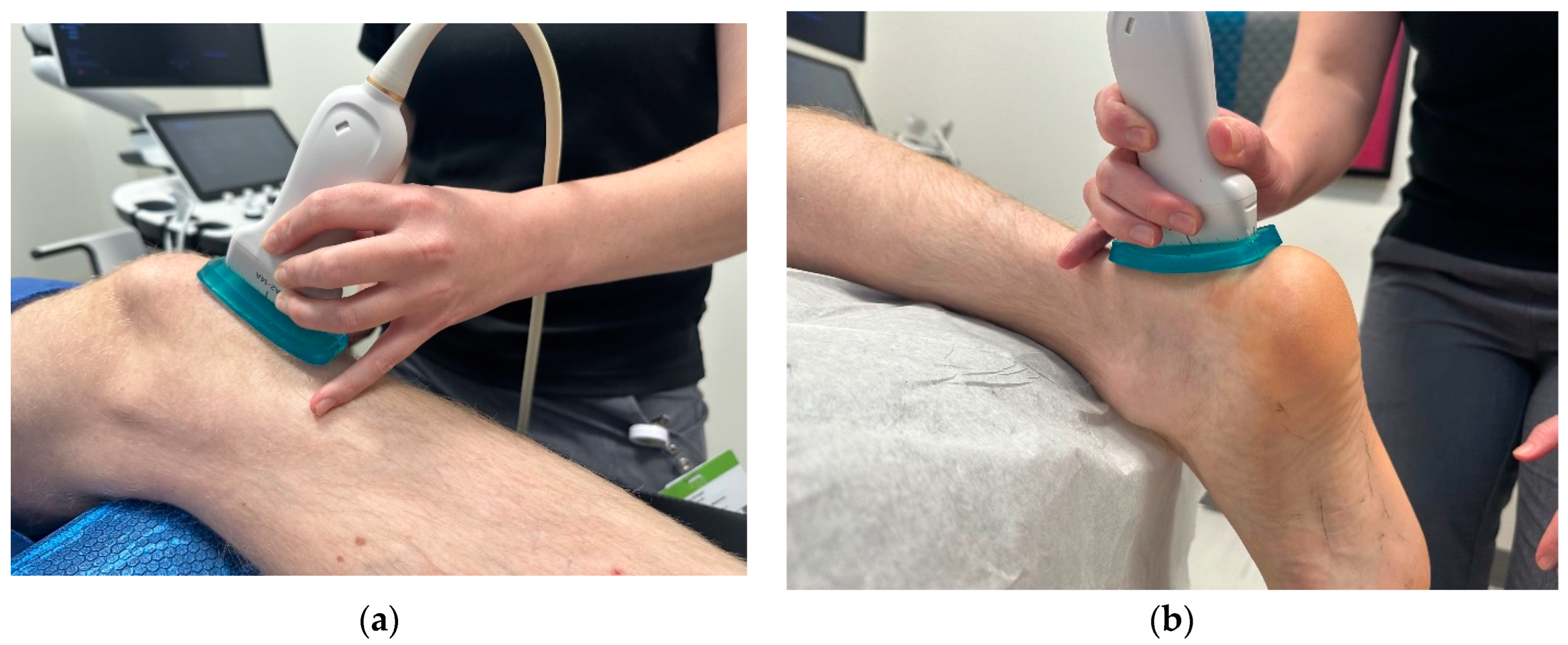
Image Acquisition: For the patellar tendon, participants were assessed supine with the knee flexed and supported at 30 degrees with the hip in a neutral position (
Figure 1a). Approximately 5mm of ultrasound gel was applied using a gel standoff pad, and images were taken without significant pressure, given that pressure directly affects SWE measures[
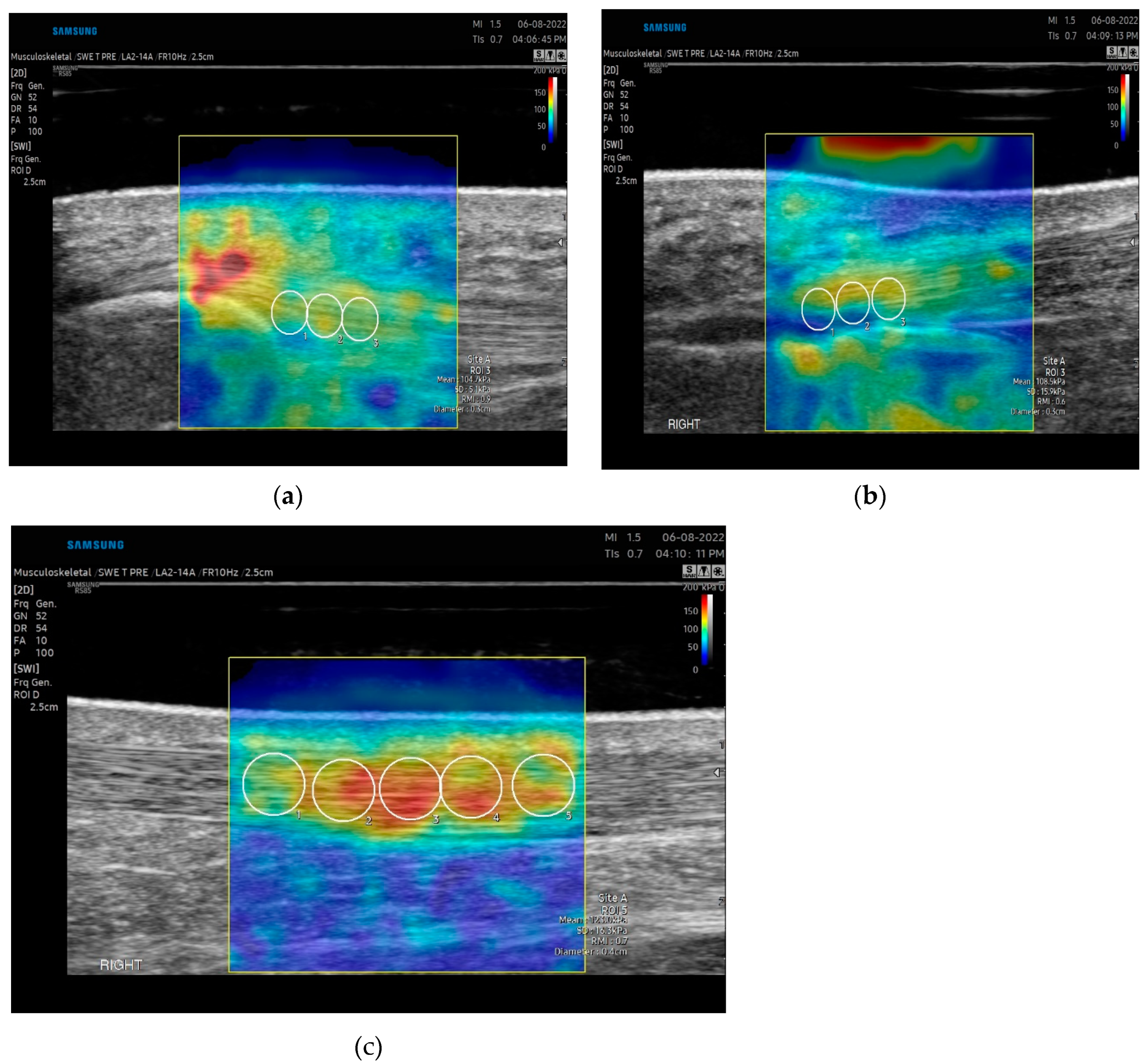
13]. The transducer was rotated into the long-axis position on the tendon. Five images were taken by the examiner to account for image artifact and reproducibility. The transducer was removed and again placed on the target tissue for each measurement. Data acquisition was obtained at the proximal portion of the patella tendon with initial measures at the peak of the lower apex of the patella. A 1.25 x 2.5cm rectangular box was used as the region of interest. A 3mm diameter q-Box was used for data analysis. Three adjacent measurements using the 3mm q-box were taken spanning from the proximal to the midportion of the patella tendon (
Figure 2a). Average values, not maximum values, were reported, as maximum values fluctuate with ROI[
13]. Images were only included if RMI (reliability of the measurement) was greater than 0.6 and IQR/Med (Interquartile/Median) ratio was under 30%. Outliers were removed if deemed an area of anisotropy based on the B mode acquired image.
For the achilles tendon, all participants were assessed in a prone position, with the knee fully extended and the foot relaxed overhanging the bed (
Figure 1b). Relaxed positioning of the tendon has been shown to have the highest reliability in assessment[
4]. Approximately 5mm of ultrasound gel was applied to maintain good contact between the ultrasound probe and skin without significant pressure using a gel standoff pad. The transducer was rotated in the long-axis position. Five images were taken by each examiner to account for image artifact and reproducibility. Data acquisition was performed at the insertion and midportion of the tendon (
Figure 2b and 2c). The midportion of the achilles tendon was determined by identifying the thickest portion of the tendon proximal to the insertion (approximately 5cm from the calcaneus) and centering this midline with the transducer. A 1.25x 2.5 cm rectangular box was used as the region of interest. A 3mm and 4-5 diameter q-Box was used for data analysis of the insertional and midportion achilles respectively. Three measurements for the insertion and five measurements for the midportion were taken spanning the tendon and the length of the q-Box.
Image analysis: In biomechanics, stiffness is defined by the proportional relationship between the stress (the external force or compression) and strain (deformation) applied to it. Transmission of a longitudinal pulse leads to tissue displacement, which is detected by pulse-echo ultrasound and allows the measurement of shear wave velocity [V in m s-1]. The shear wave velocity V is proportional to the shear modulus (μ _expressed in kPa) using the following formula: μ _= ρ.v2 (where ρ _is the tissue density, equals 1000 kg m3 in the human body). Hard tissues have a higher μ _and v than soft ones which can be directly applied to tendon assessment. Shear modulus measurements will be reported in kPa, velocity in m/s, and depth in cm. All images and data were initially assessed and then stored for further analysis.
2.2. Statistical Analysis
The intraclass correlation (ICC) was used as a measure of the magnitude of reliability agreement and was estimated with variance components from a linear model[
14]. The ICC is large (i.e., ≈1) when there is little within-subject variance. For this article, the ICC was estimated by use of within- and between-subject variance components from a two-way fixed-effects model as a measure of reliability agreement.
The repeatability coefficient (RC) was calculated by multiplying the within-subject standard deviation by 2.77 (√ 2 times 1.96). A linear regression analysis was performed to analyze the relationship between the patella and achilles tendon measurements (outcomes) with body mass index and sex as predictors. An adjusted mean was calculated for each patella and achilles tendon outcome. The adjusted mean and its 95% confidence interval for each outcome were defined as the predicted outcome value obtained by fitting the regression equation for males and for females at the mean body mass index using the same slope estimate for males and females. A one-sample t-test was used in linear regression to test the null hypothesis that the slope or the regression coefficient was equal to zero.
3. Results
Fifty-four healthy adults were recruited for this study and data were taken bilaterally for both the achilles and patella tendons of each participant. Detailed descriptive statistics are displayed in
Table 1,
Table 2 and
Table 3.
3.1. Patella Tendon
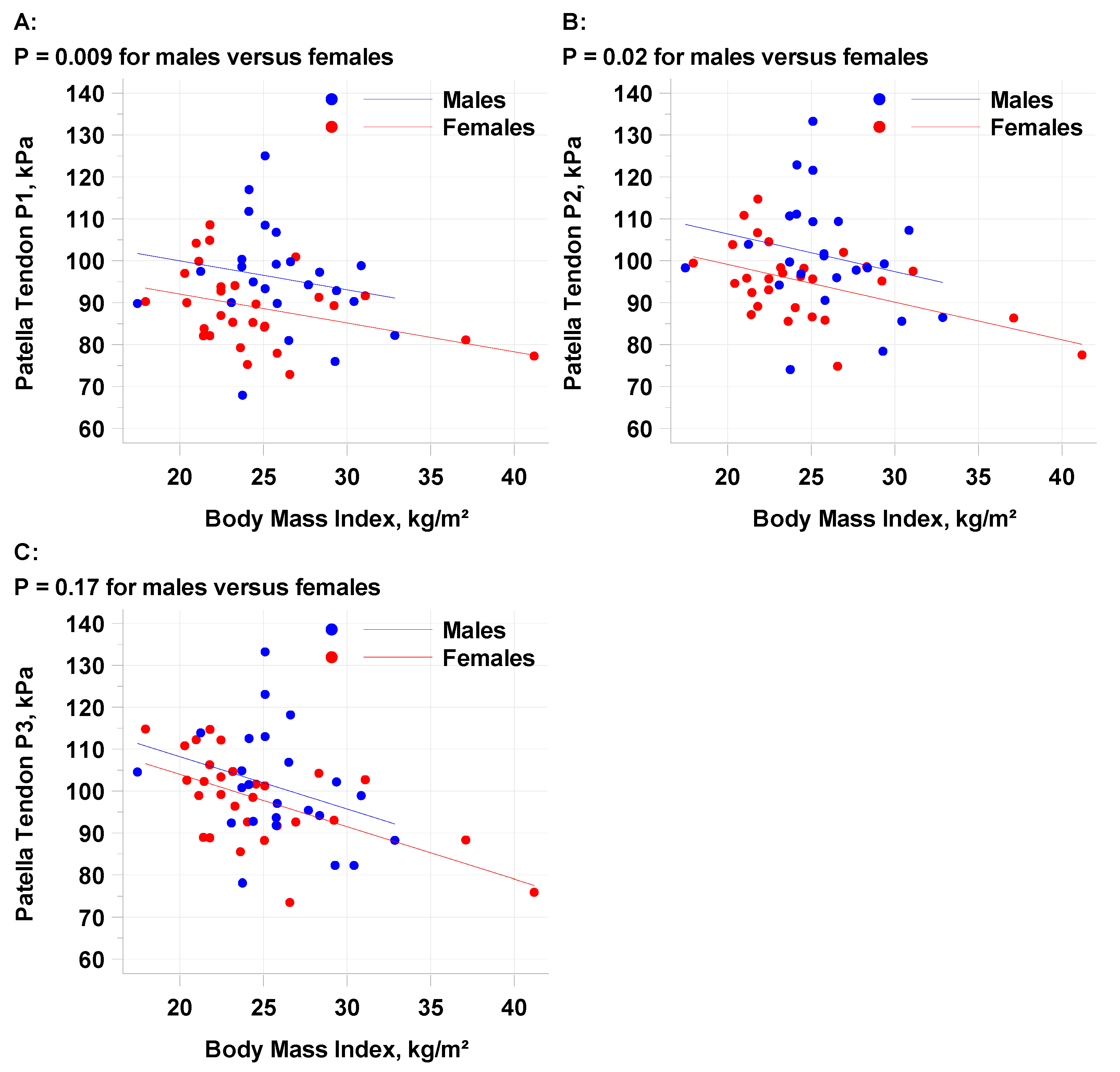
The mean SWE average of the patellar tendon (P1-P3) for all subjects was 96.3 kPA (standard deviation 10.9 kPA) with moderate reliability (intraclass correlation coefficient, ICC = 0.50, 52, 0.50 for P1, P2 and P3) (Appendix 1). The repeatability coefficient (RC) of patellar tendon 1 was 29.4 kPA suggesting the differences between multiple measurements will be less than the RC in 95% of the cases made by a single observer. Males demonstrated a statistically higher kPA than females (
Table 4) at positions P1 (p=.009) and P2 (p=.02), but not P3 (p=.17). A higher BMI was correlated with a decrease in patella tendon kPA (
Figure 3) at all points. The mean PT1 decline per 1 kg/m2 increase in BMI was -0.6894 and the decline per 5 kg/m2 increase in BMI was -3.447. There was a trend towards a higher kPA in patients age ≤29.5 (98.6 kPA versus 93.8 kPA). TAS greater than 5 had a higher kPA than lower TAS levels, but the number of individuals at each level was not enough to make statistical conclusions.
3.2. Achilles Tendon
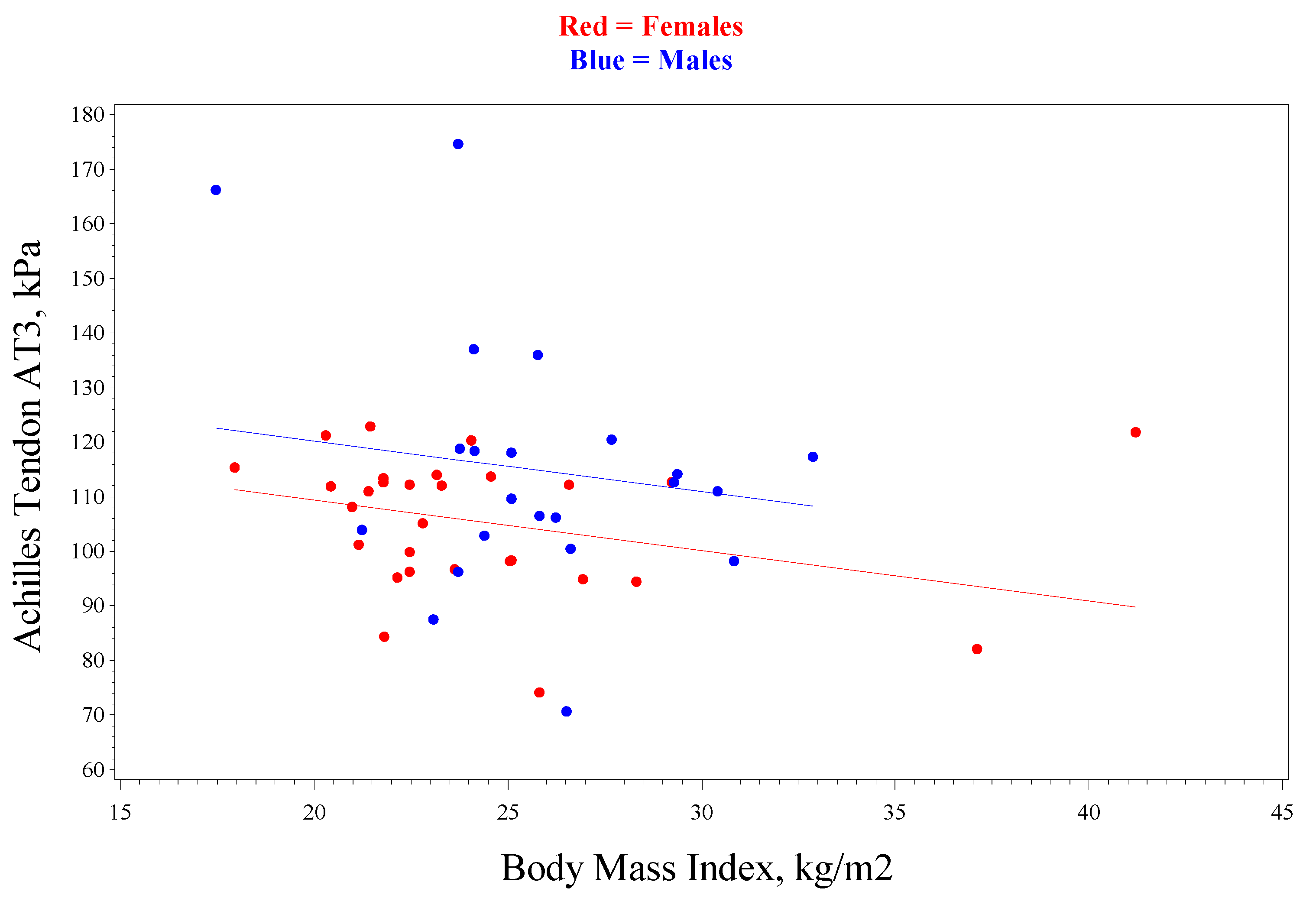
The mean SWE average of the insertional Achilles (AT1-AT3) for all subjects was 101.2 kPA (standard deviation 16.2 kPA) with moderate reliability (intraclass correlation coefficient, ICC = 0.57, 54, 0.58 for AT1, AT2 and AT3) (Appendix 2). The repeatability coefficient (RC) of insertional Achilles AT1 was 40.4 kPA suggesting the differences between multiple measurements will be less than the RC in 95% of the cases made by a single observer. The mean SWE average of the midpoint Achilles (AT1-AT3) for all subjects was 145.6 kPA (standard deviation 18.8 kPA) with poor reliability (intraclass correlation coefficient, ICC = 0.47, 0.40, 0.42, 0.43 0.40 for ATMP1 – APMP5) but moderate reliability for the left and right midpoint data. The repeatability coefficient (RC) of Achilles ATMP1 left was 47.9 kPA suggesting the differences between multiple measurements will be less than the RC in 95% of the cases made by a single observer. The insertional AT measurements were higher in males compared to females (
Table 5) but were only statistically significant for AT3 (p=04). There was a decrease in kPA for insertional achilles measurements in those with a higher BMI (
Figure 4), but this did not reach statistical significance (p=.12). There was no difference in sex or BMI observed in the midportion of the achilles. There was no trend observed with TAS scores for the insertional or midportion achilles.
4. Discussion
In this cross-sectional study of healthy volunteers, we determined the normal mechanical properties of the patella and achilles tendon assessed with ultrasound SWE on the Samsung RS85 machine. To our knowledge, this is the first article published presenting the machine, given technology for this purpose.
Different forms of elastography have been utilized to describe the elasticity of both the achilles and patella tendon. Payne et al. in a small study of 14 health volunteers found that SWE could be a reliable measure of stiffness without significant variability over a period of 5 days. Wakker et al. have also established normative values for the achilles but used acoustic radiation force impulse elastography integrated with the Siemens Acuson S3000 system. The difficulty is that there exists significant variability amongst the different machines when evaluating the results of SWE[
15]. Ultrasound systems have a diverse set of parameters and technology to produce and measure shear waves. This leads to different values and difficulties in comparing among systems. A study by
Alrashed et al. found measurement differences between systems of up to 15% for superficial structures and 38.6%-82.9% for deeper[
16]
. This sentiment was reinforced by Javed et al. calling for better standardization of systems[
17]
.
Another aspect to consider is the positioning of the tendon. Hardy et al. compared the shear modulus of the patellar tendon in two knee angles at different stress levels. Shear modulus was highest in knee flexed, then knee semi-flexed. The lowest shear wave speed was obtained at rest with the knee in a fully extended position (i.e., with the patellar tendon at full rest)[
18]. Payne et al. assessed the reproducibility of SWE of the achilles tendon from different foot positions determining that a significant difference exists between a relaxed and fixed position[
4]. In our study, we standardized the patient positioning for the patella tendon with the knee flexed to 30 degrees resting on a foam roller. For the achilles, the patient was prone with the ankle relaxed in slight plantarflexion. The positions chosen were the positions most commonly presented in the literature[
15].
Elastography is also influenced by both gel contact and manual pressure on the transducer. To standardize the amount of gel used, a gel standoff pad was implemented. To account for pressure on the probe, 5 measurements were taken sequentially, manually lifting the transducer off the position on the patient. This method produced moderate reliability, which is in line with previously published data [
4,
19,
20].
In establishing normative values, we found that participants with a higher BMI had lower tendon stiffness for both the patella tendon and insertional achilles. Females, in general, had a lower tendon stiffness than males and there was a trend towards increased stiffness in younger participants. This is comparable to previous studies for the patella tendon[
21,
22]. There is conflicting evidence, however, with the achilles. Wakker et al. concluded no change with age, sex, or BMI, while Ruan et al. determined that there is a lower elasticity with older age [
23,
24]. Further investigation is warranted to better understand this relationship. Regarding activity level, a higher stiffness was observed with an increased level of activity for the patella tendon, but not the Achilles. Our sample size was too small to make any conclusions, but differences have been noted by previous authors citing lower stiffness for the patella and higher stiffness for the achilles tendons with greater levels of activity[
25,
26].
Shear wave imaging can advance the level of diagnostic capabilities for musculoskeletal medicine. Yurdaışık et al. evaluated the accuracy of point shear wave elastography (pSWE) and two-dimensional shear wave elastography (SWE) for patella tendinopathy and compared it with magnetic resonance imaging (MRI) finding compatibility of clinical scoring[
27]. Zhang et al. compared the morphology and elastic properties of patellar tendons between athletes with and without unilateral patella tendinopathy. Athletes with tendinopathy had a higher elastic modulus[
28]. In the achilles, Gatz et al. investigated if SWE can differentiate between the symptomatic and asymptomatic side of unilateral AT. Like the patella tendon, patients with symptomatic tendinopathy displayed higher SWE values than the asymptomatic side which was also higher than healthy controls[
7]. SWE may, then, be able to serve as a more sensitive means for the diagnosis of pathology than B-mode imaging alone[
7,
29].
Another immense potential of the technology is evaluating response to treatment. Dirrichs et al. in a double-blinded longitudinal study for monitoring the treatment of tendinopathies were able to prove that SWE could depict processes associated with tendon healing that was unlike B-mode imaging and power Doppler ultrasound[
7]. This can be used in the field of ultrasound-guided procedures such as vacuum debridement, needle tenotomy, and orthobiologics to identify objective measures of change outside of patient-reported outcomes.
This study is not without limitations. Primarily, only one observer was included. Future research should address both interobserver and intra-observer agreement for each SWE outcome. In addition, another aspect to consider is the study’s lower sample size and power. Our results provide important estimates of variability (the within-subject and the between-subject standard deviation) for each endpoint that will be valuable to power future studies. These estimates are essential components for sample size calculations.
5. Conclusions
This study provides normative SWE values for the patella and achilles tendon using the Samsung RS85. These values can be used for comparison to pathological tissue by means of the same examination protocol to better understand tendon degeneration. Further studies are warranted as to how SWE can be applied to the diagnosis of tendinopathy, partial and full-thickness tears, and response to treatment.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Table S1: Estimates of the Standard Deviation and Intraclass Correlation Coefficient for Patella Tendons Measured by Shear Wave Elastography; Table S2: Estimates of the Standard Deviation and Intraclass Correlation Coefficient for Achilles Tendons Measured by Shear Wave Elastography.
Author Contributions
Conceptualization, W.B. and K.M.; methodology, W.B.; software, K.E.; formal analysis, K.E.; investigation, W.B. and K.C.; resources, W.B. and K.C.; data curation, K.C. and K.E.; writing—original draft preparation, W.B.; writing—review and editing, K.M., K.C.; visualization, K.C. and W.B; supervision, K.M.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Emory University School of Medicine with IRB identifier STUDY00003824.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
The authors would like to recognize James Gardner, MD and Bridget Doyle, MD for assistance with figures.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Estimates of the Standard Deviation and Intraclass Correlation Coefficient for Patella Tendons measured by Shear Wave Elastography (One Reader)
| Location |
Number Subjects |
Number Measurements |
Between-subject SD |
Within-Subject SD |
Intraclass Correlation Coefficient |
| |
|
|
|
|
|
| P1 |
54 |
513 |
10.6 |
10.6 |
0.50 |
| P1 Left |
54 |
257 |
11.6 |
8.6 |
0.65 |
| P1 Right |
54 |
256 |
12.6 |
8.4 |
0.69 |
| |
|
|
|
|
|
| P2 |
54 |
514 |
11.0 |
10.6 |
0.52 |
| P2 Left |
54 |
258 |
11.9 |
8.5 |
0.66 |
| P2 Right |
54 |
256 |
12.7 |
9.1 |
0.66 |
| |
|
|
|
|
|
| P3 |
54 |
514 |
11.3 |
11.4 |
0.50 |
| P3 Left |
54 |
257 |
12.5 |
8.7 |
0.68 |
| P3 Right |
54 |
257 |
13.6 |
9.7 |
0.66 |
The Interclass Correlation Coefficient (ICC) is a measure of reliability. ICC values less than 0.5, between 0.5 and 0.75, between 0.75 and 0.90, and greater than 0.90 are indicative of poor, moderate, good, and excellent reliability, respectively.
Appendix B. Estimates of the Standard Deviation and Intraclass Correlation Coefficient for Achilles Tendons measured by Shear Wave Elastography
| Location |
Number Subjects |
Number Measurements |
Between-subject SD |
Within-Subject SD |
Intraclass Correlation Coefficient |
| |
|
|
|
|
|
| AT1 |
52 |
498 |
16.6 |
14.6 |
0.57 |
| AT1 Left |
|
251 |
17.6 |
12.7 |
0.66 |
| AT1 Right |
|
247 |
19.4 |
11.7 |
0.74 |
| |
|
|
|
|
|
| AT2 |
52 |
498 |
16.5 |
15.3 |
0.54 |
| AT2 Left |
|
251 |
16.3 |
12.9 |
0.62 |
| AT2 Right |
|
247 |
20.3 |
13.1 |
0.71 |
| |
|
|
|
|
|
| AT3 |
52 |
498 |
17.5 |
14.8 |
0.58 |
| AT3 Left |
|
251 |
17.1 |
12.7 |
0.65 |
| AT3 Right |
|
247 |
21.0 |
13.2 |
0.72 |
| |
|
|
|
|
|
| ATMP1 |
52 |
505 |
19.4 |
20.7 |
0.47 |
| ATMP1 Left |
|
250 |
21.3 |
17.3 |
0.60 |
| ATMP1 Right |
|
255 |
23.1 |
17.8 |
0.63 |
| |
|
|
|
|
|
| ATMP2 |
52 |
500 |
17.7 |
21.6 |
0.40 |
| ATMP2 Left |
|
250 |
20.2 |
18.9 |
0.53 |
| ATMP2 Right |
|
250 |
22.3 |
17.1 |
0.63 |
| |
|
|
|
|
|
| ATMP3 |
52 |
500 |
17.9 |
21.0 |
0.42 |
| ATMP3 Left |
|
250 |
20.0 |
18.1 |
0.55 |
| ATMP3 Right |
|
250 |
22.6 |
16.6 |
0.65 |
| |
|
|
|
|
|
| ATMP4 |
52 |
490 |
18.7 |
20.8 |
0.45 |
| ATMP4 Left |
|
245 |
20.1 |
19.4 |
0.52 |
| ATMP4 Right |
|
245 |
20.7 |
17.3 |
0.59 |
| |
|
|
|
|
|
| ATMP5 |
52 |
480 |
17.1 |
20.9 |
0.40 |
| ATMP5 Left |
|
240 |
18.2 |
19.5 |
0.47 |
| ATMP5 Left |
|
240 |
20.1 |
17.6 |
0.57 |
The Interclass Correlation Coefficient (ICC) is a measure of reliability. ICC values less than 0.5, between 0.5 and 0.75, between 0.75 and 0.90, and greater than 0.90 are indictive of poor, moderate, good, and excellent reliability, respectively.
References
- Kujala, U.M., S. Sarna, and J. Kaprio, Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med, 2005. 15(3): p. 133-5. [CrossRef]
- Lian, O.B., L. Engebretsen, and R. Bahr, Prevalence of jumper's knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med, 2005. 33(4): p. 561-7.
- Cook, J.L. , et al., Revisiting the continuum model of tendon pathology: what is its merit in clinical practice and research? British Journal of Sports Medicine, 2016. 50(19): p. 1187-1191.
- Payne, C. , et al., Reproducibility of shear wave elastography measuresof the Achilles tendon. Skeletal Radiol, 2018. 47(6): p. 779-784. [CrossRef]
- Taljanovic, M.S. , et al., Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics, 2017. 37(3): p. 855-870. [CrossRef]
- Coombes, B.K. , et al., Achilles and patellar tendinopathy display opposite changes in elastic properties: A shear wave elastography study. Scand J Med Sci Sports, 2018. 28(3): p. 1201-1208. [CrossRef]
- Dirrichs, T. , et al., Shear Wave Elastography (SWE) for the Evaluation of Patients with Tendinopathies. Acad Radiol, 2016. 23(10): p. 1204-13. [CrossRef]
- Breda, S.J. , et al., The association between patellar tendon stiffness measured with shear-wave elastography and patellar tendinopathy-a case-control study. Eur Radiol, 2020. 30(11): p. 5942-5951. [CrossRef]
- Frankewycz, B. , et al., Changes of Material Elastic Properties during Healing of Ruptured Achilles Tendons Measured with Shear Wave Elastography: A Pilot Study. Int J Mol Sci, 2020. 21(10). [CrossRef]
- Chen, X.M. , et al., Shear wave elastographic characterization of normal and torn achilles tendons: a pilot study. J Ultrasound Med, 2013. 32(3): p. 449-55.
- Zellers, J.A. , et al., Tendon morphology and mechanical properties assessed by ultrasound show change early in recovery and potential prognostic ability for 6-month outcomes. Knee Surg Sports Traumatol Arthrosc, 2019. 27(9): p. 2831-2839. [CrossRef]
- Park, S.H. , et al., What we need to know when performing and interpreting US elastography. Clin Mol Hepatol, 2016. 22(3): p. 406-414. [CrossRef]
- Kot, B.C. , et al., Elastic modulus of muscle and tendon with shear wave ultrasound elastography: variations with different technical settings. PLoS One, 2012. 7(8): p. e44348. [CrossRef]
- Bland, J.M. and D.G. Altman, A note on the use of the intraclass correlation coefficient in the evaluation of agreement between two methods of measurement. Comput Biol Med, 1990. 20(5): p. 337-40. [CrossRef]
- Cipriano, K.J. , et al., A scoping review of methods used in musculoskeletal soft tissue and nerve shear wave elastography studies. Clinical Neurophysiology, 2022. [CrossRef]
- Alrashed, A.I. and A.M. Alfuraih, Reproducibility of shear wave elastography among operators, machines, and probes in an elasticity phantom. Ultrasonography, 2021. 40(1): p. 158-166. [CrossRef]
- Javed, H., S. O. Oyibo, and A.M. Alfuraih, Variability, Validity and Operator Reliability of Three Ultrasound Systems for Measuring Tissue Stiffness: A Phantom Study. Cureus, 2022. 14(11): p. e31731. [CrossRef]
- Hardy, A. , et al., Normal Range of Patellar Tendon Elasticity Using the Sharewave Elastography Technique: An In Vivo Study in Normal Volunteers. Surg Technol Int, 2017. 31: p. 227-230.
- Wu, J. , et al., In vivo assessment of material properties of muscles and connective tissues around the knee joint based on shear wave elastography. J Mech Behav Biomed Mater, 2020. 109: p. 103829. [CrossRef]
- Taş, S. , et al., Shear Wave Elastography Is a Reliable and Repeatable Method for Measuring the Elastic Modulus of the Rectus Femoris Muscle and Patellar Tendon. Journal of Ultrasound in Medicine, 2017. 36(3): p. 565-570. [CrossRef]
- Taş, S. , et al., Patellar tendon mechanical properties change with gender, body mass index and quadriceps femoris muscle strength. Acta Orthop Traumatol Turc, 2017. 51(1): p. 54-59. [CrossRef]
- Hsiao, M.Y. , et al., Reduced Patellar Tendon Elasticity with Aging: In Vivo Assessment by Shear Wave Elastography. Ultrasound Med Biol, 2015. 41(11): p. 2899-905. [CrossRef]
- Wakker, J. , et al., Elasticity standard values of the Achilles tendon assessed with acoustic radiation force impulse elastography on healthy volunteers: a cross section study. BMC Musculoskelet Disord, 2018. 19(1): p. 139. [CrossRef]
- Ruan, Z. , et al., Elasticity of healthy Achilles tendon decreases with the increase of age as determined by acoustic radiation force impulse imaging. Int J Clin Exp Med, 2015. 8(1): p. 1043-50.
- Siu, W.L. , et al., Sonographic evaluation of the effect of long-term exercise on Achilles tendon stiffness using shear wave elastography. J Sci Med Sport, 2016. 19(11): p. 883-887. [CrossRef]
- Zhang, Z.J., G. Y. Ng, and S.N. Fu, Effects of habitual loading on patellar tendon mechanical and morphological properties in basketball and volleyball players. Eur J Appl Physiol, 2015. 115(11): p. 2263-9. [CrossRef]
- Yurdaışık, I. , Comparison of two-dimensional shear wave elastography and point shear wave elastography techniques with magnetic resonance findings in detection of patellar tendinopathy. Eklem Hastalik Cerrahisi, 2019. 30(3): p. 275-81. [CrossRef]
- Zhang, Z.J. , et al., Changes in morphological and elastic properties of patellar tendon in athletes with unilateral patellar tendinopathy and their relationships with pain and functional disability. PLoS One, 2014. 9(10): p. e108337. [CrossRef]
- Klauser, A.S. , et al., Achilles Tendon Assessed with Sonoelastography: Histologic Agreement. Radiology, 2013. 267(3): p. 837-842. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).