Submitted:
17 October 2023
Posted:
17 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Study design and study population
2.2. Data collection and sample collection
2.3. Blood separation
2.4. Serological testing of HBV and HCV
2.5. Cytokine profile
2.6. Mitochondrial mutations
2.7. Data analysis
3. Results
3.1. Demographic and disease characteristics of study participants
3.2. Liver function of study participants
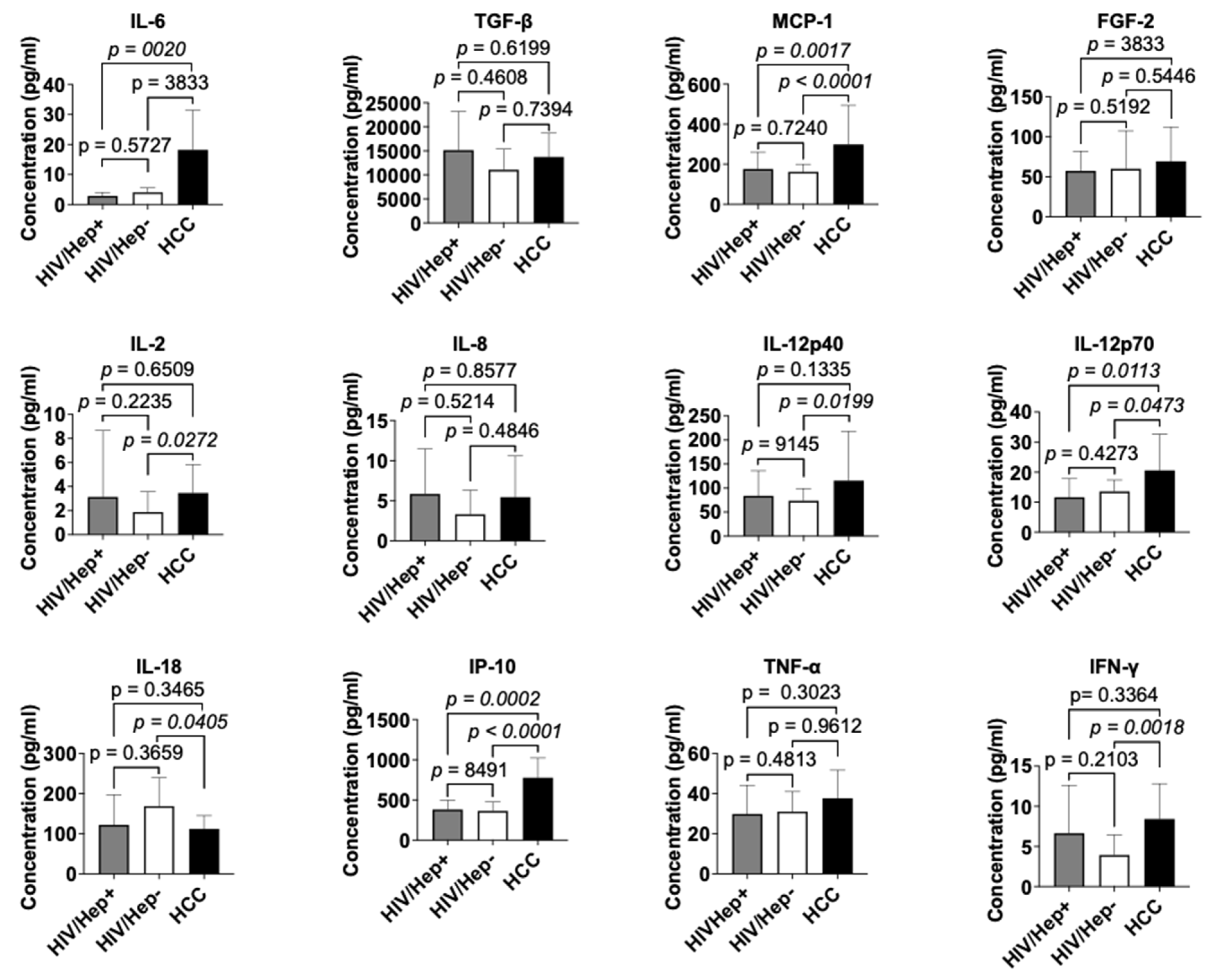
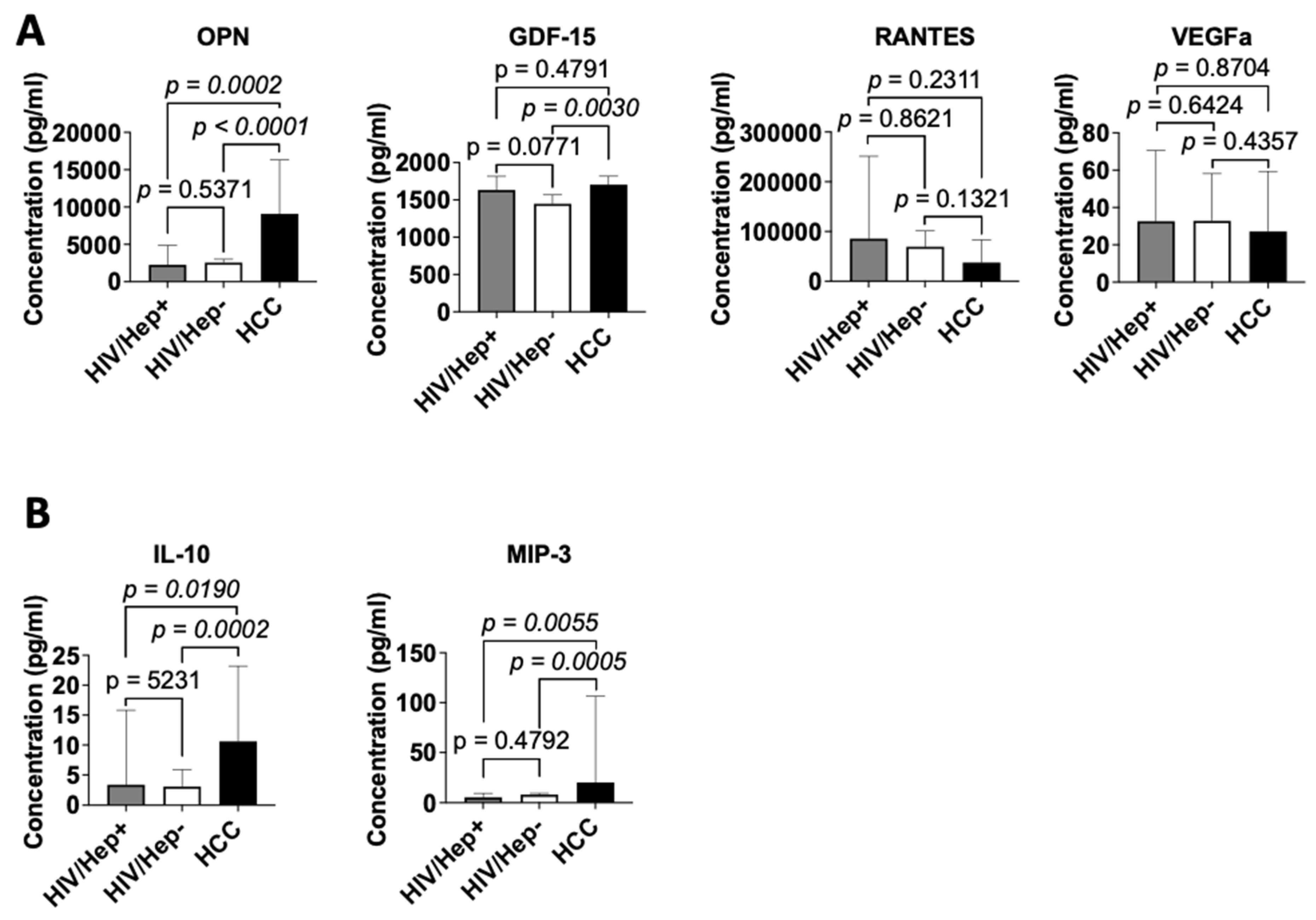
3.3. Cytokine profile of study participants
3.4. Correlation of cytokine levels in HIV+ participants
3.5. Mitochondrial mutations among the cohorts
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franceschi, S.; Lise, M.; Clifford, G. M.; Rickenbach, M.; Levi, F.; Maspoli, M.; Bouchardy, C.; Dehler, S.; Jundt, G.; Ess, S.; Bordoni, A.; Konzelmann, I.; Frick, H.; Dal Maso, L.; Elzi, L.; Furrer, H.; Calmy, A.; Cavassini, M.; Ledergerber, B.; Keiser, O. Changing Patterns of Cancer Incidence in the Early- and Late-HAART Periods: The Swiss HIV Cohort Study. Br J Cancer 2010, 103 (3), 416–422. [CrossRef]
- Yuan, T.; Hu, Y.; Zhou, X.; Yang, L.; Wang, H.; Li, L.; Wang, J.; Qian, H.-Z.; Clifford, G. M.; Zou, H. Incidence and Mortality of Non-AIDS-Defining Cancers among People Living with HIV: A Systematic Review and Meta-Analysis. eClinicalMedicine 2022, 52, 101613. [CrossRef]
- Horner, M.-J.; Shiels, M. S.; Pfeiffer, R. M.; Engels, E. A. Deaths Attributable to Cancer in the US Human Immunodeficiency Virus Population During 2001–2015. Clin Infect Dis 2020, 72 (9), e224–e231. [CrossRef]
- Smith, C. J.; Ryom, L.; Weber, R.; Morlat, P.; Pradier, C.; Reiss, P.; Kowalska, J. D.; de Wit, S.; Law, M.; el Sadr, W.; Kirk, O.; Friis-Moller, N.; Monforte, A. d’Arminio; Phillips, A. N.; Sabin, C. A.; Lundgren, J. D. Trends in Underlying Causes of Death in People with HIV from 1999 to 2011 (D:A:D): A Multicohort Collaboration. The Lancet 2014, 384 (9939), 241–248. [CrossRef]
- Rumgay, H.; Ferlay, J.; de Martel, C.; Georges, D.; Ibrahim, A. S.; Zheng, R.; Wei, W.; Lemmens, V. E. P. P.; Soerjomataram, I. Global, Regional and National Burden of Primary Liver Cancer by Subtype. European Journal of Cancer 2022, 161, 108–118. [CrossRef]
- Liver cancer statistics | World Cancer Research Fund International. WCRF International. https://www.wcrf.org/cancer-trends/liver-cancer-statistics/ (accessed 2023-01-30).
- Sun, J.; Althoff, K. N.; Jing, Y.; Horberg, M. A.; Buchacz, K.; Gill, M. J.; Justice, A. C.; Rabkin, C. S.; Goedert, J. J.; Sigel, K.; Cachay, E.; Park, L.; Lim, J. K.; Kim, H. N.; Vincent Lo Re, I. I. I.; Moore, R.; Sterling, T.; Peters, M. G.; Achenbach, C. J.; Silverberg, M.; Thorne, J. E.; Mayor, A. M.; Crane, H. M.; Kitahata, M. M.; Klein, M.; Kirk, G. D. Trends in Hepatocellular Carcinoma Incidence and Risk Among Persons With HIV in the US and Canada, 1996-2015. JAMA Network Open 2021, 4 (2). [CrossRef]
- Mehershanhi, S.; Haider, A.; Kandhi, S.; Sun, H.; Patel, H. Prevalence of Hepatocellular Carcinoma in HIV Patients Co-Infected or Triple Infected With Hepatitis B and Hepatitis C in a Community Hospital in South Bronx. Cureus 14 (6), e26089. [CrossRef]
- Otedo, A.; Simbiri, K. O.; Were, V.; Ongati, O.; Estambale, B. A. Risk Factors for Liver Cancer in HIV Endemic Areas of Western Kenya. Infect Agent Cancer 2018, 13, 41. [CrossRef]
- Mak, D.; Villiers, C. B. de; Chasela, C.; Urban, M. I.; Kramvis, A. Analysis of Risk Factors Associated with Hepatocellular Carcinoma in Black South Africans: 2000–2012. PLoS ONE 2018, 13 (5). [CrossRef]
- Nsibirwa, S. K.; Aizire, J.; Thomas, D. L.; Ocama, P.; Kirk, G. D. Rapid Progression to Death after Hepatocellular Carcinoma Diagnosis Particularly among Persons with Advanced HIV Disease in Kampala, Uganda. medRxiv June 30, 2022, p 2022.06.24.22276850. [CrossRef]
- El-Shenawy, R.; Farouk, S.; Helmy, N.; Din, N. B. E.; El-Shenawy, R.; Farouk, S.; Helmy, N.; Din, N. B. E. Risk Factors Associated with Development of Hepatocellular Carcinoma in Hepatitis C Virus Patients. In Hepatitis C - Recent Advances; IntechOpen, 2023. [CrossRef]
- Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. https://www.who.int/publications-detail-redirect/9789240027077 (accessed 2023-04-01).
- Cheng, Z.; Lin, P.; Cheng, N. HBV/HIV Coinfection: Impact on the Development and Clinical Treatment of Liver Diseases. Front Med (Lausanne) 2021, 8, 713981. [CrossRef]
- Ngcobo, S.; Molatlhegi, R. P.; Osman, F.; Ngcapu, S.; Samsunder, N.; Garrett, N. J.; Abdool Karim, S. S.; Abdool Karim, Q.; McKinnon, L. R.; Sivro, A. Pre-Infection Plasma Cytokines and Chemokines as Predictors of HIV Disease Progression. Sci Rep 2022, 12 (1), 2437. [CrossRef]
- Montanari, N. R.; Anugwom, C. M.; Boonstra, A.; Debes, J. D. The Role of Cytokines in the Different Stages of Hepatocellular Carcinoma. Cancers 2021, 13 (19). [CrossRef]
- Kany, S.; Vollrath, J. T.; Relja, B. Cytokines in Inflammatory Disease. Int J Mol Sci 2019, 20 (23), 6008. [CrossRef]
- Zamarron, B. F.; Chen, W. Dual Roles of Immune Cells and Their Factors in Cancer Development and Progression. International Journal of Biological Sciences 2011, 7 (5), 651. [CrossRef]
- Budhu, A.; Wang, X. W. The Role of Cytokines in Hepatocellular Carcinoma. Journal of Leukocyte Biology 2006, 80 (6), 1197–1213. [CrossRef]
- Lan, T.; Chen, L.; Wei, X. Inflammatory Cytokines in Cancer: Comprehensive Understanding and Clinical Progress in Gene Therapy. Cells 2021, 10 (1), 100. [CrossRef]
- Yan, B.; Wang, H.; Rabbani, Z. N.; Zhao, Y.; Li, W.; Yuan, Y.; Li, F.; Dewhirst, M. W.; Li, C.-Y. Tumor Necrosis Factor-α Is a Potent Endogenous Mutagen That Promotes Cellular Transformation. Cancer Res 2006, 66 (24), 11565–11570. [CrossRef]
- Patridge, E. F.; Bardyn, T. P. Research Electronic Data Capture (REDCap). J Med Libr Assoc 2018, 106 (1), 142–144. [CrossRef]
- Lu, Y.; Ahmed, S.; Harari, F.; Vahter, M. Impact of Ficoll Density Gradient Centrifugation on Major and Trace Element Concentrations in Erythrocytes and Blood Plasma. Journal of Trace Elements in Medicine and Biology 2015, 29, 249–254. [CrossRef]
- Skrzypkowska, M.; Stasiak, M.; Sakowska, J.; Chmiel, J.; Maciejewska, A.; Buciński, A.; Słomiński, B.; Trzonkowski, P.; Łuczkiewicz, P. Cytokines and Chemokines Multiplex Analysis in Patients with Low Disease Activity Rheumatoid Arthritis. Rheumatol Int 2022, 42 (4), 609–619. [CrossRef]
- Aztatzi-Aguilar, O. G.; Sierra-Vargas, M. P.; Ortega-Romero, M.; Jiménez-Corona, A. E. Osteopontin’s Relationship with Malnutrition and Oxidative Stress in Adolescents. A Pilot Study. PLoS ONE 2021, 16 (3), e0249057. [CrossRef]
- Chen, T.; He, J.; Shen, L.; Fang, H.; Nie, H.; Jin, T.; Wei, X.; Xin, Y.; Jiang, Y.; Li, H.; Chen, G.; Lu, J.; Bai, Y. The Mitochondrial DNA 4,977-Bp Deletion and Its Implication in Copy Number Alteration in Colorectal Cancer. BMC Med Genet 2011, 12, 8. [CrossRef]
- Srinivasan, S.; Guha, M.; Kashina, A.; Avadhani, N. G. Mitochondrial Dysfunction and Mitochondrial Dynamics-The Cancer Connection. Biochim Biophys Acta 2017, 1858 (8), 602–614. [CrossRef]
- Kowald, A.; Kirkwood, T. B. L. Transcription Could Be the Key to the Selection Advantage of Mitochondrial Deletion Mutants in Aging. Proceedings of the National Academy of Sciences 2014, 111 (8), 2972–2977. [CrossRef]
- Borges, Á. H.; Silverberg, M. J.; Wentworth, D.; Grulich, A. E.; Fätkenheuer, G.; Mitsuyasu, R.; Tambussi, G.; Sabin, C. A.; Neaton, J. D.; Lundgren, J. D. Predicting Risk of Cancer during HIV Infection: The Role of Inflammatory and Coagulation Biomarkers. AIDS 2013, 27 (9), 1433–1441. [CrossRef]
- Chen, X.; Liu, X.; Duan, S.; Tang, R.; Zhou, S.; Ye, R.; Yang, Y.; Wang, J.; Yao, S.; He, N. Plasma Inflammatory Biomarkers Associated with Advanced Liver Fibrosis in HIV–HCV-Coinfected Individuals. International Journal of Environmental Research and Public Health 2020, 17 (24), 9474. [CrossRef]
- Hu, S.; Ghabril, M.; Amet, T.; Hu, N.; Byrd, D.; Yang, K.; Vuppalanchi, R.; Saxena, R.; Desai, M.; Lan, J.; Johnson, R.; Gupta, S.; Chalasani, N.; Yu, Q. HIV-1 Coinfection Profoundly Alters Intrahepatic Chemokine but Not Inflammatory Cytokine Profiles in HCV-Infected Subjects. PLoS ONE 2014, 9 (2), e86964. [CrossRef]
- Shata, M. T. M.; Abdel-hameed, E. A.; Rouster, S. D.; Yu, L.; Liang, M.; Song, E.; Esser, M. T.; Shire, N.; Sherman, K. E. HBV and HIV/HBV Infected Patients Have Distinct Immune Exhaustion and Apoptotic Serum Biomarker Profiles. Pathogens and Immunity 2019, 4 (1), 39–65. [CrossRef]
- Falasca, K.; Ucciferri, C.; Dalessandro, M.; Zingariello, P.; Mancino, P.; Petrarca, C.; Pizzigallo, E.; Conti, P.; Vecchiet, J. Cytokine Patterns Correlate with Liver Damage in Patients with Chronic Hepatitis B and C. Ann Clin Lab Sci 2006, 36 (2), 144–150.
- Gupta, D.; Rani, M.; Khan, N.; Jameel, S. HIV-1 Infected Peripheral Blood Mononuclear Cells Modulate the Fibrogenic Activity of Hepatic Stellate Cells through Secreted TGF-β and JNK Signaling. PLoS ONE 2014, 9 (3), e91569. [CrossRef]
- Ribeiro, C. R. de A.; Beghini, D. G.; Lemos, A. S.; Martinelli, K. G.; de Mello, V. da M.; de Almeida, N. A. A.; Lewis-Ximenez, L. L.; de Paula, V. S. Cytokines Profile in Patients with Acute and Chronic Hepatitis B Infection. Microbiology and Immunology 2022, 66 (1), 31–39. [CrossRef]
- Zajkowska, M.; Mroczko, B. Chemokines in Primary Liver Cancer. Int J Mol Sci 2022, 23 (16), 8846. [CrossRef]
- da Cruz, N. S.; Pasquarelli-do-Nascimento, G.; e Oliveira, A. C. P.; Magalhães, K. G. Inflammasome-Mediated Cytokines: A Key Connection between Obesity-Associated NASH and Liver Cancer Progression. Biomedicines 2022, 10 (10), 2344. [CrossRef]
- Naseem, S.; Hussain, T.; Manzoor, S. Interleukin-6: A Promising Cytokine to Support Liver Regeneration and Adaptive Immunity in Liver Pathologies. Cytokine & Growth Factor Reviews 2018, 39, 36–45. [CrossRef]
- You, R.; Jiang, H.; Xu, Q.; Yin, G. Preintervention MCP-1 Serum Levels as an Early Predictive Marker of Tumor Response in Patients with Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Transl Cancer Res 2021, 10 (2), 966–976. [CrossRef]
- Matsui, D.; Nagai, H.; Mukozu, T.; Ogino, Y.; Sumino, Y. VEGF in Patients with Advanced Hepatocellular Carcinoma Receiving Intra-Arterial Chemotherapy. Anticancer Research 2015, 35 (4), 2205–2210.
- Sadeghi, M.; Lahdou, I.; Oweira, H.; Daniel, V.; Terness, P.; Schmidt, J.; Weiss, K.-H.; Longerich, T.; Schemmer, P.; Opelz, G.; Mehrabi, A. Serum Levels of Chemokines CCL4 and CCL5 in Cirrhotic Patients Indicate the Presence of Hepatocellular Carcinoma. Br J Cancer 2015, 113 (5), 756–762. [CrossRef]
- Choi, S. B.; Han, H. J.; Kim, W. B.; Song, T. J.; Choi, S. Y. VEGF Overexpression Predicts Poor Survival in Hepatocellular Carcinoma. Open Medicine 2017, 12 (1), 430–439. [CrossRef]
- Dong, G.; Fan, F.; He, Y.; Luo, Y.; Yu, J.; Liang, P. T-Lymphocyte Gene-Regulated CCL5 and Its Association with Extrahepatic Metastasis in Hepatocellular Carcinoma. JHC 2023, 10, 1267–1279. [CrossRef]
- Soliman, H. H.; Nagy, H.; Kotb, N.; El-Din, M. A. A. The Role of Chemokine CC Ligand 20 in Patients with Liver Cirrhosis and Hepatocellular Carcinoma. Int J Biol Markers 2012, 27 (2), 125–131. [CrossRef]
- Beckebaum, S.; Zhang, X.; Chen, X.; Yu, Z.; Frilling, A.; Dworacki, G.; Grosse-Wilde, H.; Broelsch, C. E.; Gerken, G.; Cicinnati, V. R. Increased Levels of Interleukin-10 in Serum from Patients with Hepatocellular Carcinoma Correlate with Profound Numerical Deficiencies and Immature Phenotype of Circulating Dendritic Cell Subsets. Clinical Cancer Research 2004, 10 (21), 7260–7269. [CrossRef]
- Qian, Q.; Wu, C.; Chen, J.; Wang, W. Relationship between IL10 and PD-L1 in Liver Hepatocellular Carcinoma Tissue and Cell Lines. BioMed Research International 2020, 2020, e8910183. [CrossRef]
- Rubie, C.; Frick, V. O.; Wagner, M.; Rau, B.; Weber, C.; Kruse, B.; Kempf, K.; Tilton, B.; König, J.; Schilling, M. Enhanced Expression and Clinical Significance of CC-Chemokine MIP-3α in Hepatocellular Carcinoma. Scandinavian Journal of Immunology 2006, 63 (6), 468–477. [CrossRef]
- Zhang, S.; Gan, X.; Qiu, J.; Ju, Z.; Gao, J.; Zhou, J.; Shi, C.; Zhu, Y.; Li, Z. IL-10 Derived from Hepatocarcinoma Cells Improves Human Induced Regulatory T Cells Function via JAK1/STAT5 Pathway in Tumor Microenvironment. Molecular Immunology 2021, 133, 163–172. [CrossRef]
- Ji, X.; Guo, W.; Gu, X.; Guo, S.; Zhou, K.; Su, L.; Yuan, Q.; Liu, Y.; Guo, X.; Huang, Q.; Xing, J. Mutational Profiling of MtDNA Control Region Reveals Tumor-Specific Evolutionary Selection Involved in Mitochondrial Dysfunction. eBioMedicine 2022, 80. [CrossRef]
- Li, M.; Foli, Y.; Liu, Z.; Wang, G.; Hu, Y.; Lu, Q.; Selvaraj, S.; Lam, W.; Paintsil, E. High Frequency of Mitochondrial DNA Mutations in HIV-infected Treatment-experienced Individuals. HIV Med 2017, 18 (1), 45–55. [CrossRef]
- de Mendoza, C.; Sánchez-Conde, M.; Timmermans, E.; Buitelaar, M.; de Baar, M. P.; Gonzalez-Lahoz, J.; Soriano, V. Mitochondrial Dna Depletion in HIV-Infected Patients Is More Pronounced with Chronic Hepatitis C and Enhanced Following Treatment with Pegylated Interferon plus Ribavirin. Antiviral Therapy 2005, 10 (4), 557–561. [CrossRef]
- Yu, C.; Wang, X.; Huang, L.; Tong, Y.; Chen, L.; Wu, H.; Xia, Q.; Kong, X. Deciphering the Spectrum of Mitochondrial DNA Mutations in Hepatocellular Carcinoma Using High-Throughput Sequencing. Gene Expr 2018, 18 (2), 125–134. [CrossRef]
- Vadrot, N.; Ghanem, S.; Braut, F.; Gavrilescu, L.; Pilard, N.; Mansouri, A.; Moreau, R.; Reyl-Desmars, F. Mitochondrial DNA Maintenance Is Regulated in Human Hepatoma Cells by Glycogen Synthase Kinase 3β and P53 in Response to Tumor Necrosis Factor α. PLoS ONE 2012, 7 (7), e40879. [CrossRef]
- Giampazolias, E.; Tait, S. W. G. Mitochondria and the Hallmarks of Cancer. The FEBS Journal 2016, 283 (5), 803–814. [CrossRef]
| HIV+ N (%) 60 (64.5) |
HCC+ N (%) 33 (35.5) |
Total N (%) 93 (100.0%) |
P value | |
|---|---|---|---|---|
| Gender | ||||
| Female | 43 (71.7) | 15 (45.5) | 58 (62.8) | < 0.01 |
| Male | 17 (28.3) | 18 (54.5) | 35 (27.2) | |
| Age | ||||
| < 50 | 27 (45.0) | 16 (48.5) | 43 (46.2) | 0.31 |
| ≥ 50 | 33 (55.0) | 17 (51.5) | 50 (53.8) | |
| Mean ± SD | 51 ± 11 | 50 ± 15 | 50 ± 13 | |
| Educational Level | ||||
| ≤ Junior High School | 45 (75.0) | 15 (45.5) | 60 (64.5) | < 0.01 |
| > Junior High School | 15 (25.0) | 18 (54.5) | 33 (35.5) | |
| History/Exposure | ||||
| Family history of cancer | 2 (3.3) | 5 (15.2) | 7 (13.2) | 0.04 |
| Hypertension | 33 (55.0) | 13 (39.4) | 46 (49.5) | 0.15 |
| Smoking | 1 (1.7) | 2 (6.1) | 3 (3.2) | 0.26 |
| Alcohol | 30 (50.0) | 19 (57.6) | 49 (52.7) | 0.48 |
| Virus Infection | ||||
| HCV | 6 (10.0) | 7 (21.2) | 13 (14.0) | 0.14 |
| HBV | 10 (16.7) | 31 (93.9) | 41 (44.1) | < 0.01 |
| Hepatitis B and C coinfection | 0 (0.0) | 5 (15.2) | 5 (5.4) | < 0.01 |
| Variable | HIV+ | |
|---|---|---|
| N (%) | P-value | |
| Years Diagnosed | ||
| < 10 | 26 (43.3) | 0.35 |
| ≥ 10 | 31 (51.7) | |
| Missing | 3 (5.0) | |
| Years on ART | ||
| < 10 | 28 (46.7) | 1.00 |
| ≥ 10 | 28 (46.7) | |
| Missing | 4 (6.7) | |
| HIV Stage | ||
| 1 | 24 (40.0) | < 0.01 |
| 2 | 10 (16.7) | |
| 3 | 13 (21.7) | |
| 4 | 8 (13.3) | |
| Missing | 5 (8.3) | |
| Viral Load | ||
|
≤ 50 Median (Range) |
43 (71.7) < 20 (< 20 - 30) |
< 0.01 |
| > 50 Median (Range) |
12 (20.0) 5731.5 (52 - 2.5e5) |
|
| Missing | 5 (8.3) | |
| CD4 count | ||
| < 200 Median (Range) |
5 (8.3) 159 (21 - 183) |
< 0.01 |
| 200 – 499 Median (Range) |
17 (28.3) 383 (202 - 495) |
|
| > 500 Median (Range) |
25 (41.7) 721 (502 - 1353) |
|
| Missing | 5 (100.0) | |
| Variable | Study Cohort | P value | |
|---|---|---|---|
| HIV+ | HCC+ | ||
| ALT (IU/L) | |||
| Median (Range) | 22 (7 - 123) | 62 (17 - 553) | < 0.01 |
| Normal Range | 7 to 55 | ||
| AST (IU/L) | |||
| Median (Range) | 32 (17 - 106) | 91 (19 - 870) | < 0.01 |
| Normal Range | 8 to 48 | ||
| ALP (IU/L) | |||
| Median (Range) | 99.5 (0 - 323) | 207 (91 - 1581) | < 0.01 |
| Normal Range | 44 to 147 | ||
| Albumin (g/L) | |||
| Median (Range) | 41 (4.3 - 267) | 35 (21 - 46) | 0.04 |
| Normal Range | 34 to 55 | ||
| Bilirubin (µmol/L) | |||
| Median (Range) | 7.9 (0 - 73) | 23.1 (4 - 680) | < 0.01 |
| Normal Range | 1.7 to 20.5 | ||
| HIV+ | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | TGFb | MCP1 | FGF-2 | VEGF | OPN | GDF-15 | RANTES | IL-10 | MIP-3 | IP-10 | IL-2 | IL-8 | IL-12p70 | IL-12p40 | IL-18 | TNF-α | IFN-γ | ||
| HIV+ | IL-6 | 1 | |||||||||||||||||
| TGF-β | 0.52 | 1 | |||||||||||||||||
| MCP1 | 0.27 | -0.06 | 1 | ||||||||||||||||
| FGF-2 | 0.56 | 0.69 | 0.05 | 1 | |||||||||||||||
| VEGF | 0.37 | 0.60 | 0.06 | 0.46 | 1 | ||||||||||||||
| OPN | 0.41 | 0.29 | -0.02 | 0.11 | 0.23 | 1 | |||||||||||||
| GDF-15 | -0.13 | -0.33 | 0.17 | -0.18 | -0.29 | -0.26 | 1 | ||||||||||||
| RANTES | 0.37 | 0.87 | 0.00 | 0.55 | 0.66 | 0.30 | -0.35 | 1 | |||||||||||
| IL-10 | 0.22 | 0.01 | 0.17 | -0.03 | 0.02 | 0.42 | 0.16 | -0.03 | 1 | ||||||||||
| MIP-3 | 0.22 | 0.09 | 0.00 | 0.02 | 0.11 | 0.09 | -0.12 | 0.02 | -0.07 | 1 | |||||||||
| IP-10 | 0.08 | -0.03 | 0.38 | 0.01 | 0.00 | 0.03 | 0.13 | -0.03 | 0.31 | 0.02 | 1 | ||||||||
| IL-2 | 0.57 | 0.20 | 0.43 | 0.42 | 0.29 | 0.21 | 0.00 | 0.22 | 0.24 | -0.06 | 0.21 | 1 | |||||||
| IL-8 | 0.43 | 0.64 | -0.28 | 0.63 | 0.32 | 0.20 | -0.28 | 0.49 | -0.14 | 0.09 | -0.07 | -0.12 | 1 | ||||||
| IL-12p70 | 0.56 | 0.24 | 0.52 | 0.25 | 0.33 | 0.29 | 0.02 | 0.26 | 0.47 | -0.10 | 0.32 | 0.76 | -0.10 | 1 | |||||
| IL-12p40 | 0.59 | 0.26 | 0.52 | 0.22 | 0.25 | 0.34 | -0.04 | 0.23 | 0.40 | -0.08 | 0.29 | 0.72 | -0.12 | 0.89 | 1 | ||||
| IL-18 | 0.24 | 0.22 | 0.05 | 0.11 | 0.07 | -0.12 | 0.06 | 0.17 | -0.01 | -0.11 | 0.13 | 0.03 | 0.35 | 0.11 | 0.02 | 1 | |||
| TNF-α | 0.52 | 0.85 | -0.08 | 0.65 | 0.56 | 0.23 | -0.19 | 0.68 | -0.01 | 0.06 | 0.08 | 0.08 | 0.68 | 0.12 | 0.16 | 0.27 | 1 | ||
| IFN-γ | 0.38 | 0.06 | 0.04 | 0.46 | 0.22 | 0.05 | 0.06 | 0.07 | 0.06 | -0.04 | 0.10 | 0.73 | 0.00 | 0.38 | 0.26 | 0.12 | 0.00 | 1 | |
| Variable | Study Cohort | |||
|---|---|---|---|---|
| HIV+ | HCC+ | Total | P value | |
| mtDNA mutation | ||||
| Present | 36 (60.0) | 21 (63.6) | 57 (61.3) | 0.730 |
| Absent | 24 (40.0) | 12 (36.4) | 36 (38.7) | |
| P value | 0.03 | 0.03 | < 0.01 | |
| Age | ||||
| 18-39 | 3/7 (42.9) | 4/7 (57.1) | 7/14 (50.0) | 0.59 |
| 40-49 | 9/20 (45.0) | 5/9 (55.6) | 14/29 (48.3) | 0.60 |
| ≥ 50 | 24/33 (72.7) | 12/17 (70.5) | 36/50 (72.0) | 0.87 |
| P value | 0.13 | 0.42 | 0.07 | |
| Sex | ||||
| Female | 22/43 (51.2) | 9/15 (60.0) | 31/58 (53.4) | 0.55 |
| Male | 14/17 (82.4) | 12/18 (66.7) | 26/35 (74.3) | 0.29 |
| P value | 0.03 | 0.69 | 0.05 | |
| Alcohol history | ||||
| Yes | 18/30 (60.0) | 10/19 (52.6) | 28/49 (57.1) | 0.61 |
| No | 18/30 (60.0) | 11/14 (78.5) | 29/44 (65.9) | 0.23 |
| P value | 1.00 | 0.13 | 0.39 | |
| Variable | mtDNA deletion | OR (CI) | P value | |
|---|---|---|---|---|
| Yes (%) | No (%) | |||
| Age | ||||
| < 50 | 12 (44.4) | 15 (55.6) | 1 | |
| ≥ 50 | 24 (72.7) | 9 (27.3) | 3.3 (1.1 – 9.8) | 0.03 |
| Sex | ||||
| Female | 22 (51.2) | 21 (48.8) | 1 | |
| Male | 14 (82.4) | 3 (17.6) | 4.5 (1.1 – 17.8) | 0.03 |
| Years Diagnosed | ||||
| < 10 | 11 (42.3) | 15 (57.7) | 1 | |
| ≥ 10 | 22 (71.0) | 9 (29.0) | 3.3 (1.1 – 10.0) | 0.03 |
| Years on ART | ||||
| < 10 | 14 (50.0) | 14 (50.0) | 1 | |
| ≥ 10 | 19 (67.9) | 9 (32.1) | 2.1 (0.7 – 6.2) | 0.18 |
| Viral Load | ||||
| ≤ 50 | 25 (58.1) | 18 (41.9) | 1 | |
| > 50 | 9 (75.0) | 3 (25.0) | 2.2 (0.5 – 9.1) | 0.29 |
| CD4 count | ||||
| < 200 | 1 (20.0) | 4 (80.0) | 0.1 (0.0 – 1.5) | 0.10 |
| 200 – 499 | 13 (76.5) | 4 (23.5) | 1.8 (0.5 – 7.3) | 0.39 |
| > 500 | 16 (64.0) | 9 (36.0) | 1 | |
| Hepatitis status | ||||
| Positive | 11 (68.8) | 5 (21.2) | 1.7 (0.5 -5.6) | 0.47 |
| Negative | 25 (56.8) | 19 (43.2) | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





