Submitted:
16 October 2023
Posted:
17 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting and Ethical Issues
2.2. Subjects
2.3. Data Collection
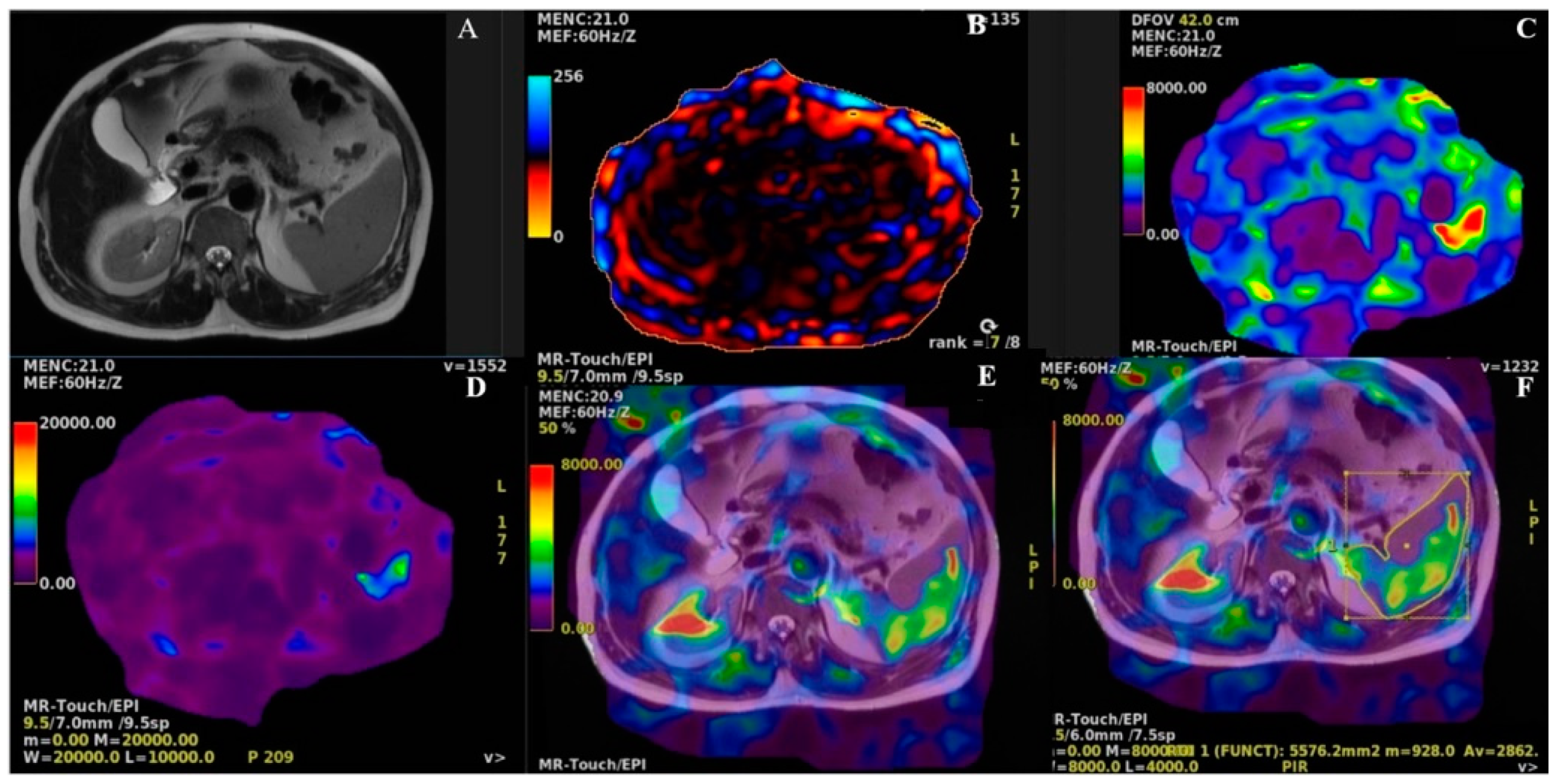
2.4. MRI and MRE Protocols
2.5. Outcomes
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Pawluś, A.; Inglot, M.S.; Szymańska, K.; Kaczorowski, K.; Markiewicz, B.D.; Kaczorowska, A.; Gąsiorowski, J.; Szymczak, A.; Inglot, M.; Bladowska, J.; et al. Shear wave elastography of the spleen: evaluation of spleen stiffness in healthy volunteers. Abdom Radiol (NY). 2016, 41, 2169–2174. [Google Scholar] [CrossRef]
- Ma, X.; Wang, L.; Wu, H.; Feng, Y.; Han, X.; Bu, H.; Zhu, Q. Spleen Stiffness Is Superior to Liver Stiffness for Predicting Esophageal Varices in Chronic Liver Disease: A Meta-Analysis. PLoS One. 2016, 11, e0165786. [Google Scholar] [CrossRef]
- Manatsathit, W.; Samant, H.; Kapur, S.; Ingviya, T.; Esmadi, M.; Wijarnpreecha, K.; Mccashland, T. Accuracy of liver stiffness, spleen stiffness, and LS-spleen diameter to platelet ratio score in detection of esophageal varices: Systemic review and meta-analysis. J Gastroenterol Hepatol. 2018, 33, 1696–1706. [Google Scholar] [CrossRef]
- Marasco, G.; Dajti, E.; Ravaioli, F.; Alemanni, L.V.; Capuano, F.; Gjini, K.; Colecchia, L.; Puppini, G.; Cusumano, C.; Renzulli, M.; et al. Spleen stiffness measurement for assessing the response to β-blockers therapy for high-risk esophageal varices patients. Hepatol Int. 2020, 14, 850–857. [Google Scholar] [CrossRef]
- Colecchia, A.; Colli, A.; Casazza, G.; Mandolesi, D.; Schiumerini, R.; Reggiani, L.B.; Marasco, G.; Taddia, M.; Lisotti, A.; Mazzella, G.; et al. Spleen stiffness measurement can predict clinical complications in compensated HCV-related cirrhosis: a prospective study. J Hepatol. 2014, 60, 1158–1164. [Google Scholar] [CrossRef]
- Buechter, M.; Manka, P.; Theysohn, J.M.; Reinboldt, M.; Canbay, A.; Kahraman, A. Spleen stiffness is positively correlated with HVPG and decreases significantly after TIPS implantation. Dig Liver Dis. 2018, 50, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, O.; Ozgokce, M.; Turko, E.; Merter, M. Spleen Stiffness Measurement by Using Shear-Wave Elastography as a Predictor of Progression to Secondary Myelofibrosis. Ultrasound Q. 2021, 37, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Abraldes, J.G.; Reverter, E.; Berzigotti, A. Spleen stiffness: toward a noninvasive portal sphygmomanometer? Hepatology. 2013, 57, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, E.; Server, S. The relationship of spleen stiffness value measured by shear wave elastography with age, gender, and spleen size in healthy volunteers. J Med Ultrason (2001). 2019, 46, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M.; Merkel, C.; Sacerdoti, D.; Nava, V.; Gatta, A. Role of spleen enlargement in cirrhosis with portal hypertension. Dig Liver Dis. 2002, 34, 144–150. [Google Scholar] [CrossRef]
- Fraquelli, M.; Giunta, M.; Pozzi, R.; Rigamonti, C.; Della Valle, S.; Massironi, S.; Conti, C.B.; Aghemo, A.; Ronchi, G.; Iurlo, A.; et al. Feasibility and reproducibility of spleen transient elastography and its role in combination with liver transient elastography for predicting the severity of chronic viral hepatitis. J Viral Hepat. 2014, 21, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Takuma, Y.; Nouso, K.; Morimoto, Y.; Tomokuni, J.; Sahara, A.; Toshikuni, N.; Takabatake, H.; Shimomura, H.; Doi, A.; Sakakibara, I.; et al. Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology. 2013, 144, 92–101.e102. [Google Scholar] [CrossRef]
- Singh, R.; Wilson, M.P.; Katlariwala, P.; Murad, M.H.; Mcinnes MD, F.; Low, G. Accuracy of liver and spleen stiffness on magnetic resonance elastography for detecting portal hypertension: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021, 32, 237–245. [Google Scholar] [CrossRef]
- Zykus, R.; Jonaitis, L.; Petrenkienė, V.; Pranculis, A.; Kupčinskas, L. Liver and spleen transient elastography predicts portal hypertension in patients with chronic liver disease: a prospective cohort study. BMC Gastroenterol. 2015, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Procopet, B.; Berzigotti, A.; Abraldes, J.G.; Turon, F.; Hernandez-Gea, V.; García-Pagán, J.C.; Bosch, J. Real-time shear-wave elastography: applicability, reliability and accuracy for clinically significant portal hypertension. J Hepatol. 2015, 62, 1068–1075. [Google Scholar] [CrossRef]
- Calvaruso, V.; Di Marco, V.; Bronte, F.; Licata, G.; Simone, F.; Butera, G.; Pecoraro, G.; Cabibbi, D.; Alessi, N.; Cammà, C.J.J.O.H. Spleen stiffness correlates with portal hypertension and increases the accuracy of detection of esophageal varices in HCV cirrhosis. J Hepatol. 2010, S159–S160. [Google Scholar] [CrossRef]
- Mannelli, L.; Godfrey, E.; Joubert, I.; Patterson, A.J.; Graves, M.J.; Gallagher, F.A.; Lomas, D.J. MR elastography: Spleen stiffness measurements in healthy volunteers--preliminary experience. AJR Am J Roentgenol. 2010, 195, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Serai, S.D.; Elsingergy, M.M.; Hartung, E.A.; Otero, H.J. Liver and spleen volume and stiffness in patients post-Fontan procedure and patients with ARPKD compared to normal controls. Clin Imaging. 2022, 89, 147–154. [Google Scholar] [CrossRef]
- Talwalkar, J.A.; Yin, M.; Venkatesh, S.; Rossman, P.J.; Grimm, R.C.; Manduca, A.; Romano, A.; Kamath, P.S.; Ehman, R.L. Feasibility of in vivo MR elastographic splenic stiffness measurements in the assessment of portal hypertension. AJR Am J Roentgenol. 2009, 193, 122–127. [Google Scholar] [CrossRef]
- Kassym, L.; Nounou, M.A.; Zhumadilova, Z.; Dajani, A.I.; Barkibayeva, N.; Myssayev, A.; Rakhypbekov, T.; Abuhammour, A.M. New combined parameter of liver and splenic stiffness as determined by elastography in healthy volunteers. Saudi J Gastroenterol. 2016, 22, 324–330. [Google Scholar] [CrossRef]
- Bhatia, A.; Bhatia, H.; Saxena, A.K.; Lal, S.B.; Sodhi, K.S. Shear wave elastography of the spleen using elastography point quantification: stiffness values in healthy children. Abdom Radiol (NY). 2022, 47, 2128–2134. [Google Scholar] [CrossRef]
- Hirooka, M.; Ochi, H.; Koizumi, Y.; Kisaka, Y.; Abe, M.; Ikeda, Y.; Matsuura, B.; Hiasa, Y.; Onji, M. Splenic elasticity measured with real-time tissue elastography is a marker of portal hypertension. Radiology. 2011, 261, 960–968. [Google Scholar] [CrossRef]
- Tanaka, H.; Iijima, H.; Nishimura, J.; Takashima, T.; Ishii, A.; Sakai, Y.; Iwata, K.; Ikeda, N.; Iwata, Y.; Enomoto, H. (Eds.) Could spleen stiffness measurement using Virtual Touch tissue quantification be a good predictor of the presence of varices, including large esophageal varices? Hepatology. NJ USA: Wiley-Blackwell, 2012.
- Yasar, T.K.; Wagner, M.; Bane, O.; Besa, C.; Babb, J.S.; Kannengiesser, S.; Fung, M.; Ehman, R.L.; Taouli, B. Interplatform reproducibility of liver and spleen stiffness measured with MR elastography. J Magn Reson Imaging. 2016, 43, 1064–1072. [Google Scholar] [CrossRef]
- Hines, C.D.; Bley, T.A.; Lindstrom, M.J.; Reeder, S.B. Repeatability of magnetic resonance elastography for quantification of hepatic stiffness. J Magn Reson Imaging. 2010, 31, 725–731. [Google Scholar] [CrossRef]
- Bota, S.; Herkner, H.; Sporea, I.; Salzl, P.; Sirli, R.; Neghina, A.M.; Peck-Radosavljevic, M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013, 33, 1138–1147. [Google Scholar] [CrossRef]
- Ferraioli, G.; Tinelli, C.; Lissandrin, R.; Zicchetti, M.; Bernuzzi, S.; Salvaneschi, L.; Filice, C. Ultrasound point shear wave elastography assessment of liver and spleen stiffness: effect of training on repeatability of measurements. Eur Radiol. 2014, 24, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Eaton, J.E.; Murad, M.H.; Tanaka, H.; Iijima, H.; Talwalkar, J.A. Accuracy of spleen stiffness measurement in detection of esophageal varices in patients with chronic liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014, 12, 935–945.e934. [Google Scholar] [CrossRef] [PubMed]
- Shire, N.J.; Yin, M.; Chen, J.; Railkar, R.A.; Fox-Bosetti, S.; Johnson, S.M.; Beals, C.R.; Dardzinski, B.J.; Sanderson, S.O.; Talwalkar, J.A.; et al. Test-retest repeatability of MR elastography for noninvasive liver fibrosis assessment in hepatitis C. J Magn Reson Imaging. 2011, 34, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Morisaka, H.; Motosugi, U.; Ichikawa, S.; Sano, K.; Ichikawa, T.; Enomoto, N. Association of splenic MR elastographic findings with gastroesophageal varices in patients with chronic liver disease. J Magn Reson Imaging. 2015, 41, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.; Tzschätzsch, H.; Schwahofer, F.; Haas, M.; Bayerl, C.; Muche, M.; Klatt, D.; Majumdar, S.; Uyanik, M.; Hamm, B.; et al. Diagnostic performance of tomoelastography of the liver and spleen for staging hepatic fibrosis. Eur Radiol. 2020, 30, 1719–1729. [Google Scholar] [CrossRef]
- Wagner, M.; Hectors, S.; Bane, O.; Gordic, S.; Kennedy, P.; Besa, C.; Schiano, T.D.; Thung, S.; Fischman, A.; Taouli, B. Noninvasive prediction of portal pressure with MR elastography and DCE-MRI of the liver and spleen: Preliminary results. J Magn Reson Imaging. 2018, 48, 1091–1103. [Google Scholar] [CrossRef]
- Cho, Y.S.; Lim, S.; Kim, Y.; Sohn, J.H.; Jeong, J.Y. Spleen Stiffness Measurement Using 2-Dimensional Shear Wave Elastography: The Predictors of Measurability and the Normal Spleen Stiffness Value. J Ultrasound Med. 2019, 38, 423–431. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, M.J.; Han, K.H.; Yoon, C.S. Age-related changes in liver, kidney, and spleen stiffness in healthy children measured with acoustic radiation force impulse imaging. Eur J Radiol. 2013, 82, e290–e294. [Google Scholar] [CrossRef]
- Arda, K.; Ciledag, N.; Aktas, E.; Aribas, B.K.; Köse, K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. AJR Am J Roentgenol. 2011, 197, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, F.; Schmidberger, J.; Schlingeloff, P.; Binzberger, A.; Kratzer, W. Comparison of point and two-dimensional shear wave elastography of the spleen in healthy subjects. World J Radiol. 2021, 13, 137–148. [Google Scholar] [CrossRef]
- Amin, B.; Bowser, B.L.; Robinson, R.a.S. Quantitative proteomics to study aging in rabbit spleen tissues. Exp Gerontol. 2022, 167, 111908. [Google Scholar] [CrossRef]
- Cesta, M.F. Normal Structure, Function, and Histology of the Spleen. Toxicol Pathol. 2006, 34, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Alex, L.; Rajan, M.L.; Xavier, B.; Jacob, P.; Rani, K.D.; Lakshmi, G.V. Microscopic study of human spleen in different age groups. Int J Res Med Sci. 2015, 1701–1706. [Google Scholar] [CrossRef]
- Losco, P. Normal development, growth, and aging of the spleen. Pathology of the Aging Rat. 1992, 75–94. [Google Scholar]
- Hogenesch, H.; Hahn, F. The lymphoid organs: anatomy, development, and age-related changes. Pathobiology of the Aging Dog. 2001, 1, 127e135. [Google Scholar]
- Madden, K.S.; Bellinger, D.L.; Felten, S.Y.; Snyder, E.; Maida, M.E.; Felten, D.L. Alterations in sympathetic innervation of thymus and spleen in aged mice. Mech Ageing Dev. 1997, 94, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Slagboom, P.E.; De Leeuw, W.J.; Vijg, J. Messenger RNA levels and methylation patterns of GAPDH and beta-actin genes in rat liver, spleen and brain in relation to aging. Mech Ageing Dev. 1990, 53, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.T.; Nadakavukaren, M.J. Age-dependent changes in the cellularity and ultrastructure of the spleen of Fischer F344 rats. Mech Ageing Dev. 1983, 22, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Rifai, K.; Sebagh, M.; Karam, V.; Saliba, F.; Azoulay, D.; Adam, R.; Castaing, D.; Bismuth, H.; Reynès, M.; Samuel, D.; et al. Donor age influences 10-year liver graft histology independently of hepatitis C virus infection. J Hepatol. 2004, 41, 446–453. [Google Scholar] [CrossRef]
- Stefanescu, H.; Grigorescu, M.; Lupsor, M.; Procopet, B.; Maniu, A.; Badea, R. Spleen stiffness measurement using Fibroscan for the noninvasive assessment of esophageal varices in liver cirrhosis patients. J Gastroenterol Hepatol. 2011, 26, 164–170. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Souza, F.; Muñoz, C.; Augustin, S.; Loo, N.; Deng, Y.; Ciarleglio, M.; Garcia-Tsao, G. Liver and Spleen Stiffness Measurements by Point Shear Wave Elastography via Acoustic Radiation Force Impulse: Intraobserver and Interobserver Variability and Predictors of Variability in a US Population. J Ultrasound Med. 2016, 35, 2373–2380. [Google Scholar] [CrossRef]
- Mannelli, L.; Godfrey, E.; Graves, M.J.; Patterson, A.J.; Beddy, P.; Bowden, D.; Joubert, I.; Priest, A.N.; Lomas, D.J. Magnetic resonance elastography: feasibility of liver stiffness measurements in healthy volunteers at 3T. Clin Radiol. 2012, 67, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, F.; Tzschätzsch, H.; Hirsch, S.; Barnhill, E.; Braun, J.; Sack, I.; Guo, J. Tomoelastography of the abdomen: Tissue mechanical properties of the liver, spleen, kidney, and pancreas from single MR elastography scans at different hydration states. Magn Reson Med. 2017, 78, 976–983. [Google Scholar] [CrossRef]
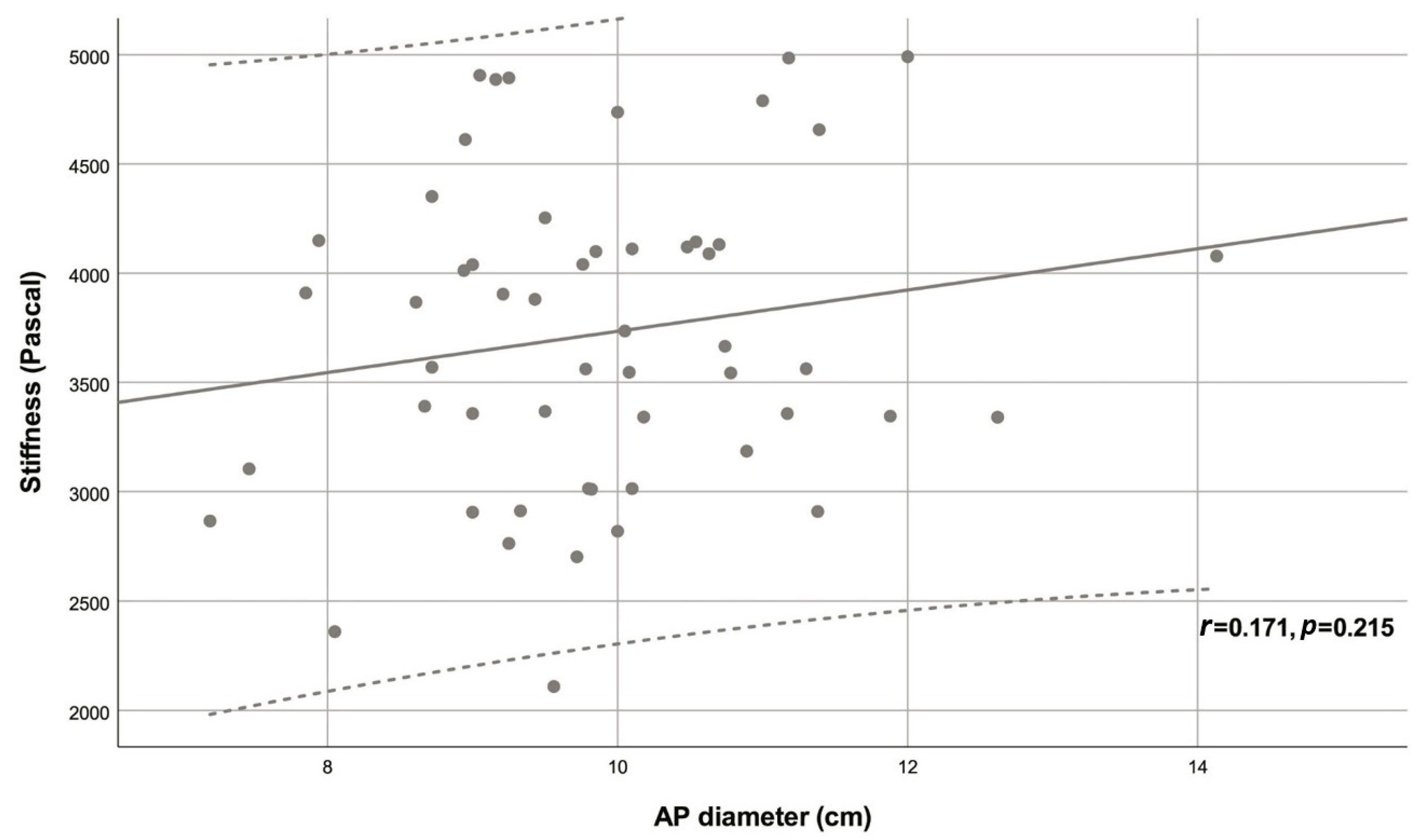
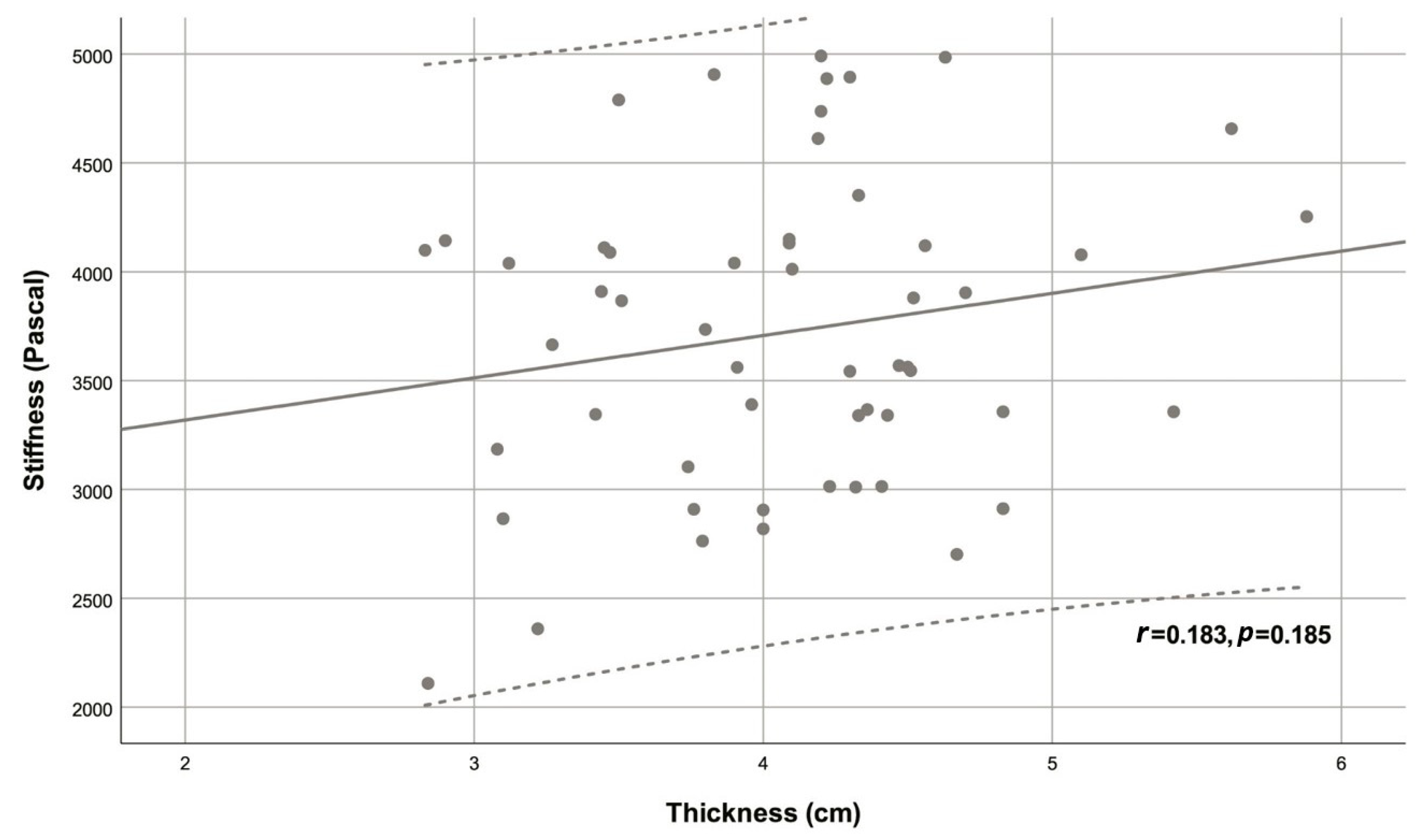
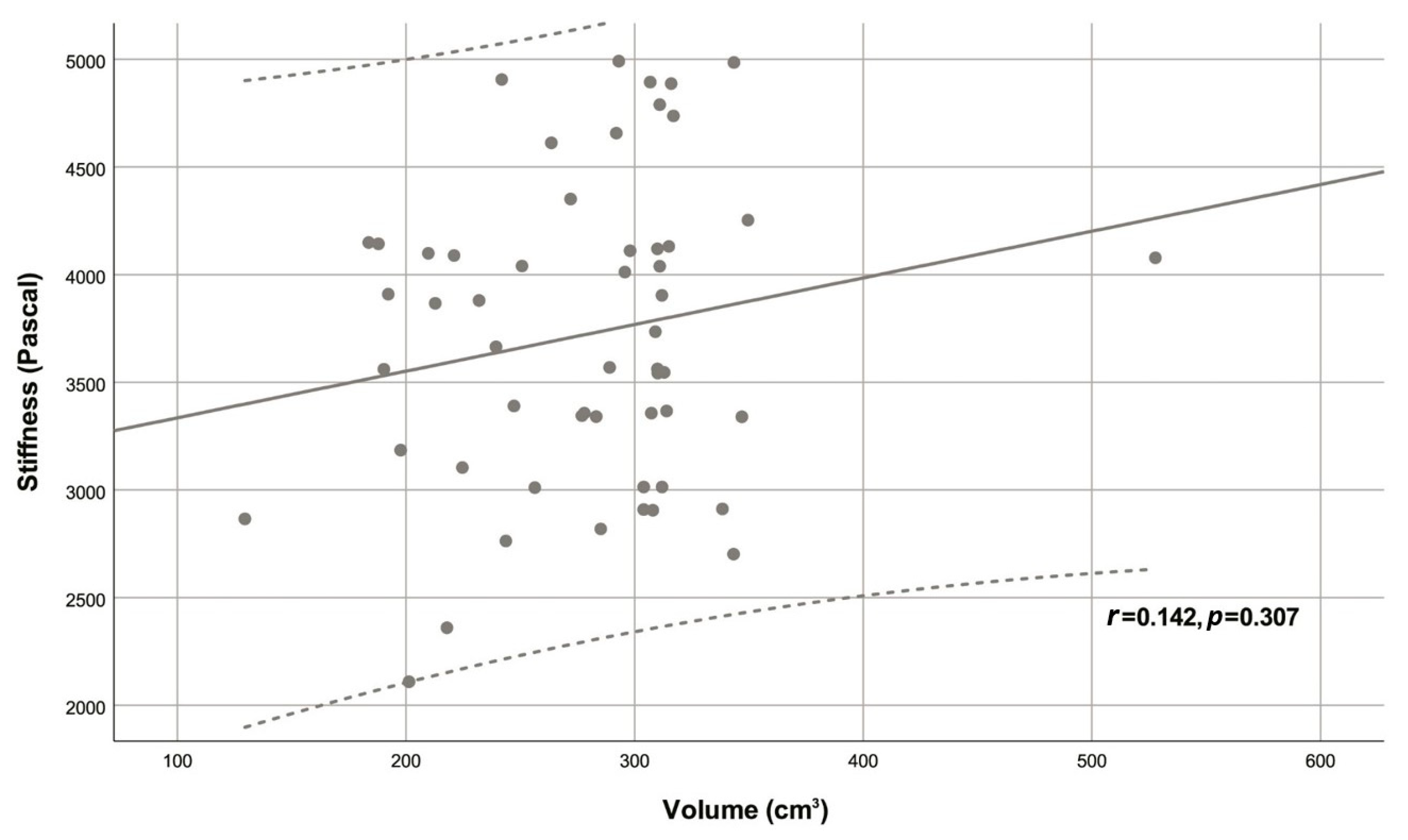
| Variable | Descriptive statistic | r | p |
|---|---|---|---|
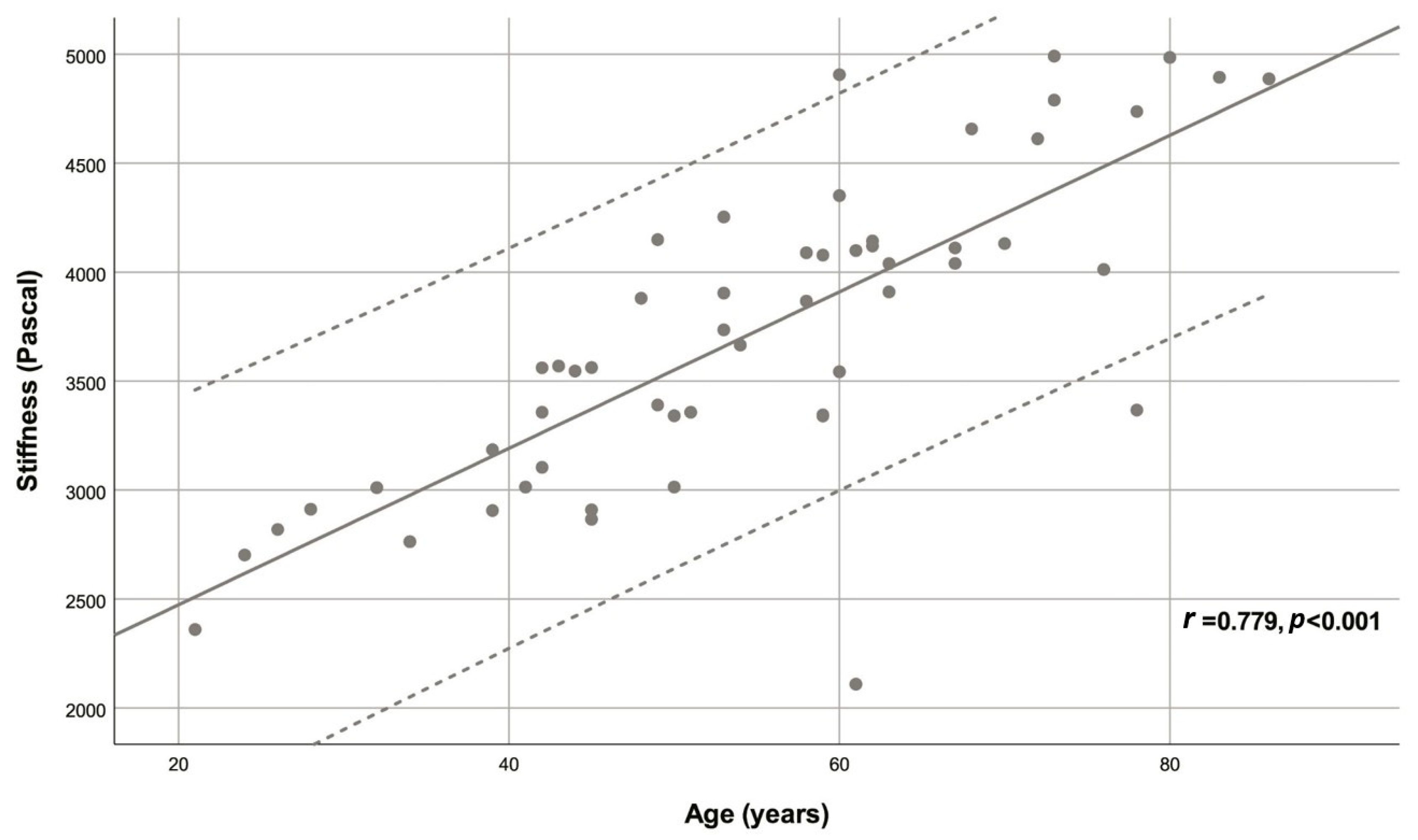
| Age (years) | 54.78 ± 15.40 | 0.779 | <0.001 |
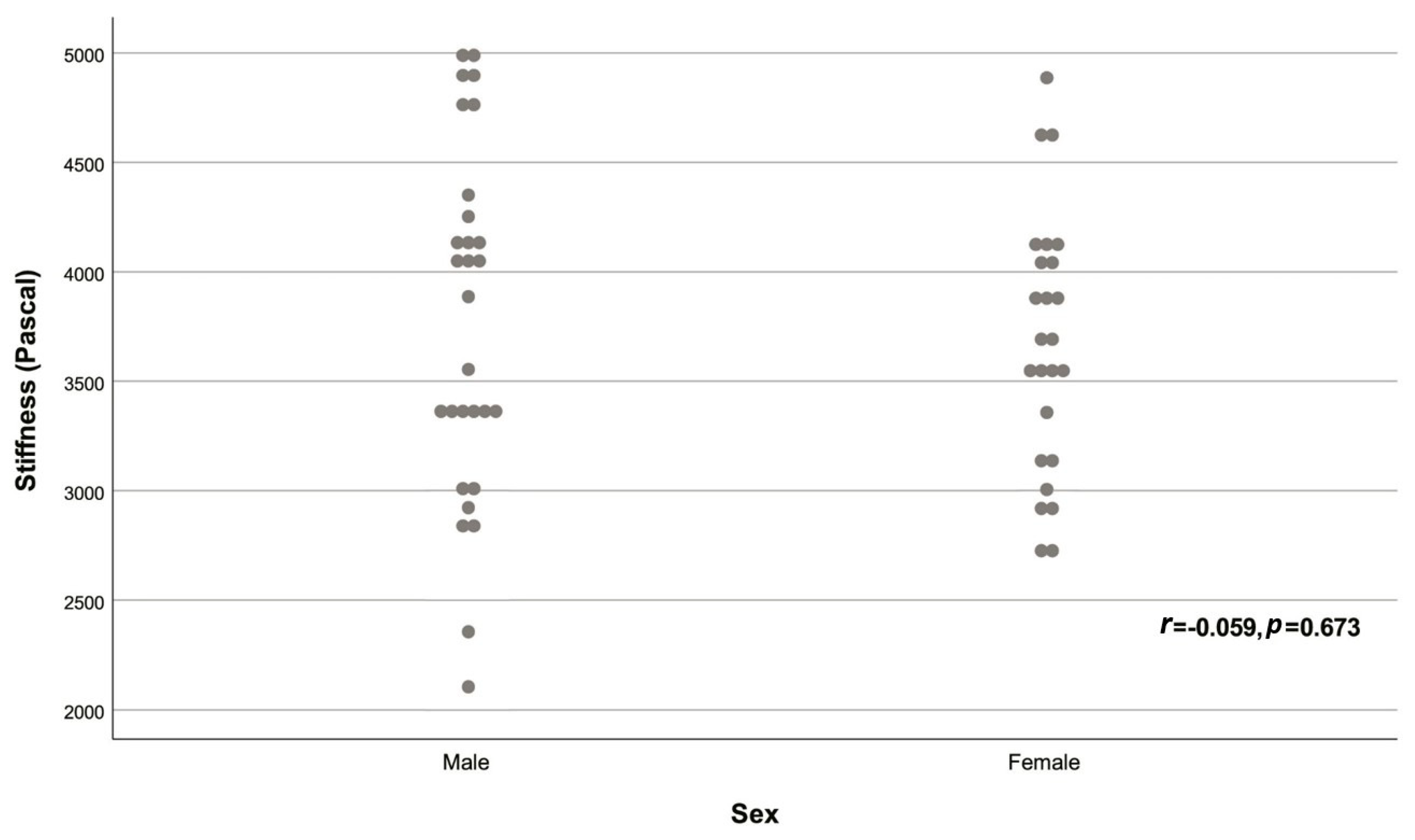
| Sex | |||
| Male | 29 (53.70%) | -0.059 | 0.673 |
| Female | 25 (46.30%) | ||
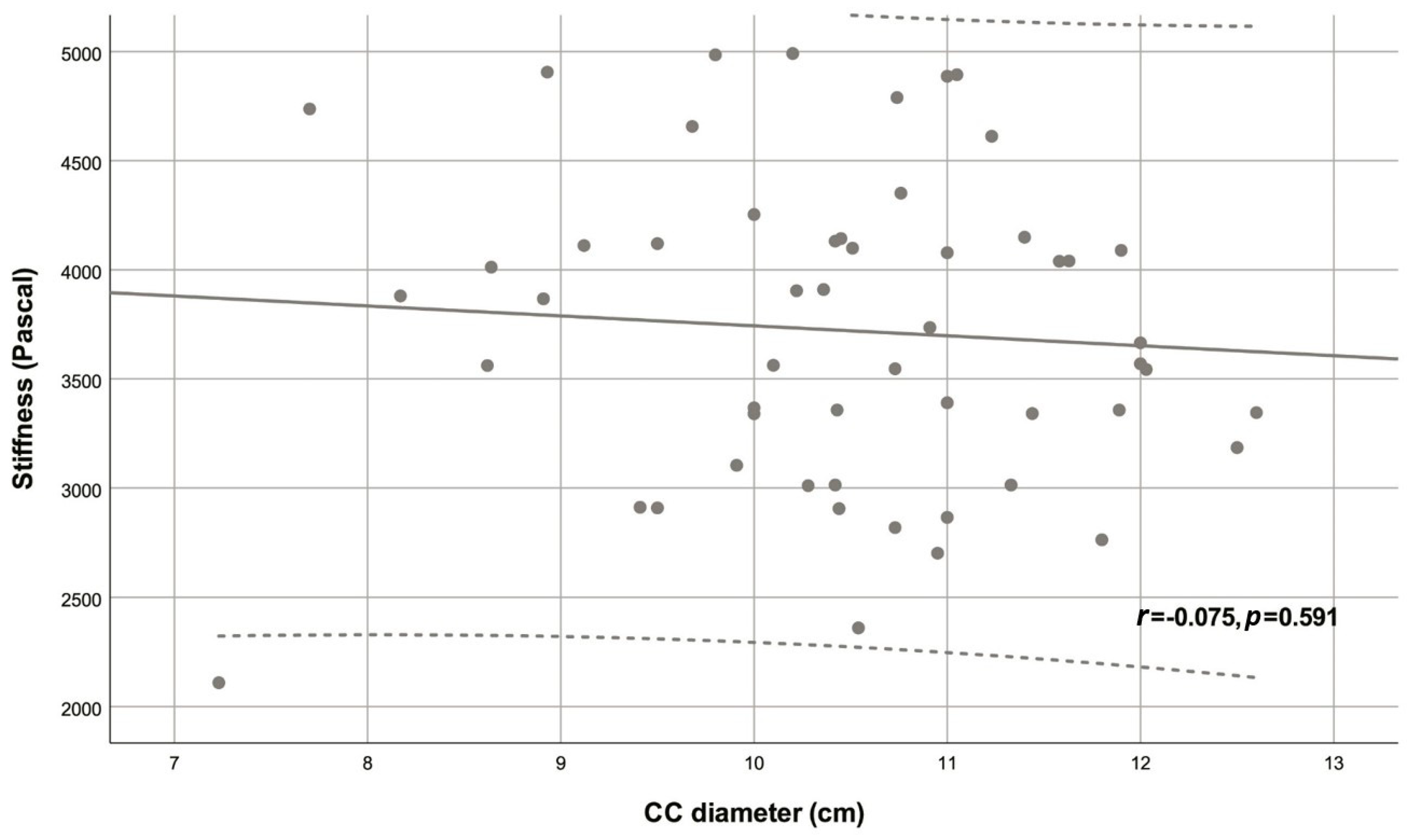
| CC diameter (cm) | 10.46 ± 1.16 | -0.075 | 0.591 |
| AP diameter (cm) | 9.88 ± 1.29 | 0.171 | 0.215 |
| Thickness (cm) | 4.08 ± 0.67 | 0.183 | 0.185 |
| Volume (cm3) | 292.54 (239.40 - 311.00) | 0.142 | 0.307 |
| Stiffness (Pascal) | 3721.94 ± 709.69 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




