Introduction
Meconium obstruction of prematurity (MOP) is a common cause of neonatal intestinal obstruction, especially in preterm infants
[1,2]. The incidence of MOP ranges between 20 and 30% in extremely low birth weight (ELBW) infants [
2,
3,
4]. Delayed diagnosis and treatment of MOP can affect morbidity, mortality, hospital stay, and the treatment cost of affected infants [
4,
5,
6]. Delay in establishing enteral feeding can lead to prolonged use of total parental nutrition (TPN) with an increased risk of its associated cholestasis and prolonged use of a percutaneous inserted central catheter (PICC), which increases the rate of central line-associated sepsis. MOP can lead to sub-acute/acute intestinal obstruction, necessitating the use of contrast enema and laparotomy, both of which increase the risks of necrotizing enterocolitis (NEC) and intestinal perforation. The need for surgical intervention and anesthesia has a potentially negative impact on the infant’s future neurodevelopment [
6,
7]. All these interventions require multidisciplinary inputs from surgeons, anesthetists, and radiologists, potentially escalating the cost of care and financial burden for patients.
We previously conducted a pilot study that demonstrated the efficacy of a twice-daily SE in reducing the time to achieve full enteral feeds in infants with delayed meconium passage [
8]. This study showed a statistically significant reduction in the time required to achieve full enteral feeding of the ELBW infants who received twice-daily SE in those with moderate to severe meconium retention compared to controls. The study led to the development of a protocol that allowed formally accredited, specially trained nurses to perform the SE procedure to address the manpower requirement and reduce the procedure-related cost. The current study compares nurse-administered with physician-administered SE. We hypothesized that nurse to physician-administered twice-daily SE is comparable in administration, catheter insertion length, the volume of enema, daily maximum meconium output, treatment failure, and safety outcomes.
Methods
Study design
The data for this cohort study was obtained by secondary analysis of data from the intervention arm of a randomized control trial (RCT) conducted by our team [
8] (
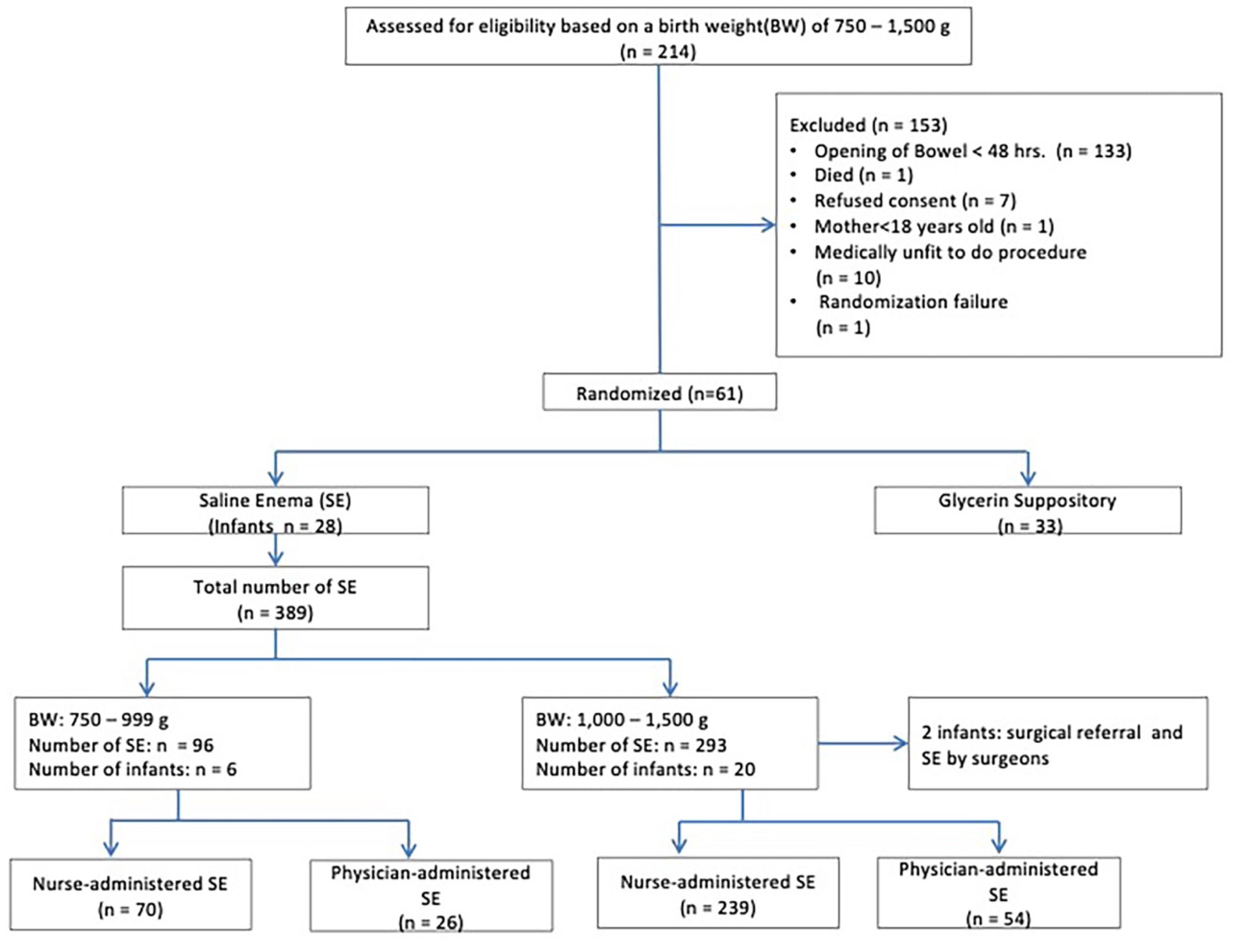
Figure 1).
Participants, settings, sample size
Ethical approval was obtained from the SingHealth Centralized Institutional Review Board (2013/441/E). Informed consent was obtained from all subjects involved in the study. The study was conducted at the neonatal intensive care unit (NICU) (level III C) of KK Women’s and Children’s Hospital in Singapore between November 2013 and October 2015 in 61 infants, of whom 28 were given twice daily SE (intervention arm) and 33 were controls treated conventionally with a glycerin suppository (GS) (2,000mg, a quarter unit, four doses, twice daily over 48h). Infants who failed to pass meconium within 48h of birth were randomized to receive either twice-daily normal SE or a GS until they reached full enteral feeds (110 ml/kg/day). The data for the current cohort study was obtained by secondary analysis of data from the intervention arm of the RCT involving 28 infants.
Nurse Accreditation and Intervention
Graduate nurses eligible to administer SE underwent a formal training and accreditation process, which involved attending a teaching session conducted by a pediatric surgical specialist on gastrointestinal anatomy, physiology, and motility in preterm infants. All participants observed the procedures two or three times and performed the procedure under supervision. Satisfactory performance of the procedure on at least two occasions by meeting a comprehensive competency checklist involving a preparation and performance phase was required for certification and accreditation. The training protocol is published under supplementary material (Supplementary Document 1). Key features of the protocol include prewarming the saline and advancing the catheter (5 or 6 French feeding catheters) 10(-15(<1000g) cm into the colon commensurate with birth weight in the absence of resistance during advancement. A total saline volume of 10-20 ml/kg and 20-40 ml/kg per SE was used in <1000g and ≥1000g infants, respectively. Three nurses completed the accreditation process (Supplementary Document 1).
Outcomes
Data on efficacy, safety, process, and resource outcomes were monitored. The primary outcome was the comparability of SE performed by the nurses with physician-administered SE regarding catheter insertion length, enema volume, the frequency of nurse-administered saline enema, daily maximum meconium output, and treatment failure. Adverse events (AE) (colonic perforation, rectal bleeding, significant clinical deterioration during SE leading to resuscitation, or major morbidity including intraventricular hemorrhage (IVH), sepsis, NEC, and significant electrolyte abnormalities) were also recorded.
Sample size
The saline enema data from 28 infants on the pilot RCT intervention arm was analyzed for this study.
Statistical analysis
We performed Intention to Treat (ITT) and Per Protocol (PP) analyses. Clinical variables and rectal washout intervention parameters were summarized as mean (SD) and median (IQR) stratified by weight group. These were compared using a two-sided two-sample t-test and Wilcoxon rank-sum test. Median time (days) to yellow stool survival curves were estimated using the Kaplan-Meier product-limit estimator and compared using the log-rank test. Binary outcome variables (treatment failure, surgical referral, contrast administered, and laparotomy) were compared between SE and GS groups within each birth weight stratum using Fisher's exact test. The proportion of infants in the SE and GS arms that experienced NEC, sepsis, and IVH was computed, and the absolute difference in proportion in each of these AEs was used. The profile was deemed comparable if the difference in proportion was no more than +3% or a difference of no more than one infant with an event. The proportion of infants encountering any AE was monitored and tolerated if an absolute difference in proportion was no more than + 10%. SAS Software version 9.4 (SAS Institute, Cary, North Carolina, USA) was used in the analyses. Zero serious AE secondary to saline enema was tolerated.
Results
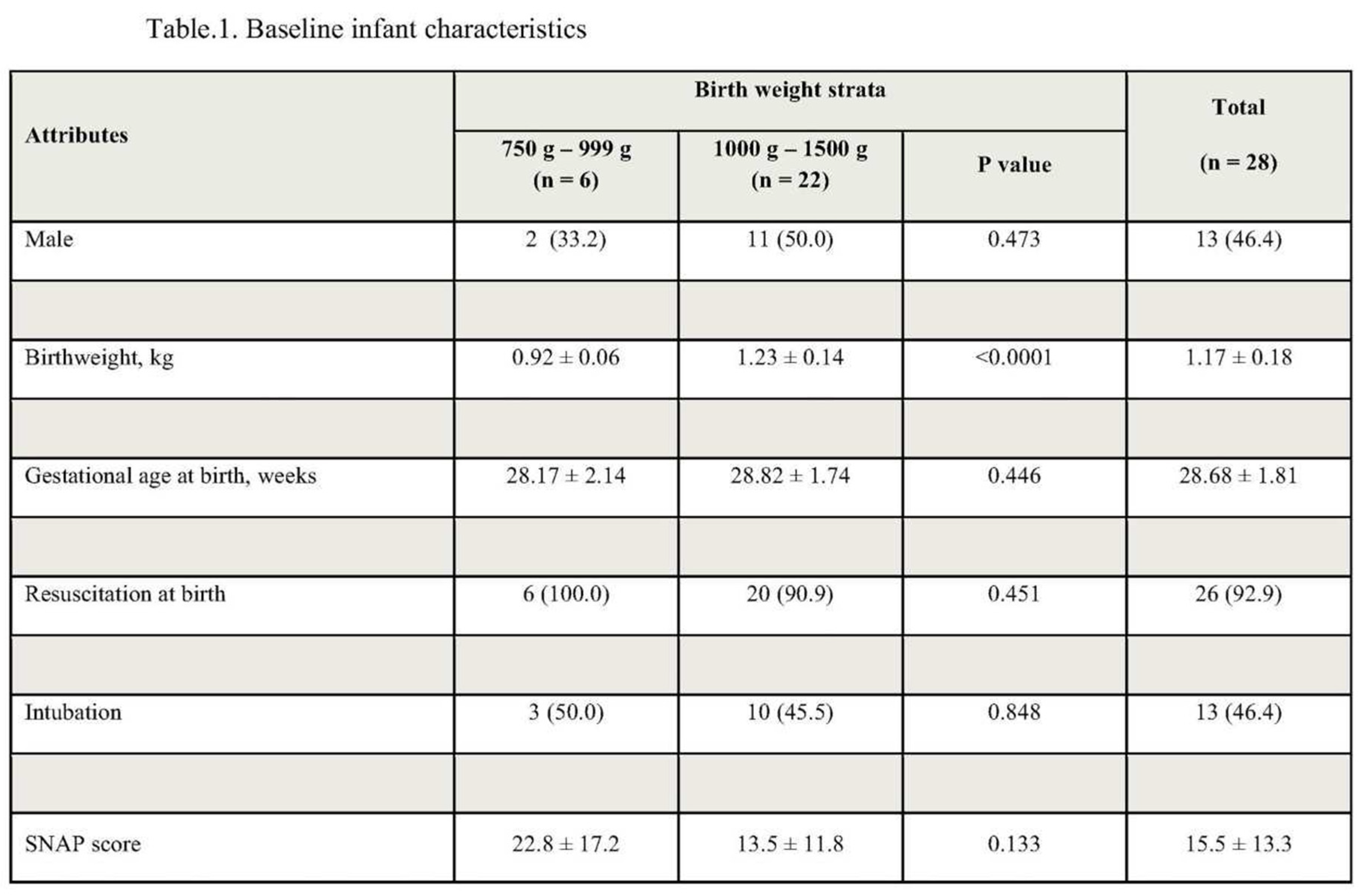
During the two years, 28 infants underwent saline enema administration. Baseline characteristics are described in
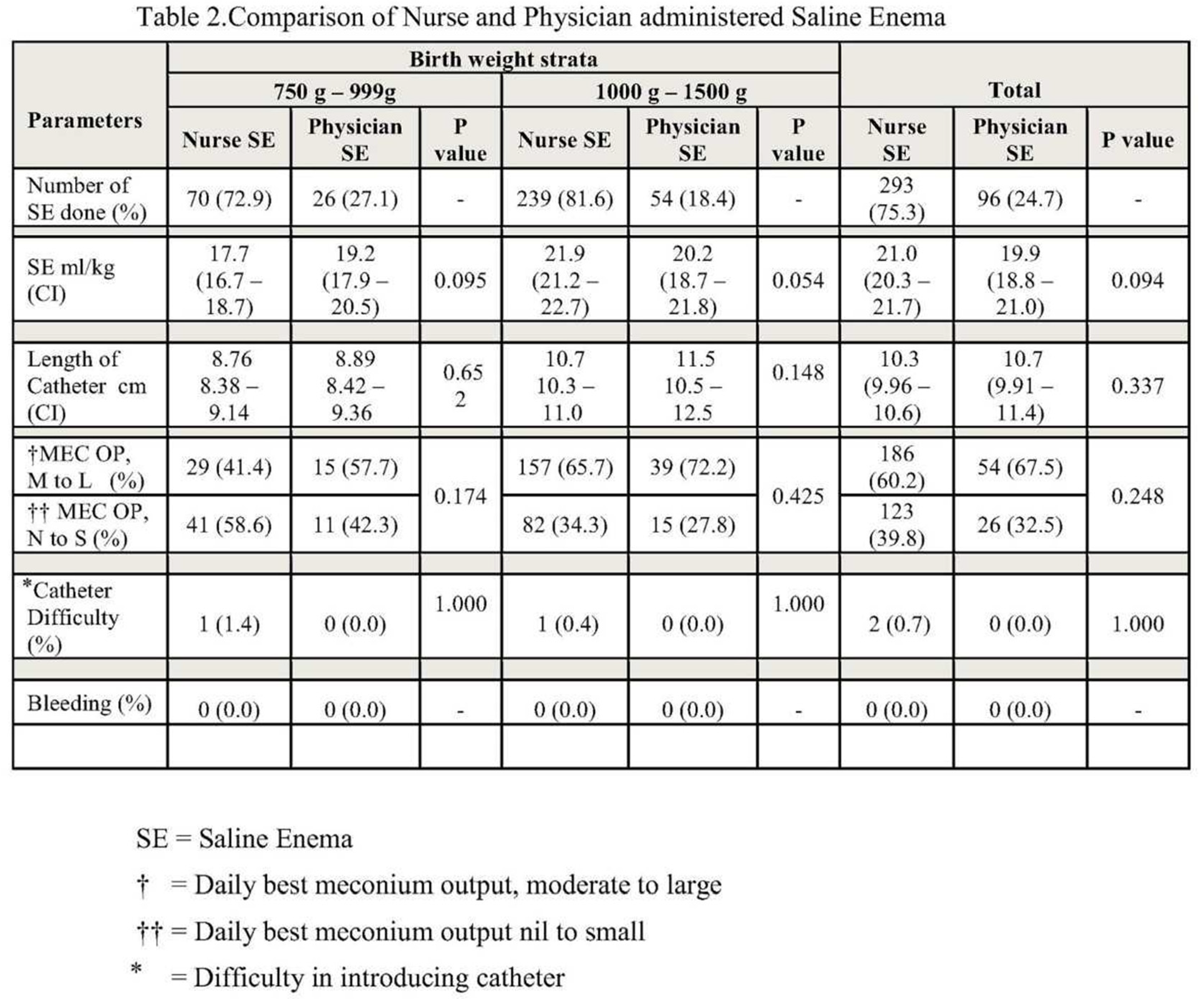
Table 1. Three hundred and eighty-nine enemas were administered, and the nurses administered most of them. There were no reported AEs in the SE arm of the study. Saline enema intervention parameters and treatment failure data are summarized in
Table 2. The mean number of rectal washouts performed was 15.8 and 14.1(p=0.611) in the <1000g and ≥1000g infants, respectively. Feeding catheter sizes of 5 or 6 French were used in the procedure, with 6 French sizes used in more than 99% of the cases. 389 SEs were performed, 96 and 293 in <1000g and ≥1000g, respectively. The nurses administered 70 (72.9%) and 239 (81.6%) of SEs in the <1000g and ≥1000g groups, respectively
. The mean length of insertion of the catheter past the anal margin was 8.7 and 8.8 cm (p=0.65) for nurses and doctors in <1000g infants and 10.7 and 11.5 cm (p=0.14), respectively, for nurses and doctors in ≥1000g infants, respectively. The mean volume of saline was 17.7 and 19.2 ml/kg (p=0.09) for nurses and doctors, respectively, in <1000g and 21.9 and 20.2 ml/kg (p=0.05), respectively, in ≥1000g infants. No significant difference was reported between nurses and doctors for the difficulty in passing the catheter and for the daily best result for meconium output (
Table 2).
There was no treatment failure in the SE arm. All infants in both weight groups were successfully managed with SE alone. Two infants, one from each of the two weight subgroups in the SE arm, were referred to surgeons, one with difficulty passing the catheter and the other for blood-stained meconium. However, both infants were successfully managed with saline enema administered by pediatric surgeons.
Discussion
Our study examined the comparability of nurse-administered, twice-daily SE with physician-administered SE in treating MOP. The performance of SE was similar between trained nurses and physicians regarding catheter insertion length, enema volume, and daily meconium output. There was no treatment failure, and the procedure was safe [
8,
9].
There was a case of grade IV IVH among infants <1000g on day four of life. Based on a formalized quality assurance checklist, the authors and safety committee concluded that this morbidity was unrelated to the intervention, and the infant continued to receive SE despite the IVH. Nevertheless, we advocate gentle handling of infants, especially in the first five days of life. None of the infants in <1000g developed NEC. In this pilot study, we demonstrated that NEC does not appear to be a significant concern with high-volume saline enema compared to other interventions such as gastrografin or glycerin [
10,
11].
To our knowledge, this cohort study is the first to compare the nurse-administered SE with a physician-administered saline enema for treating MOP [
13]. The findings may have significant implications for preterm infants with meconium obstruction. Trained nurses can safely and effectively administer the SE. Our primary study showed that when administered early, this intervention can reduce the length of stay in the NICU, parenteral nutrition days, PICC days, and risk of sepsis [
8]. The cumulative impact of these benefits and trained nursing manpower can reduce the cost of care and enhance the availability of skilled human resources in managing premature infants with MOP.
Current interventions from the published literature for managing MOP are invasive [
6,
7]. Conventional management for the established condition in most centers is a gastrografin enema. This treatment has been associated with a risk of NEC due to the high osmolality of the contrast agent. Intestinal perforation is another known complication of gastrografin. Gastrografin also entails radiation exposure with its inherent risk [
7]. Infants who fail to respond to contrast enema are managed with laparotomy under general anesthesia. Most retrospective studies indicate that general anesthesia in preterm and term infants is associated with a higher risk of learning disability, behavioral disorders, and language and cognition issues in children [
13]. Moreover, the cost of treatment with either a gastrografin enema or laparotomy is considerably higher than SE, as described in our study. In addition to cost-saving, the ease of administration of an SE, safety, and effectiveness are shown by our study results [
8].
This report demonstrates the comparability and safety of nurse and physician-administered SE in treating infants with MOP. However, our study has limitations in that it did not include infants with birth weight <750g nor cases of MOP with atypical presentations such as those infants who initially pass meconium but subsequently develop sub-acute intestinal obstruction manifesting as abdominal distension, emesis, feed intolerance and inability to pass meconium, without external intervention [
1,
9]. The definition of MOP needs further refinement. In addition to no bowel opening for 48 h, one of the following criteria needs to be added to the definition: a) feed intolerance and b) clinical or radiological features of abdominal distention. The intervention was not randomized between nurses and physicians. The number of infants who underwent a SE was small, requiring caution in interpreting safety outcomes.
The study design of future trials employing nurse-administered saline enema as a treatment for MOP can be further revised to accommodate the limitations of this study. In addition, the definition of treatment failure can be further refined by using the following criteria: a) no bowel opening for three days despite twice daily SE, b) constant clear return after two days of SE, and c) clinical deterioration with worsening abdominal distension, bowel loop distension and increase in the gastric aspirate. This helps in timely referral to surgeons and improves patient safety and referral decision objectivity. It is noteworthy that none of our study infants encountered these situations. Ultrasound-guided catheter insertion for SE may improve safety and ensure the saline into the terminal ileum. Still, it may affect the skin integrity, predispose to infection, and increase the cost of care. However, ultrasound-guided hydrostatic saline enema requires serious consideration in < 750g infants to mitigate the risks [
14,
15]. Initial catheterization by pediatric surgeons may further improve the safety of the intervention and should be considered in complex cases.
Conclusions
The outcome of our study suggests that nurses administered twice daily SE is a productive and safe strategy for MOP. The outcome of the nurse-administered SE was comparable to that of the physician. The study design of future trials may be refined to address the limitations of this study, such as sample size, including infants with birth weight less than 750g, infants with an atypical presentation, and refinement of MOP and treatment failure criteria.
Supplementary Materials
Supplementary document No 1. SE Performance protocol and competency assessment document.
Author Contributions
IT and LW conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised it. YBMA, LS, YPL, WC, and AJ designed the data collection instruments, collected data, conducted the initial analyses, and reviewed and revised the manuscript. VSR and SC conceptualized and designed the study and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work. YBMA and LS contributed equally to this work.
Funding Source
Khoo Pilot Award / DUKE-NUS.
Financial Disclosures
The authors have no financial relationship relevant to this article to disclose.
Data Availability Statement
Data is available on request and is stored under SingHealth guidelines. Contact person: Dr John Allen. john.allen@duke-nus.edu.sg.
Acknowledgments
We thank the following individuals for their contribution to the publication of the study: Dr. Eddy Saputra Leman (Duke-NUS Medical School); Prof. A John Rush (Duke-NUS Medical School); Prof. Haresh Kirpalani (The Children’s Hospital of Philadelphia); Prof. Anette Sundfor Jacobsen (KKH); A/Prof. Low Yee (KKH); Dr. Abdul Alim Abdul Haium (KKH); Dr. Ann Wright (KKH); Dr. Sridhar Arunachalam (SGH). We extend our thanks to the following organizations for their support of the success of the study:The Duke-NUS Graduate Medical School Singapore; “Estate of Tan Sri Khoo Teck Puat” for the funding support; National University of Singapore
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Clinical Trial Registration
References
- Siddiqui MM, Drewett M, Burge DM. Meconium obstruction of prematurity. Arch Dis Child Fetal Neonatal Ed 2012, 97, F147–F150. [Google Scholar] [CrossRef] [PubMed]
- Juang D, Snyder CL. Neonatal bowel obstruction. Surg Clin North Am 2012, 92, 685–711. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, MM. Chapter 81 - Meconium Ileus A2 - Grosfeld, Jay L. In: O'Neill JA, Coran AG, Fonkalsrud EW, Caldamone AA, editors. Pediatric Surgery (Sixth Edition). Philadelphia: Mosby; 2006. pp. 1289–1303.
- Kim YJ, Kim EK, Kim ES, Kim HS, Choi JH, Cheon JE, Kim WS, Kim IO, Park KW. Recognition, diagnosis, and treatment of meconium obstruction in extremely low birth weight infants. Neonatology. 2012, 3, 172–178. [Google Scholar]
- Garza-Cox S, Keeney SE, Angel CA, Thompson LL, Swischuk LE. Meconium obstruction in the very low birth weight premature infant. Pediatrics 2004, 114, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Cho HH, Cheon JE, Choi YH, Lee SM, Kim WS, Kim IO, Shin SM, Kim EK, Kim HS, Choi JH, et al. Ultrasound-guided contrast enema for meconium obstruction in very low birth weight infants: Factors that affect treatment success. Eur J Radiol. 2015, 84, 2024–2031. [Google Scholar] [CrossRef] [PubMed]
- Haiden N, Norooz F, Klebermass-Schrehof K, Horak AS, Jilma B, Berger A, Repa A. The effect of an osmotic contrast agent on complete meconium evacuation in preterm infants. Pediatrics. 2012, 130, e1600–e1606. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim T, Li Wei C, Bautista D, Sriram B, Xiangzhen Fay L, Rajadurai VS. Saline Enemas versus Glycerin Suppositories to Promote Enteral Feeding in Premature Infants: A Pilot Randomized Controlled Trial. Neonatology 2017, 112, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Paradiso VF, Briganti V, Oriolo L, Coletta R, Calisti A. Meconium obstruction in the absence of cystic fibrosis in low-birth-weight infants: an emerging challenge from increasing survival. Ital J Pediatr 2011, 37, 55. [Google Scholar] [CrossRef] [PubMed]
- Livingston MH, Shawyer AC, Rosenbaum PL, Williams C, Jones SA, Walton JM. Glycerin enemas and suppositories in premature infants: a meta-analysis. Pediatrics 2015, 135, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Anabrees J, Shah VS, AlOsaimi A, AlFaleh K. Glycerin laxatives for prevention or treatment of feeding intolerance in very low birth weight infants. Cochrane Database Syst Rev 2015, 9, CD010464. [Google Scholar]
- Sáenz de Pipaón Marcos M, Teresa Montes Bueno M, Sanjosé B, Gil M, Parada I, Amo P. Randomized controlled trial of prophylactic rectal stimulation and enemas on stooling patterns in extremely low birth weight infants. J Perinatol 2013, 33, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Vinson AE, Houck CS. Neurotoxicity of Anesthesia in Children: Prevention and Treatment. Curr Treat Options Neurol 2018, 20, 51. [Google Scholar] [CrossRef] [PubMed]
- Nakaoka T, Nishimoto S, Tsukazaki Y, Santo K, Higashio A, Kamiyama M, Uehara S, Yoneda A, Tanaka Y, Ichiba H. Ultrasound-guided hydrostatic enema for meconium obstruction in extremely low birth weight infants: a preliminary report. Pediatr Surg Int. 2017, 33, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T. Saline Enema Administration in Meconium Obstruction of Prematurity and Impact on the Resolution, Feeds, Microbiome, and Gut-brain Axis.ClinicalTrials.gov identifier: NCT06048614. Available online: https://www.clinicaltrials.gov/ct2/show/NCT06048614 (accessed on 4 October 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).