Submitted:
17 October 2023
Posted:
18 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Diabetes
3. Functional Beverages
3.1. Definition and Types
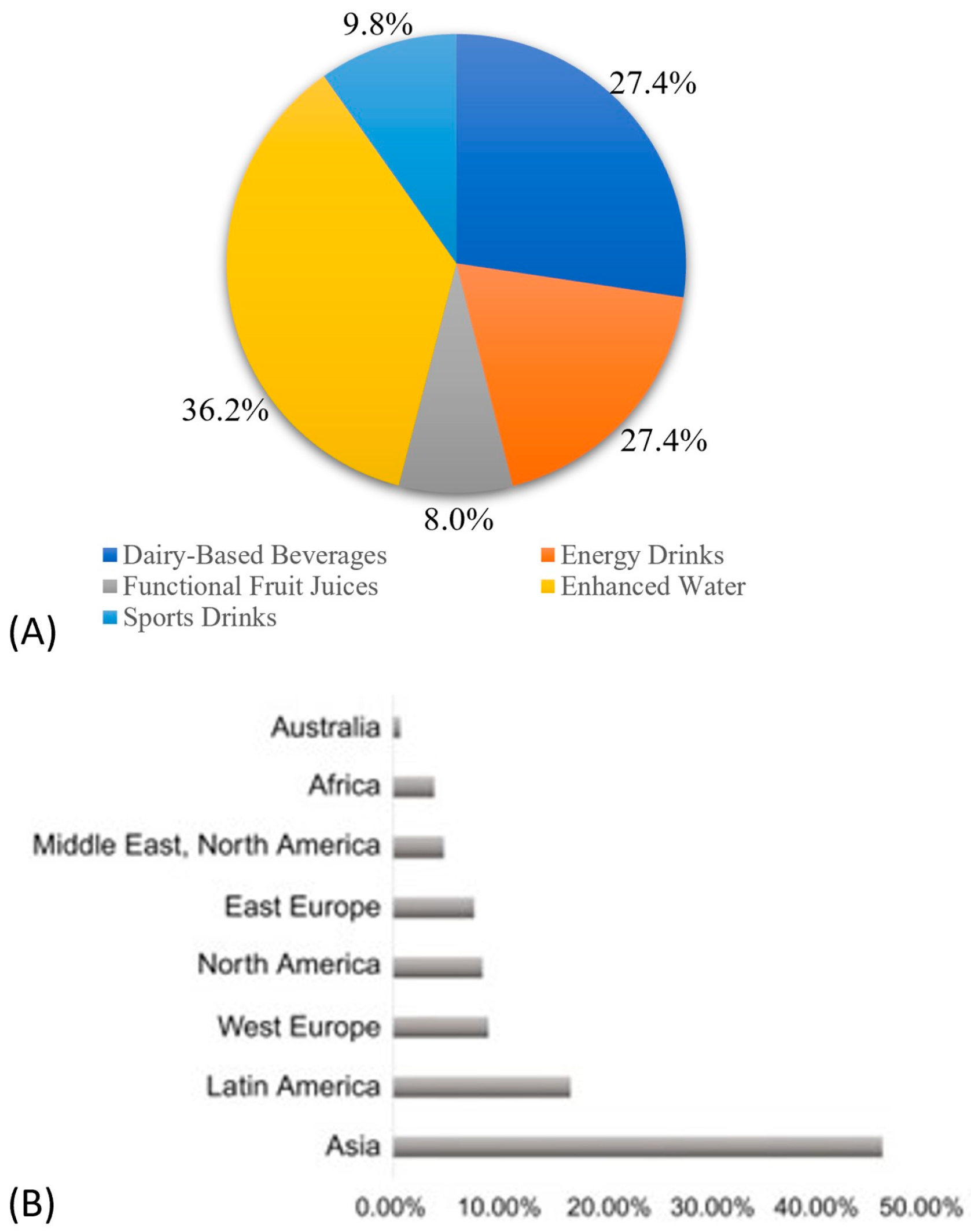
3.2. Market of Functional Foods and Beverages
4. The Role of Fruit and Vegetable-Based Functional Foods and Beverages against Diabetes
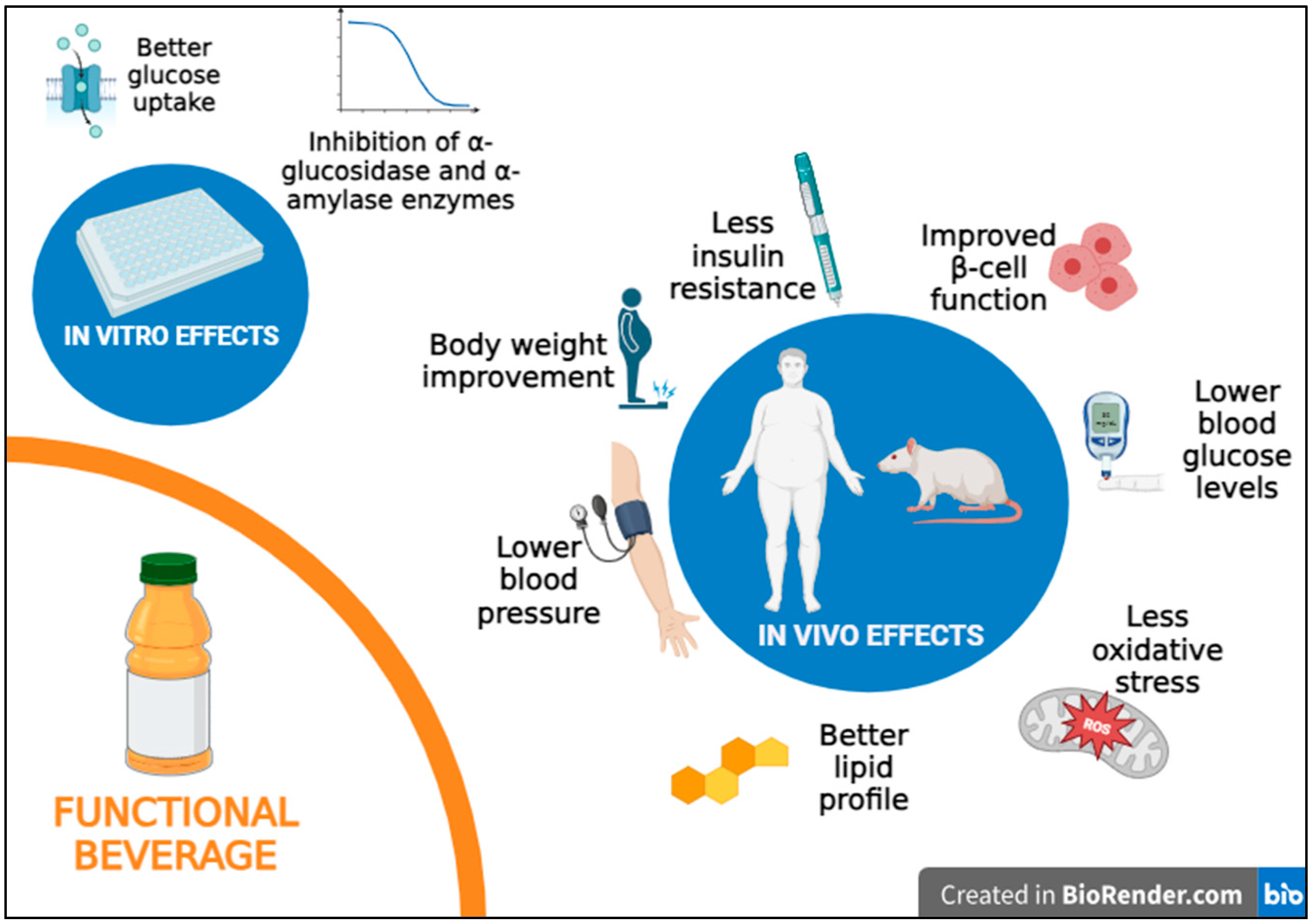
4.1. In Vitro Studies with Functional Beverages and Diabetes
| Beverage | Assays | Results | Reference |
|---|---|---|---|
| Fermented bitter gourd juice | α-glucosidase inhibition (measured as glucose production reduction) | ↓ glucose production = 14.5 - 19.2 % | [60] |
| Prunus fruit smoothies | α-amylase and α-glucosidase inhibition | IC50 amy = <1.00 - 8.03 mg/mL IC50 gluco = 1.20 - 6.94 mg/mL |
[62] |
| Bitter gourd fruit juice | Glucose uptake by diaphragms from diabetic rats | Glucose uptake (absence of insulin): ↑ 1.40 mg/g tissue Glucose uptake (presence of insulin): ↑ 4.08 mg/g tissue |
[68] |
| Apple and blackcurrant polyphenol-rich drinks | Glucose uptake by Caco-2 cells | ↓ glucose uptake (apple polyphenols) = 46 – 51% IC50 (blackcurrant polyphenols) = 0.51 – 0.63 mg/mL |
[70] |
| Fermented sprouted quinoa yoghurt beverages | α-amylase inhibition | IC50 amy (100 µL) = 30.48 - 39.36 mg/mL IC50 amy (200 µL) = 39.44 - 51.57 mg/mL IC50 amy (400 µL) = 50.06 - 71.28 mg/mL |
[63] |
| Tigernut beverages fortified with extracts of Vernonia amygdalina and Momordica charantia | α-amylase and α-glucosidase inhibition | Inhibition amy = 20.59 - 60.14 % Inhibition gluco = 38.82 - 75.54 % |
[61] |
| Probiotics-fermented blueberry juices |
α-amylase and α-glucosidase inhibition Glucose uptake by HepG2 cells |
IC50 amy = 0.25 - 2.67 mg/mL IC50 gluco = 1 - 40.68 mg/mL ↑ glucose uptake ≈ 1 mmol/L |
[64] |
| Sea-buckthorn based smoothies | α-amylase, α-glucosidase and pancreatic lipase inhibition | Inhibition amy = 20.03 - 49.82 % Inhibition gluco = 6.12 – 98.61 % Inhibition lipase = 50.80 – 96.31 % |
[13] |
4.2. In Vivo Studies and Clinical Trials with Functional Beverages and Diabetes
| Beverage | Administration | Relevant results | Reference |
|---|---|---|---|
| Emblica officinalis fruit juice | 1 ml/kg, daily, 8 weeks in STZ-DR | ↓ serum glucose, FBG, TAG, TC, VLDL-C ↑ serum insulin, FBI, HDL-C, LDL-C |
[83] |
| Fermented noni fruit juice | 1.5 µL/kg, 2xday, 12 weeks in HFD-OR | ↓ body weight, FBG, insulin resistance ↑ insulin, glucose tolerance |
[77] |
| Musa sapientum lyophilized stem juice | 50 mg/kg, daily, 2 weeks in STZ-DR | ↓ FPG, PPG, HbA1c, TC, LDL-C, VLDL-C, TAG, G6P, HMG-CoA ↑ insulin, HDL-C, GCK activity |
[84] |
| Processed tomato-vinegar beverage | 14 ml/kg, daily, 6 weeks in HFD-OR | ↓ TAG, body weight, insulin resistance ↑ glucose tolerance, HDL-C, GCK activity |
[78] |
| Fresh pomegranate juice | 1.5 mL/kg, once, in T2D patients | ↑ β-cell function ↓ FPG, insulin resistance |
[96] |
| Bittergourd fermented beverage | 45 ml, daily, for 1 and 6 months in diabetic patients | ↓ FBG and PPBS (1 month) ↓ FBG and PPBS, = blood lipid profile, ↑ HbA1c (6 months) |
[97] |
| Grapefruit sweetened juices | 2-3 ml, daily, for 2 weeks in HFD-OR | ↓ body weight, FBG, FSI, liver TAG | [88] |
| Vaccinium corymbosum infusion | Cup of juice, daily, for 2 years, in a pre-diabetic | ↓ serum glucose, HbA1c, insulin resistance | [100] |
| Palm fruit juice | 170-720 mg GAE/kg, daily, for 4-36 weeks in CS-DR | ↓ blood glucose, TAG, TC, liver lipids = body weight |
[89] |
| Palm fruit juice | HC diet + 5.4 g GAE/kg, 4 weeks in DR | upregulation of 71 genes (HDL apolipoproteins hepatic detoxification) downregulation of 108 genes (insulin signalling and fibrosis) |
[93] |
| Apple and blackcurrant polyphenol-rich drinks | 200 g, once, in healthy patients | ↓ PPG, insulin, C-peptide, GIP = TAG |
[70] |
| Fermented Syzygium cumini stem | 4ml/kg, daily, for 30 days in STZ-DR | ↓ FBG, TC, LDL-C, serum TAG, AI, VLDL-C ↑ serum insulin, HDL-C |
[85] |
| Momordica charantia fruit juice | 10 ml/kg, 14 days before diabetes and 21 days after, in STZ-DR | ↓ serum glucose, insulin resistance, serum TC, TAG, pancreatic MDA ↑ serum insulin, β-cell function, HDL-C, TAOC, pancreatic GSH |
[68] |
| Strawberry and cranberry polyphenols beverage | 333 mg polyphenols, daily, for 6 weeks, in insulin-resistant patients | ↑ insulin sensitivity = lipids and markers of inflammation and oxidative stress |
[98] |
| Cowpea juice, tomato juice and green apple juices combined | Combinations, daily, for 28 days in ALL-DR | ↓ FBG | [33] |
| Pigeon pea beverage dilluted in water | 2.7 g/kg, daily, for 2 weeks in DHR | ↓ plasma glucose, TC, MDA = body weight |
[74] |
| Açaí beverage | 2x325 mL, daily, for 12 weeks, in patients with metabolic syndrome | ↓ IFN-γ plasma level, 8-isoprostane = lipid and glucose metabolism markers |
[99] |
| Fermented Momordica charantia juice | 10 mL/kg, daily, for 4 weeks in STZ-DR | ↓ body weight loss, blood glucose, FBG, serum insulin, insulin resistance, TC, LDL-C, TAG, MDA ↑ HDL-C |
[79] |
| Fermented jackfruit leaf beverage | 1.5 mL/kg, daily, for 28 days in STZ-DR | ↓ FBG, body weight loss, relative organ weights | [32] |
| Emblica officinalis fruit juice | 2 ml/kg, daily, for 42 days in DR; for 4 weeks in HF-DR | ↓ body weight, FBG, insulin resistance, HbA1c, TAG, blood pressure; TC = HDL-C |
[76] |
| Citrus concentrate enriched with b-cryptoxanthin, hesperidin and pectin | 2 mL, daily, for 8 weeks in HF-PDR | ↑ glucose tolerance ↓ plasma glucose, plasma insulin, TAG, LDL-C, VLDL-C, blood pressure = TC, HDL-C |
[92] |
| P. angulata fruit extract | 1 and 2 mL/kg, daily, for 2 weeks in STZ-DR | ↓ FBG, upregulation of GLUT-4, restoration of damaged organs = body weight |
[90] |
| Yogurt bengkuang tape ketan hitam | 200 mL, daily, for 2 weeks in T2D patients | = FBG ↓ plasma MDA |
[101] |
5. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Gayathry, K.S.; John, J.A. Functional Beverages: Special Focus on Anti-Diabetic Potential. J. Food Process. Preserv. 2021, 45, 1–12. [Google Scholar] [CrossRef]
- World Health Organization. Noncommunicable diseases. World Health Organization. https://www.who.int/en/news-room/fact-sheets/detail/noncommunicable-diseases (accessed 2023-07-20). /: https.
- Olaiya, C.O.; Soetan, K.O.; Esan, A.M. The Role of Nutraceuticals, Functional Foods and Value Added Food Products in the Prevention and Treatment of Chronic Diseases. African J. Food Sci. 2016, 10, 185–193. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine; 2019. [Google Scholar]
- World Health Organization. Global Report on Diabetes; 2016; https://www.who.int/publications/i/item/9789241565257.
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; Lindstedt, P.A.; Aali, A.; Abate, Y.H.; Abate, M.D.; Abbasian, M.; Abbasi-Kangevari, Z.; Abbasi-Kangevari, M.; Abd ElHafeez, S.; Abd-Rabu, R.; Abdulah, D.M.; Abdullah, A.Y.M.; Abedi, V.; Abidi, H.; Aboagye, R.G.; Abolhassani, H.; Abu-Gharbieh, E.; Abu-Zaid, A.; Adane, T.D.; Adane, D.E.; Addo, I.Y.; Adegboye, O.A.; Adekanmbi, V.; Adepoju, A.V.; Adnani, Q.E.S.; Afolabi, R.F.; Agarwal, G.; Aghdam, Z.B.; Agudelo-Botero, M.; Aguilera Arriagada, C.E.; Agyemang-Duah, W.; Ahinkorah, B.O.; Ahmad, D.; Ahmad, R.; Ahmad, S.; Ahmad, A.; Ahmadi, A.; Ahmadi, K.; Ahmed, A.; Ahmed, A.; Ahmed, L.A.; Ahmed, S.A.; Ajami, M.; Akinyemi, R.O.; Al Hamad, H.; Al Hasan, S.M.; AL-Ahdal, T.M.A.; Alalwan, T.A.; Al-Aly, Z.; AlBataineh, M.T.; Alcalde-Rabanal, J.E.; Alemi, S.; Ali, H.; Alinia, T.; Aljunid, S.M.; Almustanyir, S.; Al-Raddadi, R.M.; Alvis-Guzman, N.; Amare, F.; Ameyaw, E.K.; Amiri, S.; Amusa, G.A.; Andrei, C.L.; Anjana, R.M.; Ansar, A.; Ansari, G.; Ansari-Moghaddam, A.; Anyasodor, A.E.; Arabloo, J.; Aravkin, A.Y.; Areda, D.; Arifin, H.; Arkew, M.; Armocida, B.; Ärnlöv, J.; Artamonov, A.A.; Arulappan, J.; Aruleba, R.T.; Arumugam, A.; Aryan, Z.; Asemu, M.T.; Asghari-Jafarabadi, M.; Askari, E.; Asmelash, D.; Astell-Burt, T.; Athar, M.; Athari, S.S.; Atout, M.M.W.; Avila-Burgos, L.; Awaisu, A.; Azadnajafabad, S.; B, D.B.; Babamohamadi, H.; Badar, M.; Badawi, A.; Badiye, A.D.; Baghcheghi, N.; Bagheri, N.; Bagherieh, S.; Bah, S.; Bahadory, S.; Bai, R.; Baig, A.A.; Baltatu, O.C.; Baradaran, H.R.; Barchitta, M.; Bardhan, M.; Barengo, N.C.; Bärnighausen, T.W.; Barone, M.T.U.; Barone-Adesi, F.; Barrow, A.; Bashiri, H.; Basiru, A.; Basu, S.; Basu, S.; Batiha, A.-M. M.; Batra, K.; Bayih, M.T.; Bayileyegn, N.S.; Behnoush, A.H.; Bekele, A.B.; Belete, M.A.; Belgaumi, U.I.; Belo, L.; Bennett, D.A.; Bensenor, I.M.; Berhe, K.; Berhie, A.Y.; Bhaskar, S.; Bhat, A.N.; Bhatti, J.S.; Bikbov, B.; Bilal, F.; Bintoro, B.S.; Bitaraf, S.; Bitra, V.R.; Bjegovic-Mikanovic, V.; Bodolica, V.; Boloor, A.; Brauer, M.; Brazo-Sayavera, J.; Brenner, H.; Butt, Z.A.; Calina, D.; Campos, L.A.; Campos-Nonato, I.R.; Cao, Y.; Cao, C.; Car, J.; Carvalho, M.; Castañeda-Orjuela, C.A.; Catalá-López, F.; Cerin, E.; Chadwick, J.; Chandrasekar, E.K.; Chanie, G.S.; Charan, J.; Chattu, V.K.; Chauhan, K.; Cheema, H.A.; Chekol Abebe, E.; Chen, S.; Cherbuin, N.; Chichagi, F.; Chidambaram, S.B.; Cho, W.C.S.; Choudhari, S.G.; Chowdhury, R.; Chowdhury, E.K.; Chu, D.-T.; Chukwu, I.S.; Chung, S.-C.; Coberly, K.; Columbus, A.; Contreras, D.; Cousin, E.; Criqui, M.H.; Cruz-Martins, N.; Cuschieri, S.; Dabo, B.; Dadras, O.; Dai, X.; Damasceno, A.A.M.; Dandona, R.; Dandona, L.; Das, S.; Dascalu, A.M.; Dash, N.R.; Dashti, M.; Dávila-Cervantes, C.A.; De la Cruz-Góngora, V.; Debele, G.R.; Delpasand, K.; Demisse, F.W.; Demissie, G.D.; Deng, X.; Denova-Gutiérrez, E.; Deo, S.V.; Dervišević, E.; Desai, H.D.; Desale, A.T.; Dessie, A.M.; Desta, F.; Dewan, S.M.R.; Dey, S.; Dhama, K.; Dhimal, M.; Diao, N.; Diaz, D.; Dinu, M.; Diress, M.; Djalalinia, S.; Doan, L.P.; Dongarwar, D.; dos Santos Figueiredo, F.W.; Duncan, B.B.; Dutta, S.; Dziedzic, A.M.; Edinur, H.A.; Ekholuenetale, M.; Ekundayo, T.C.; Elgendy, I.Y.; Elhadi, M.; El-Huneidi, W.; Elmeligy, O.A.A.; Elmonem, M.A.; Endeshaw, D.; Esayas, H.L.; Eshetu, H.B.; Etaee, F.; Fadhil, I.; Fagbamigbe, A.F.; Fahim, A.; Falahi, S.; Faris, M.E.M.; Farrokhpour, H.; Farzadfar, F.; Fatehizadeh, A.; Fazli, G.; Feng, X.; Ferede, T.Y.; Fischer, F.; Flood, D.; Forouhari, A.; Foroumadi, R.; Foroutan Koudehi, M.; Gaidhane, A.M.; Gaihre, S.; Gaipov, A.; Galali, Y.; Ganesan, B.; Garcia-Gordillo, M.; Gautam, R.K.; Gebrehiwot, M.; Gebrekidan, K.G.; Gebremeskel, T.G.; Getacher, L.; Ghadirian, F.; Ghamari, S.-H.; Ghasemi Nour, M.; Ghassemi, F.; Golechha, M.; Goleij, P.; Golinelli, D.; Gopalani, S.V.; Guadie, H.A.; Guan, S.-Y.; Gudayu, T.W.; Guimarães, R.A.; Guled, R.A.; Gupta, R.; Gupta, K.; Gupta, V.B.; Gupta, V.K.; Gyawali, B.; Haddadi, R.; Hadi, N.R.; Haile, T.G.; Hajibeygi, R.; Haj-Mirzaian, A.; Halwani, R.; Hamidi, S.; Hankey, G.J.; Hannan, M.A.; Haque, S.; Harandi, H.; Harlianto, N.I.; Hasan, S.M.M.; Hasan, S.S.; Hasani, H.; Hassanipour, S.; Hassen, M.B.; Haubold, J.; Hayat, K.; Heidari, G.; Heidari, M.; Hessami, K.; Hiraike, Y.; Holla, R.; Hossain, S.; Hossain, M.S.; Hosseini, M.-S.; Hosseinzadeh, M.; Hosseinzadeh, H.; Huang, J.; Huda, M.N.; Hussain, S.; Huynh, H.-H.; Hwang, B.-F.; Ibitoye, S.E.; Ikeda, N.; Ilic, I.M.; Ilic, M.D.; Inbaraj, L.R.; Iqbal, A.; Islam, S.M.S.; Islam, R.M.; Ismail, N.E.; Iso, H.; Isola, G.; Itumalla, R.; Iwagami, M.; Iwu, C.C.D.; Iyamu, I.O.; Iyasu, A.N.; Jacob, L.; Jafarzadeh, A.; Jahrami, H.; Jain, R.; Jaja, C.; Jamalpoor, Z.; Jamshidi, E.; Janakiraman, B.; Jayanna, K.; Jayapal, S.K.; Jayaram, S.; Jayawardena, R.; Jebai, R.; Jeong, W.; Jin, Y.; Jokar, M.; Jonas, J.B.; Joseph, N.; Joseph, A.; Joshua, C.E.; Joukar, F.; Jozwiak, J.J.; Kaambwa, B.; Kabir, A.; Kabthymer, R.H.; Kadashetti, V.; Kahe, F.; Kalhor, R.; Kandel, H.; Karanth, S.D.; Karaye, I.M.; Karkhah, S.; Katoto, P.D.; Kaur, N.; Kazemian, S.; Kebede, S.A.; Khader, Y.S.; Khajuria, H.; Khalaji, A.; Khan, M.A.; Khan, M.; Khan, A.; Khanal, S.; Khatatbeh, M.M.; Khater, A.M.; Khateri, S.; Khorashadizadeh, F.; Khubchandani, J.; Kibret, B.G.; Kim, M.S.; Kimokoti, R.W.; Kisa, A.; Kivimäki, M.; Kolahi, A.-A.; Komaki, S.; Kompani, F.; Koohestani, H.R.; Korzh, O.; Kostev, K.; Kothari, N.; Koyanagi, A.; Krishan, K.; Krishnamoorthy, Y.; Kuate Defo, B.; Kuddus, M.; Kuddus, M.A.; Kumar, R.; Kumar, H.; Kundu, S.; Kurniasari, M.D.; Kuttikkattu, A.; La Vecchia, C.; Lallukka, T.; Larijani, B.; Larsson, A.O.; Latief, K.; Lawal, B.K.; Le, T.T.T.; Le, T.T.B.; Lee, S.W.H.; Lee, M.; Lee, W.-C.; Lee, P.H.; Lee, S.; Lee, S.W.; Legesse, S.M.; Lenzi, J.; Li, Y.; Li, M.-C.; Lim, S.S.; Lim, L.-L.; Liu, X.; Liu, C.; Lo, C.-H.; Lopes, G.; Lorkowski, S.; Lozano, R.; Lucchetti, G.; Maghazachi, A.A.; Mahasha, P.W.; Mahjoub, S.; Mahmoud, M.A.; Mahmoudi, R.; Mahmoudimanesh, M.; Mai, A.T.; Majeed, A.; Majma Sanaye, P.; Makris, K.C.; Malhotra, K.; Malik, A.A.; Malik, I.; Mallhi, T.H.; Malta, D.C.; Mamun, A.A.; Mansouri, B.; Marateb, H.R.; Mardi, P.; Martini, S.; Martorell, M.; Marzo, R.R.; Masoudi, R.; Masoudi, S.; Mathews, E.; Maugeri, A.; Mazzaglia, G.; Mekonnen, T.; Meshkat, M.; Mestrovic, T.; Miao Jonasson, J.; Miazgowski, T.; Michalek, I.M.; Minh, L.H.N.; Mini, G.; Miranda, J.J.; Mirfakhraie, R.; Mirrakhimov, E.M.; Mirza-Aghazadeh-Attari, M.; Misganaw, A.; Misgina, K.H.; Mishra, M.; Moazen, B.; Mohamed, N.S.; Mohammadi, E.; Mohammadi, M.; Mohammadian-Hafshejani, A.; Mohammadshahi, M.; Mohseni, A.; Mojiri-forushani, H.; Mokdad, A.H.; Momtazmanesh, S.; Monasta, L.; Moniruzzaman, M.; Mons, U.; Montazeri, F.; Moodi Ghalibaf, A.; Moradi, Y.; Moradi, M.; Moradi Sarabi, M.; Morovatdar, N.; Morrison, S.D.; Morze, J.; Mossialos, E.; Mostafavi, E.; Mueller, U.O.; Mulita, F.; Mulita, A.; Murillo-Zamora, E.; Musa, K.I.; Mwita, J.C.; Nagaraju, S.P.; Naghavi, M.; Nainu, F.; Nair, T.S.; Najmuldeen, H.H.R.; Nangia, V.; Nargus, S.; Naser, A.Y.; Nassereldine, H.; Natto, Z.S.; Nauman, J.; Nayak, B.P.; Ndejjo, R.; Negash, H.; Negoi, R.I.; Nguyen, H.T.H.; Nguyen, D.H.; Nguyen, P.T.; Nguyen, V.T.; Nguyen, H.Q.; Niazi, R.K.; Nigatu, Y.T.; Ningrum, D.N.A.; Nizam, M.A.; Nnyanzi, L.A.; Noreen, M.; Noubiap, J.J.; Nzoputam, O.J.; Nzoputam, C.I.; Oancea, B.; Odogwu, N.M.; Odukoya, O.O.; Ojha, V.A.; Okati-Aliabad, H.; Okekunle, A.P.; Okonji, O.C.; Okwute, P.G.; Olufadewa, I.I.; Onwujekwe, O.E.; Ordak, M.; Ortiz, A.; Osuagwu, U.L.; Oulhaj, A.; Owolabi, M.O.; Padron-Monedero, A.; Padubidri, J.R.; Palladino, R.; Panagiotakos, D.; Panda-Jonas, S.; Pandey, A.; Pandey, A.; Pandi-Perumal, S.R.; Pantea Stoian, A.M.; Pardhan, S.; Parekh, T.; Parekh, U.; Pasovic, M.; Patel, J.; Patel, J.R.; Paudel, U.; Pepito, V.C.F.; Pereira, M.; Perico, N.; Perna, S.; Petcu, I.-R.; Petermann-Rocha, F.E.; Podder, V.; Postma, M.J.; Pourali, G.; Pourtaheri, N.; Prates, E.J.S.; Qadir, M.M.F.; Qattea, I.; Raee, P.; Rafique, I.; Rahimi, M.; Rahimifard, M.; Rahimi-Movaghar, V.; Rahman, M.O.; Rahman, M.A.; Rahman, M.H.U.; Rahman, M.; Rahman, M.M.; Rahmani, M.; Rahmani, S.; Rahmanian, V.; Rahmawaty, S.; Rahnavard, N.; Rajbhandari, B.; Ram, P.; Ramazanu, S.; Rana, J.; Rancic, N.; Ranjha, M.M.A.N.; Rao, C.R.; Rapaka, D.; Rasali, D.P.; Rashedi, S.; Rashedi, V.; Rashid, A.M.; Rashidi, M.-M.; Ratan, Z.A.; Rawaf, S.; Rawal, L.; Redwan, E.M.M.; Remuzzi, G.; Rengasamy, K.R.; Renzaho, A.M.N.; Reyes, L.F.; Rezaei, N.; Rezaei, N.; Rezaeian, M.; Rezazadeh, H.; Riahi, S.M.; Rias, Y.A.; Riaz, M.; Ribeiro, D.; Rodrigues, M.; Rodriguez, J.A.B.; Roever, L.; Rohloff, P.; Roshandel, G.; Roustazadeh, A.; Rwegerera, G.M.; Saad, A.M.A.; Saber-Ayad, M.M.; Sabour, S.; Sabzmakan, L.; Saddik, B.; Sadeghi, E.; Saeed, U.; Saeedi Moghaddam, S.; Safi, S.; Safi, S.Z.; Saghazadeh, A.; Saheb Sharif-Askari, N.; Saheb Sharif-Askari, F.; Sahebkar, A.; Sahoo, S.S.; Sahoo, H.; Saif-Ur-Rahman, K.; Sajid, M.R.; Salahi, S.; Salahi, S.; Saleh, M.A.; Salehi, M.A.; Salomon, J.A.; Sanabria, J.; Sanjeev, R.K.; Sanmarchi, F.; Santric-Milicevic, M.M.; Sarasmita, M.A.; Sargazi, S.; Sathian, B.; Sathish, T.; Sawhney, M.; Schlaich, M.P.; Schmidt, M.I.; Schuermans, A.; Seidu, A.-A.; Senthil Kumar, N.; Sepanlou, S.G.; Sethi, Y.; Seylani, A.; Shabany, M.; Shafaghat, T.; Shafeghat, M.; Shafie, M.; Shah, N.S.; Shahid, S.; Shaikh, M.A.; Shanawaz, M.; Shannawaz, M.; Sharfaei, S.; Shashamo, B.B.; Shiri, R.; Shittu, A.; Shivakumar, K.M.; Shivalli, S.; Shobeiri, P.; Shokri, F.; Shuval, K.; Sibhat, M.M.; Silva, L.M.L.R.; Simpson, C.R.; Singh, J.A.; Singh, P.; Singh, S.; Siraj, M.S.; Skryabina, A.A.; Sohag, A.A.M.; Soleimani, H.; Solikhah, S.; Soltani-Zangbar, M.S.; Somayaji, R.; Sorensen, R.J.D.; Starodubova, A.V.; Sujata, S.; Suleman, M.; Sun, J.; Sundström, J.; Tabarés-Seisdedos, R.; Tabatabaei, S.M.; Tabatabaeizadeh, S.-A.; Tabish, M.; Taheri, M.; Taheri, E.; Taki, E.; Tamuzi, J.J.L.; Tan, K.-K.; Tat, N.Y.; Taye, B.T.; Temesgen, W.A.; Temsah, M.-H.; Tesler, R.; Thangaraju, P.; Thankappan, K.R.; Thapa, R.; Tharwat, S.; Thomas, N.; Ticoalu, J.H.V.; Tiyuri, A.; Tonelli, M.; Tovani-Palone, M.R.; Trico, D.; Trihandini, I.; Tripathy, J.P.; Tromans, S.J.; Tsegay, G.M.; Tualeka, A.R.; Tufa, D.G.; Tyrovolas, S.; Ullah, S.; Upadhyay, E.; Vahabi, S.M.; Vaithinathan, A.G.; Valizadeh, R.; van Daalen, K.R.; Vart, P.; Varthya, S.B.; Vasankari, T.J.; Vaziri, S.; Verma, M. verma; Verras, G.-I.; Vo, D.C.; Wagaye, B.; Waheed, Y.; Wang, Z.; Wang, Y.; Wang, C.; Wang, F.; Wassie, G.T.; Wei, M.Y.W.; Weldemariam, A.H.; Westerman, R.; Wickramasinghe, N.D.; Wu, Y.; Wulandari, R.D.; Xia, J.; Xiao, H.; Xu, S.; Xu, X.; Yada, D.Y.; Yang, L.; Yatsuya, H.; Yesiltepe, M.; Yi, S.; Yohannis, H.K.; Yonemoto, N.; You, Y.; Zaman, S. Bin; Zamora, N.; Zare, I.; Zarea, K.; Zarrintan, A.; Zastrozhin, M.S.; Zeru, N.G.; Zhang, Z.-J.; Zhong, C.; Zhou, J.; Zielińska, M.; Zikarg, Y.T.; Zodpey, S.; Zoladl, M.; Zou, Z.; Zumla, A.; Zuniga, Y.M.H.; Magliano, D.J.; Murray, C.J.L.; Hay, S.I.; Vos, T. Global, Regional, and National Burden of Diabetes from 1990 to 2021, with Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Ojo, O. Nutrition and Chronic Conditions. Nutrients 2019, 11, 459. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional Beverages : The Emerging Side of Functional Foods Commercial Trends, Research, and Health Implications. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Selcuk, M.Y.; Aygen, B.; Dogukan, A.; Tuzcu, Z.; Akdemir, F.; Komorowski, J.R.; Atalay, M.; Sahin, K. Chromium Picolinate and Chromium Histidinate Protects against Renal Dysfunction by Modulation of NF-B Pathway in High-Fat Diet Fed and Streptozotocin-Induced Diabetic Rats. Nutr. Metab. 2012, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Rafighi, Z.; Shiva, A.; Arab, S.; Mohd Yousof, R. Association of Dietary Vitamin C and E Intake and Antioxidant Enzymes in Type 2 Diabetes Mellitus Patients. Glob. J. Health Sci. 2013, 5, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Yamada, M.; Miura, T.; Nagashima, K.; Ogura, K.; Inagaki, N.; Maeda-Yamamoto, M. Chronic Administration of Apple Polyphenols Ameliorates Hyperglycaemia in High-Normal and Borderline Subjects: A Randomised, Placebo-Controlled Trial. Diabetes Res. Clin. Pract. 2017, 129, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, M. High-Methoxyl Pectin Has Greater Enhancing Effect on Glucose Uptake in Intestinal Perfused Rats. Nutrition 2005, 21, 372–377. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Nowicka, P. Anti-Diabetic, Anti-Cholinesterase, and Antioxidant Potential, Chemical Composition and Sensory Evaluation of Novel Sea Buckthorn-Based Smoothies. Food Chem. 2021, 338, 128105. [Google Scholar] [CrossRef] [PubMed]
- Dey, G.; Sireswar, S. Tailoring Functional Beverages from Fruits and Vegetables for Specific Disease Conditions-Are We There Yet? Crit. Rev. Food Sci. Nutr. 2021, 61, 2034–2046. [Google Scholar] [CrossRef]
- Liu, J.; Ren, Z.H.; Qiang, H.; Wu, J.; Shen, M.; Zhang, L.; Lyu, J. Trends in the Incidence of Diabetes Mellitus: Results from the Global Burden of Disease Study 2017 and Implications for Diabetes Mellitus Prevention. BMC Public Health 2020, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- United Nations. In Political Declaration of the High-Level Meeting of the General Assemblyon the Prevention and Control of Noncommunicable Diseases; 2011; https://digitallibrary.un.org/record/710899/?ln=en.
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; 2013. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Ryan, L. Improving Public Health?: The Role of Antioxidant-Rich Fruit and Vegetable Beverages. Food Res. Int. 2011, 44, 3135–3148. [Google Scholar] [CrossRef]
- Zhou, B.; Lu, Y.; Hajifathalian, K.; Bentham, J.; Di Cesare, M.; Danaei, G.; Bixby, H.; Cowan, M.J.; Ali, M.K.; Taddei, C.; Lo, W.C.; Reis-Santos, B.; Stevens, G.A.; Riley, L.M.; Miranda, J.J.; Bjerregaard, P.; Rivera, J.A.; Fouad, H.M.; Ma, G.; Mbanya, J.C.N.; McGarvey, S.T.; Mohan, V.; Onat, A.; Pilav, A.; Ramachandran, A.; Ben Romdhane, H.; Paciorek, C.J.; Bennett, J.E.; Ezzati, M.; Abdeen, Z.A.; Kadir, K.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Adams, R.; Aekplakorn, W.; Aguilar-Salinas, C.A.; Agyemang, C.; Ahmadvand, A.; Al-Othman, A.R.; Alkerwi, A.; Amouyel, P.; Amuzu, A.; Bo Andersen, L.; Anderssen, S.A.; Anjana, R.M.; Aounallah-Skhiri, H.; Aris, T.; Arlappa, N.; Arveiler, D.; Assah, F.K.; Avdicová, M.; Azizi, F.; Balakrishna, N.; Bandosz, P.; Barbagallo, C.M.; Barceló, A.; Batieha, A.M.; Baur, L.A.; Benet, M.; Bernabe-Ortiz, A.; Bharadwaj, S.; Bhargava, S.K.; Bi, Y.; Bjertness, E.; Bjertness, M.B.; Björkelund, C.; Blokstra, A.; Bo, S.; Boehm, B.O.; Boissonnet, C.P.; Bovet, P.; Brajkovich, I.; Breckenkamp, J.; Brenner, H.; Brewster, L.M.; Brian, G.R.; Bruno, G.; Bugge, A.; De León, A.C.; Can, G.; Cåndido, A.P.C.; Capuano, V.; Carlsson, A.C.; Carvalho, M.J.; Casanueva, F.F.; Casas, J.P.; Caserta, C.A.; Castetbon, K.; Chamukuttan, S.; Chaturvedi, N.; Chen, C.J.; Chen, F.; Chen, S.; Cheng, C.Y.; Chetrit, A.; Chiou, S.T.; Cho, Y.; Chudek, J.; Cifkova, R.; Claessens, F.; Concin, H.; Cooper, C.; Cooper, R.; Costanzo, S.; Cottel, D.; Cowell, C.; Crujeiras, A.B.; D’Arrigo, G.; Dallongeville, J.; Dankner, R.; Dauchet, L.; De Gaetano, G.; De Henauw, S.; Deepa, M.; Dehghan, A.; Deschamps, V.; Dhana, K.; Di Castelnuovo, A.F.; Djalalinia, S.; Doua, K.; Drygas, W.; Du, Y.; Dzerve, V.; Egbagbe, E.E.; Eggertsen, R.; El Ati, J.; Elosua, R.; Erasmus, R.T.; Erem, C.; Ergor, G.; Eriksen, L.; Escobedo-De La Peña, J.; Fall, C.H.; Farzadfar, F.; Felix-Redondo, F.J.; Ferguson, T.S.; Fernández-Bergés, D.; Ferrari, M.; Ferreccio, C.; Feskens, E.J.M.; Finn, J.D.; Föger, B.; Foo, L.H.; Forslund, A.S.; Francis, D.K.; Do Carmo Franco, M.; Franco, O.H.; Frontera, G.; Furusawa, T.; Gaciong, Z.; Garnett, S.P.; Gaspoz, J.M.; Gasull, M.; Gates, L.; Geleijnse, J.M.; Ghasemian, A.; Ghimire, A.; Giampaoli, S.; Gianfagna, F.; Giovannelli, J.; Giwercman, A.; González-Gross, M.M.; Rivas, J.P.G.; Gorbea, M.B.; Gottrand, F.; Grafnetter, D.; Grodzicki, T.; Grøntved, A.; Gruden, G.; Gu, D.; Guan, O.P.; Guerrero, R.; Guessous, I.; Guimaraes, A.L.; Gutierrez, L.; Hambleton, I.R.; Hardy, R.; Kumar, R.H.; Hata, J.; He, J.; Heidemann, C.; Herrala, S.; Hihtaniemi, I.T.; Ho, S.Y.; Ho, S.C.; Hofman, A.; Hormiga, C.M.; Horta, B.L.; Houti, L.; Howitt, C.; Htay, T.T.; Htet, A.S.; Htike, M.M.T.; Hu, Y.; Hussieni, A.S.; Huybrechts, I.; Hwalla, N.; Iacoviello, L.; Iannone, A.G.; Ibrahim, M.M.; Ikeda, N.; Ikram, M.A.; Irazola, V.E.; Islam, M.; Iwasaki, M.; Jacobs, J.M.; Jafar, T.; Jamil, K.M.; Jasienska, G.; Jiang, C.Q.; Jonas, J.B.; Joshi, P.; Kafatos, A.; Kalter-Leibovici, O.; Kasaeian, A.; Katz, J.; Kaur, P.; Kavousi, M.; Keinänen-Kiukaanniemi, S.; Kelishadi, R.; Kengne, A.P.; Kersting, M.; Khader, Y.S.; Khalili, D.; Khang, Y.H.; Kiechl, S.; Kim, J.; Kolsteren, P.; Korrovits, P.; Kratzer, W.; Kromhout, D.; Kujala, U.M.; Kula, K.; Kyobutungi, C.; Laatikainen, T.; Lachat, C.; Laid, Y.; Lam, T.H.; Landrove, O.; Lanska, V.; Lappas, G.; Laxmaiah, A.; Leclercq, C.; Lee, J.; Lee, J.; Lehtimäki, T.; Rampal, L.; León-Muñoz, L.M.; Li, Y.; Lim, W.Y.; Lima-Costa, M.F.; Lin, H.H.; Lin, X.; Lissner, L.; Lorbeer, R.; Lozano, J.E.; Luksiene, D.; Lundqvist, A.; Lytsy, P.; Machado-Coelho, G.L.L.; Machi, S.; Maggi, S.; Magliano, D.J.; Makdisse, M.; Rao, K.M.; Manios, Y.; Manzato, E.; Margozzini, P.; Marques-Vidal, P.; Martorell, R.; Masoodi, S.R.; Mathiesen, E.B.; Matsha, T.E.; McFarlane, S.R.; McLachlan, S.; McNulty, B.A.; Mediene-Benchekor, S.; Meirhaeghe, A.; Menezes, A.M.B.; Merat, S.; Meshram, I.I.; Mi, J.; Miquel, J.F.; Mohamed, M.K.; Mohammad, K.; Mohammadifard, N.; Mohd Yusoff, M.F.; Møller, N.C.; Molnár, D.; Mondo, C.K.; Morejon, A.; Moreno, L.A.; Morgan, K.; Moschonis, G.; Mossakowska, M.; Mostafa, A.; Mota, J.; Motta, J.; Mu, T.T.; Muiesan, M.L.; Müller-Nurasyid, M.; Mursu, J.; Nagel, G.; Námešná, J.; Nang, E.E.K.; Nangia, V.B.; Navarrete-Muñoz, E.M.; Ndiaye, N.C.; Nenko, I.; Nervi, F.; Nguyen, N.D.; Nguyen, Q.N.; Nieto-Martínez, R.E.; Ning, G.; Ninomiya, T.; Noale, M.; Noto, D.; Al Nsour, M.; Ochoa-Avilés, A.M.; Oh, K.; Ordunez, P.; Osmond, C.; Otero, J.A.; Owusu-Dabo, E.; Pahomova, E.; Palmieri, L.; Panda-Jonas, S.; Panza, F.; Parsaeian, M.; Peixoto, S.V.; Peltonen, M.; Peters, A.; Peykari, N.; Pham, S.T.; Pitakaka, F.; Piwonska, A.; Piwonski, J.; Plans-Rubió, P.; Porta, M.; Portegies, M.L.P.; Poustchi, H.; Pradeepa, R.; Price, J.F.; Punab, M.; Qasrawi, R.F.; Qorbani, M.; Radisauskas, R.; Rahman, M.; Raitakari, O.; Rao, S.R.; Ramke, J.; Ramos, R.; Rampal, S.; Rathmann, W.; Redon, J.; Reganit, P.F.M.; Rigo, F.; Robinson, S.M.; Robitaille, C.; Rodríguez-Artalejo, F.; Del CristoRodriguez-Perez, M.; Rodríguez-Villamizar, L.A.; Rojas-Martinez, R.; Ronkainen, K.; Rosengren, A.; Rubinstein, A.; Rui, O.; Ruiz-Betancourt, B.S.; Horimoto, A.R.V.R.; Rutkowski, M.; Sabanayagam, C.; Sachdev, H.S.; Saidi, O.; Sakarya, S.; Salanave, B.; Salonen, J.T.; Salvetti, M.; Sánchez-Abanto, J.; Santos, D.; Dos Santos, R.N.; Santos, R.; Saramies, J.L.; Sardinha, L.B.; Sarrafzadegan, N.; Saum, K.U.; Scazufca, M.; Schargrodsky, H.; Scheidt-Nave, C.; Sein, A.A.; Sharma, S.K.; Shaw, J.E.; Shibuya, K.; Shin, Y.; Shiri, R.; Siantar, R.; Sibai, A.M.; Simon, M.; Simons, J.; Simons, L.A.; Sjostrom, M.; Slowikowska-Hilczer, J.; Slusarczyk, P.; Smeeth, L.; Snijder, M.B.; So, H.K.; Sobngwi, E.; Söderberg, S.; Solfrizzi, V.; Sonestedt, E.; Soumare, A.; Staessen, J.A.; Stathopoulou, M.G.; Steene-Johannessen, J.; Stehle, P.; Stein, A.D.; Stessman, J.; Stöckl, D.; Stokwiszewski, J.; Stronks, K.; Strufaldi, M.W.; Sun, C.A.; Sundström, J.; Sung, Y.T.; Suriyawongpaisal, P.; Sy, R.G.; Tai, E.S.; Tamosiunas, A.; Tang, L.; Tarawneh, M.; Tarqui-Mamani, C.B.; Taylor, A.; Theobald, H.; Thijs, L.; Thuesen, B.H.; Tolonen, H.K.; Tolstrup, J.S.; Topbas, M.; Torrent, M.; Traissac, P.; Trinh, O.T.H.; Tulloch-Reid, M.K.; Tuomainen, T.P.; Turley, M.L.; Tzourio, C.; Ueda, P.; Ukoli, F.A.M.; Ulmer, H.; Uusitalo, H.M.T.; Valdivia, G.; Valvi, D.; Van Rossem, L.; Van Valkengoed, I.G.M.; Vanderschueren, D.; Vanuzzo, D.; Vega, T.; Velasquez-Melendez, G.; Veronesi, G.; Verschuren, W.M.M.; Verstraeten, R.; Viet, L.; Vioque, J.; Virtanen, J.K.; Visvikis-Siest, S.; Viswanathan, B.; Vollenweider, P.; Voutilainen, S.; Vrijheid, M.; Wade, A.N.; Wagner, A.; Walton, J.; Wan Mohamud, W.N.; Wang, F.; Wang, M.D.; Wang, Q.; Wang, Y.X.; Wannamethee, S.G.; Weerasekera, D.; Whincup, P.H.; Widhalm, K.; Wiecek, A.; Wijga, A.H.; Wilks, R.J.; Willeit, J.; Wilsgaard, T.; Wojtyniak, B.; Wong, T.Y.; Woo, J.; Woodward, M.; Wu, F.C.; Wu, S.L.; Xu, H.; Yan, W.; Yang, X.; Ye, X.; Yoshihara, A.; Younger-Coleman, N.O.; Zambon, S.; Zargar, A.H.; Zdrojewski, T.; Zhao, W.; Zheng, Y.; Cisneros, J.Z. Worldwide Trends in Diabetes since 1980: A Pooled Analysis of 751 Population-Based Studies with 4.4 Million Participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 Diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of Diabetes and Diabetes-Related Complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C.C.; Harjutsalo, V.; Rosenbauer, J.; Neu, A.; Cinek, O.; Skrivarhaug, T.; Rami-Merhar, B.; Soltesz, G.; Svensson, J.; Parslow, R.C.; Castell, C.; Schoenle, E.J.; Bingley, P.J.; Dahlquist, G.; Jarosz-Chobot, P.K.; Marčiulionytė, D.; Roche, E.F.; Rothe, U.; Bratina, N.; Ionescu-Tirgoviste, C.; Weets, I.; Kocova, M.; Cherubini, V.; Rojnic Putarek, N.; DeBeaufort, C.E.; Samardzic, M.; Green, A. Trends and Cyclical Variation in the Incidence of Childhood Type 1 Diabetes in 26 European Centres in the 25 Year Period 1989–2013: A Multicentre Prospective Registration Study. Diabetologia 2019, 62, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C.C.; Dahlquist, G.G.; Gyürüs, E.; Green, A.; Soltész, G.; Group, E.S. Incidence Trends for Childhood Type 1 Diabetes in Europe during 1989-2003 and Predicted New Cases 2005-20: A Multicentre Prospective Registration Study. Lancet 2009, 373, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, K.; Chiu, H.F.; Wang, C.K. Popular Functional Foods and Herbs for the Management of Type-2-Diabetes Mellitus: A Comprehensive Review with Special Reference to Clinical Trials and Its Proposed Mechanism. J. Funct. Foods 2019, 57, 425–438. [Google Scholar] [CrossRef]
- Li, P.; Tang, Y.; Liu, L.; Wang, D.; Zhang, L.; Piao, C. Therapeutic Potential of Buckwheat Hull Flavonoids in Db/Db Mice, a Model of Type 2 Diabetes. J. Funct. Foods 2019, 52, 284–290. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk Factors Contributing to Type 2 Diabetes and Recent Advances in the Treatment and Prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Tinajero, M.G.; Malik, V.S. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol. Metab. Clin. North Am. 2021, 50, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, M.; Long, Z.; Ning, H.; Li, J.; Cao, Y.; Liao, Y.; Liu, G.; Wang, F.; Pan, A. Global Burden of Type 2 Diabetes in Adolescents and Young Adults, 1990-2019: Systematic Analysis of the Global Burden of Disease Study 2019. Bmj 2022, 379, e072385. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diabetes. https://www.who.int/europe/health-topics/diabetes#tab=tab_1 (accessed 2023-07-26).
- CDC. Diabetes Tests. Centers for Disease Control and Prevention. https://www.cdc.gov/diabetes/basics/getting-tested.html (accessed 2023-07-07). /: Disease Control and Prevention. https.
- Saad Masood Butt. Management and Treatment of Type 2 Diabetes. Int. J. Comput. Inf. Manuf. 2022, 2, 15–27. [Google Scholar] [CrossRef]
- Koh, S.P.; Maarof, S.; Sew, Y.S.; Sabidi, S.; Abdullah, R.; Mohd Danial, A.; Nur Diyana, A.; Mustaffa, R. Fermented Jackfruit Leaf Beverage Offers New Affordable and Effective Diabetes Therapy. Food Res. 2020, 4, 19–25. [Google Scholar] [CrossRef]
- Naim, A.; Anisa, L.; Marjoni, R. Antidiabetes Effects - Combination of Cowpea Juice (Vigna Sinensis L.), Tomato Juice (Solanum lycopersicum L.), and Green Apple Juice (Malus sylvestris Mill.) in White Male Mice. Int. J. Green Pharm. 2018, 12, S633–S637. [Google Scholar]
- Gupta, A.; Sanwal, N.; Bareen, M.A.; Barua, S.; Sharma, N.; Joshua Olatunji, O.; Prakash Nirmal, N.; Sahu, J.K. Trends in Functional Beverages: Functional Ingredients, Processing Technologies, Stability, Health Benefits, and Consumer Perspective. Food Res. Int. 2023, 170, 113046. [Google Scholar] [CrossRef]
- Manousi, N.; Sarakatsianos, I.; Samanidou, V. Extraction Techniques of Phenolic Compounds and Other Bioactive Compounds From Medicinal and Aromatic Plants. In Engineering Tools in the Beverage Industry; Elsevier, 2019; pp. 283–314. [Google Scholar] [CrossRef]
- Raman, M.; Ambalam, P.; Doble, M. Probiotics, Prebiotics, and Fibers in Nutritive and Functional Beverages. In Nutrients in Beverages; Elsevier, 2019; pp. 315–367. [Google Scholar] [CrossRef]
- European Parliament and the Council of the European Union. Regulation (EC) 1924/2006 on Nutrition and Health Claims Made on Foods; 2006; pp. 1–15. [Google Scholar]
- Gonçalves, A.C.; Nunes, A.R.; Flores-Félix, J.D.; Alves, G.; Silva, L.R. Cherries and Blueberries-Based Beverages: Functional Foods with Antidiabetic and Immune Booster Properties. Molecules 2022, 27, 1–44. [Google Scholar] [CrossRef]
- Cong, L.; Bremer, P.; Mirosa, M. Functional Beverages in Selected Countries of Asia Pacific Region: A Review. Beverages 2020, 6, 1–17. [Google Scholar] [CrossRef]
- Henry, C.J. Functional Foods. Eur. J. Clin. Nutr. 2010, 64, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Sugajski, M.; Buszewska-Forajta, M.; Buszewski, B. Functional Beverages in the 21st Century. Beverages 2023, 9, 27. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Kelleher, A.H.; Maykish, A.; Kristo, A.S. Non-Alcoholic Beverages, Old and Novel, and Their Potential Effects on Human Health, with a Focus on Hydration and Cardiometabolic Health. Medicina (B. Aires). 2020, 56, 490. [Google Scholar] [CrossRef]
- Functional and Medicinal Beverages Volume 11: The Science of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Charlotte Cockle, 2019. [Google Scholar]
- Technavio. Functional Foods and Beverages Market by Product, Distribution Channel, and Geography - Forecast and Analysis 2023-2027. Technavio. https://www.technavio.com/report/functional-foods-and-beverages-market-industry-analysis (accessed 2023-05-30).
- Research, K. Global Functional Beverages Market By End User (Fitness Lifestyle Users, Athletes and Others), By Type (Energy Drinks, Sports Drinks, Juices, Dairy-Based Beverages and Others), By Distribution Channel (Supermarket/Hypermarket, Specialty Stores, E-Commerce; 2021; https://www.kbvresearch.com/functional-beverages-market/.
- Company, T.B.R. Functional Beverages Global Market Report 2023; 2023; https://www.reportlinker.com/p06284496/Functional-Beverages-Global-Market-Report.html?utm_source=GNW#summary.
- Ashaolu, T.J.; Adeyeye, S.A.O. African Functional Foods and Beverages: A Review. J. Culin. Sci. Technol. 2022, 1–36. [Google Scholar] [CrossRef]
- Mirmiran, P. Functional Foods-Based Diet as a Novel Dietary Approach for Management of Type 2 Diabetes and Its Complications: A Review. World J. Diabetes 2014, 5, 267. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1–18. [Google Scholar] [CrossRef]
- 50. WHO; FAO. Diet, Nutrition and the Prevention of Chronic Diseases. World Heal. Organ. - Tech. Rep. Ser. 2003, No. 916. [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; Afarideh, M.; Aggarwal, A.; Agrawal, S.; Akinyemiju, T.; Alahdab, F.; Bacha, U.; Bachman, V.F.; Badali, H.; Badawi, A.; Bensenor, I.M.; Bernabe, E.; Biadgilign, S.K.K.; Biryukov, S.H.; Cahill, L.E.; Carrero, J.J.; Cercy, K.M.; Dandona, L.; Dandona, R.; Dang, A.K.; Degefa, M.G.; El Sayed Zaki, M.; Esteghamati, A.; Esteghamati, S.; Fanzo, J.; Farinha, C.S. e. S.; Farvid, M.S.; Farzadfar, F.; Feigin, V.L.; Fernandes, J.C.; Flor, L.S.; Foigt, N.A.; Forouzanfar, M.H.; Ganji, M.; Geleijnse, J.M.; Gillum, R.F.; Goulart, A.C.; Grosso, G.; Guessous, I.; Hamidi, S.; Hankey, G.J.; Harikrishnan, S.; Hassen, H.Y.; Hay, S.I.; Hoang, C.L.; Horino, M.; Islami, F.; Jackson, M.D.; James, S.L.; Johansson, L.; Jonas, J.B.; Kasaeian, A.; Khader, Y.S.; Khalil, I.A.; Khang, Y.H.; Kimokoti, R.W.; Kokubo, Y.; Kumar, G.A.; Lallukka, T.; Lopez, A.D.; Lorkowski, S.; Lotufo, P.A.; Lozano, R.; Malekzadeh, R.; März, W.; Meier, T.; Melaku, Y.A.; Mendoza, W.; Mensink, G.B.M.; Micha, R.; Miller, T.R.; Mirarefin, M.; Mohan, V.; Mokdad, A.H.; Mozaffarian, D.; Nagel, G.; Naghavi, M.; Nguyen, C.T.; Nixon, M.R.; Ong, K.L.; Pereira, D.M.; Poustchi, H.; Qorbani, M.; Rai, R.K.; Razo-García, C.; Rehm, C.D.; Rivera, J.A.; Rodríguez-Ramírez, S.; Roshandel, G.; Roth, G.A.; Sanabria, J.; Sánchez-Pimienta, T.G.; Sartorius, B.; Schmidhuber, J.; Schutte, A.E.; Sepanlou, S.G.; Shin, M.J.; Sorensen, R.J.D.; Springmann, M.; Szponar, L.; Thorne-Lyman, A.L.; Thrift, A.G.; Touvier, M.; Tran, B.X.; Tyrovolas, S.; Ukwaja, K.N.; Ullah, I.; Uthman, O.A.; Vaezghasemi, M.; Vasankari, T.J.; Vollset, S.E.; Vos, T.; Vu, G.T.; Vu, L.G.; Weiderpass, E.; Werdecker, A.; Wijeratne, T.; Willett, W.C.; Wu, J.H.; Xu, G.; Yonemoto, N.; Yu, C.; Murray, C.J.L. Health Effects of Dietary Risks in 195 Countries, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Fruit and Vegetables for Health. Rep. a Jt. FAO/WHO Work. 2004, 10, 1–46. [Google Scholar]
- Li, M.; Fan, Y.; Zhang, X.; Hou, W.; Tang, Z. Fruit and Vegetable Intake and Risk of Type 2 Diabetes Mellitus: Meta-Analysis of Prospective Cohort Studies. BMJ Open 2014, 4, e005497. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Broadhurst, C.L.; Polansky, M.M.; Schmidt, W.F.; Khan, A.; Flanagan, V.P.; Schoene, N.W.; Graves, D.J. Isolation and Characterization of Polyphenol Type-A Polymers from Cinnamon with Insulin-like Biological Activity. J. Agric. Food Chem. 2004, 52, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K.; Bhargav, V.K. Influence of Different Solvents in Extraction of Phenolic Compounds from Vegetable Residues and Their Evaluation as Natural Sources of Antioxidants. J. Food Sci. Technol. 2014, 51, 2568–2575. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; Chen, H.; Qin, W.; Wu, H.; Chen, S. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Survay, N.S.; Ko, E.; Upadhyay, C.P.; Mi, J.; Park, S.W.; Lee, D.; Jung, Y.-S.; Yoon, D.-Y.; Hong, S. Hypoglycemic Effects of Fruits and Vegetables in Hyperglycemic Rats for Prevention of Type-2 Diabetes. Korean J. Hortic. Sci. Technol. 2010, 28, 850–856. [Google Scholar]
- Jayaprakasam, B.; Vareed, S.K.; Olson, L.K.; Nair, M.G. Insulin Secretion by Bioactive Anthocyanins and Anthocyanidins Present in Fruits. J. Agric. Food Chem. 2005, 53, 28–31. [Google Scholar] [CrossRef]
- Wedick, N.M.; Pan, A.; Cassidy, A.; Rimm, E.B.; Sampson, L.; Rosner, B.; Willett, W.; Hu, F.B.; Sun, Q.; Van Dam, R.M. Dietary Flavonoid Intakes and Risk of Type 2 Diabetes in US Men and Women. Am. J. Clin. Nutr. 2012, 95, 925–933. [Google Scholar] [CrossRef]
- Mazlan, F.A.; Suffian, M.; Sharifuddin, Y. Biotransformation of Momordica charantia Fresh Juice by Lactobacillus plantarum BET003 and Its Putative Anti-Diabetic Potential. PeerJ 2015, 2015, 1–18. [Google Scholar] [CrossRef]
- Badejo, A.A.; Falarunu, A.J.; Duyilemi, T.I.; Fasuhanmi, O.S. Antioxidative and Anti-Diabetic Potentials of Tigernut (Cyperus esculentus) Sedge Beverages Fortified with Vernonia amygdalina and Momordica charantia. J. Food Meas. Charact. 2020, 14, 2790–2799. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Samoticha, J. Evaluation of Phytochemicals, Antioxidant Capacity, and Antidiabetic Activity of Novel Smoothies from Selected Prunus Fruits. J. Funct. Foods 2016, 25, 397–407. [Google Scholar] [CrossRef]
- Ujiroghene, O.J.; Liu, L.; Zhang, S.; Lu, J.; Zhang, C.; Pang, X.; Lv, J. Potent α-Amylase Inhibitory Activity of Sprouted Quinoa-Based Yoghurt Beverages Fermented with Selected Anti-Diabetic Strains of Lactic Acid Bacteria. RSC Adv. 2019, 9, 9486–9493. [Google Scholar] [CrossRef]
- Zhong, H.; Abdullah; Zhao, M.; Tang, J.; Deng, L.; Feng, F. Probiotics-Fermented Blueberry Juices as Potential Antidiabetic Product: Antioxidant, Antimicrobial and Antidiabetic Potentials. J. Sci. Food Agric. 2021, 101, 4420–4427. [CrossRef]
- Etxeberria, U.; De La Garza, A.L.; Campin, J.; Martnez, J.A.; Milagro, F.I. Antidiabetic Effects of Natural Plant Extracts via Inhibition of Carbohydrate Hydrolysis Enzymes with Emphasis on Pancreatic Alpha Amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef]
- Rubilar, M.; Jara, C.; Poo, Y.; Acevedo, F.; Gutierrez, C.; Sineiro, J.; Shene, C. Extracts of Maqui (Aristotelia chilensis) and Murta (Ugni molinae Turcz.): Sources of Antioxidant Compounds and α-Glucosidase/α-Amylase Inhibitors. J. Agric. Food Chem. 2011, 59, 1630–1637. [Google Scholar] [CrossRef]
- Costamagna, M.S.; Zampini, I.C.; Alberto, M.R.; Cuello, S.; Torres, S.; Pérez, J.; Quispe, C.; Schmeda-Hirschmann, G.; Isla, M.I. Polyphenols Rich Fraction from Geoffroea decorticans Fruits Flour Affects Key Enzymes Involved in Metabolic Syndrome, Oxidative Stress and Inflammatory Process. Food Chem. 2016, 190, 392–402. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; El Ashry, F.E.Z.Z.; El Maraghy, N.N.; Fahmy, A. Studies on the Antidiabetic Activities of Momordica charantia Fruit Juice in Streptozotocin-Induced Diabetic Rats. Pharm. Biol. 2017, 55, 758–765. [Google Scholar] [CrossRef]
- Vhora, N.; Naskar, U.; Hiray, A.; Kate, A.S.; Jain, A. Recent Advances in In-Vitro Assays for Type 2 Diabetes Mellitus: An Overview. Rev. Diabet. Stud. 2020, 16, 13–23. [Google Scholar] [CrossRef]
- Castro-Acosta, M.L.; Stone, S.G.; Mok, J.E.; Mhajan, R.K.; Fu, C.I.; Lenihan-Geels, G.N.; Corpe, C.P.; Hall, W.L. Apple and Blackcurrant Polyphenol-Rich Drinks Decrease Postprandial Glucose, Insulin and Incretin Response to a High-Carbohydrate Meal in Healthy Men and Women. J. Nutr. Biochem. 2017, 49, 53–62. [Google Scholar] [CrossRef]
- Kottaisamy, C.P.D.; Raj, D.S.; Prasanth Kumar, V.; Sankaran, U. Experimental Animal Models for Diabetes and Its Related Complications—a Review. Lab. Anim. Res. 2021, 37, 1–14. [Google Scholar] [CrossRef]
- Hu, J.; Nie, S.; Xie, M. Antidiabetic Mechanism of Dietary Polysaccharides Based on Their Gastrointestinal Functions. J. Agric. Food Chem. 2018, 66, 4781–4786. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2009, 32, S62–S67. [Google Scholar] [CrossRef]
- Ariviani, S.; Affandi, D.R.; Listyaningsih, E.; Handajani, S. The Potential of Pigeon Pea (Cajanus cajan) Beverage as an Anti-Diabetic Functional Drink. IOP Conf. Ser. Earth Environ. Sci. 2018, 102, 012054. [Google Scholar] [CrossRef]
- O’Neill, H.M. AMPK and Exercise : Glucose Uptake and Insulin Sensitivity. Diabetes Metab 2013, 37, 1–21. [Google Scholar] [CrossRef]
- Variya, B.C.; Bakrania, A.K.; Patel, S.S. Antidiabetic Potential of Gallic Acid from Emblica officinalis: Improved Glucose Transporters and Insulin Sensitivity through PPAR-γ and Akt Signaling. Phytomedicine 2020, 73, 152906. [Google Scholar] [CrossRef]
- Nerurkar, P.V.; Nishioka, A.; Eck, P.O.; Johns, L.M.; Volper, E.; Nerurkar, V.R. Regulation of Glucose Metabolism via Hepatic Forkhead Transcription Factor 1 (FoxO1) by Morinda citrifolia (Noni) in High-Fat Diet-Induced Obese Mice. Br. J. Nutr. 2012, 108, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Lee, J.; Choi, R.; Lee, H.; Lee, J.; Jeong, Y.; Kim, M.; Lee, M. Anti-Obesity and Anti-Insulin Resistance Effects of Tomato Vinegar Beverage in Diet-Induced Obese Mice. Food Funct. 2014, 5, 1579–1586. [Google Scholar] [CrossRef]
- Gao, H.; Wen, J.-J.; Hu, J.-L.; Nie, Q.-X.; Chen, H.H.; Xiong, T.; Nie, S.-P.; Xie, M.-Y. Fermented Momordica charantia L. Juice Modulates Hyperglycemia, Lipid Profile, and Gut Microbiota in Type 2 Diabetic Rats. Food Res. Int. 2019, 121, 367–378. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between Insulin Resistance and the Development of Cardiovascular Disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Goldstein, B.J. Insulin Resistance as the Core Defect in Type 2 Diabetes Mellitus. Am. J. Cardiol. 2002, 90, 3G–10G. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Doble, M. Mechanism of Action of Natural Products Used in the Treatment of Diabetes Mellitus. Chin. J. Integr. Med. 2011, 17, 563–574. [Google Scholar] [CrossRef]
- Patel, S.S.; Goyal, R.K. Prevention of Diabetes-Induced Myocardial Dysfunction in Rats Using the Juice of the Emblica officinalis Fruit. Exp. Clin. Cardiol. 2011, 16, 87–91. [Google Scholar] [PubMed]
- Dikshit, P.; Shukla, K.; Tyagi, M.K.; Garg, P.; Gambhir, J.K.; Shukla, R. Antidiabetic and Antihyperlipidemic Effects of the Stem of Musa sapientum Linn. in Streptozotocin-Induced Diabetic Rats. J. Diabetes 2012, 4, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Swami, U.; Rishi, P.; Soni, S.K. Anti-diabetic, hypolipidemic and hepato-renal protective effect of a novel fermented beverage from Syzygium cumini stem. IJPSR 2017, 8, 1336–1345. [Google Scholar] [CrossRef]
- Koebnick, C.; Garcia, A.L.; Dagnelie, P.C.; Strassner, C.; Lindemans, J.; Katz, N.; Leitzmann, C.; Hoffmann, I. Long-Term Consumption of a Raw Food Diet Is Associated with Favorable Serum LDL Cholesterol and Triglycerides but Also with Elevated Plasma Homocysteine and Low Serum HDL Cholesterol in Humans. J. Nutr. 2005, 135, 2372–2378. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, G.; Su, X.; Yang, H.; Zhang, J. Antiobesity Action of a Daidzein Derivative on Male Obese Mice Induced by a High-Fat Diet. Nutr. Res. 2009, 29, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Chudnovskiy, R.; Thompson, A.; Tharp, K.; Hellerstein, M.; Napoli, J.L.; Stah, A. Consumption of Clarified Grapefruit Juice Ameliorates High-Fat Diet Induced Insulin Resistance and Weight Gain in Mice. PLoS One 2014, 9, e108408. [Google Scholar] [CrossRef] [PubMed]
- Bolsinger, J.; Pronczuk, A.; Sambanthamurthi, R.; Hayes, K.C. Anti-Diabetic Effects of Palm Fruit Juice in the Nile Rat (Arvicanthis niloticus). J. Nutr. Sci. 2014, 3, 1–11. [Google Scholar] [CrossRef]
- Iwansyah, A.C.; Luthfiyanti, R.; Ardiansyah, R.C.E.; Rahman, N.; Andriana, Y.; Hamid, H.A. Antidiabetic Activity of Physalis angulata L. Fruit Juice on Streptozotocin-Induced Diabetic Rats. South African J. Bot. 2022, 145, 313–319. [Google Scholar] [CrossRef]
- Ullah, A.; Khan, A.; Khan, I. Diabetes Mellitus and Oxidative Stress –– A Concise Review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Dhuique-mayer, C.; Gence, L.; Portet, K.; Tousch, D.; Poucheret, P. Preventive Action of Retinoids in Metabolic Syndrome/Type 2 Diabetic Rats Fed with Citrus Functional Food Enriched in β-Cryptoxanthin Claudie. Food Funct. 2020, 11, 9263–9271. [Google Scholar] [CrossRef] [PubMed]
- Leow, S.; Bolsinger, J.; Pronczuk, A.; Hayes, K.C.; Sambanthamurthi, R. Hepatic Transcriptome Implications for Palm Fruit Juice Deterrence of Type 2 Diabetes Mellitus in Young Male Nile Rats. Genes Nutr. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Czech, M.P. The GLUT4 Glucose Transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef]
- L. Kouznetsova, V.; Hauptschein, M.; Tsigelny, I.F. Glucose and Lipid Transporters Roles in Type 2 Diabetes. Integr. Obes. Diabetes 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Banihani, S.A.; Makahleh, S.M.; El-Akawi, Z.; Al-Fashtaki, R.A.; Khabour, O.F.; Gharibeh, M.Y.; Saadah, N.A.; Al-Hashimi, F.H.; Al-Khasieb, N.J. Fresh Pomegranate Juice Ameliorates Insulin Resistance, Enhances β-Cell Function, and Decreases Fasting Serum Glucose in Type 2 Diabetic Patients. Nutr. Res. 2014, 34, 862–867. [Google Scholar] [CrossRef]
- Devaki, C.S.; Premavalli, K.S. Evaluation of Supplementation of Bittergourd Fermented Beverage to Diabetic Subjects. J. Pharm. Nutr. Sci. 2014, 4, 27–36. [Google Scholar] [CrossRef]
- Paquette, M.; Medina Larqué, A.S.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonné, S.; Marette, A.; Jacques, H. Strawberry and Cranberry Polyphenols Improve Insulin Sensitivity in Insulin-Resistant, Non-Diabetic Adults: A Parallel, Double-Blind, Controlled and Randomised Clinical Trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Simbo, S.Y.; Fang, C.; McAlister, L.; Roque, A.; Banerjee, N.; Talcott, S.T.; Zhao, H.; Kreider, R.B.; Mertens-Talcott, S.U. Açaí (Euterpe oleracea Mart.) Beverage Consumption Improves Biomarkers for Inflammation but Not Glucose- or Lipid-Metabolism in Individuals with Metabolic Syndrome in a Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Food Funct. 2018, 9, 3097–3103. [Google Scholar] [CrossRef] [PubMed]
- Aktan, A.; Ozcelik, A.; Cure, E.; Cure, M.; Yuce, S. Profound Hypoglycemia-Induced by Vaccinium corymbosum Juice and Laurocerasus Fruit. Indian J. Pharmacol. 2014, 46, 446–447. [Google Scholar] [CrossRef] [PubMed]
- Hasniyati, R.; Yuniritha, E.; Fadri, R.A. The Efficacy of Therapeutic-Diabetes Mellitus Functional Drink on Blood Glucose and Plasma Malondialdehyde (MDA) Levels of Type 2 Diabetes Mellitus Patients. 1st Lekantara Annu. Conf. Nat. Sci. Environ. 2022, 1097, 012021. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified Anthocyanin Supplementation Reduces Dyslipidemia, Enhances Antioxidant Capacity, and Prevents Insulin Resistance in Diabetic Patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, S.; Zhou, T.; Zhang, P.; Li, H. Bin. Alcoholic Beverage Consumption and Chronic Diseases. Int. J. Environ. Res. Public Health 2016, 13. [Google Scholar] [CrossRef]
- Conigrave, K.M.; Rimm, E.B. Alcohol for the Prevention of Type 2 Diabetes Mellitus? Treat. Endocrinol. 2003, 2, 145–152. [Google Scholar] [CrossRef]
- SANZ, M. Inositols and Carbohydrates in Different Fresh Fruit Juices. Food Chem. 2004, 87, 325–328. [Google Scholar] [CrossRef]
- Lifschitz, C.H. Carbohydrate Absorption From Fruit Juices in Infants. Pediatrics 2000, 105, e4–e4. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Li, T.Y.; Joshipura, K.J.; Hu, F.B. Intake of Fruit, Vegetables, and Fruit Juices and Risk of Diabetes in Women. Diabetes Care 2008, 31, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Caswell, H. The Role of Fruit Juice in the Diet: An Overview. Nutr. Bull. 2009, 34, 273–288. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).