1. Introduction
The introduction should briefly The 2018 European Society of Cardiology (ESC)/European Society of Hypertension (ESH) as well the latest ESH 2023 Guideline for the management of arterial hypertension support lifestyle changes as the initial therapeutic approach for individuals with high-normal BP and grade 1 hypertension [systolic and diastolic blood pressure (SBP, DBP) up to 159 and/or 99 mmHg, respectively] with low cardiovascular (CV) risk [
1,
2]. Lifestyle changes to lower BP values include reducing dietary calories, sodium and alcohol intake, adequate potassium integration and physical activity, aiming also at reducing body weight and waist circumference in overweight/obese patients [
3]. Although it has been proven that a healthy lifestyle can lower BP by approximately 4–5 mmHg [
4], treatment strategies based on non-pharmacological interventions are strongly limited by higher costs (many healthcare providers do not reimburse lifestyle strategies, such as a personalized diet by a dietician) and low adherence/persistence to the prescribed measures as they may also interfere with home or working life habits and needs [
5]. Alongside lifestyle changes, some dietary components, both as natural “functional” foods or as products in nutraceutical formulations, may have potential therapeutic properties in preventing or treating diseases [
6] and are often complementarily administered despite the low quality and strength of the supporting evidence (few or none RCTs, small sample sizes, short durations of follow-up, surrogate biomarkers rather than patient outcomes) [
7]. Nevertheless, a position document of the ESH stated that nutraceuticals support lifestyle improvement in lowering BP without significant side effects [
8]. Especially in the setting of cardiovascular disease (CVD) prevention, where canonical pharmacological treatments and their possible adversities often assume a quod vitam connotation, when a nutraceutical is also included, the adherence to overall lifestyle interventions may improve, even if cost-effectiveness is detrimental to the long-term [
9]. In hypertension, nutritional supplements rich in inorganic nitrates (NO3-) have been found to exert antihypertensive properties. Among functional foods and nutraceuticals as sources of nitrates, beetroot juice and its by-products have received considerable attention with the presence in literature of several studies, including placebo-controlled double-blinded RCTs and their metanalysis exploring the effect of dietary nitrates beyond BP profile [
10,
11,
12,
13,
14]. On these bases, we evaluated the effectiveness in clinical practice of 2018 ESC/ESH guideline-derived written lifestyle advice together with a beetroot-based nutraceutical on the 24-hour BP profile by ambulatory blood pressure monitoring (ABPM) in a population with high-normal BP and low-moderate CV risk from two distinct ESH Hypertension Excellence Centres.
2. Materials and Methods
2.1. Study design and population
We performed a bicentric longitudinal observational pilot study on consecutive patients referred to two Italian “Hypertension Excellence Centres” of the ESH as part of the Internal Medicine and Geriatrics, IRCCS INRCA (Ancona, Italy) and the Internal Medicine Clinical Unit, Rovigo General Hospital (Rovigo, Italy) from December 2021 to May 2022. We applied the following inclusion criteria: age ≥ 18 years, high-normal office BP (SBP 130-139 mmHg and/or DBP 85-89 mmHg) as defined by the 2018 ESC/ESH guidelines [
1], individual low CV risk, no history of CV events, no antihypertensive drug treatment at home, willingness to adhere to the proposed lifestyle and suggested nutraceutical according to clinical practice. We defined “individual low-moderate CV risk” patients < 50 years with a calculated SCORE2 < 2.5%, patients 50-69 years with SCORE2 < 5%, and patients ≥ 70 years with SCORE2-OP < 7.5% according to the 2021 ESC Guidelines on cardiovascular disease prevention [
15]. Individual CV risk was also double-checked using a web app,
www.humtelemed.it. All participants gave their informed consent, and clinical investigations were conducted according to the principles of the Declaration of Helsinki and its later amendments. This study was approved by the local institutional ethics committee (Comitato Etico INRCA).
2.2. Clinical parameters
The patient’s complete medical history, anthropometric measurements, laboratory parameters, and ABPM parameters were collected. Smoking habit was defined as current smoking or previous smoking of at least 100 cigarettes in a lifetime [
16]. Body mass index (BMI) was defined as the body mass divided by the square of the body height and was expressed in units of kg per square meter (BMI <25 normal weight, BMI 25-30 overweight, and BMI ≥30 obesity). After fasting blood sampling, the following laboratory parameters were considered: glycaemia, creatinine and estimation of glomerular filtration rate (eGFR, using CKD-EPI equation), lipid profile including total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG). Low-density lipoprotein cholesterol (LDL-C) was calculated using the modified Friedewald equation proposed by Martin [
17].
2.3. Blood pressure measurements
We performed three sequential oscillometric automatic BP measurements on both arms during office evaluation using validated devices (Microlife
® model BP3MQ1-2D and BP A200 AFib, Widnau, Switzerland). Correct cuff sizes (range 22–32 cm or 32-42 cm) were selected according to arm circumference, and BP measurements were performed after at least 5 min of rest in the sitting position. The patient’s arm was kept at the heart level during the measurement. The higher average BP value between arms was used for the analysis and to position the ABPM, thus avoiding errors due to interarm BP differences [
18]. A 24-hour ABPM was performed at baseline in each enrolled patient, using Spacelab devices model 90227 (Spacelab Healthcare, Snoqualmie, WA, United States) and TM-2430 ambulatory blood pressure monitors (A&D Company, Tokyo, Japan) with appropriate cuff dimensions according to the arm circumference. Twenty-four-hour BP, daytime BP (defined as the BP values from 06:00 to 22:00 hour), and night-time BP (defined as the BP values from 22:00 to 06:00 hour) were considered. The definitions of “day” and “night” periods were based on the most common answers to a questionnaire in which patients were asked about their sleeping behaviour. Moreover, the medical staff verified the correct positioning of the brachial cuff and its proper functioning. Minimum quality criteria for a satisfactory ABPM recording were based on recommendations by Omboni et al.[
19]. Patients with mean 24-hour BP < 130/80 mmHg, mean daytime BP < 135/85 mmHg, and mean night-time BP < 120/70 mmHg were defined as normotensive/controlled. We considered ‘dippers’ those patients with a mean SBP reduction equal to or greater than 10% from day to night.
2.4. Lifestyle advice, nutraceutical and follow-up
At baseline, each enrolled subject received a schematic summary with dietary and behavioural advice extrapolated from the 2018 ESH Guidelines on the management of arterial hypertension [
1], written in simple, schematic Italian language for easy comprehension by any patient (see Supplement 1 online, a traduction from the original in Italian administered to the patients). Such written advice was orally explained to the patients once, at the first visit. Furthermore, a once-daily (in the morning) nutraceutical containing red beetroot dry extract (500 mg of Beta Vulgaris L.) was suggested as an integrated part of lifestyle advice. The advice to add a nutraceutical to lifestyle adjustments was intended as part of common good clinical practice (GCP) [
8]. After three months, a second 24-hour ABPM was performed in each enrolled patient to re-evaluate the 24-hour BP profile. The incidence of any adverse event was also evaluated at follow-up. All enrolled patients were managed according to guidelines and routine clinical practice; no other procedures or interventions were performed.
2.5. Statistical analysis
Data were analyzed using the Statistical Package for Social Science version 21 (SPSS Inc., Chicago, Illinois, USA). A p-value less than 0.05 was defined as statistically significant. Continuous variables were checked for normality and expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) if markedly skewed. Paired t-test, McNemar test and repeated measures analysis of covariance (ANCOVA) were used to assess the differences between the selected variables at the specified time intervals.
3. Results
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3.1. Baseline characteristics of the study population
We enrolled a cohort of 43 caucasian patients with a mean age of 50 ± 11 years and a prevalence of males (54%), overweight/obese (58%), and baseline mean office BP of 135±3/85±3 mmHg. Twenty-two patients were enrolled in the Hypertension Centre of Ancona and 21 in Rovigo. At baseline, the ABPM reported a mean 24-hour SBP of 127±7 mmHg, a mean 24-hour DBP of 80±6 mmHg, a mean daytime SBP of 131±8 mmHg, a mean daytime DBP of 83±6 mmHg, a mean night-time SBP of 118±8 mmHg, and a mean night-time DBP of 70±5 mmHg. Thus, ABPM identified 49% and 30% of patients with BP values compatible with hypertension according to the mean 24-hour and daytime cutoffs, respectively, despite repeated measurements revealing only high-normal OBP before ABPM. The main clinical characteristics of the study population, the basal anthropometric measurements, and the laboratory parameters are reported in
Table 1.
3.2. Changes in the ABPM at follow-up
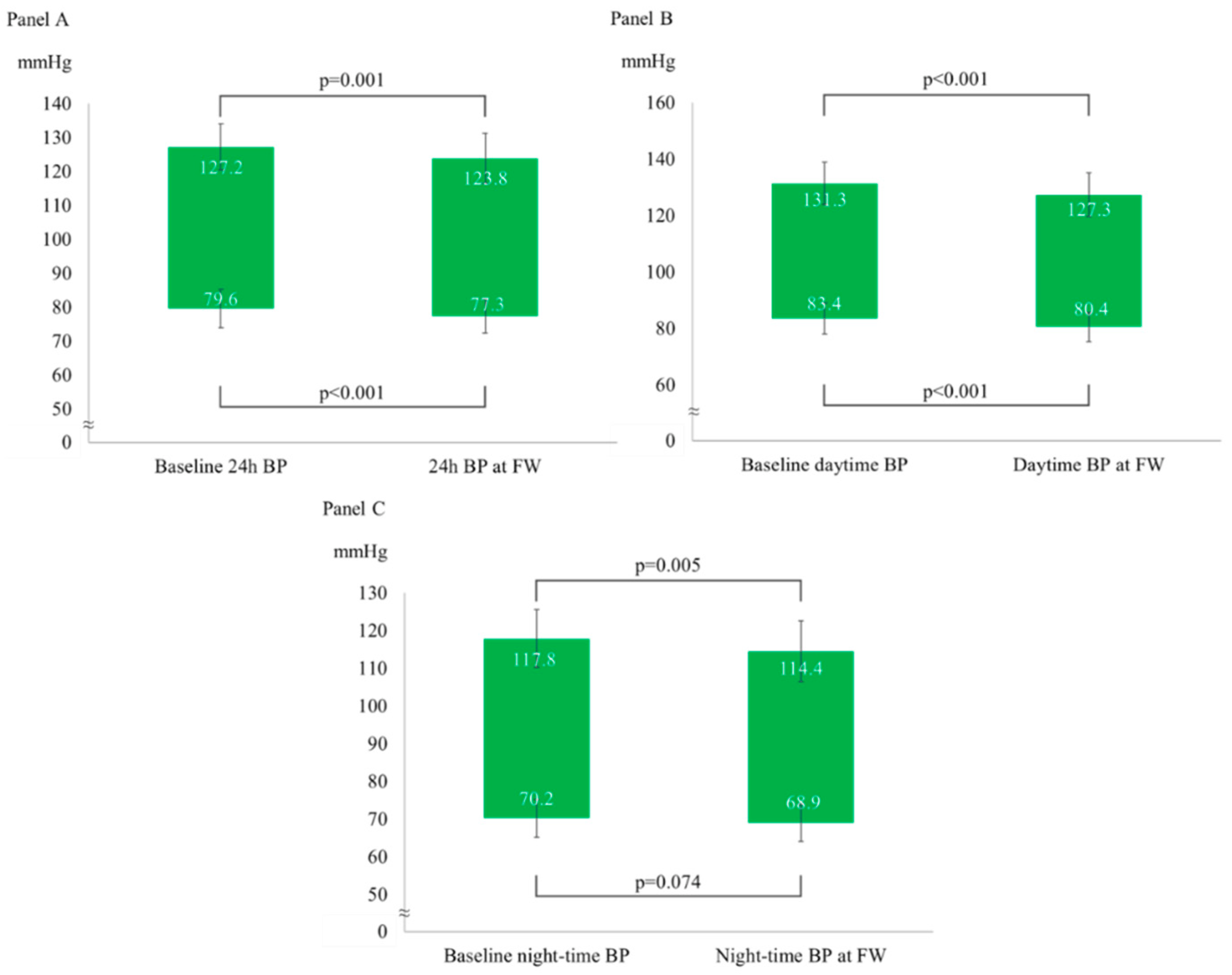
After a median follow-up of 98 (92-121) days, the overall BP profile improved, as shown by a statistically significant decrease in all parameters measured by the ABPM, except for mean night-time DBP (-3±6/-2±4 mmHg for 24-hour BP; -4±6/-3±4 mmHg for daytime BP and -3±7/-1±5 mmHg for night-time BP),
Figure 1. The prevalence of patients with 24-hour and daytime BP values compatible with normotension increased to 75% and 86%, respectively. The population had a non-clinically meaningful weight loss (∆weight = -0.4±1.2 Kg; p=0.05) at follow-up. In the subgroup analyses, ABP decreased independently from gender (p for interaction for 24-hour SBP = 0.873; for 24 DBP = 0.991; for daytime SBP = 0.862; for daytime DBP = 0.909; for night-time SBP = 0.965; for night-time DBP = 0.772), baseline BP level (high-normal BP vs hypertensive) (p for interaction for 24-hour SBP = 0.319; for 24 DBP = 0.586; for daytime SBP = 0.591; for daytime DBP = 0.273; for night-time SBP = 0.172; for night-time DBP = 0.655), basal BMI (<25 vs ≥25 Kg/mq) (p for interaction for 24-hour SBP = 0.635; for 24 DBP = 0.997; for daytime SBP = 0.798; for daytime DBP = 0.900; for night-time SBP = 0.595; for night-time DBP = 0.981) and smoking status (p for interaction for 24-hour SBP = 0.311; for 24 DBP = 0.312; for daytime SBP = 0.229; for daytime DBP = 0.151; for night-time SBP = 0.447; for night-time DBP = 0.808).
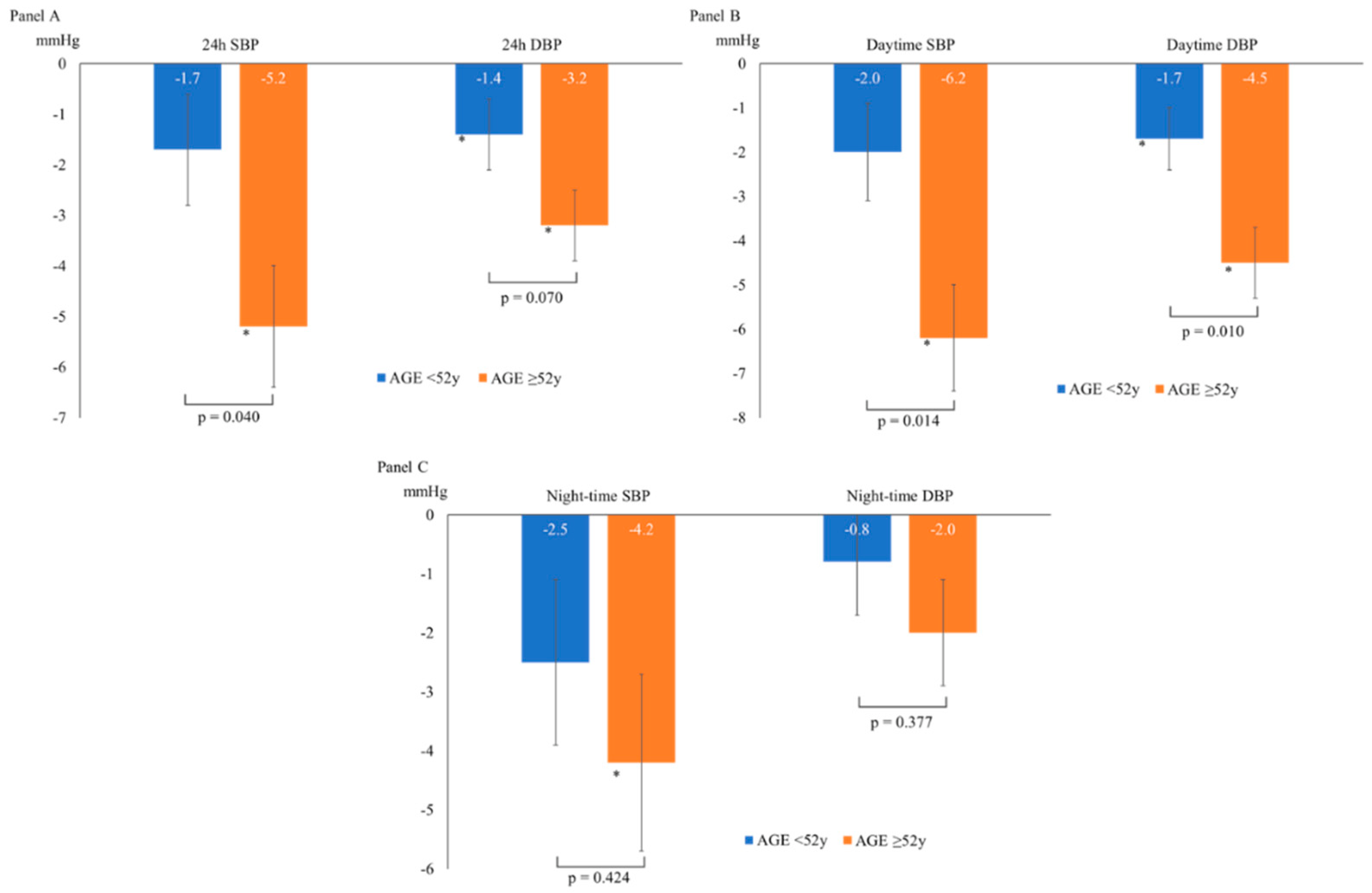
Table 2 shows the ABPM trends at follow-up according to gender, baseline BP level, BMI and smoking status. Conversely, compared to the younger subjects (aged <52 years, with 52 being the median age of the study population), a more significant reduction in 24-hour SBP, daytime SBP and daytime DBP was observed in the older subjects (aged ≥52 years) (p for interaction for 24-hour SBP = 0.040; for 24 DBP = 0.070; for daytime SBP = 0.014; for daytime DBP = 0.010; for night-time SBP = 0.424; for night-time DBP = 0.377), as shown in
Figure 2. No significant difference in the percentage of non-dipper subjects was present between baseline and follow-up (44.2% vs 48.8%, p=0.688). All the enrolled subjects completed the follow-up without any adverse events reported. All patients confirmed their willingness to adhere to the simple written lifestyle advice after the observational period.
4. Discussion
Lifestyle modifications have been proven safe and effective, with benefits beyond CV health. Regarding CVD, they are recommended as first-line preventive and treatment strategies for managing hypertension, dyslipidemias, and type 2 diabetes mellitus (DM2), regardless of their severity and the individual basal CV risk [
15]. In some cases, mainly through weight and visceral adiposity reduction, a healthy lifestyle can completely reverse the dysmetabolic and neuro-hormonal derangement that characterizes CV risk factors, avoiding further progression and reducing the need for pharmacological treatments. In the specific setting of hypertension, adequate lifestyle interventions should be suggested for high-normal BP patients, representing nearly 30% of the general population [
20]. Intercepting patients at these early stages is pivotal because it has been demonstrated that overt hypertension may develop in up to 65% of non-treated high-normal BP cases within the following two to four years [
20] and because high-normal BP has been associated with hypertension-related CV risk and target organ damage, similar to that typically found in frankly hypertensive individuals [
21,
22]. The 2018 ESC/ESH guidelines for managing arterial hypertension propose general lifestyle recommendations, including suggestions on preferable food groups, limited salt assumption, moderate alcohol consumption, stop smoking and regular physical activity [
2]. However, evidence of the usefulness of these simple generic approaches in clinical practice is scarce in the absence of a personalized diet accompanied by an exercise program [
23]. Our study showed how this simple approach could effectively reduce BP in the appropriately selected patient. A significant reduction in ABP emerged at 3-month follow-up. This BP drop is not strictly attributable to simple weight loss (there was no clinically significant change in body weight at three months) but is probably linked to the multiple generic advice given to the patient in a simple, non-personalized written form.
Previous observational investigations, RCTs and their meta-analyses evaluating beetroot-based nutraceuticals’ efficacy and safety in lowering BP in low CV-risk patients came up with encouraging data [
12,
24,
25]. In particular, beetroot consumption in the form of juice seemed to be associated with significant dose-dependent changes in SBP (mean reduction -4 mmHg, 95% CI -6 to -3) but not in DBP (-1 mm Hg (95% CI -2.2, 0.1) in a meta-analysis of RCTs with variable duration (2h–15 days) and different daily doses ranging from 321 to 2790 mg [
10]. Conversely, other investigations were consistent with a significant DBP reduction that the pressure measurement techniques could explain. In such cases, BP was measured early after beetroot ingestion, when significantly increasing plasma nitrate and nitrite concentrations occurred [
14,
26]. As mentioned, BP changes in response to beetroot or other dietary sources of nitrates administration evaluated with ABPM led to appreciably variable results [
13,
14]. Our real-life findings regarding the BP profile changes were comparable to those reported by a previous trial with a similar population and design (mean age 59 years, 12 weeks of daily 400 mg of beetroot extract or nitrates-rich vegetable diet, basal and follow-up ABPM). In particular, the DBP profile did not improve, while the SBP profile improved in the diet group [
27].
Regarding the subgroup analyses, those with initial ABPM profiles compatible with hypertension and non-smokers showed a greater BP decrease but were not statistically significant. At the same time, the more aged individuals had a more considerable drop in BP (
Table 2 and
Figure 2). The study design only allows us to make limited hypotheses about these findings. Smoking and ageing partly share the mechanisms sustaining hypertension as both lead to loss of elasticity, increased arterial stiffness and reduced endothelium-dependent vasodilation. The known “regression to the mean” effect can also have a role. Alongside more adherence to lifestyle advice than the younger group, different arterial responses to vasodilating stimuli by restoring endothelial function and enhancing NO bioavailability could have brought out this additional finding, as reported by recent experimental and clinical investigations [
28,
29,
30]. Thus, middle-aged pre-hypertensive and non-smoking patients could be the preferable target of this approach based on generic lifestyle advice and nitrate supplementation [
27].
Regarding safety, no adverse events were notified, and all subjects declared good adherence to the proposed antihypertensive non-pharmacological strategy that included a nutraceutical as a complementary part of the lifestyle interventions. Accordingly, a recent study considering and comparing lipid-lowering therapies (LLTs) revealed a 30% more persistence at two years of non-reimbursed nutraceuticals versus statins, independently of age, adverse events during treatment, and estimated CV risk [
31]. In our real-life study, we did not intentionally use a precise tool to evaluate adherence to both lifestyle advice (e.g., 24-hour urinary sodium) and nutraceuticals (e.g., rating scales) because they would affect the patient’s behaviour during follow-up, while our study primarily aimed to investigate effectiveness in everyday clinical practice of written generic advice.
4.1. Study strengths and limitations
A strength of our study was the original but straightforward design based on ESC/ESH guidelines and the use of ABPM to evaluate BP profiles at basal and follow-up, allowing greater accuracy in assessing BP variations and control [
32]. The main limitation is the small sample size, which characterizes this study as exploratory. The design did not contemplate a placebo-control group without a nutraceutical, given that estimating the effect on BP exerted by the nutraceutical alone was out of our purpose, as previously stated. However, we could not isolate from the comprehensive lifestyle approach the net effect of the beetroot-based nutraceutical on the BP profile in our population.
5. Conclusions
Our study showed that written generic lifestyle interventions extrapolated from ESC/ESH 2018 guidelines and “reinforced” by the prescription of a beetroot-based nutraceutical could lead to a mid-term ABP profile normalization in individuals with high-normal BP, reducing progression to overt “office” hypertension and the need of pharmacological treatment. To our knowledge, no previous study has evaluated the effectiveness of simple written, generic, non-personalized lifestyle advice in clinical practice. In contrast, most studies on hypertensives are instead focused on specific, standardized or personalized diets. Despite the limitations mentioned earlier, our findings support that generic lifestyle advice associated with a nutraceutical source of nitric oxide may be a valid initial non-pharmacological therapy in subjects with high-normal BP and low CV risk, many of which proved to be grade 1 hypertensive at ABPM. These results align with the growing evidence that lifestyle interventions are effective and safe and should be intended broadly, with some nutraceuticals playing a complementary role in enhancing the effectiveness of more conventional approaches and helping overcome limitations such as poor adherence.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Little Steps to a Healthy Lifestyle…
Author Contributions
M.L. and F.S. made the analyses, wrote the manuscript, and elaborated tables and figures. R.S. and A.M. planned and supervised the whole study and revised the manuscript. All the other authors contributed equally to the data collection and preparation of the dataset. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mancia Chairperson G, Kreutz Co-Chair R, Brunström M, Burnier M, Grassi G, Januszewicz A, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023.
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-104.
- Fu J, Liu Y, Zhang L, Zhou L, Li D, Quan H, et al. Nonpharmacologic Interventions for Reducing Blood Pressure in Adults With Prehypertension to Established Hypertension. J Am Heart Assoc. 2020;9(19):e016804.
- Pazoki R, Dehghan A, Evangelou E, Warren H, Gao H, Caulfield M, et al. Genetic Predisposition to High Blood Pressure and Lifestyle Factors: Associations With Midlife Blood Pressure Levels and Cardiovascular Events. Circulation. 2018;137(7):653-61.
- Dhakal A, K CT, Neupane M. Adherence to lifestyle modifications and its associated factors in hypertensive patients. J Clin Nurs. 2022;31(15-16):2181-8.
- Aronson, JK. Defining ‘nutraceuticals’: neither nutritious nor pharmaceutical. Br J Clin Pharmacol. 2017;83(1):8-19.
- Penson PE, Banach M. Natural compounds as anti-atherogenic agents: Clinical evidence for improved cardiovascular outcomes. Atherosclerosis. 2021;316:58-65.
- Borghi C, Tsioufis K, Agabiti-Rosei E, Burnier M, Cicero AFG, Clement D, et al. Nutraceuticals and blood pressure control: a European Society of Hypertension position document. J Hypertens. 2020;38(5):799-812.
- Catapano AL, Barrios V, Cicero AF, Pirro M. Lifestyle interventions and nutraceuticals: Guideline-based approach to cardiovascular disease prevention. Atherosclerosis Supplements. 2019;39:100003.
- Siervo M, Lara J, Ogbonmwan I, Mathers JC. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: a systematic review and meta-analysis. J Nutr. 2013;143(6):818-26.
- Beirne AM, Mitchelmore O, Palma S, Andiapen M, Rathod KS, Hammond V, et al. NITRATE-CIN Study: Protocol of a Randomized (1:1) Single-Center, UK, Double-Blind Placebo-Controlled Trial Testing the Effect of Inorganic Nitrate on Contrast-Induced Nephropathy in Patients Undergoing Coronary Angiography for Acute Coronary Syndromes. J Cardiovasc Pharmacol Ther. 2021;26(4):303-9.
- Coles LT, Clifton PM. Effect of beetroot juice on lowering blood pressure in free-living, disease-free adults: a randomized, placebo-controlled trial. Nutr J. 2012;11:106.
- Gilchrist M, Winyard PG, Aizawa K, Anning C, Shore A, Benjamin N. Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free Radic Biol Med. 2013;60:89-97.
- Hobbs DA, Kaffa N, George TW, Methven L, Lovegrove JA. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br J Nutr. 2012;108(11):2066-74.
- Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227-337.
- Giulietti F, Filipponi A, Rosettani G, Giordano P, Iacoacci C, Spannella F, et al. Pharmacological Approach to Smoking Cessation: An Updated Review for Daily Clinical Practice. High Blood Press Cardiovasc Prev. 2020;27(5):349-62.
- Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. Jama. 2013;310(19):2061-8.
- Spannella F, Giulietti F, Fedecostante M, Ricci M, Balietti P, Cocci G, et al. Interarm blood pressure differences predict target organ damage in type 2 diabetes. J Clin Hypertens (Greenwich). 2017;19(5):472-8.
- Omboni S, Palatini P, Parati G. Standards for ambulatory blood pressure monitoring clinical reporting in daily practice: recommendations from the Italian Society of Hypertension. Blood Press Monit. 2015;20(5):241-4.
- Egan BM, Stevens-Fabry S. Prehypertension--prevalence, health risks, and management strategies. Nat Rev Cardiol. 2015;12(5):289-300.
- Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345(18):1291-7.
- Cuspidi C, Sala C, Tadic M, Gherbesi E, Facchetti R, Grassi G, et al. High-normal blood pressure and abnormal left ventricular geometric patterns: a meta-analysis. J Hypertens. 2019;37(7):1312-9.
- Nicoll R, Henein MY. Hypertension and lifestyle modification: how useful are the guidelines? Br J Gen Pract. 2010;60(581):879-80.
- Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65(2):320-7.
- Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51(3):784-90.
- Hobbs DA, Goulding MG, Nguyen A, Malaver T, Walker CF, George TW, et al. Acute ingestion of beetroot bread increases endothelium-independent vasodilation and lowers diastolic blood pressure in healthy men: a randomized controlled trial. J Nutr. 2013;143(9):1399-405.
- van der Avoort CMT, Ten Haaf DSM, Bongers C, van Oorschot F, Verdijk LB, van Loon LJC, et al. Increasing Nitrate-Rich Vegetable Intake Lowers Ambulatory Blood Pressure in (pre)Hypertensive Middle-Aged and Older Adults: A 12-Wk Randomized Controlled Trial. J Nutr. 2021;151(9):2667-79.
- Virdis A, Giannarelli C, Neves MF, Taddei S, Ghiadoni L. Cigarette smoking and hypertension. Curr Pharm Des. 2010;16(23):2518-25.
- Tawa M, Nakagawa K, Ohkita M. Effects of beetroot juice supplementation on vascular functional and structural changes in aged mice. Physiol Rep. 2023;11(12):e15755.
- de Jongh RT, Serné EH, RG IJ, de Vries G, Stehouwer CD. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109(21):2529-35.
- Cicero AF, Derosa G, Parini A, Baronio C, Borghi C. Factors associated with 2-year persistence in fully non reimbursed lipid-lowering treatments. Atherosclerosis. 2014;235(1):81-3.
- Balietti P, Spannella F, Giulietti F, Rosettani G, Bernardi B, Cocci G, et al. Ten-year changes in ambulatory blood pressure: The prognostic value of ambulatory pulse pressure. J Clin Hypertens (Greenwich). 2018;20(9):1230-7.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).