1. Introduction
Mycosis fungoides (MF) is the most common form of cutaneous T-cell lymphoma (CTCL) [
1]. MF is a rare disease and more common in males than females (ratio 1.4–1.6) [
2,
3]. The prevalence of MF is higher in Europe than in North America [
4] and the proportion of patients with MF in relation to the overall CTCL population varies between countries: e.g., Germany (62%) [
5] and Italy (64%) [
6] versus France (41%) [
7], the United Kingdom [55%] [
8] and Spain [59%] [
9]. According to the International Society for Cutaneous Lymphomas (ISCL) and European Organisation for Research and Treatment of Cancer (EORTC; ISCL/EORTC), patients with MF are classified into early-stage (IA–IIA) and advanced-stage (IIB–IVB) [
10]. Most patients are diagnosed with early disease at a median age of 58 years [
11]. Five-year overall survival (OS) rates vary according to disease stage in patients with MF [
3], ranging from 93.6% and 83.8% at stages IA and IB, to 60%, 45.9% and 44.9% at stages IIB, IIIB and IV, respectively [
12]. Compared with patients at stage IA, the age- and sex-adjusted relative risks of death for patients at stages IB and IIA are 1.3 and 3.5, respectively [
11].
Due to the heterogeneous and progressive nature of MF, treatment plans are multimodal and vary depending on clinical stage, subtype and location of involvement [
13,
14]. In Europe, treatment of MF is usually based on the EORTC consensus recommendations [
15,
16]. However, achieving and maintaining a durable response remains challenging, particularly in patients with advanced-stage MF. Most patients who receive front-line therapy, which includes skin-directed (earlystage MF) and systemic treatments (e.g., chemotherapy in advanced-stage MF) alone or in combination, eventually relapse or develop treatment-refractory disease [
14]. In such cases, several novel therapies are available to these patients, including monoclonal antibodies (mogamulizumab) [
17], histone deacetylase inhibitors (vorinostat, belinostat, romidepsin) [
18] and antibody-drug conjugates, such as brentuximab vedotin [
19,
20]. Nevertheless, among patients with relapsed or refractory (R/R) MF, a knowledge gap exists regarding patient characteristics, treatment patterns and outcomes with these novel treatments. This study aimed to assess current treatment patterns and outcomes among patients with R/R CTCL in a real-world setting. We previously reported the results from 157 patients with R/R CTCL after systemic therapy across five European countries, with a median follow-up time of 5.6 years from diagnosis [
20]. Here, we report real-world treatment patterns and OS from the same study in the subgroup of patients with MF.
2. Materials and Methods
Study Design and Patients
The study design has been previously described [
20]. Briefly, this was a retrospective, multicentre, chart review study involving 27 participating clinical European sites in France, Germany, Italy, Spain, and the UK. Sites were carefully selected to include a representative sample considering size, type (academic versus community), and geography (spread across each country) to capture potential variability in treatment practices. Within the retrospective design, patient charts were based on dates of diagnosis of R/R disease, allowing all patients to be included regardless of their outcome to prevent immortal time bias. Given the rarity of patients with MF, no other sampling techniques to reduce bias were considered. Eligible patients were aged ≥18 years with a diagnosis of CTCL, including MF, with R/R disease after a first course of systemic therapy before 1 January 2016, 8 within routine practice and according to the local standard of care. The index date was defined as the date of diagnosis of R/R disease (1984–2016). Patients were excluded if they received systemic therapy before having R/R disease as part of a clinical trial. While the primary study included three lines of therapies [
20], the current analysis focused on patients who received second- and third-line therapies. Data from patients’ medical charts were extracted by the study sites into an electronic data capture system (September 2017–November 2018) and included patient demographics and clinical characteristics (CTCL subtype and disease stage at index), treatments and responses to treatment. The study was conducted in accordance with the International Society for Pharmacoepidemiology (ISPE) guidelines for Good Pharmacoepidemiology Practice [
21] and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [
22]. Patient data were pseudonymised following both central and local ethics committee approvals in accordance with local regulations as specified previously [
20].
Outcome Measures
Outcomes included treatments received by line of therapy (systemic therapy, radiotherapy, skin therapy and other therapies [e.g., surgery, transplant]); response to treatment, both global and local (including time from start of a line of therapy to R/R disease); stage at index date in patients who received second-line treatment; OS and progression-free survival (PFS). MF stages I–IV were determined according to the ISCL/EORTC classification [
10]. Global response assessment was based on tumour-node-metastasis-blood (TNMB) staging (i.e., skin, lymph nodes, viscera and blood); where global response assessment was not available in patients’ charts, skin (local) response alone was recorded. Although safety was an outcome measure in the previously published full CTCL population [
20], it was not specifically analysed in the MF sub-population.
Statistical Analyses
As the study was descriptive, no formal sample size calculation was performed. Death, to assess OS, and death or progression, to assess PFS, were estimated from the index date using Kaplan–Meier estimates with 95% confidence intervals (CI). Progression was defined as progression from early- to advanced-stage MF, developing R/R MF or initiation of subsequent therapy after treatment for the first R/R MF. All other analyses were summarised using descriptive statistics (mean and standard deviation [SD] or median and range [minimum–maximum] for continuous variables; numbers and percentages for categorical variables). Dates needed to have both the year and month included in the time interval calculation; if a date was missing the day, 15 was imputed as the day.
3. Results
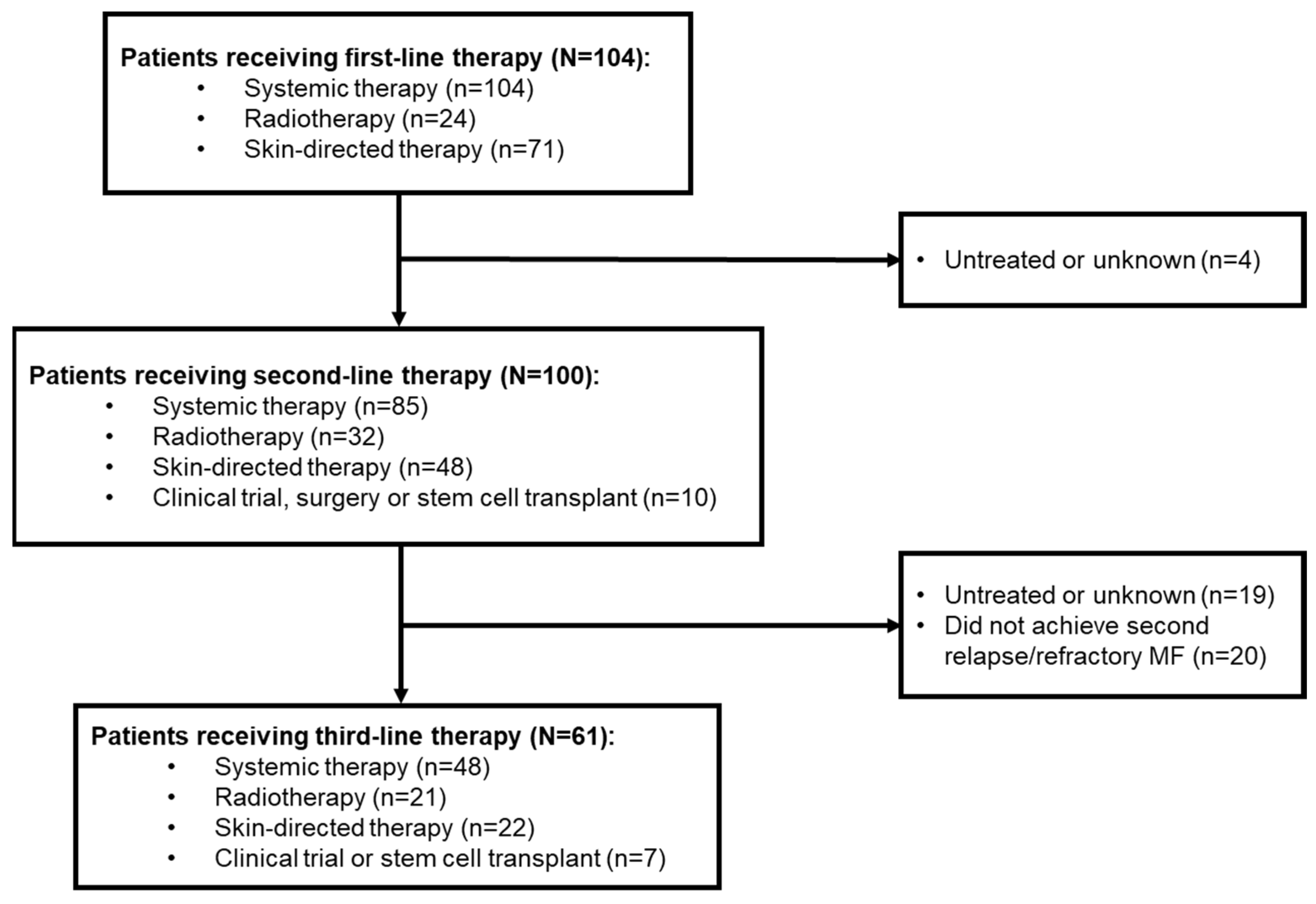
In total, 104 patients with MF who had received first-line therapy were included in this retrospective study. Median age was 54.5 years, and most patients were male (60.6%;
Table 1).
3.1. Disease Stage at Diagnose or First R/R Disease
The most common ISCL/EORTC stages reported at diagnosis or at time of first R/R disease IB or IIB (
Table 2).
Of these 104 patients, 100 went on to receive second-line therapy, and 61 received third-line therapy (
Figure 1).
Of 81 patients with available global response assessment to first-line systemic therapy or radiotherapy, seven (6.7%) had a complete response (CR) and 30 (28.8%) had a partial response (PR). Local response assessments were available for 13 patients; one had a CR (1.0%) while eight (7.7%) had PRs (
Table 3).
New lesions were present in 64 (64.0%) patients with first R/R disease. The median (range) time from diagnosis to first R/R disease was 11.2 (0.3–166.5) months. The median (range) time from diagnosis of the first R/R disease to the last known status was 3.5 (0.0–20.7) years.
3.2. Second-Line Treatment Patterns
Among 100 patients who received second-line therapy, 85 (85.0%) received systemic treatment, 32 (32.0%) radiotherapy, 48 (48.0%) skin-directed therapies, and 10 (10.0%) other treatments. Of those receiving systemic therapy, 20 (23.5%) were treated with single-agent chemotherapy and 11 (12.9%) with combination chemotherapy. Radiotherapy and skin-directed therapy were mostly delivered via electron beam (n=16 [50.0%]) and topical steroids (n=35 [72.9%]), respectively (
Table 4).
3.3. Response to Second-Line Treatment
Overall, 94 patients received second-line systemic therapy or radiotherapy. Of these, 69 had available global response assessment; 12 (12.8%) had CRs and 31 (33.0%) had PRs. Local response assessment was available for 14 patients; one (1.1%) had a CR and seven (7.4%) had PRs (
Table 3). Of the total population (n=100) with MF who received second-line therapy, 80 (80.0%) patients had a second R/R MF. Median (range) time from first to second R/R MF was 13.5 (0.0–174.6) months (
Table 5).
In patients who received systemic therapy or radiotherapy as second-line treatments, 31/94 (33.0%) discontinued treatment, mainly due to disease progression (n=7; 22.6%) and toxicity (n=6; 19.4%;
Table 6).
3.4. Third-Line Treatment Patterns
Of the 80 patients with R/R MF after second-line treatment, 61 (76.3%) received third-line treatment and 19 (23.8%) remained untreated during data collection. Forty-eight (78.7%) patients received systemic therapy, 21 (34.4%) radiotherapy and 22 (36.1%) skin-directed therapy. Among those receiving systemic therapy, 14 (29.2%) received single-agent chemotherapy and six (12.5%) combination chemotherapy. Radiotherapy and skin-directed therapy were mostly delivered via electron beam (n=13, 61.9%) and topical steroids (n=19, 86.4%), respectively (
Table 4).
3.5. Response to Third-Line Treatment
Of the 61 patients who received a third-line therapy, 38 (62.3%) subsequently relapsed or became refractory to treatment. In total, 56 (91.8%) patients received third-line systemic therapy or radiotherapy. Of these, 39 had available global response assessment; 7 (12.5%) had CRs and 15 (26.8%) had PRs. Local response assessment was available for 10 patients; five (8.9%) had PRs (
Table 3). Median (range) time from second to third R/R MF was 9.1 (0.0–39.0) months (
Table 5). Overall, 20/56 (35.7%) patients who received systemic or radiotherapy as a third-line treatment discontinued therapy (
Table 6). Survival outcomes At last follow-up (11th March 2019), 39 (37.5%) patients had died at the mean (SD) age of 65.2 (11.6) years, primarily due to CTCL (n=18, 46.2%) and CTCL-related complication or toxicity (n=10, 25.6%); the remaining deaths were due to other causes (n=7, 17.9%) or were unknown (n=4, 10.3%). Of the 63 (60.6%) patients known to be alive at the time of analysis, 57 (90.5%) had unresolved CTCL. Median 21 OS (95% CI) in patients with first relapsed/refractory MF was 11.5 years (6.5–not reached [NR]). Patients receiving non-chemotherapy regimens had prolonged survival (n=54; NR [12.1–NR] years) versus patients treated with chemotherapy (n=31; 6.5 [2.7–NR] years) (
Figure 2).
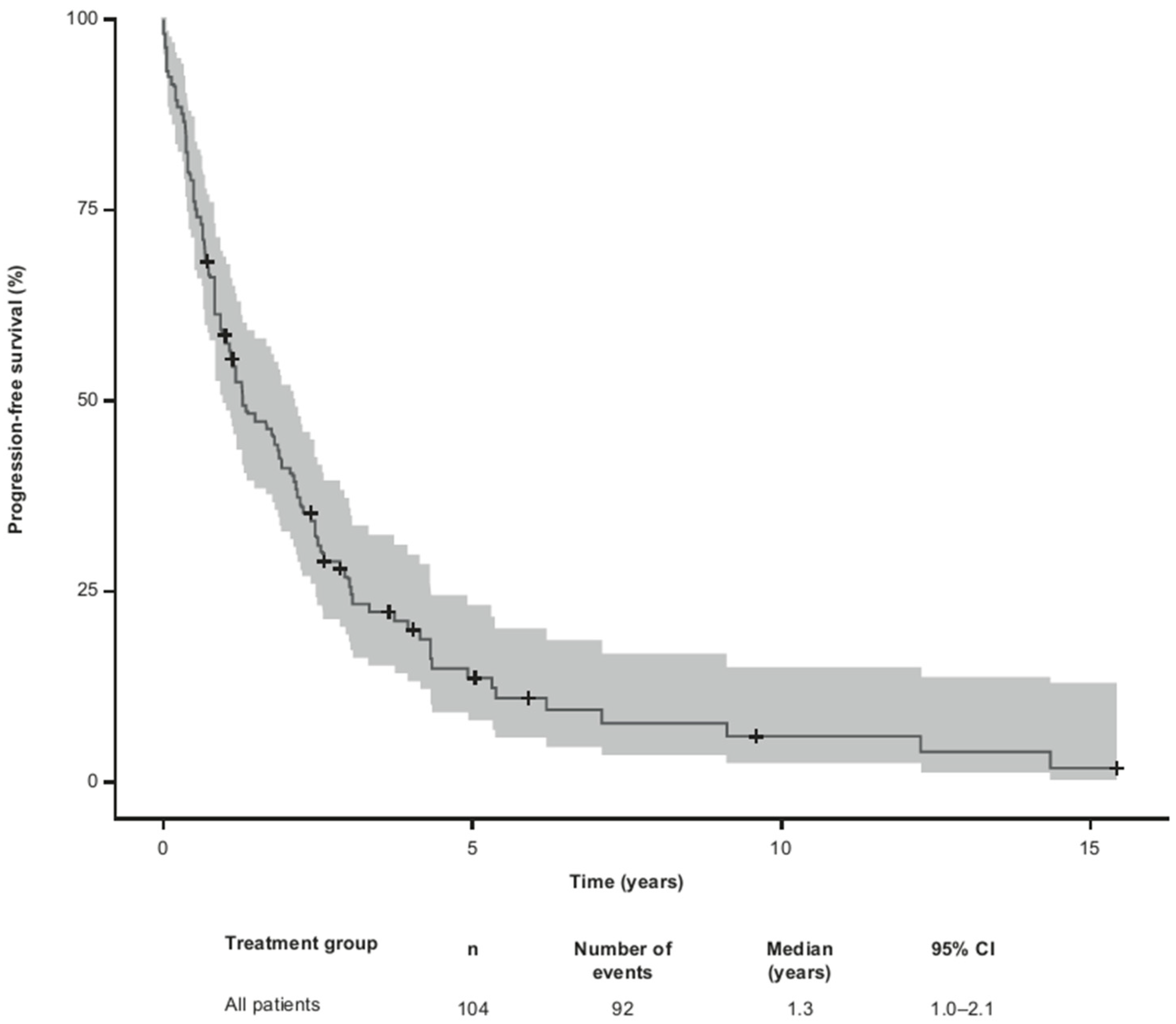
Median PFS for all 104 patients from the time of first R/R MF was 1.3 (95% CI 1.0– 2.1) years (
Figure 3).
4. Discussion
Existing clinical research in MF focuses on advanced-stage disease, with a paucity of data on R/R MF. As such, we conducted this observational, retrospective cohort study to examine real-world treatment patterns and outcomes of patients with R/R MF across 27 clinics in Europe. Our data show that following first-line systemic treatment, 48% of patients relapsed and 52% developed refractory disease, and despite the administration of chemotherapy as second-line treatment, 80% of patients progressed to second R/R MF and approximately 60% experienced a third relapse or became refractory within 1 year. Treatment options at second and third lines were heterogenous, including systemic therapies in the majority of patients, radiotherapy (almost a third in both lines), and topical therapies. The high rates of relapse and refractory disease were consistent with our previous analyses in similar cohorts in patients with CTCL and MF [
20,
23] and with a retrospective analysis of patients with MF and Sézary syndrome receiving a median of three lines of systemic therapies [
24]. In line with our current data, despite the use of chemotherapy (more than two-thirds of patients at second and third line), patients progressed after treatment for their first and second R/R MF within approximately 1 year. This is supported by reports of the limited durability of singleor multi-agent chemotherapy at early treatment lines in controlling MF (median time to subsequent therapy, 3.9 months) [
24]. For early-stage MF, EORTC recommends skin-directed therapies (e.g., topical corticosteroids, ultraviolet irradiation and mechlorethamine) for first-line treatment and systemic therapies (e.g., retinoids, interferon α-2b and low-dose methotrexate), possibly in combination with skin-directed therapy, for second-line treatment. These systemic therapies, in addition to others such as chemotherapy (gemcitabine, pegylated liposomal doxorubicin and cyclophosphamide, hydroxydaunomycin, vincristine, prednisolone [CHOP]), alemtuzumab and allogeneic stem cell transplant, are recommended for the first-line treatment of advanced-stage MF; single-agent and combination chemotherapy and allogeneic stem cell transplant are used as second-line therapies [
13,
16].
In our study, combination chemotherapy was used in approximately 13% of patients as second and third lines. This heterogeneity, particularly in R/R MF, was higher than expected per European guidelines [
15,
16]. Combination chemotherapy has limited durability in MF disease control and is best followed by allogeneic hematopoietic cell transplantation [
14]. Almost a quarter of patients with second R/R MF were untreated or had unknown third-line treatment at the time of data extraction. This suggests that patients either relapse but do not show aggressive disease and remain untreated, or that there is a lack of systemic treatments with an acceptable benefit-risk ratio beyond second-line treatment. Based on recent clinical trial data, such as the MAVORIC and ALCANZA post-hoc analysis studies, using targeted therapies resulted in beneficial outcomes and a manageable safety profile in patients with advanced or R/R MF [
19,
25,
26,
27,
28,
29]. In addition, compared with other CTCL subtypes, patients with MF have been reported to have a longer OS from the time of first R/R MF [
20].
Here, patients with MF who received non-chemotherapy treatments had a longer OS than patients who received chemotherapy, suggesting that non-chemotherapy options may be more beneficial to patients. Our results indicate that the clinical burden of CTCL was likely to be considerable in the five European countries at the time of data collection. However, it is notable that this study spanned a period when using novel targeted therapies, such as mogamulizumab or brentuximab vedotin, was not common clinical practice in the R/R setting (1984–2016). Indeed, more recently approved targeted agents have been shown to improve outcomes in this patient population [
19,
25,
27,
28,
29,
30]. For example, in the ALCANZA trial, patients with MF had global response rates of 50% with brentuximab vedotin versus 10% with methotrexate or bexarotene [
29]; in the MAVORIC trial, treatment with mogamulizumab resulted in superior PFS of 7.7 months versus 3.1 months with vorinostat in patients with R/R MF or Sézary syndrome [
25]. Other targeted agents with novel mechanisms of action, such as checkpoint inhibitors and JAK inhibitors, are currently being explored in ongoing clinical trials [
31]. Additional observational studies including targeted therapies are warranted to inform on the real-world impact of these novel treatments.
Also contributing to the heterogeneity of treatment approaches at the different disease stages was that patients were managed by multiple physicians with diverse specialities, including dermatologists, oncologists and haematologists, as well as a variety of reference guidelines (e.g., EORTC, the European Society for Medical Oncology [ESMO] [
32] and the National Institute for Health and Care Excellence [NICE]) [33]. Limitations are related to the retrospective nature of the study. Since the managing physicians collected the data for purposes other than to address the objectives of this study, specific clinical characteristics, treatments and outcomes might not have been standardized, as is the case in clinical trials. The level of detail of clinical records might vary depending on the chart recording practices of each study site. The small number of patients receiving second- and third-line therapies prevented the performance of sub-analyses on some outcomes (e.g., stratifying OS by types of chemotherapy or disease stage).
5. Conclusions
This observational study demonstrated a high rate of R/R MF following second- and third-line treatments in real-world settings in Europe, with a longer OS in patients receiving non-chemotherapy versus chemotherapy. This suggests that the clinical burden of R/R MF is significant in Europe. Adhering to standard management of MF and using recently approved targeted therapies may improve patient outcomes, including prolonging OS in advanced-stage MF
Author Contributions
Conceptualization, N.W. and M.D.; methodology, N.W., M.H., M.D.; validation, N.W., M.D.; formal analysis, M.H.; investigation, N.W. and T.L.; resources, N.W.; data curation, M. H..; writing—original draft preparation, N.W.; writing—review and editing, C.A., M.B., A.Z., N.B., M.L., N.P., P.O. and T.I.; project administration, N.W. and T.L.; funding acquisition, M.D. All authors have read and agreed to the published version of the manuscript.”
Funding
This research was funded by Takeda Development Center Americas, Inc. (TDCA), Lexington, MA.
Institutional Review Board Statement
List of ethics committees in Germany, Spain and Italy: Local ethics commissions: Landesärztekammer Baden-Württemberg, Sächsische Landesärztekammer, Eberhard Karls Universität Tübingen, Fachbereich Medizin Frankfurt Goethe Universität and Medizinische Fakultät der Ruhr-Universität Bochum.. Local ethics commission: Clinica del Hospital Clinic de Barcelona, Investigación con medicamentos de Euskadi, Hospital Universitario 12 de Octubre, Hospital de la Santa Creu I Sant Pau, Parc de Salut MAR, Clinica del Hospital Universitari de Bellvitge and Servicio Canario de la Salud. Local ethics commission: Fondazione IRCCS Ca’ Granda Ospedale Magiorre Policlinico, Fondazione Policlinico Tor Vergata, Azienda Ospedaliero Universitaria Città della Salute e della Scienza di Torino, Ospedale Papa Giovanni XXIII di Bergamo, Istituto Tumori Giovanni Paolo II Bari, Task Force Clinica della Area Vasta Centro Regione Toscana, and Comitato Etico Regionale della Marche Ancona.
Informed Consent Statement
Patient consent was waived due to anonymous retrospective data acquisition in agreement with ethical approval from listed ethics committee above prior to conducting the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank the patients who participated in this study and their families. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Sam Hijazi, PhD, of Ashfield MedComms, an Inizio company, funded by Takeda Pharmaceuticals U.S.A., Inc., Lexington, MA, and complied with the Good Publication Practice (GPP) guidelines (DeTora LM, et al. Ann Intern Med 2022;175:1298–1304).
Conflicts of Interest
CA: advisory board fees from 4SC, Helsinn, Innate Pharma, Kyowa Kirin, Recordati Rare Diseases and Takeda. TMI: consultancy/advisory for Takeda. NW, MH and TL: employees of ICON. AZ, NBE and MD: employees of Takeda. ML: employee of Agios. PLOR: advisory board or presentation fees from 4SC, Actelion, Bioprofarma, Helsinn, Kyowa Kirin, Innate Pharma, Mallinckrodt, MIRAGEN, Recordati Rare Diseases and Takeda; research support from MEDA (Viatris); patent Phospholipase C gamma 1 (PLCG1). NP: advisory board fees from Kyowa Kirin, Novartis, Pierre Fabre, Sanofi and Takeda; speakers’ bureau fees from Kyowa Kirin, Recordati Rare Diseases and Takeda; research project funding from BMS and Novartis. MB: advisory board fees from Innate Pharma, Kyowa Kirin, Helsinn, Recordati Rare Diseases and Takeda.
References
- Olsen, EA. Evaluation, diagnosis, and staging of cutaneous lymphoma. Dermatol Clin 2015, 33, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Kaufman AE, Patel K, Goyal K, O'Leary D, Rubin N, Pearson D; et al. Mycosis fungoides: Developments in incidence, treatment and survival. J Eur Acad Dermatol Venereol 2020, 34, 2288–2294. [Google Scholar] [CrossRef]
- Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S; et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: Validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol 2010, 28, 4730–4739. [Google Scholar] [CrossRef]
- Dobos G, Pohrt A, Ram-Wolff C, Lebbé C, Bouaziz JD, Battistella M; et al. Epidemiology of cutaneous T-cell lymphomas: A systematic review and meta-analysis of 16,953 patients. Cancers 2020, 12, 2921. [Google Scholar] [CrossRef] [PubMed]
- Assaf C, Gellrich S, Steinhoff M, Nashan D, Weisse F, Dippel E; et al. Cutaneous lymphomas in Germany: An analysis of the Central Cutaneous Lymphoma Registry of the German Society of Dermatology (DDG). J Dtsch Dermatol Ges 2007, 5, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Maurelli M, Tessari G, Colato C, Schena D, Girolomoni G. Incidence and ten-year follow-up of primary cutaneous lymphomas: A single-centre cohort study. Eur J Dermatol 2018, 28, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Dobos G, de MA, Ram-Wolff C, Beylot-Barry M, Pham-Ledard A, Ortonne N; et al. Epidemiological changes in cutaneous lymphomas: An analysis of 8593 patients from the French Cutaneous Lymphoma Registry. Br J Dermatol 2021, 184, 1059–1067. [Google Scholar] [CrossRef]
- National Cancer Registration and Analysis Service (NCRAS). National Cancer Intelligence Network (NCIN) data briefings. 2016. Available at: http://www.ncin.org.uk/publications/data_briefings [Accessed 17 January 2023].
- Peñate Y, Servitje O, Machan S, Fernández-de-Misa R, Estrach MT, Acebo E; et al. The first year of the AEVD primary cutaneous lymphoma registry. Actas Dermosifiliogr (Engl Ed) 2018, 109, 610–616. [Google Scholar]
- Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R; et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007, 110, 1713–1722. [Google Scholar]
- Maguire A, Puelles J, Raboisson P, Chavda R, Gabriel S, Thornton S. Early-stage mycosis fungoides: Epidemiology and prognosis. Acta Derm Venereol 2020, 100, adv00013. [Google Scholar]
- Mourad M, Gniadecki R. Overall survival and prognosis in mycosis fungoides: A systematic review and meta-analysis. Available at: https://www.wcd2019milan-dl.org/abstract-book/documents/abstracts/39-skin-cancer/overall-survival-and-prognosis-in-2681.pdf [Accessed 23 November 2023].
- Jonak C, Tittes J, Brunner PM, Guenova E. Mycosis fungoides and Sézary syndrome. J Dtsch Dermatol Ges 2021, 19, 1307–1334. [Google Scholar]
- Kamijo H, Miyagaki T. Mycosis fungoides and Sézary syndrome: Updates and review of current therapy. Curr Treat Options Oncol 2021, 22, 10. [Google Scholar] [CrossRef]
- Trautinger F, Knobler R, Willemze R, Peris K, Stadler R, Laroche L; et al. EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome. Eur J Cancer 2006, 42, 1014–1030. [Google Scholar] [CrossRef]
- Trautinger F, Eder J, Assaf C, Bagot M, Cozzio A, Dummer R; et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome - Update 2017. Eur J Cancer 2017, 77, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Duvic M, Pinter-Brown LC, Foss FM, Sokol L, Jorgensen JL, Challagundla P; et al. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood 2015, 125, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- Yoon S, Eom GH. HDAC and HDAC inhibitor: From cancer to cardiovascular diseases. Chonnam Med J 2016, 52, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim YH, Tavallaee M, Sundram U, Salva KA, Wood GS, Li S; et al. Phase II investigator-initiated study of brentuximab vedotin in mycosis fungoides and Sézary syndrome with variable CD30 expression level: A multi-institution collaborative project. J Clin Oncol 2015, 33, 3750–3758. [Google Scholar] [CrossRef] [PubMed]
- Assaf C, Waser N, Bagot M, He M, Li T, Dalal M; et al. Contemporary treatment patterns and response in relapsed/refractory cutaneous T-cell lymphoma (CTCL) across five European countries. Cancers 2021, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- International Society for Pharmacoepidemiology (ISPE). Guidelines for Good Pharmacoepidemiology Practices (GPP). 2015. Available at: https://www.pharmacoepi.org/resources/policies/guidelines-08027/ [Accessed 17 January 2023].
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med 2007, 147, 573–577. [Google Scholar] [CrossRef]
- Bagot M, Illidge T, Waser NA, He M, Li T, Sambrook R; et al. Survival among a patient cohort of relapsed/refractory mycosis fungoides in France, Germany, Italy, Spain and the United Kingdom. Hematol Oncol 2019, 37, 485–486. [Google Scholar] [CrossRef]
- Hughes CF, Khot A, McCormack C, Lade S, Westerman DA, Twigger R; et al. Lack of durable disease control with chemotherapy for mycosis fungoides and Sézary syndrome: A comparative study of systemic therapy. Blood 2015, 125, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Kim YH, Bagot M, Pinter-Brown L, Rook AH, Porcu P, Horwitz SM; et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): An international, open-label, randomised, controlled phase 3 trial. Lancet Oncol 2018, 19, 1192–1204. [Google Scholar] [CrossRef]
- Watanabe R, Teague JE, Fisher DC, Kupper TS, Clark RA. Alemtuzumab therapy for leukemic cutaneous T-cell lymphoma: Diffuse erythema as a positive predictor of complete remission. JAMA Dermatol 2014, 150, 776–779. [Google Scholar] [CrossRef]
- Duarte RF, Boumendil A, Onida F, Gabriel I, Arranz R, Arcese W; et al. Long-term outcome of allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sézary syndrome: A European society for blood and marrow transplantation lymphoma working party extended analysis. J Clin Oncol 2014, 32, 3347–3348. [Google Scholar] [CrossRef]
- Lechowicz MJ, Lazarus HM, Carreras J, Laport GG, Cutler CS, Wiernik PH; et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant 2014, 49, 1360–1365. [Google Scholar] [CrossRef]
- Horwitz SM, Scarisbrick JJ, Dummer R, Whittaker S, Duvic M, Kim YH; et al. Randomized phase 3 ALCANZA study of brentuximab vedotin vs physician's choice in cutaneous T-cell lymphoma: Final data. Blood Adv 2021, 5, 5098–5106. [Google Scholar] [CrossRef] [PubMed]
- Dai J, Duvic M. Cutaneous T-Cell Lymphoma: Current and Emerging Therapies. Oncology (Williston Park) 2023, 37, 55–62. [Google Scholar]
- Willemze R, Hodak E, Zinzani PL, Specht L, Ladetto M. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018, 29, iv30–iv40. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). A list of all our products on blood and bone marrow cancers. 2022. Available at: https://www.nice.org.uk/guidance/conditions-and-diseases/blood-and-immune-system-conditions/blood-and-bone-marrow-cancers/products?ProductType=Guidance&Status=Published (accessed on 17 January 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).