Submitted:
19 October 2023
Posted:
20 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
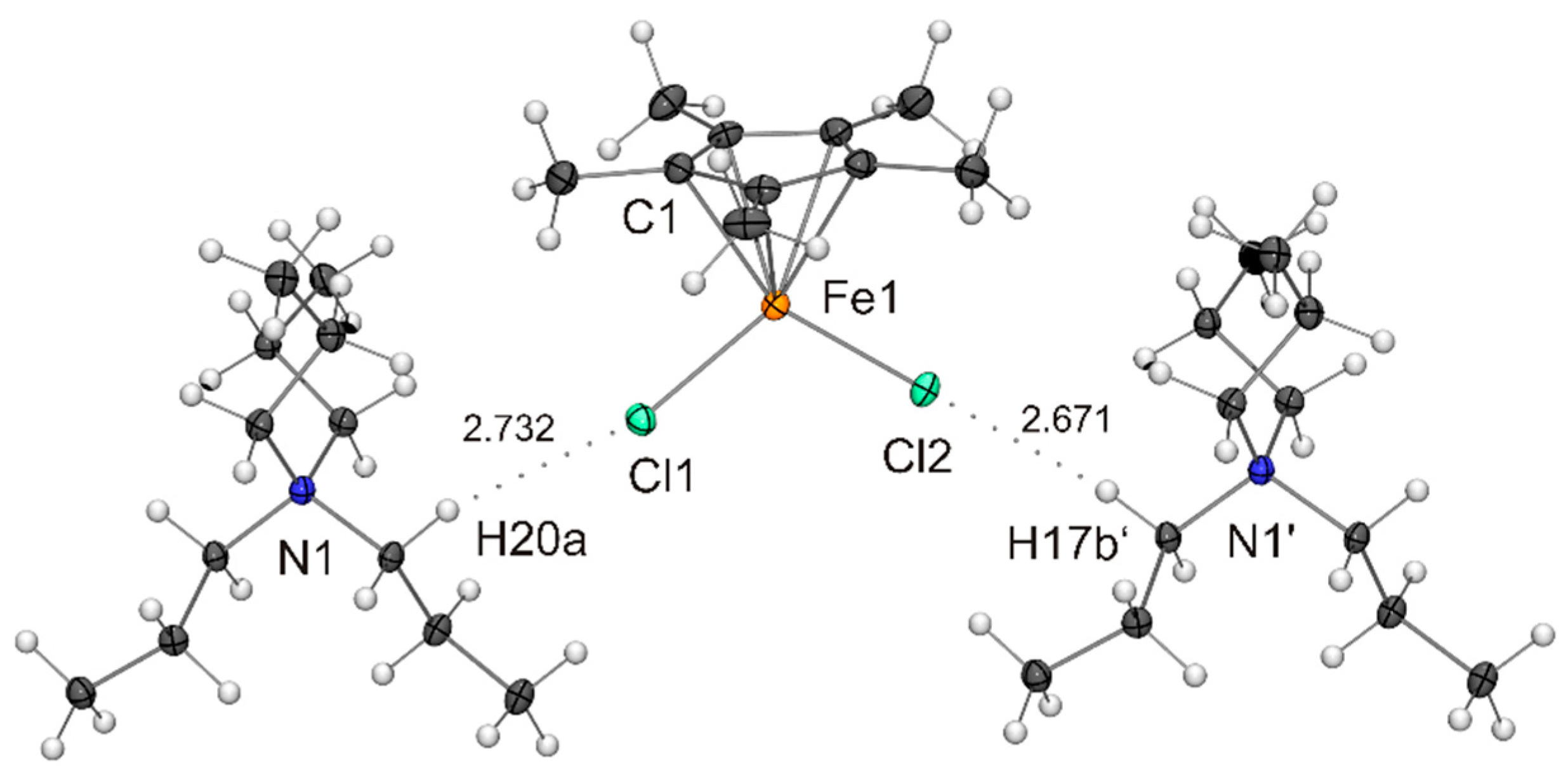
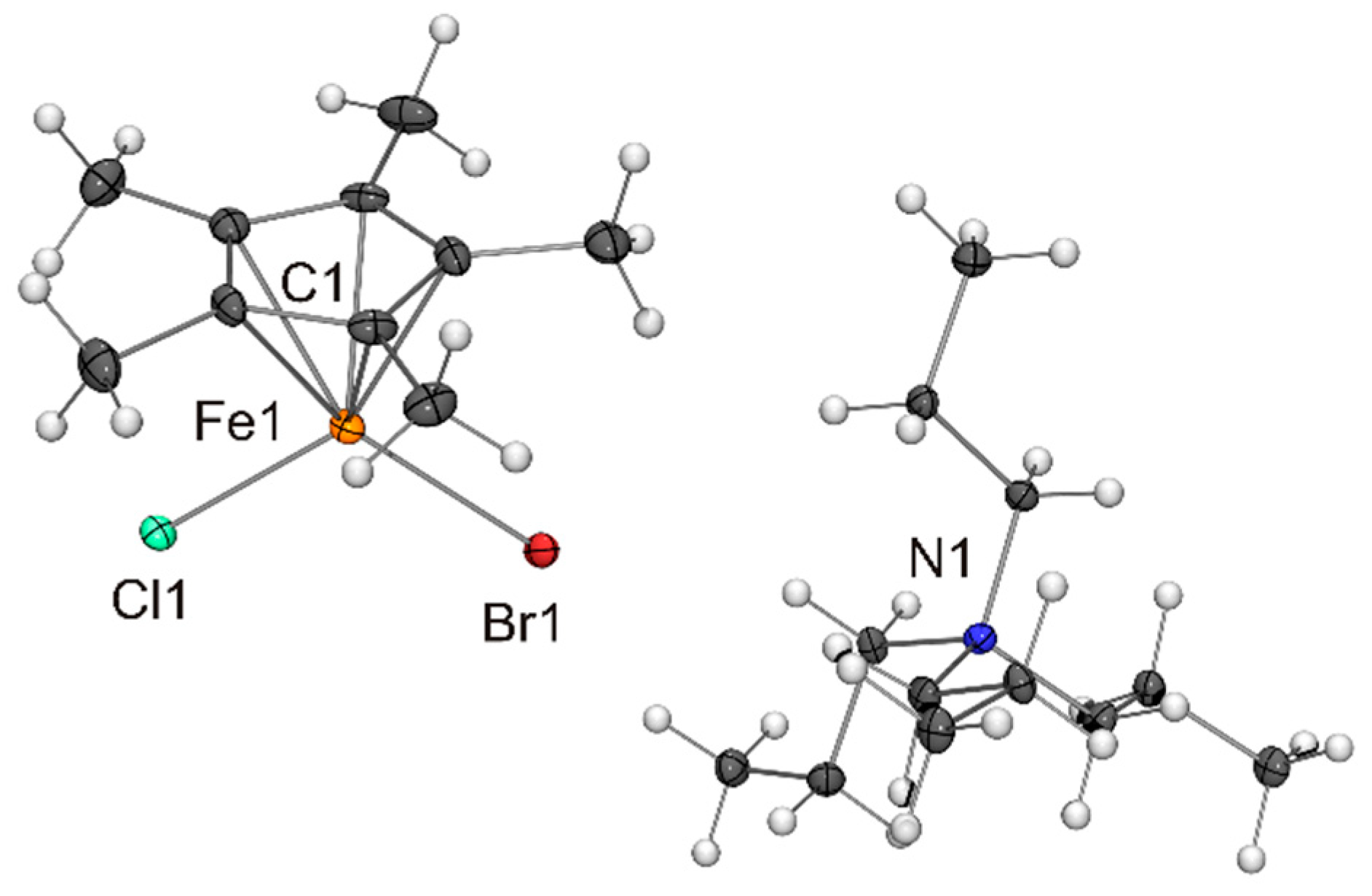
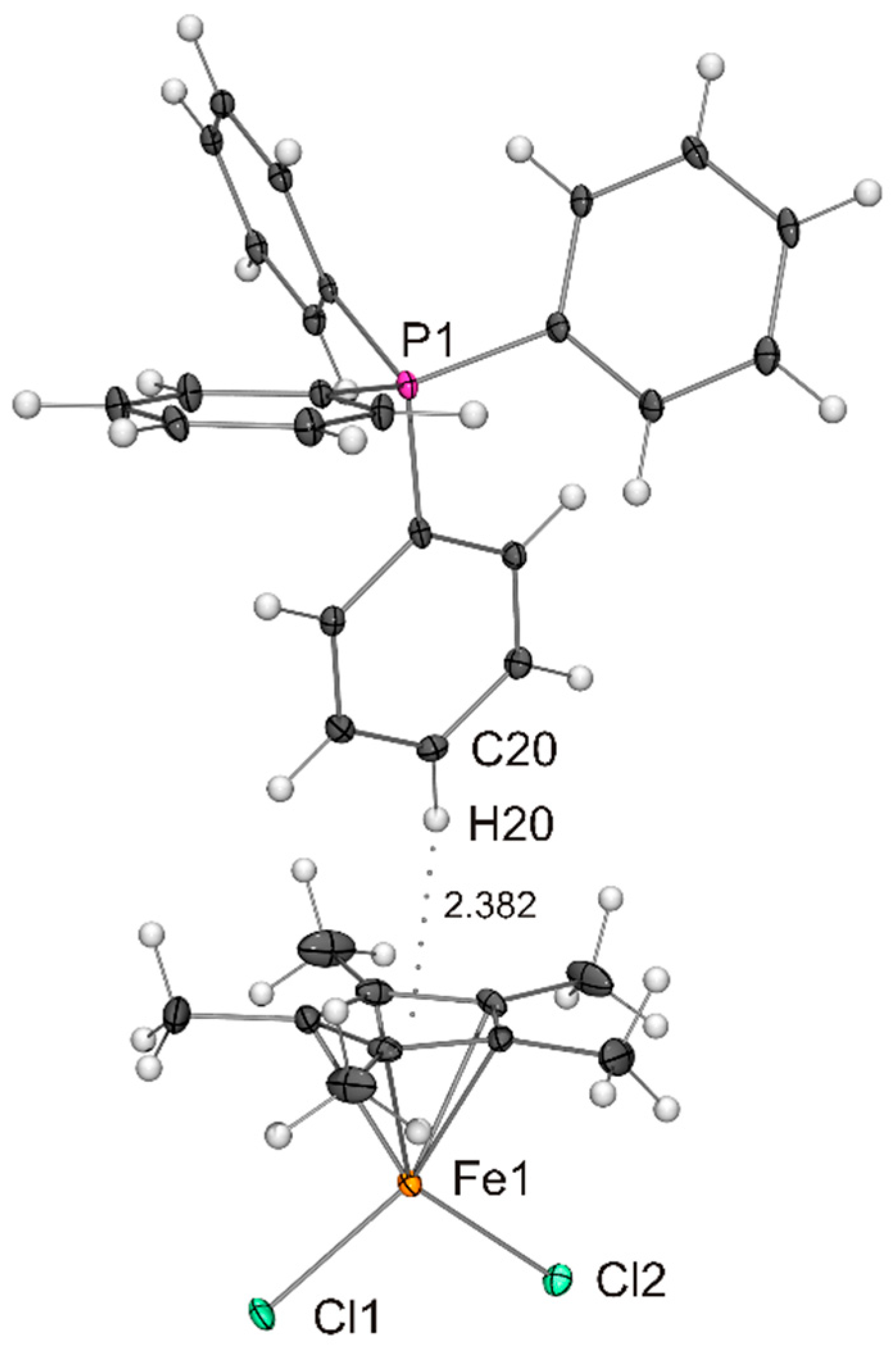
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Münster, K.; Walter, M.D. Monocyclopentadienyl and Other Half-Sandwich Complexes of Iron. In Comprehensive Organometallic Chemistry, 4th ed.; Parkin, G., Meyer, K., O’Hare, D., Eds.; Elsevier: Kidlington, UK, 2022; Volume 7, pp. 46–184. [Google Scholar] [CrossRef]
- Kölle, U.; Fuss, B.; Khouzami, F.; Gersdorf, J. Pentamethylcyclopentadienyl-Übergangsmetall-Komplexe: X. Halbsandwichkomplexe des Fe und Ni als reaktive Zwischenprodukte. J. Organomet. Chem. 1985, 290, 77–83. [Google Scholar] [CrossRef]
- Sun, R.; Deng, W.-H.; Yu, B.; Lu, Y.; Zhai, X.; Liao, R.-Z.; Tung, C.-H.; Wang, W. Hydroboration of the (C5Me5)Fe(1,2-Ph2PC6H4) System To Derive Hydridoborate and Hydridosilicate Complexes. Organometallics 2022, 41, 2504–2512. [Google Scholar] [CrossRef]
- Sun, T.; Xu, S.; Yang, D.; Su, L.; Wang, B.; Qu, J. Catalytic Disproportionation of Hydrazine Promoted by Biomimetic Diiron Complexes with Benzene-1,2-Dithiolate Bridge Modified by Different Substituents. Eur. J. Inorg. Chem. 2020, 4263–4269. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Yang, D.; Li, Y.; Sun, P.; Wang, B.; Qu, J. Synthesis and Reactivity of Thioether-Dithiolate-Bridged Multi-iron Complexes. Organometallics 2015, 34, 1661–1667. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Wang, B.; Luo, Y.; Yang, D.; Tong, P.; Zhao, J.; Luo, L.; Zhou, Y.; Chen, S.; Cheng, F.; Qu, J. Ammonia formation by a thiolate-bridged diiron amide complex as a nitrogenase mimic. Nat. Chem. 2013, 5, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, S.; Ogura, S.-i.; Yo, H.; Hosokoshi, Y.; Kamikawa, K.; Matsuzaka, H. Diiron Amido−Imido Complex [(Cp*Fe)2(μ2-NHPh)(μ2-NPh)]: Synthesis and a Net Hydrogen Atom Abstraction Reaction To Form a Bis(imido) Complex. Inorg. Chem. 2006, 45, 4871–4873. [Google Scholar] [CrossRef] [PubMed]
- Shintani,R. ; Fu, G.C. Copper-Catalyzed Enantioselective Conjugate Addition of Diethylzinc to Acyclic Enones in the Presence of Planar-Chiral Phosphaferrocene-Oxazoline Ligands. Org. Lett. 2002, 4, 3699–3702. [Google Scholar] [CrossRef]
- Hettrich, R.; Kaschke, M.; Wadepohl, H.; Weinmann, W.; Stephan, M.; Pritzkow, H.; Siebert, W.; Hyla-Kryspin, I.; Gleiter, R. Electron-poor 2,3-Dihydro-1,3-diborolyl Complexes of Iron and Ruthenium: Synthesis, Reactivity, and Crystal and Electronic Structures of an Iron Sandwich Complex. Chem. Eur. J. 1996, 2, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Stephan, M.; Müller, P.; Zenneck, U.; Pritzkow, H.; Siebert, W.; Grimes, R.N. Organotransition-Metal Metallacarboranes. 37. Paramagnetic Iron−Cobalt and Dicobalt Triple-Decker Sandwich Complexes. Inorg. Chem. 1995, 34, 2058–2067. [Google Scholar] [CrossRef]
- Jonas, K.; Klusmann, P.; Goddard, R. Pentamethylcyclopentadienylbis(ethen)eisen − ein 17e-Halbsandwichkomplex mit leicht verdrängbaren Ethenliganden, Z. Naturforsch. B 1995, 50, 394–404. [Google Scholar] [CrossRef]
- Schneider, J.J.; Spickermann, D.; Lehmann, C.W.; Magull, J.; Krüger, H.-J.; Ensling, J.; Gütlich, P. Decacyclene as Complexation Manifold: Synthesis, Structure and Properties of Its Fe2 and Fe4 Slipped Triple-Decker Complexes. Chem. Eur. J. 2006, 12, 1427–1435. [Google Scholar] [CrossRef]
- Groß, O.A.; Lauk, S.; Müller, C.; Gidt, W.; Sun, Y.; Demeshko, S.; Meyer, F.; Sitzmann, H. Iron(II) High-Spin and Low-Spin Complexes from Pentaisopropylcyclopentadienyliron(II) Bis(trimethylsilyl)amide. Eur. J. Inorg. Chem. 2017, 3635–3643. [Google Scholar] [CrossRef]
- Liang, Q.; Song, D. Syntheses and Reactivity of Piano-Stool Iron Complexes of Picolyl-Functionalized N-Heterocyclic Carbene Ligands. Organometallics 2021, 40, 3943–3951. [Google Scholar] [CrossRef]
- Takahashi, H.; Watanabe, T.; Tobita, H. Bifunctional Iron-Amino Complexes: Highly Efficient Catalysts for Dehydrogenation of Ammonia-Borane. Chem. Lett. 2018, 47, 296–299. [Google Scholar] [CrossRef]
- Liang, Q.; Osten, K.M.; Song, D. Iron-Catalyzed gem-Specific Dimerization of Terminal Alkynes. Angew. Chem. Int. Ed. 2017, 56, 6317–6320. [Google Scholar] [CrossRef]
- Ohki, Y.; Hatanaka, T.; Tatsumi, K. C−H Bond Activation of Heteroarenes Mediated by a Half-Sandwich Iron Complex of N-Heterocyclic Carbene. J. Am. Chem. Soc. 2008, 130, 17174–17186. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Suárez, A.; Nelson, D.J.; Nolan, S.P. Quantifying and understanding the steric properties of N-heterocyclic carbenes. Chem. Commun. 2017, 53, 2650–2660. [Google Scholar] [CrossRef] [PubMed]
- Clavier, H.; Nolan, S.P. Percent buried volume for phosphine and N-heterocyclic carbene ligands: steric properties in organometallic chemistry. Chem. Commun. 2010, 46, 841–861. [Google Scholar] [CrossRef] [PubMed]
- Lauk, S.; Schäfer, A. Pentaisopropyl Cyclopentadienyl: An Overview across the Periodic Table. Eur. J. Inorg. Chem. 2021, 5026–5036. [Google Scholar] [CrossRef]
- Field, L.D.; Lindall, C.M.; Masters, A.F.; Clentsmith, G.K.B. Penta-arylcyclopentadienyl complexes, Coord. Chem. Rev. 2011, 255, 1733–1790. [Google Scholar] [CrossRef]
- Janiak, C.; Schumann, H. Bulky or Supracyclopentadienyl Derivatives in Organometallic Chemistry. Adv. Organomet. Chem. 1991, 33, 291–393. [Google Scholar] [CrossRef]
- Bauer, H.; Weismann, D.; Wolmershäuser, G.; Sun, Y.; Sitzmann, H. Iron-Mediated Coupling of Two Ethyl Anions to Form a 2-Butyne Ligand. Eur. J. Inorg. Chem. 2014, 3072–3084. [Google Scholar] [CrossRef]
- Wallasch, M.; Wolmershäuser, G.; Sitzmann, H. Phenolate Complexes of Iron(II) in Different Spin States. Angew. Chem. Int. Ed. 2005, 44, 2597–2599. [Google Scholar] [CrossRef]
- Walter, M.D.; White, P.S. [Cp′FeI]2 as convenient entry into iron-modified pincer complexes: bimetallic η6,κ1-POCOP-pincer iron iridium compounds. New J. Chem. 2011, 35, 1842–1854. [Google Scholar] [CrossRef]
- Chakraborty, U.; Modl, M.; Mühldorf, B.; Bodensteiner, M.; Demeshko, S.; van Velzen, N.J.C.; Scheer, M.; Harder, S.; Wolf, R. Pentaarylcyclopentadienyl Iron, Cobalt, and Nickel Halides. Inorg. Chem. 2016, 55, 3065–3074. [Google Scholar] [CrossRef]
- Reiners, M.; Maekawa, M.; Baabe, D.; Zaretzke, M.-K.; Schweyen, P.; Daniliuc, C.G.; Freytag, M.; Raeder, J.; Hohenberger, J.; Sutter, J.; Meyer, K.; Walter, M.D. Monomeric Fe(III) half-sandwich complexes [Cp′FeX2] – synthesis, properties and electronic structure. Dalton Trans. 2018, 47, 10517–10526. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Chilton, N.F.; Kumar, A.; Colebatch, A.L.; Whittell, G.R.; Sparkes, H.A.; Weller, A.S.; Manners, I. Iron Precatalysts with Bulky Tri(tert-butyl)cyclopentadienyl Ligands for the Dehydrocoupling of Dimethylamine-Borane. Chem. Eur. J. 2018, 24, 14127–14136. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, G.Y.; Wallasch, M.W.; Saurenz, D.; Eger, T.R.; Bauer, H.; Wollmershäuser, G.; Prosenc, M.H.; Sitzmann, H. Benzylidyne Bridges from Diphenylacetylene and a Methylidyne Bridge from Methylmagnesium Chloride. Organometallics 2015, 34, 644–652. [Google Scholar] [CrossRef]
- Malischewski, M.; Seppelt, K.; Sutter, J.; Munz, D.; Meyer, K. A Ferrocene-Based Dicationic Iron(IV) Carbonyl Complex. Angew. Chem. Int. Ed. 2018, 57, 14597–14601. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Giansiracusa, M.J.; Greer, S.M.; Nicholas, H.M.; Evans, P.; Vonci, M.; Hill, S.; Chilton, N.F.; Mills, D.P. Isolation and electronic structures of derivatized manganocene, ferrocene and cobaltocene anions. Nat. Chem. 2021, 13, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Siemeling, U.; Vorfeld, U.; Neumann, B.; Stammler, H.-G. Bis(trimethylsilyl)amido](η5-pentamethylcyclopentadienyl)-iron(II): A Diamagnetic 14-Electron Complex with a “Pogo-Stick” Structure. Organometallics 1998, 17, 483–484. [Google Scholar] [CrossRef]
- Pauling, L.; Huggins, M.L. Covalent Radii of Atoms and Interatomic Distances in Crystals containing Electron-Pair Bonds. Z. Kristallogr. 1934, 87, 205–238. [Google Scholar] [CrossRef]
- van den Berg, J.-A.; Seddon, K.R. Critical Evaluation of C−H···X Hydrogen Bonding in the Crystalline State. Cryst. Growth Des. 2003, 3, 643–661. [Google Scholar] [CrossRef]
- Thallapally, P.K.; Nangia, A. A Cambridge Structural Database analysis of the C–H⋯Cl interaction: C–H⋯Cl− and C–H⋯Cl–M often behave as hydrogen bonds but C–H⋯Cl–C is generally a van der Waals interaction. CrystEngComm 2001, 3, 114–119. [Google Scholar] [CrossRef]
- Hwang, J.w.; Li, P.; Shimizu, K.D. Synergy between experimental and computational studies of aromatic stacking interactions. Org. Biomol. Chem. 2017, 15, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.A.; Castellano, R.K.; Diederich, F. Interactions with Aromatic Rings in Chemical and Biological Recognition. Angew. Chem. Int. Ed. 2003, 42, 1210–1250. [Google Scholar] [CrossRef] [PubMed]
- Tummanapelli, A.K.; Vasudevan, S. Response to “Comment on ‘Communication: Benzene dimer—The free energy landscape’”. J. Chem. Phys. 2014, 140, 227102. [Google Scholar] [CrossRef]
- van der Avoird, A.; Podeszwa, R.; Ensing, B.; Szalewicz, K. Comment on “Communication: Benzene dimer—The free energy landscape”. J. Chem. Phys. 2014, 140, 227101. [Google Scholar] [CrossRef]
- Tummanapelli, A.K.; Vasudevan, S. Benzene dimer—The free energy landscape. J. Chem. Phys. 2013, 139, 201102. [Google Scholar] [CrossRef]
- Schnell, M.; Erlekam, U.; Bunker, P.R.; von Helden, G.; Grabow, J.-U.; Meijer, G.; van der Avoird, A. Structure of the Benzene Dimer—Governed by Dynamics. Angew. Chem. Int. Ed. 2013, 52, 5180–5183. [Google Scholar] [CrossRef]
- Sinnokrot, M.O.; Sherrill, C.D. High-Accuracy Quantum Mechanical Studies of π−π Interactions in Benzene Dimers. J. Phys. Chem. A 2006, 110, 10656–10668. [Google Scholar] [CrossRef] [PubMed]
- Podeszwa, R.; Bukowski, R.; Szalewicz, K. Potential Energy Surface for the Benzene Dimer and Perturbational Analysis of π−π Interactions. J. Phys. Chem. A 2006, 110, 10345–10354. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.K.; Venkatnarayan, R. Substituents’ influence on the C–H···π interaction in the T-shaped benzene dimer. Theor. Chem. Acc. 2018, 137, 72. [Google Scholar] [CrossRef]
- Jutzi, P.; Mix, A. Oxidative Addition von Halogenpentamethylcyclopentadienen an Metallcarbonyle: ein einfaches Verfahren zur Synthese von (η5-Pentamethylcyclopentadienyl)metallhalogeniden. Chem. Ber. 1990, 123, 1043–1045. [Google Scholar] [CrossRef]
- Clegg, W.; Compton, N.A.; Errington, R.J.; Norman, N.C. Synthesis and Structural Characterisation of some Cyclopentadienyliron–Bismuth Complexes. J. Chem. Soc. Dalton Trans. 1988, 1671–1678. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
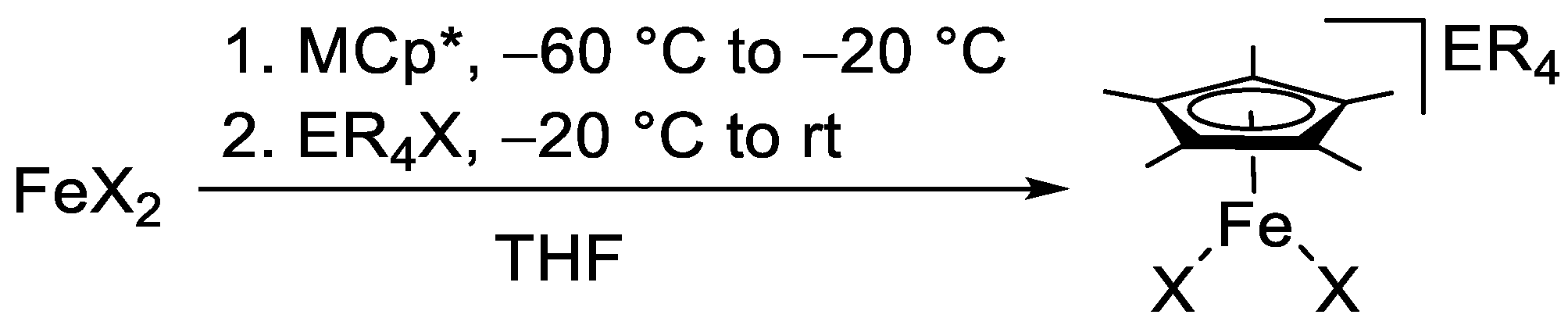
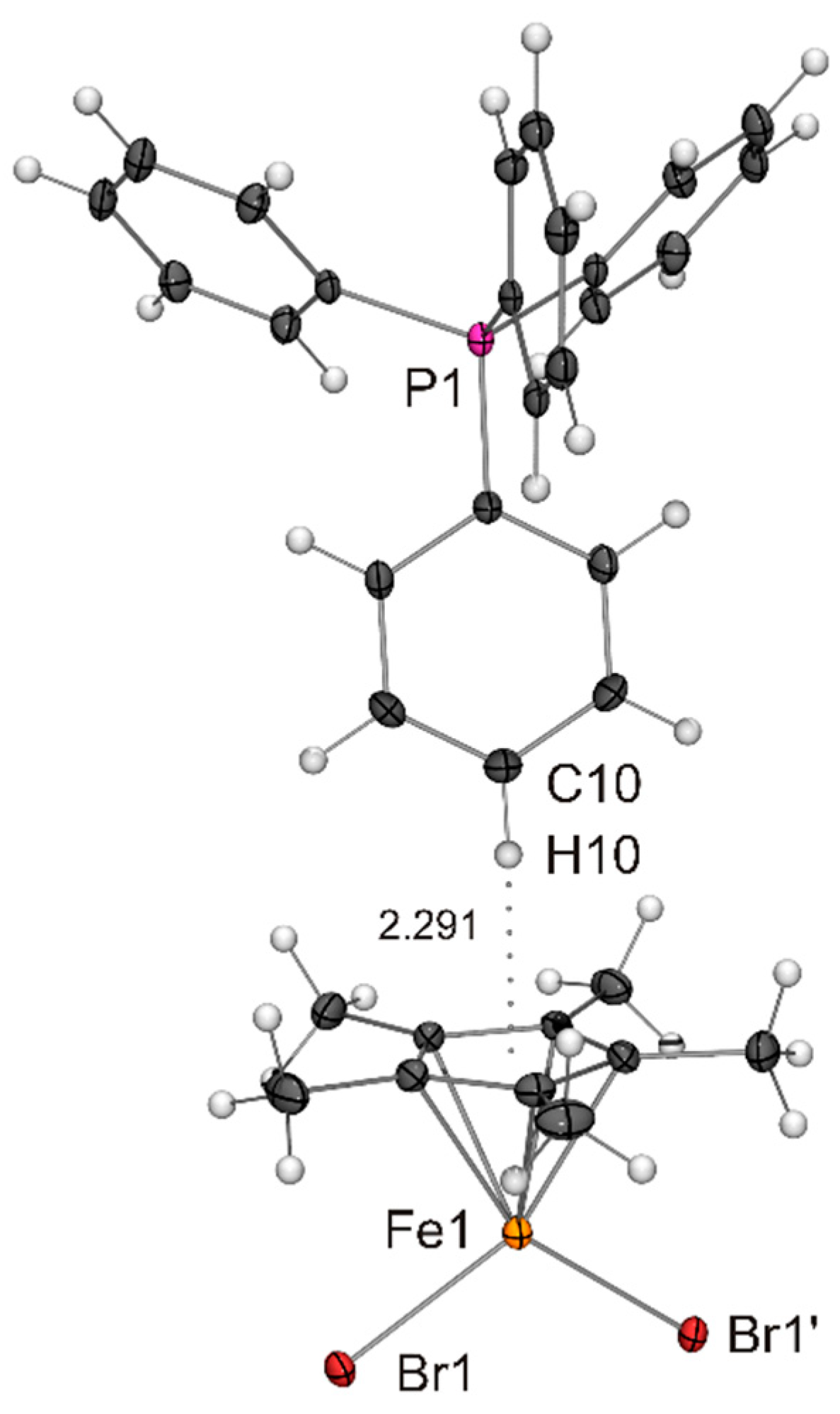
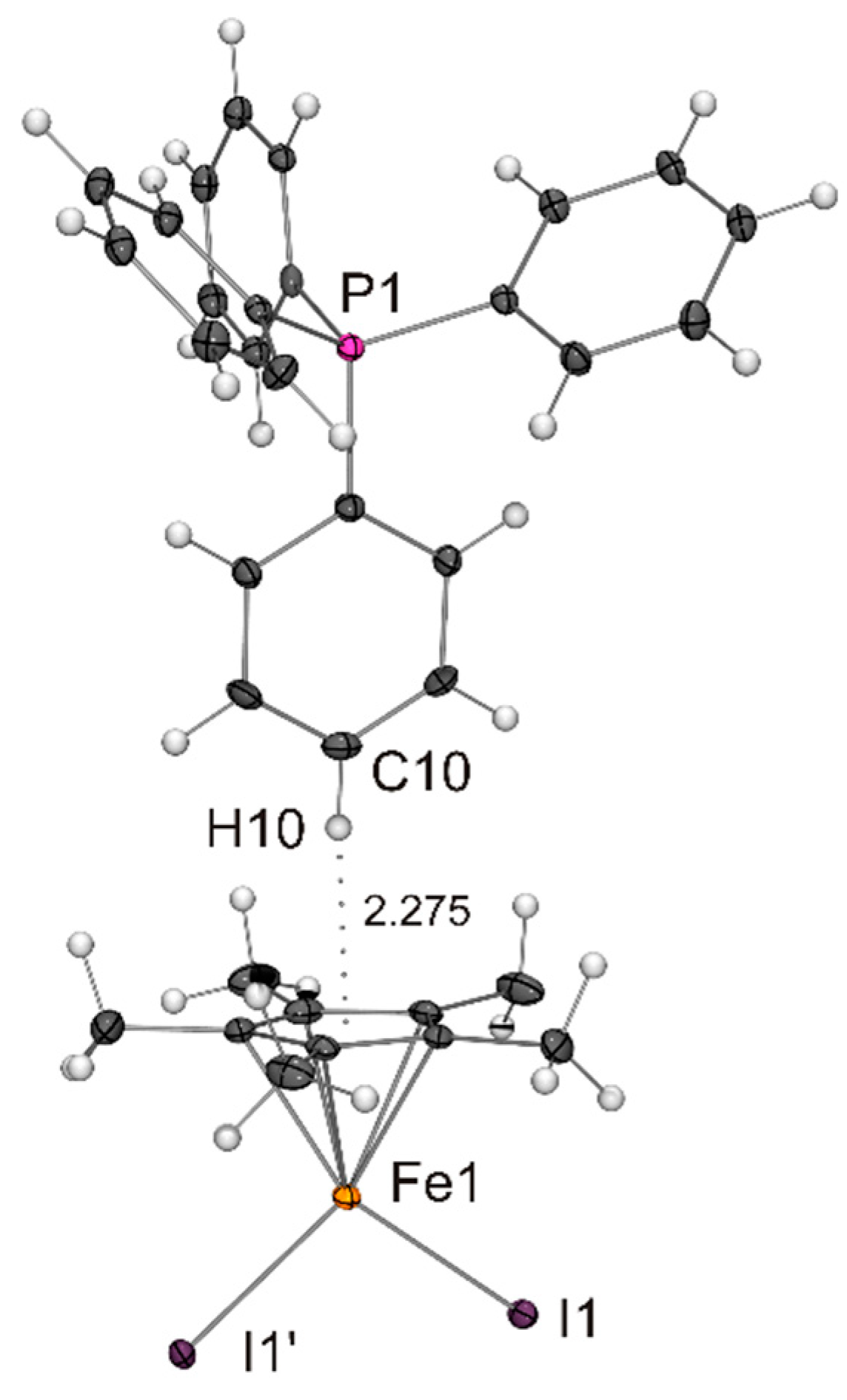
| Fe–Cp*centroid | Fe–X | X–Fe–X | |
|---|---|---|---|
| NnPr4[Fe(η5-Cp*)Cl2] | 1.975 | 2.2953(8) 2.2814(8) |
106.27(3) |
| NnPr4[Fe(η5-Cp*)BrCl] 1 | 1.970 | 2.27(2) 2 2.357(9) 3 |
109.0(6) |
| NnPr4[Fe(η5-Cp*)Br2] 4, 5 | 1.958 1.961 1.999 1.995 |
2.432(5) 2.406(5) 2.404(5) 2.446(5) 2.415(5) 2.431(5) 2.405(5) 2.415(5) |
103.8(2) 103.8(2) 104.8(2 103.6(2) |
| PPh4[Fe(η5-Cp*)Cl2] | 1.988 | 2.288(2) 2.284(2) |
107.07(7) |
| PPh4[Fe(η5-Cp*)Br2] | 1.972 | 2.4278(8) | 107.86(5) |
| PPh4[Fe(η5-Cp*)I2] | 1.958 | 2.6201(5) | 106.56(3) |
| NnBu4[Fe(η5-C5H2-1,2,4-tBu3)I2] 6 | 1.989 | 2.7003(6) 2.6144(6) |
102.20(2) |
| [Na(DME)2][Fe(η5-C5H2-1,2,4-tBu3)Br2] 7 | 1.967 | 2.4633(7) 2.4316(7) |
102.36(2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





