Submitted:
20 October 2023
Posted:
20 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animal, Experimental Design, diet, and Management
2.2. Body weight and feed intake
2.3. Blood collection and analyses
2.4. Collection and measurements of fecal samples
2.5. Statistical analysis
3. Results
3.1. Growth performance
3.2. Apparent nutrient digestibility
3.3. Growth-related hormones
3.4. Serum immunoglobulin and cytokine
3.5. Gut microbiota community
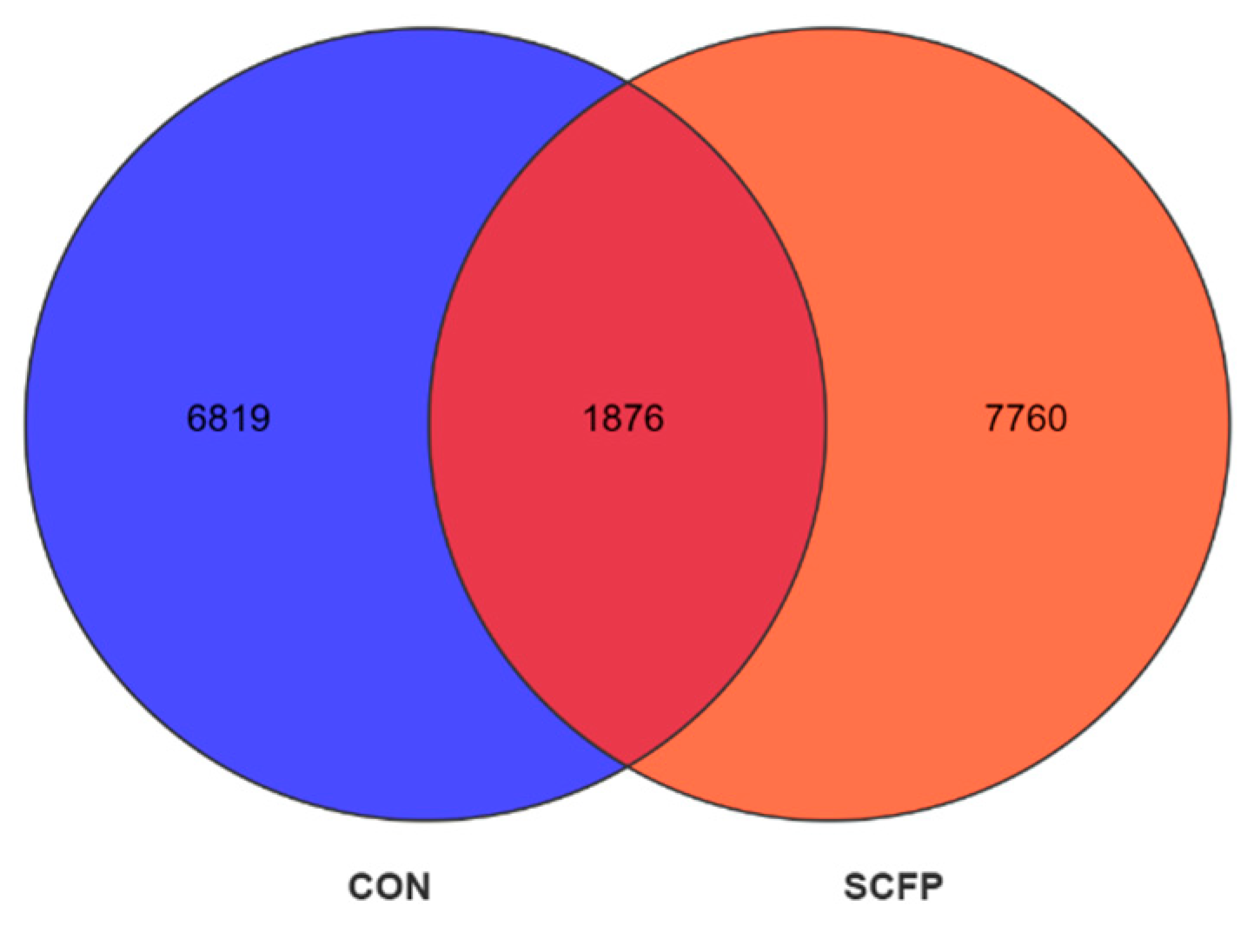
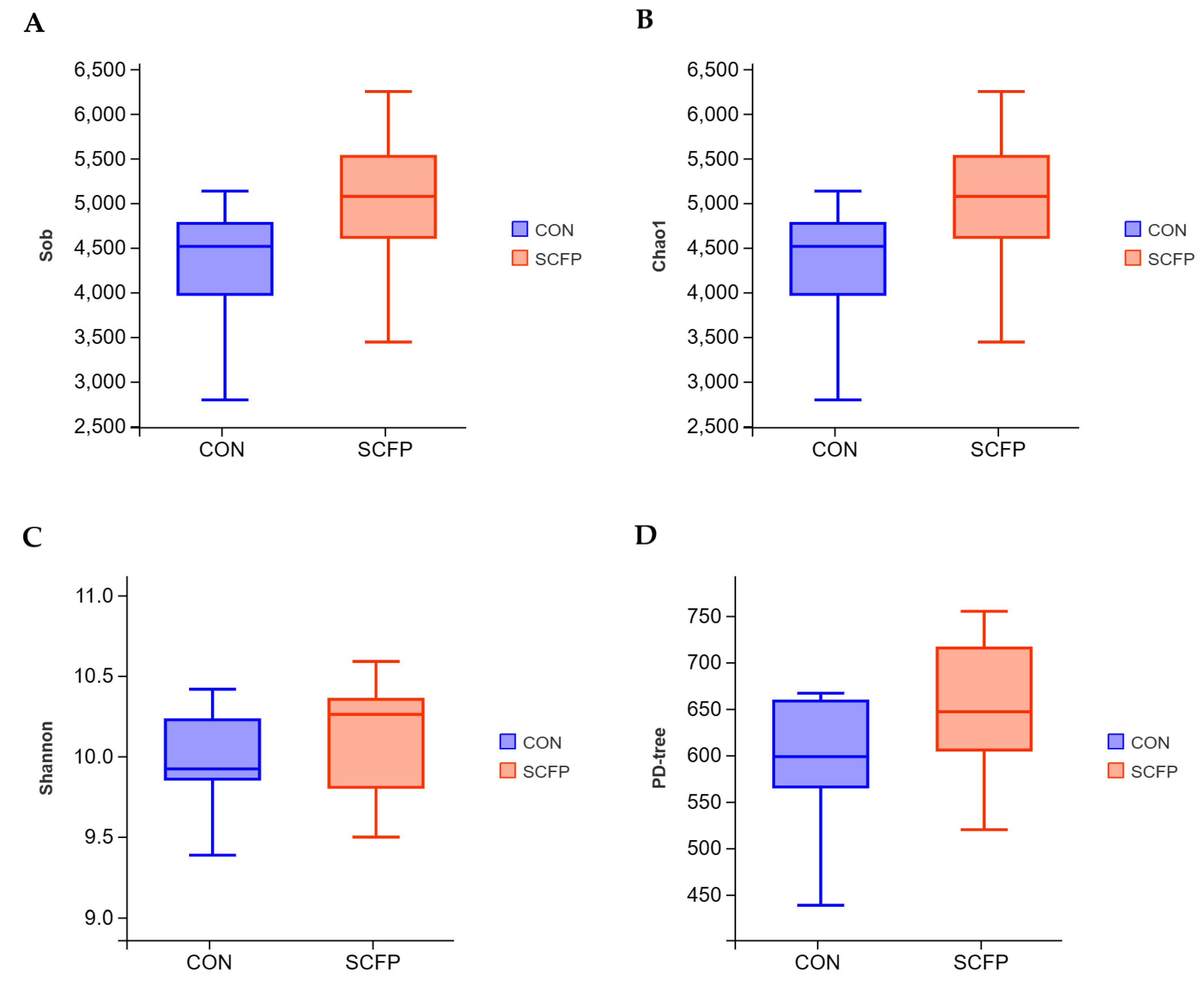
3.5.1. OTU Statistics
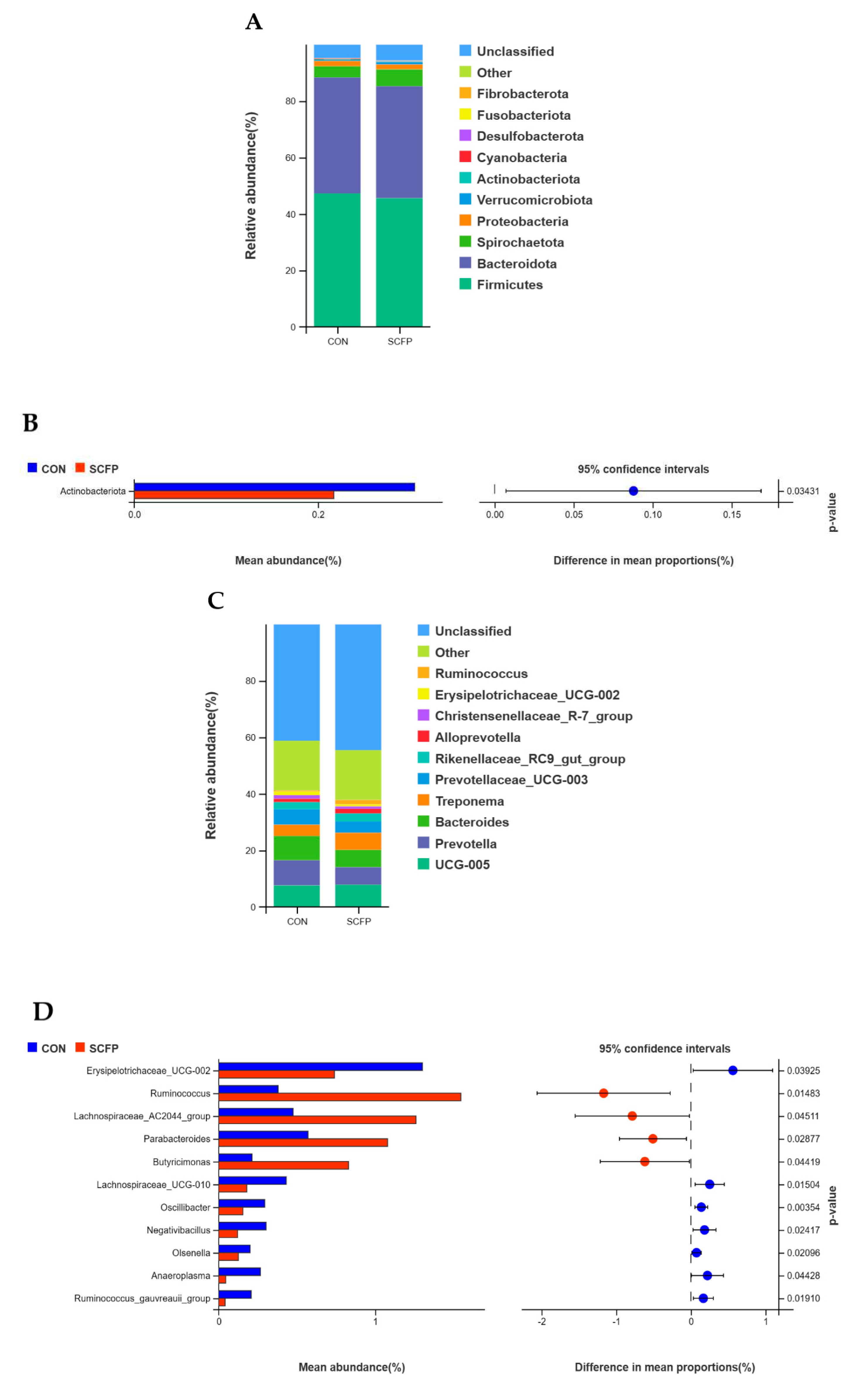
3.5.2. Species composition analysis
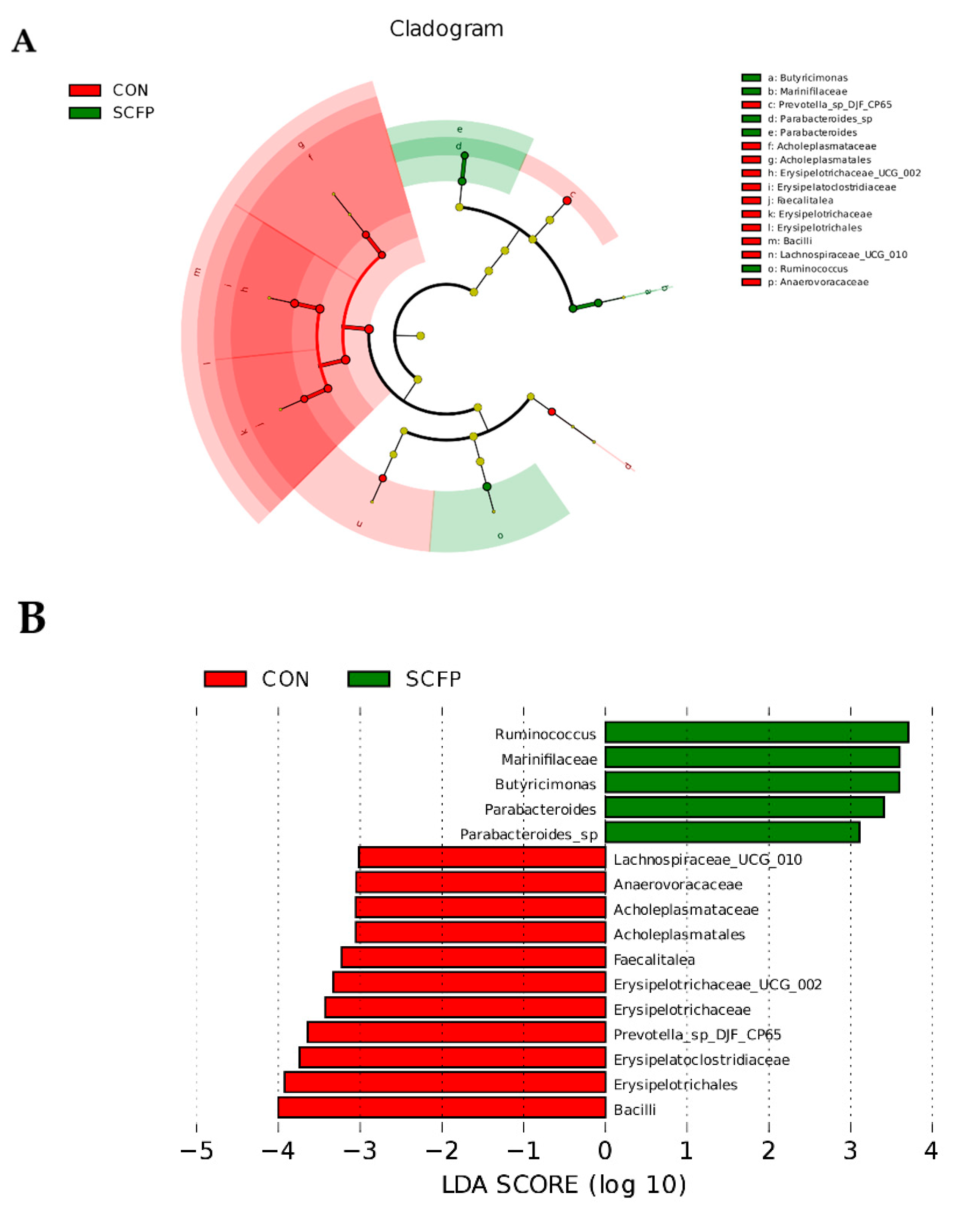
3.5.3. LEfSe analysis of gut microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim HS.; Whon TW.; Sung H..; Jeong YS.; Jung.; ES.; Shin NR.; et al. Longitudinal evaluation of fecal microbiota transplantation for ameliorating calf diarrhea and improving growth performance. Nat Commun. 2021, 12, 161.
- Chuang, S.T.; Chen, C.T.; Hsieh, J.C.; Li, K.Y.; Ho, S.T.; Chen, M.J. Development of Next-Generation Probiotics by Investigating the Interrelationships between Gastrointestinal Microbiota and Diarrhea in Preruminant Holstein Calves. Animals. 2022, 12, 695. [Google Scholar] [CrossRef] [PubMed]
- Malmuthuge, N.; Guan, L.L. Understanding the gut microbiome of dairy calves: Opportunities to improve early-life gut health. J. Dairy Sci. 2017, 100, 5996–6005. [Google Scholar] [CrossRef]
- Arshad, M.A.; Hassan, F.U.; Rehman, M.S.; Huws, S.A.; Cheng, Y.; Din, A.U. Gut microbiome colonization and development in neonatal ruminants: Strategies, prospects, and opportunities. Anim. Nutr. 2021, 7, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.; Seifert, J. Dynamic progression of the calf’s microbiome and its influence on host health. Comput Struct Biotechnol J. 2021, 19, 989–1001. [Google Scholar] [CrossRef]
- Du, Y.; Gao, Y.; Hu, M.; Hou, J.; Yang, L.; Wang, X.; Du, W.; Liu, J.; Xu, Q. Colonization and development of the gut microbiome in calves. J Anim Sci Biotechnol. 2023, 14, 46. [Google Scholar] [CrossRef]
- Yan, X.; Si, H.; Zhu, Y.; Li, S.; Han, Y.; Liu, H.; Du, R.; Pope, P.B.; Qiu, Q.; Li, Z. Integrated multi-omics of the gastrointestinal microbiome and ruminant host reveals metabolic adaptation underlying early life development. Microbiome. 2022, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]
- Welch, C.B.; Ryman, V.E.; Pringle, T.D.; Lourenco, J.M. Utilizing the Gastrointestinal Microbiota to Modulate Cattle Health through the Microbiome-Gut-Organ Axes. Microorganisms. 2022, 10, 1391. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Griebel, P.J.; Guan, L L. Malmuthuge, N.; Griebel, P.J.; Guan, L L. The Gut Microbiome and Its Potential Role in the Development and Function of Newborn Calf Gastrointestinal Tract. Front Vet Sci. 2015, 2, 36.
- Angelakis, E. Weight gain by gut microbiota manipulation in productive animals. Microb Pathog. 2017, 106, 162–170. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; D Lieber, A.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; Schweizer, W.; Zheng, X.; Contreras, M.; Dominguez-Bello, M.G.; Blaser, M.J. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Olaniyan, O.T.; Dash, R.; Varma, A. Roles of Beneficial Microorganisms for the Effective Production of Commercial Animal Feed. Animal Manure. 2022, 64, 285–296. [Google Scholar]
- Pang, Y.; Zhang, H.; Wen, H.; Wan, H.; Wu, H.; Chen, Y.; Li, S.; Zhang, L.; Sun, X.; Li, B.; Liu, X. Yeast Probiotic and Yeast Products in Enhancing Livestock Feeds Utilization and Performance: An Overview. J Fungi. 2022, 8, 1191. [Google Scholar] [CrossRef] [PubMed]
- Klopp, R.N.; Centeno-Martinez, R.E.; Yoon, I.; Johnson, T.A.; Boerman, J.P. Effects of feeding Saccharomyces cerevisiae fermentation products on the health and growth performance of Holstein dairy calves. JDS Commun. 2022, 3, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Klopp, R.N.; Yoon, I.; Eicher, S.; Boerman, J.P. Effects of feeding Saccharomyces cerevisiae fermentation products on the health of Holstein dairy calves following a lipopolysaccharide challenge. J. Dairy Sci. 2022, 105, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Deters, E.L.; Stokes, R.S.; Genther-Schroeder, O.N.; Hansen, S.L. Effects of a Saccharomyces cerevisiae fermentation product in receiving diets of newly weaned beef steers. II. Digestibility and response to a vaccination challenge1. J Anim Sci 2018, 96, 3906–3915. [Google Scholar] [CrossRef]
- Alugongo, G.M.; Xiao, J.X.; Chung, Y.H.; Dong, S.Z.; Li, S.L.; Yoon, I.; Wu, Z.H.; Cao, Z.J. Effects of Saccharomyces cerevisiae fermentation products on dairy calves: Performance and health. J. Dairy Sci. 2017, 100, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Centeno-Martinez, R.E.; Dong, W.; Klopp, R.N.; Yoon, I.; Boerman, J.P.; Johnson, T.A. Effects of feeding Saccharomyces cerevisiae fermentation postbiotic on the fecal microbial community of Holstein dairy calves. Anim Microbiome. 2023, 5, 13. [Google Scholar] [CrossRef]
- Ji, H.; Tan, D.; Chen, Y.; Cheng, Z.; Zhao, J.; Lin, M. Effects of different manganese sources on nutrient digestibility.; fecal bacterial community.; and mineral excretion of weaning dairy calves. Front Microbiol. 2023, 14, 1163468. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, J.; Mir, L.; Seradj, A.R.; Morazán, H.; Balcells, J.; Babot, D. Nutritional strategies to cope with reduced litter weight gain and total tract digestibility in lactating sows. J. Anim. Physiol. Anim. Nutr. 2017, 101, 914–924. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016, 13, 581–3. [Google Scholar] [CrossRef] [PubMed]
- Baker, LM.; Kraft, J.; Karnezos, T.P.; Greenwood, S.L. Review: The effects of dietary yeast and yeast-derived extracts on rumen microbiota and their function. Anim. Feed Sci. Technol. 2022, 294, 115476. [Google Scholar] [CrossRef]
- Desnoyers, M.; Giger-Reverdin, S.; Bertin, G.; Duvaux-Ponter, C. .; Sauvant, D. Meta-analysis of the influence of Saccharomyces cerevisiae supplementation on ruminal parameters and milk production of ruminants. J. Dairy Sci. 2009, 92, 1620–32. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.K.; Heinrichs, A.J. Feeding various forages and live yeast culture on weaned dairy calf intake.; growth.; nutrient digestibility.; and ruminal fermentation. J. Dairy Sci. 2020, 103, 8880–8897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, J.; Yu, Z.; Zhou, G.; Yao, J. Effects of supplementation with Saccharomyces cerevisiae products on dairy calves: A meta-analysis. J. Dairy Sci. 2022, 105, 7386–7398. [Google Scholar] [CrossRef] [PubMed]
- Li, L.P.; Qu, L.; L, T. Supplemental dietary Selenohomolanthionine affects growth and rumen bacterial population of Shaanbei white cashmere wether goats. Front Microbiol. 2022, 13, 942848. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, C.; Niu, J.; Cui, Z.; Zhao, X.; Li, W.; Zhang, Y.; Yang, Y.; Gao, P.; Guo, X.; Li, B.; Kim, S.W.; Cao, G. Impacts of dietary fiber level on growth performance, apparent digestibility, intestinal development, and colonic microbiota and metabolome of pigs. J Anim Sci. 2023, 101, 174. [Google Scholar] [CrossRef] [PubMed]
- D’Ercole, A.J.; Stiles, A.D.; Underwood, LE. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci U S A. 1984, 81, 935–9. [Google Scholar] [CrossRef] [PubMed]
- Thissen, J.P.; Pucilowska, J.B.; Underwood, LE. Differential regulation of insulin-like growth factor I (IGF-I) and IGF binding protein-1 messenger ribonucleic acids by amino acid availability and growth hormone in rat hepatocyte primary culture. Endocrinology. 1994, 134, 1570–6. [Google Scholar] [CrossRef]
- Gosteli-Peter, M.A.; Winterhalter, K.H.; Schmid, C.E.; Froesch, R.; Zapf, J. Expression and regulation of insulin-like growth factor-I (IGF-I) and IGF-binding protein messenger ribonucleic acid levels in tissues of hypophysectomized rats infused with IGF-I and growth hormone. Endocrinology. 1994, 135, 2558–2567. [Google Scholar] [CrossRef]
- Giustina, A.; Mazziotti, G.; Canalis, E. Growth hormone.; insulin-like growth factors.; and the skeleton. Endocrine reviews.; 2008, 29, 535–559.
- Graham, T.W.; Breher, J.E.; Farver, T.B.; Cullor, J.S.; Kehrli, M.E.; Oberbauer.; A.M. Biological markers of neonatal calf performance: the relationship of insulin-like growth factor-I.; zinc.; and copper to poor neonatal growth. J Anim Sci. 2010, 88, 2585-93. [CrossRef]
- Jensen, E.A.; Young, J.A.; Mathes, S.C.; List, E.O.; Carroll, R.K.; Kuhn, J.; Onusko, M.; Kopchick, J.J.; Murphy, E.R.; Berryman, D.E. Crosstalk between the growth hormone/insulin-like growth factor-1 axis and the gut microbiome: A new frontier for microbial endocrinology. Growth Horm IGF Res. 2020, 53-54, 101333. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.A.; Young, J.A.; Jackson, Z.; Busken, J.; List, E.O.; Carroll, R.K.; Kopchick, J.J.; Murphy, E.R.; Berryman, D.E. Growth Hormone Deficiency and Excess Alter the Gut Microbiome in Adult Male Mice. Endocrinology. 2020, 161, 026. [Google Scholar] [CrossRef] [PubMed]
- Burrin, D.G.; Stoll, B.; Guan, X. Glucagon-like peptide 2 function in domestic animals. Domest. Anim. Endocrinol. 2003, 24, 103–22. [Google Scholar] [CrossRef] [PubMed]
- Elsabagh, M.; Inabu, Y.; Obitsu, T.; Sugino, T. Response of plasma glucagon-like peptide-2 to feeding pattern and intraruminal administration of volatile fatty acids in sheep. Domest. Anim. Endocrinol. 2017, 60, 31–41. [Google Scholar] [CrossRef]
- Inabu, Y.; Saegusa, A.; Inouchi, K.; Koike, S.; Oba, M.; Sugino, T. Plasma concentrations of glucagon-like peptide 1 and 2 in calves fed calf starters containing lactose. J. Dairy Sci. 2017, 100, 9361–9371. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Vande Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; Muccioli, G.G.; Delzenne, N.M. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009, 58, 1091–103. [Google Scholar] [CrossRef]
- Kim, E.T.; Lee, H.G.; Kim, D.H.; Son, J.K.; Kim, B.W.; Joo, S.S.; Park, D.S.; Park, Y.J.; Lee, S.Y.; Kim.; M.H. Hydrolyzed Yeast Supplementation in Calf Starter Promotes Innate Immune Responses in Holstein Calves under Weaning Stress Condition. Animals. 2020, 10, 1468.
- Alugongo, G.M.; Xiao, J.; Wu, Z.; Li, S.; Wang, Y.; Cao, Z. Review: Utilization of yeast of Saccharomyces cerevisiae origin in artificially raised calves. J. Anim. Sci. Biotechnol. 2017, 8, 34. [Google Scholar] [CrossRef]
- Sun, P.; Wang, J.Q.; Zhang, H.T. Effects of Bacillus subtilis natto on performance and immune function of preweaning calves. J. Dairy Sci. 2010, 93, 5851–5. [Google Scholar] [CrossRef]
- Beiranvand, H.; Khani, M.; Ahmadi, F.; Omidi-Mirzaei, H.; Ariana, M.; Bayat, A.R. Does adding water to a dry starter diet improve calf performance during winter. Animal. 2019, 13, 959–967. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.; Wang, Z.; Cao, G.; Hu, R.; Wang, X.; Zou, H.; Kang, K.; Peng, Q.; Xue, B.; Wang, L.; Zhu, Y.; Zhu, X. Active dry yeast supplementation improves the growth performance.; rumen fermentation.; and immune response of weaned beef calves. Anim. Nutr. 2021, 7, 1352–1359. [Google Scholar] [CrossRef]
- Callaway, E.S.; Martin, S.A. Effects of a Saccharomyces cerevisiae culture on ruminal bacteria that utilize lactate and digest cellulose. J. Dairy Sci. 1997, 80, 2035–44. [Google Scholar] [CrossRef]
- Kim, H.B.; Isaacson, R.E. The pig gut microbial diversity: Understanding the pig gut microbial ecology through the next generation high throughput sequencing. Vet. Microbiol. 2015, 177, 242–51. [Google Scholar] [CrossRef]
- Cao, Z.; Xiao, J.; Alugongo, G.M.; Ji, S.; Wu, Z.; Dong, S.; Li, S.; Yoon, I.; Chung, R. Effects of Saccharomyces Cerevisiae Fermentation Products on the Microbial Community throughout the Gastrointestinal Tract of Calves. Animals. 2018.; 9, 959.
- Yu, X.; Wu, X.; Qiu, L.; Wang, D.; Gan, M.; Chen, X.; Wei, H.; Xu, F. Analysis of the intestinal microbial community structure of healthy and long-living elderly residents in Gaotian Village of Liuyang City. Appl. Microbiol. Biotechnol. 2015, 99, 9085–95. [Google Scholar] [CrossRef]
- Davila, A.M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.H.; Sanz, Y.; Tomé, D. Re-print of “Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host”. Pharmacol. Res. 2013, 69, 114–26. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science. 2005, 307, 1915–20. [Google Scholar] [CrossRef]
- Xu, J.; Bjursell, M.K.; Himrod, J.; Deng, S.; Carmichael, L.K.; Chiang, H.C.; Hooper, L.V.; Gordon, J.I. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003, 299, 2074–6. [Google Scholar] [CrossRef] [PubMed]
- Aricha, H.; Simujide, H.; Wang, C.; Zhang, J.; Lv, W.; Jimisi, X.; Liu, B.; Chen, H.; Zhang.; C.; He, L.; Cui, Y.; Gao, R.; Aorigele, C. Comparative Analysis of Fecal Microbiota of Grazing Mongolian Cattle from Different Regions in Inner Mongolia, China. Animals. 2021, 11, 1938.
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, C.; Simujide, H.; Aricha, H.; Zhang, J.; Liu, B.; Aorigele, C. Effects of Pathogenic Escherichia coli Infection on the Flora Composition, Function, and Content of Short-Chain Fatty Acids in Calf Feces. Animals, 2022, 12, 959.
- Li, J.; Chu, R.; Wang, C.; Li, Y.; Wu, B.; Wan, J. Microbiome characteristics and Bifidobacterium longum in colorectal cancer patients pre- and post-chemotherapy. Transl. Cancer Res. 2020, 9, 2178–2190. [Google Scholar] [CrossRef] [PubMed]
- Tun, H.M.; Li, S.; Yoon, I.; Meale, S.J.; Azevedo, P.A.; Khafipour, E.; Plaizier, J.C. Saccharomyces cerevisiae fermentation products (SCFP) stabilize the ruminal microbiota of lactating dairy cows during periods of a depressed rumen pH. BMC Vet. Res. 2020, 16, 237. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Guo, C.; Gong, Y.; Sun, X.; Wang, W.; Wang, Y.; Yang, H.; Cao, Z.; Li, S. Rumen Fermentation, Digestive Enzyme Activity, and Bacteria Composition between Pre-Weaning and Post-Weaning Dairy Calves. Animals. 2021, 11, 2527. [Google Scholar] [CrossRef]
- Fonty, G.; Gouet, P.; Jouany, J. P.; Senaud, J. Establishment of the microflora and anaerobic fungi in the rumen of lambs. Microbiology. 1987, 133, 1835–1843. [Google Scholar] [CrossRef]
- Smet, A. M. D.; Boever, J. L. D.; Brabander, D. L. D.; Vanacker, J. M.; Ch.V. Boucqué. Investigation of dry matter degradation and acidotic effect of some feedstuffs by means of in sacco and in vitro incubations. Anim. Feed Sci. Technol. 1995, 51, 297-315.
- Kang, J.; Zeng, B.; Tang, S.; Wang, M.; Han, X.; Zhou, C.; Yan, Q.; Liu, J.; Tan, Z. ; Effects of Momordica charantia polysaccharide on in vitro ruminal fermentation and cellulolytic bacteria. Ital. J. Anim. Sci. 2017, 16, 226–233. [Google Scholar] [CrossRef]
- Sakamoto, M.; Tanaka, Y.; Benno, Y.; Ohkuma, M. Butyricimonas faecihominis sp. nov. and Butyricimonas paravirosa sp. Nov., isolated from human faeces, and emended description of the genus Butyricimonas. Int. J. Syst. Evol. Microbiol. 2014, 64, 2992–2997. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Takagaki, A.; Matsumoto, K.; Kato, Y.; Goto, K.; Benno, Y. Butyricimonas synergistica gen. nov., sp. nov. and Butyricimonas virosa sp. nov., butyric acid-producing bacteria in the family 'Porphyromonadaceae' isolated from rat faeces. Int. J. Syst. Evol. Microbiol. 2009, 59, 1748–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; An, J.; Kim, J.; Choi, D.; Song, Y.; Lee, C.K.; Kong, H.; Kim, S.B.; Kim, K. A Novel Bacterium.; Butyricimonas virosa, Preventing HFD-Induced Diabetes and Metabolic Disorders in Mice via GLP-1 Receptor. Front Microbiol. 2022. 13, 858192.
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; Liu, S.J.; Liu, H. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235. [Google Scholar] [CrossRef]
- Wu.; T.R.; Lin.; C.S.; Chang.; C.J.; Lin.; T.L.; Martel.; J.; Ko.; Y.F.; Ojcius.; D.M.; Lu.; C.C.; Young.; J.D.; Lai.; H.C. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut. 2019, 68, 248-262.
| Items | Starter | Oat hay | SCFP |
|---|---|---|---|
| DM | 94.00 | 89.00 | 91.60 |
| CP | 21.07 | 8.52 | 18.73 |
| EE | 3.25 | 5.27 | 1.25 |
| Ash | 9.94 | 9.30 | 11.92 |
| NDF | 22.99 | 47.33 | 31.47 |
| ADF | 14.85 | 32.06 | 22.20 |
| Ca | 1.12 | 0.47 | 0.67 |
| P | 0.62 | 0.28 | 0.76 |
| NaCl | 0.60 | ― | 0.15 |
| MOS | ― | ― | 2.00 |
| Items | CON1 | SCFP2 | SEM | p |
|---|---|---|---|---|
| BW (kg) | ||||
| d 1 | 52.36 | 53.18 | 2.23 | 0.717 |
| d 15 | 60.27 | 63.55 | 1.87 | 0.096 |
| d 30 | 70.68 | 74.00 | 2.76 | 0.244 |
| d 45 | 86.41 | 92.36 | 2.83 | 0.048 |
| ADG (g/d) | ||||
| d 1-15 | 527.27 | 690.91 | 77.58 | 0.048 |
| d 15-30 | 693.94 | 696.97 | 69.67 | 0.966 |
| d 30-45 | 1048.49 | 1224.24 | 78.55 | 0.037 |
| d 1-45 | 756.57 | 870.71 | 34.09 | 0.003 |
| ADMI (g/d) | ||||
| d 1-15 | 121.42 | 142.86 | 27.25 | 0.461 |
| d 15-30 | 916.04 | 919.81 | 126.11 | 0.977 |
| d 30-45 | 2472.73 | 2455.74 | 153.59 | 0.916 |
| d 1-45 | 1330.34 | 1340.95 | 111.32 | 0.927 |
| FCR (g/g) | ||||
| d 1-15 | 4.41 | 5.18 | 1.09 | 0.512 |
| d 15-30 | 0.74 | 0.79 | 0.18 | 0.791 |
| d 30-45 | 0.43 | 0.65 | 0.04 | 0.027 |
| d 1-45 | 0.57 | 0.65 | 0.04 | 0.103 |
| Digestibility | CON | SCFP | SEM | p |
|---|---|---|---|---|
| DM | 71.03 | 76.21 | 0.93 | <0.001 |
| CP | 60.00 | 62.33 | 1.10 | 0.047 |
| Fat | 59.63 | 64.85 | 2.11 | 0.022 |
| DNF | 50.32 | 52.72 | 2.55 | 0.357 |
| ADF | 43.28 | 49.09 | 2.69 | 0.043 |
| Ca | 61.83 | 66.99 | 1.65 | 0.005 |
| P | 54.91 | 65.45 | 3.89 | 0.014 |
| Items | CON | SCFP | SEM | p |
|---|---|---|---|---|
| GH (ng/mL) | ||||
| d 15 | 9.66 | 12.75 | 0.90 | 0.003 |
| d 45 | 11.61 | 16.45 | 1.57 | 0.007 |
| IGF-1 (ng/mL) | ||||
| d 15 | 171.55 | 226.65 | 10.29 | <0.001 |
| d 45 | 210.87 | 257.12 | 5.82 | <0.001 |
| GLP-1 (pmol/L) | ||||
| d 15 | 26.45 | 29.40 | 1.99 | 0.157 |
| d 45 | 25.07 | 33.53 | 1.56 | <0.001 |
| GLP-2 (pmol/L) | ||||
| d 15 | 417.07 | 439.15 | 30.24 | 0.475 |
| d 45 | 411.89 | 487.35 | 39.01 | 0.070 |
| Items | CON | SCFP | SEM | p |
|---|---|---|---|---|
| IgA (g/L) | ||||
| d 15 | 0.033 | 0.047 | 0.006 | 0.029 |
| d 45 | 0.045 | 0.048 | 0.002 | 0.138 |
| IgM (g/L) | ||||
| d 15 | 0.018 | 0.019 | 0.004 | 0.884 |
| d 45 | 0.027 | 0.026 | 0.002 | 0.643 |
| TNF-α (pg/mL) | ||||
| d 15 | 64.107 | 48.309 | 2.217 | <0.001 |
| d 45 | 54.473 | 40.962 | 2.109 | <0.001 |
| IL-1β (pg/mL) | ||||
| d 15 | 26.764 | 20.067 | 0.950 | <0.001 |
| d 45 | 23.449 | 17.396 | 1.036 | <0.001 |
| IL-6 (pg/mL) | ||||
| d 15 | 135.530 | 108.411 | 4.079 | <0.001 |
| d 45 | 118.935 | 94.387 | 2.549 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





