1. Introduction
It is generally believed that medicines (including the natural ones) do not have to taste good, but they must be good for our health. The least pleasant taste for many people is the bitter taste of both food and medicines. Type 2 taste receptors (T2Rs) are G-protein coupled receptors (GPCRs) that were first identified in the mouth as receptors of bitter taste. It has been assumed that, responding to the pressure of food selection, different species have evolved with different numbers of T2Rs. Recently, there has been a growing interest in assessing the role of T2Rs in humans [
1,
2,
3], where the T2R gene family consists of 25 functional receptor-encoding genes, many of which are polymorphic. We already know that these receptors, which have a relatively simple structure (compared to the receptors representing other tastes, such as sweet, sour or salty) [
4], are widely distributed in the human body, also beyond the tongue and oral cavity. In fact, T2Rs were found in many different tissues and organs, e.g. in the digestive tract, immune system, nervous system, pulmonary tract [
2,
3], and throughout the body T2Rs are sentinels, that monitor environmental challenges and coordinate defensive endocrine, behavioral, and immunological responses [
3].
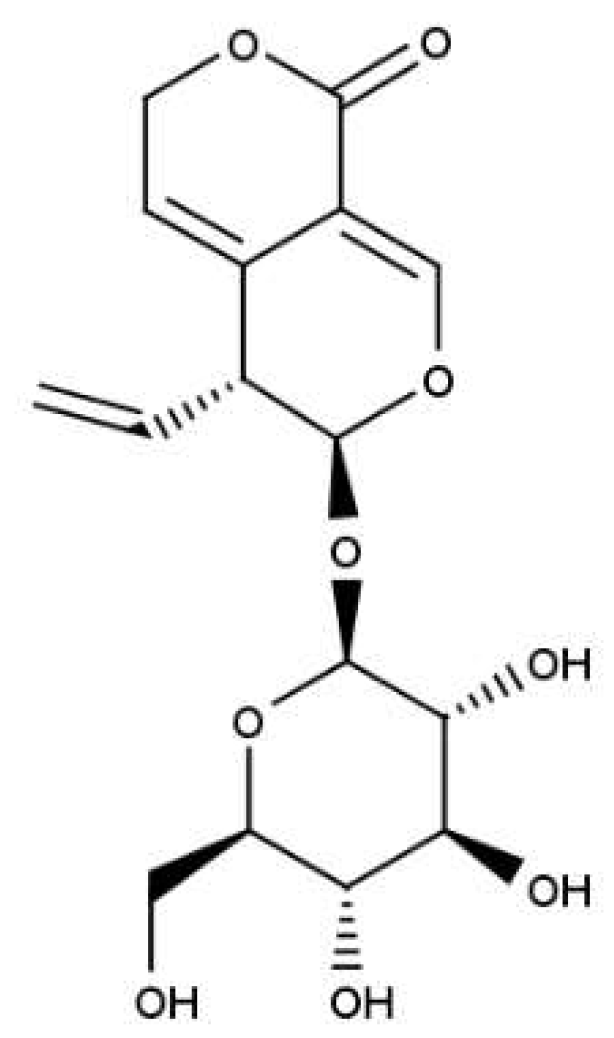
As already mentioned, many plants used as medicines very often interact with human body through a bitter taste, which is one of the most important signals that a person receives from an external environment, informing about potentially dangerous changes that result in dysregulation of the body's homeostasis. Bitter-tasting plant compounds represent many different chemical structures, including: alkaloids, polyphenolic compounds (coumarins, flavonoids), glucosinolates and terpenoids. Belonging to the last group secoiridoid glucoside gentiopicroside (gentiopicrin, GPS, CAS No 20831-76-9;
Figure 1) occurs mainly in Gentianales order, in the botanical family Gentianaceae [
5]. Interestingly, GPS has been also found in plant species from the other botanical families; in
Aster auriculatus Franch (Asteraceae) [
6],
Artocarpus heterophyllus Lam. (Moraceae) [
7]), and
Cephalaria kotschyi (Dipsaceae) [
8]. In Gentianaceae family GPS is present in genus
Swertia,
Centaurium and commonly in the genus
Gentiana, which contains about 400 species that are native in Europe, Asia and North America [
9].
Gentian has been used in natural medicine for centuries, including traditional Chinese medicine (TCM) and Ayurvedic medicine. Plants of the
Gentiana genus are also listed in many modern Pharmacopoeias, including the 10th edition of the European Pharmacopoeia, where a bitterness index of not less than 10,000 is required for the root of
Gentiana lutea L. (compared to the bitterness index of quinine hydrochloride of 200,000) [
10]. In this valuable medicinal plant, gentiopicroside is one of the best-known active substances and was first isolated from this plant source in 1862 [
5]. Since then, this compound has been extensively studied for biological activity in various
in vitro and
in vivo models, as well as in several clinical trials. GPS was found to be non-toxic at the doses used and relatively stable, although it is not readily bioavailable.
In this review, we intend to analyze and highlight the pharmacological potential of GPS as a biologically active compound of natural origin. Based on available literature data, we will discuss in vitro, in vivo experiments with GPS as an active metabolite isolated from a natural source and tested as a single compound (this review will not consider the effects of the whole plant extracts containing GPS). We would like to encourage researchers to explore the possible effects and interactions of GPS with various target tissues in hopes for a better understanding of the role of bitter compounds and their benefits on human health that may be discovered in the future.
3. Bioavailability, Biotransformation and Stability of GPS
As mentioned above, GPS is an ingredient of numerous dietary supplements and plant drugs that are prepared from different gentian species and are successfully used to improve digestion or to exert anti-inflammatory, antioxidant or analgesic properties. Based on these applications it can be concluded that GPS is most often administered orally and because of this fact, this molecule undergoes the typical ADME processes characterizing the transport of a substance in a living organism, namely the liberation, absorption, distribution, metabolism and excretion.
Some of the GPS metabolites were determined by Zeng
et al. in their UPLC/Q-TOF MS based study on gentiopicroside isolated from
Gentiana scabra [
11]. The authors proposed a possible metabolic pathway of GPS that was induced by ß-glucosidase in their
in vitro assay. According to the authors hydrolysis was an initial step to trigger biotransformation and produce an intermediate aglycone, which was next converted to four metabolites (M1–M4)
via isomerization, reduction and oxidation reactions. M1 (identified as gentiopicral) and M2 (identified as erythrocentaurin) were the main metabolic products formed by means of different isomerization paths at the hemiacetal part of aglycone. Metabolite M4 was identified as 5-(hydroxymethyl)-1H -isochromen-1-one, and M3 was proposed to be the lactone ring open metabolite of M1.
Additionally, the authors tested different concentrations of the obtained metabolites M1 and M2 and GPS itself (different concentrations 2.5, 5, 10 and 25 µM / 24 h) for their hepatoprotective activities on human embryonic hepatocytes (L02 cells) that were H2O2 - injured. The results showed that the hepatoprotective activities of metabolites were higher than that of GPS.
The formation of GPS metabolites was attributed to the intestinal flora as well. The anaerobic incubation of the compound with each one of 24 strains of bacteria isolated from human feces resulted in the transformation of GPS to at least 5 metabolites in different ratios. The products of bacterial metabolism were extracted by ethyl acetate and analyzed by TLC-densitometry by El-Sedawy
et al. [
12]. In their study, GPS was hydrolyzed by the bacterial ß-glucosidase enzyme to deliver an unstable aglycone in a hemiacetal form, that was converted to two types of compounds: isochroman and pyrano[3,4-
c]pyran derivatives. The aldehydes of both types were later next reduced to the respective alcohols. Finally, the metabolites of GPS were identified as G1 – erythrocentaurin, G2 – gentiopicral (gentiogenal) that had been previously reported to have antibacterial, antifungal and antitumor activities, G3 – 5-hydroxymethylisochromen-1-on, G4 - 5-hyroxymethylisochroman-1-on, and G5 - 5,6-dihydro-5-hydroxymethyl-6-methyl-1H,3H-pyrano[3,4-c]pyran-1-one.
Next to the metabolism of GPS in the digestive tract, several studies on the intestinal absorption of the compound of interest were performed. The uptake, transepithelial transport and efflux of gentiopicroside together with the mechanism of its absorption was explored in the HPLC-based experiments in Caco-2 cells conducted by Zhang
et al. [
13]. The effects of time, temperature and P-glycoprotein (P-gp) inhibitors on the absorption of GPS were tested in this human intestinal epithelial cell model. The authors claimed that the absorption of GPS was mainly by passive diffusion process and was correlated positively to time and negatively to temperature. There was no significant change between different concentrations of GPS. The inhibitors of P-gp such as cyclosporine A and verapamil, enhanced significantly the uptake and transport of this secoiridoid glucoside, and, as expected, P-gp had strong efflux effects on the absorption of GPS.
The stability of GPS in the intestinal fluids was investigated
in vitro in the study of Chen
et al. [
14]. For this purpose total gentian glycosides were dissolved in phosphate buffer solutions and diluted in hydrochloric acid at different pH values, in liver and mucosa homogenates, and in plasma and gastrointestinal contents at 37°C. The degradation of GPS measured by HPLC at certain time intervals showed that the degradation of GPS did not follow apparent first-order kinetics equation. After 48 h of incubation, GPS was degraded by 2.6%, 7.9%, 20.2% and 34.4%, (at pH 1.2 diluted hydrochloric acid, pH 6.8, pH 7.4 and pH 8.0 phosphate buffer solutions, respectively). The degradation profile of GPS in gastric contents and mucosa homogenates, small intestinal contents, intestinal mucosa homogenates and liver homogenates was similar to that in the aqueous buffer. After 12 h only 20% of the initial concentration of GPS was detected in the large intestine’s content, and after 24 h – as much as 53% in plasma. GTP was stable in stomach, small intestine and liver, however, blood and large intestine were possible sites where GPS was be degraded.
4. Pharmacological Properties of GPS In Vitro
Vast traditional applications of GPS containing plants encouraged the researchers to study the biological potential of GPS more extensively and broadly. Multitude of scientific reports denoting marked biological potential of this secoiridoid were published. Here below the pharmacological potential of the compound proved in the in vitro tests was reviewed.
4.1. Anti-Inflammatory Activity of GPS and Its Semi-Synthetic Analogues
GPS, isolated from
Gentiana officinalis, was tested for its anti-inflammatory properties by Zhang and co-investigators [
15]. The concentrations of 25, 50 and 100 μg/mL were set to examine the anti-inflammatory effects of GPS in mouse macrophages cell line RAW 264.7 stimulated with lipopolysaccharide (LPS).
In vitro results showed that GPS inhibited nitric oxide (NO), prostaglandin E2 (PGE2), and interleukin-6 (IL-6) production in RAW 264.7. The overproduction of NO and PGE2 was associated with the overexpression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in cells, so GPS could inhibit the expression of iNOS and COX-2. Molecular docking of COX-2 and iNOS by GPS (with a lower docking score; -13.329 and -11.386 kcal/mol respectively) showed that tested compound displayed satisfactory anti-inflammatory activities. Hydrogen bonds (H-bonds) were formed between the sugar fragments in GPS structure with Tyr355, Ser353, Leu352, Ser530, Arg120, and His90 of COX-2, and Glu377, Asp382, Tyr373, Tyr347, Gln263, Asn370, and Gly371 of iNOS. MTT assay showed that GPS had no cytotoxicity within 100 μg/mL concentration.
In another study the expression of COX-2 in J774A.1 cell line (BALB/c murine macrophages) was tested using Western blot assay with increasing concentrations of GPS (1–3–10 μM / 24 h), after the LPS (100 ng/mL / 24 h) treatment. A positive binding with cyclooxygenase-2 (COX-2), alpha-1-antichymotrypsin (AATC), and alpha-1-acid glycoprotein (orosomucoid, ORM) emerged from the computational experiments and the outcomes from the promising interaction with COX-2 were confirmed by Western blot. It was revealed that GPS exerts anti-inflammatory activity by decreasing COX-2 expression in J774A.1 cells [
16].
Chang
et al. [
17] studied an influence of GPS on cell survival using the ethyl alcohol-induced gastric mucosal cell injury model in ethanol treated human gastric mucosal cell line GES-1. It was found that GPS promoted cell survival and suppressed inflammatory cytokine production. The ELISA assay showed an inhibition of TNF-α, IL-1β, and IL-8 production and enhanced IL-10 production (restored IL-10 expression, which was reduced by ethanol) in the GES-1 cell culture supernatant by GPS. Also the regulation of matrix metallopeptidase MPP-10 expression and pERK1/2 signaling were involved in GPS mechanism of action, which suggests that the secoiridoid might be considered a promising therapeutic drug in ethanol-induced gastritis.
Sepsis - a life-threatening systemic inflammatory response to infection – that can be enhanced by the LPS – the Gram-negative bacteria and bacterial RNA or DNA, proceeds with the occurrence of pan-inflammation.
The anti-inflammatory properties of GPS were helpful in soothing inflammatory conditions in the studies of Wang
et al. [
18]. As the result it was found that
in vitro GPS reduced the inflammatory cytokine production of BMMs (primary bone marrow-derived macrophages) stimulated by (LPS)/IFN-γ and ameliorated the phosphorylation of IKKα/β and p65, the degradation of IκBα, and the translocation of p65 into the nucleus. NF-κB inhibitor and p65 siRNAs eliminated the inhibitory effect of GPS confirming that GPS prevented LPS/IFN-γ-induced inflammatory cytokine production by macrophages through the NF-κB signaling pathway
in vitro, and might exert a protective role against LPS-induced sepsis by suppressing pro-inflammatory cytokine production by activated macrophages.
Due to the presence of a sugar moiety in the GPS structure this compound is highly hydrophilic. This fact explains its reduced oral bioavailability, quick metabolism and a short biological half-life, and therefore a limited efficacy. In their experiments Zhang
et al. [
19] attempted to reduce the compound’s polarity and keep the biological activity of GPS simultaneously. They introduced hydrophobic cyclic acetals into the structure of GPS to enhance its lipophilicity, aiming to obtain 26 novel derivatives of GPS with excellent anti-inflammatory activity. The
in vitro anti-inflammatory activities were evaluated against NO, PGE2, and IL-6 production in the mouse macrophage cell line RAW264.7 stimulated by LPS. The most potent compound (P23) with the 4-difluoromethoxyphenyl moiety was more active from GPS, and the inhibitory potential of P23 (57.26%) was exceeded the one of the positive control drug celecoxib (46.05%) tested at dose of 0.28 mmol/kg. Also, other derivatives substituted with the following groups: NO
2 , F, Cl, CH
3 , CF
3 , and OCF
2H showed a satisfactory activity. It was concluded that the
para- substitution with electron-withdrawing groups might be beneficial for obtaining the anti-inflammatory activity of GPS derivatives. Their mechanism of action was possibly associated with the downregulation of inflammatory cytokines: NO, PGE2, by the production of IL-6 and by the suppression of iNOS and COX-2. These compounds may represent a novel class of selective COX-2 and iNOS inhibitors as new anti-inflammatory agents.
4.2. Wound Healing Effect of GPS
Wound healing properties of GPS alone and as the main component of the plant extract (
Gentiana lutea ssp. symphyandra) were evaluated in comparison to the dexpanthenol on cultured chicken embryonic fibroblasts from fertilised eggs. The influence of GPS on collagen production, mitotic ability, and cells biological changes observed using microscopy studied by Öztürk
et al. [
20] confirmed that GPS contribute in a wound healing ability of gentian by increasing mitotic ability and delaying cell death of threated cells. GPS (0.4 and 2 µg/mL) was also effective in the stimulation of collagen production.
4.3. Anti-Rheumatic Activity of GPS
Rheumatoid arthritis (RA) is a highly debilitating, chronic form of systemic autoimmunological disease associated with the progressive destruction of bone and cartilage and synovitis.
Gentiana macrophylla is a plant that is used for the treatment of RA in traditional Chinese medicine. Zhang
et al. [
21] investigated the effect of GPS (isolated from
G. macrophylla) on the expression of inflammatory factors in TNF-α stimulated human fibroblast-like synoviocytes (RA-FLS) and evaluated the mechanism involved.
The treatment with GPS significantly and dose-dependently decreased the levels of IL-1β and IL-6 mRNAs, and down-regulated the expression of p-p38MAPK and NF-κB-p65 proteins in the TNF-α cell group. GPS exhibited a therapeutic potential in RA through a mechanism involving the inhibition of p38MAPK/NF-κB pathway. The treatment with 5-25 μM of GPS did not affect the number of viable cells in the MTT assay, however, at 50 and 100 μM, the number of viable cells were significantly increased.
Wang
et al. [
22] found that GPS was able to suppress the TNF-α-induced proliferation and migration of RA-FLS cells. This suppression was attributed to the ability of GPS to block NOD-like receptor protein 3 (NLRP3), apoptosis-associated speck-like protein containing a CARD (ASC), and caspase-1, thereby disrupting the activation of the NLRP3 inflammasome. Consistent with such suppression, GPS led to a significant decrease in IL-1ß secretion by the treated cells. The reduction in NLRP3 inflammasome activation was also associated with a decrease in the activation of nuclear factor (NF-kB), the production of reactive oxygen species (ROS), and the expression of inflammatory IL-6. These findings indicate that GPS can suppress the ROS-NF-kB-NLRP3 axis to alleviate RA symptoms, and may therefore have value as a novel therapeutic agent that can treat RA via inhibiting this ROS-NF-kB-NLRP3 inflammasome axis.
4.4. Promotion of the Osteogenic Effect by GPS in Bone Mesenchymal Stem Cells
Osteoporosis is a systemic disease characterized by bone loss
via destruction of their microstructure and increasing its fragility. The primary cause of osteoporosis is due to a decrease in osteoblasts’ number that leads to a reduced bone formation, and an increase in osteoclasts number resulting in an enhanced osteolysis. Osteoblasts are mainly differentiated from bone mesenchymal stem cells (BMSCs). BMSCs can differentiate into several cell types (chondrocytes, osteoblasts, adipocytes and endothelial cells) depending on the specific conditions. Jiang
et al. [
23] in their experiments were trying to find whether GPS could promote the osteogenic differentiation of BMSCs and to explore the molecular mechanism induced by GPS in differentiating BMSCs. As BMSCs release several osteogenic factors such as osteocalcin (OCN), osterix (OSX), osteopontin (OPN) and runt-related transcription factor 2 (Runx2), the influence of GPS on this mediators was evaluated
in vitro.
Female C57BL/6 mice (4-week-old) were sacrificed to obtain BMSCs from the bone marrow of the femur for in vitro experiments. Cells were cultured and exposed to GPS at different doses (0, 10, 20, 40 and 80 μM / 7 days) and the cytotoxicity test was performed. It was found that GPS was not harmful to BMSCs at the concentrations of 10, 20 and 40 μM, however, 80 μM GPS significantly inhibited the proliferation of BMSCs. Therefore alkaline phosphatase (ALP) activity measurement, quantitative PCR and Western blot analyses were performed after exposition on GPS (0, 10, 20, 40 μM / 14 days). The mRNA expression of the osteogenic genes (ALP, Runx2, OSX, OCN, OPN and BMP2) was significantly increased by GPS in a concentration-dependent manner. Also the expression of osteogenic proteins (Runx2, OCN and BMP2) was promoted by GPS and reached a maximum at 40 μM of GPS. The in vitro results showed that GPS enhanced the activity of ALP, increased the number of calcified nodules in BMSCs and up-regulated the osteogenic factors (Runx2, OSX, OCN, OPN and BMP2) which suggests that GPS strengthened the osteogenic differentiation in BMSCs.
4.5. Neurogenic and Neuroprotective Activitity of GPS
Chiba
et al. [
24] tested the ability of several secoiridoids, including GPS, on their neurotrophic factor-like activity (neurotrophic factors contribute to the growth, survival, and function of brain neurons) in PC12h cells (a subclone of rat pheochromocytoma cells). GPS (1,5,10,20, 50 and 100 µM) obtained from the whole plant of
Swertica japonica, induced significant neurite outgrowth in PC12h in a dose dependent manner. No toxic effects were observed for GPS at a concentration of 100 µM for 2 days. As compared to other secoiridoids, GPS was found the most active. It was clear that its action was not related to the hyrophobicidy, and mono-glucosides were more active than diglucosides.
GPS was also proved to exhibit neuroprotective properties. The anti-apoptotic effects of GPS in neonatal rat hippocampal neurons following oxygen-glucose deprivation and reperfusion injury was tested by Wang
et al. [
25] in an
in vitro model of ischemia and reperfusion. In their studies the cells were pretreated with GPS 24 h before being exposed to oxygen-glucose deprivation. Pretreatment with GPS (10, 20, 40 mg/L) significantly attenuated neuronal damage, decreased neuronal apoptosis rate (which was visualised by Hoechst 33342 staining) and nerve cell lactate dehydrogenase (LDH) leakage rate. GPS at the highest tested dose effectively down regulated the expression of caspase-3 and Bax in mRNA and protein level, and up regulated the expression of Bcl-2 level. By these mechanisms GPS preconditioning prevented neurons from oxygen-glucose deprivation and reperfusion injury.
4.6. Antiaging Properties of GPS
Aging is a time-dependent functional decline that is the primary risk factor for many diseases, therefore, studies on prolonging the health lifespan have been intensively conducted worldwide. Yeast as a simple model which is amenable to genetic and molecular manipulations, is widely used to study aging mechanisms and screen potential drug candidates for attenuating this process. Autophagy, particularly mitophagy, is a degenerative process that degrades cellular components, such as the mitochondria, for recycling into amino acids and other metabolites. Autophagy and aging are closely related.
Liu
et al. [
26] studied if GPS isolated from
Gentiana rigescens Franch, a traditional Chinese medicine, prolonged the replicative and chronological lifespans of yeast. As was found, GPS increased the survival rate of yeast under oxidative stress condition, enhanced the activities of catalase, superoxide dismutase, and glutathione peroxidase, and decreased the levels of reactive oxygen species and MDA (malondialdehyde - the product of lipid peroxidation of polyunsaturated fatty acids). GPS at the concentration of 1 μM significantly induced authophagy and mitophagy in yeast. The PCR analysis result showed that the expression of
ATG32 gene was increased by GPS at concentrations of 1 and 3 μM. GPS extended the replicative lifespan through the regulation of mitophagy that requires
ATG32. These results indicated that autophagy, especially mitophagy, and antioxidative stress are involved in the antiaging effect. GPS can significantly prolong the replicative lifespan of K6001 yeast cells at concentrations of 1, 3, and 10 μM (p < 0:01, p < 0:05, and p < 0:05, respectively), and significantly increased the survival rate of YOM36-GFP-Atg8 yeast at concentrations of 1 and 3 μM (p < 0:001, p < 0:01, respectively).
4.7. Anti-Diabetic, Hypoglycemic and Hypolipidemic Effect of GPS
The influence of GPS on gluconeogenesis was investigated
in vitro by Yang
et al. [
27]. In this study human L02 liver cells isolated from healthy adults were considered to possess similar functions in glucose and lipid metabolism as primary liver cells. Analysis was performed on insulin resistant (IR) and in normal cells respectively (NC). It was concluded that GPS stimulated glucose uptake with stronger effect on IR than NC cells. This compound also suppressed gluconeogenesis in both types of cells. It indicates that GPS exerts the insulin receptor independent effects. Moreover, GPS regulated gluconeogenesis via AKT-FoxO1 pathway by activating the phosphorylation of AKT (serine/threonine-specific protein kinase), which down-regulates transcriptional activity of FoxO1 leading to an inhibited expression of key regulatory enzymes of gluconeogenesis, namely glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK). GPS did not exert cytotoxic effects on L02 cells at concentrations as high as 5 mmol/L, as was found in cell viability test and maximum concentration of the tested glucoside was set at 100 µmol/L.
Abnormalities in lipid and glucose metabolism occurring in type 2 diabetes (T2DM) were ameliorated by GPS. Considering the key role of fibroblast growth factor receptor 1/phosphatidylinositol 3-kinase/protein kinase B (FGFR1/PI3K/AKT) pathway in T2DM, Xu
et al. [
28] explored the possible mechanism of GPS on lipid and glucose metabolism through its effects on FGFR1/PI3K/AKT pathway. GPS at the concentration lower than 320 µM showed no cytotoxicity on HepG2 cells. Starting from 20 µM GPS promoted glycogen synthesis and glucose consumption. Thus, the concentrations of 20, 40 and 80 µM were selected in the experiments. GPS treatment significantly induced glycogen storage, glucose consumption and reduced lipid accumulation in PA-induced HepG2 cells (induced by 0.25 mm PA-palmitic acid / 24 h). The investigations of the effects of GPS on PI3K/AKT pathway in PA-induced HepG2 cells showed that GPS significantly increased the expressions and promoted the phosphorylation of PI3K, AKT and FoxO1. GPS ameliorated glucose and lipid metabolism disorders in PA-induced HepG2 cells by the activation of FGFR1/PI3K/AKT pathway.
In further experiments conducted by Xiao
et al. [
29] on HepG2 cells that were treated with GPS (5, 20, 40, 80, 160 and 320 µmol/L) for 24 h it was found that 80 µmol/L GPS showed similar effects as 250 µmol/L metformin in an increased glucose utilization. The treatement resulted in improved glycolipid metabolism with no toxicity below. According to the authors GPS inhibited the interaction between adipoQ receptor 3 (PAQR3) and the PI3K catalytic subunit to restore PI3K/AKT signaling pathway. GPS directly bound with amino acids of the PAQR3 NH
2-terminus (including Leu40, Asp42, Glu69, Tyr125 and Ser129) which is an important binding site for GPS. Also, GPS treatment ameliorated high-fat diet (HFD) and palmitic acid (PA)-induced hepatic insulin resistance by promoting PI3K/AKT axis activation. This findings provide a rationale for the potential application of GPS to restore insulin sensitivity in diabetes.
In diabetic nephropathy the pathological changes may occur, resulting in renal fibrosis which is a combination of glomerulosclerosis and renal tubulointerstitial fibrosis (TIF) due to the excessive deposition of the extracellular matrix (ECM). In experiments conducted by Xu
et al. [
30] GPS (12.5, 25, and 50 μM) inhibited TIF, which may be related to glucose-lowering activity of GPS. The secoiridoid may also directly inhibit the CK2/NF-κB inflammatory signaling pathway
via angiotensin II type 1 receptor (AT1R) to ameliorate TIF in diabetes. As was confirmed, the effects of GPS were observed
in vitro in HG-stimulated NRK-52E cells (rat kidney epithelial cells cultured with 30 mM high glucose) were reversed by AT1R over-expression.
4.8. Hepatoprotective Effect of GPS
Novel traditional Chinese drug named “Gentiopicroside Injection” was approved for the treatment of acute jaundice and chronic active hepatitis by Saudi Food and Drug Authority (SFDA), however, the inhibitory and inducible effects of GPS on the activity of cytochrome P450 (CYP450) need to be clarified. Deng
et al. [
31] in
in vitro study used Human primary hepatocytes obtained from 34-year old Mongolian man, who was homozygotous for the CYP2D6 wild type of CYP450. Pooled human liver microsomes (n=16, mixed gender) were used for investigation of the ability of GPS to inhibit the major drug metabolizing P450 enzymes. To evaluate GPS (absence or presence of GPS at concentration from 1.0 to 1000 µg/mL) as a direct-acting inhibitor, pooled human liver microsomes were incubated with P450 marker substrates, and reaction of the O-dealkylation (Phenacetin); 6β-; 4’-, 6-, hydroxylation (Testosterone, Diclophenac, Coumarin, S-Mephenytoin,, Chloroxazone) and O-demetylation (Dextromethorphan) were monitored by LC-MS.
The direct-inhibition of CYP2A6 and CYP2E1 activity by GPS was observed in a concentration dependent manner. The enzyme the most potently inhibited by GPS in vitro was CYP2A6 (activity of the enzyme decreased by 80% compared to the control). The evaluated IC50 was 21.80 µg/mL, which was close to the value observed in clinical studies (19.50 µg/mL). This indicates CYP2A6 inhibition by the pharmacologically relevant concentrations of GPS, and that GPS had a little effect on CYP2E1 or CYP3A4, which have been reported to be involved in the mechanism of hepatitis therapy. Therefore, it seems that the hepato-protective effect of GPS is unrelated to its effect on P450s.
Alcoholic liver disease (ALD) is a result of long-term alcohol consumption. Curing this disease is important due to a loss of liver function
via liver steatosis, fibrosis, and cirrhosis which frequently resulted in liver cancer. Several studies reported that AMP-activated protein kinase (AMPK) is a lipid metabolism-regulating kinase having an important role in ALD pathogenesis. AMPK activity decreased during ethanol intake and simultaneously ethanol increased the acetyl-CoA carboxylase (ACC) activity. Consequently, this change exacerbated the imbalance of lipid metabolism. Therefore, inhibiting lipid accumulation or promoting lipolysis may prevent liver steatosis from further developing into liver fibrosis. Hepatic stellate cells (HSCs), normally rich in vitamin A, when targeted by ethanol or viruses lose vitamin A and are activated. It results in a secretion of large amounts of liver fibrosis markers, such as collagen I, smooth muscle α-actin (α-SMA) and transforming growth factor-β (TGF-β). Thus controlling the activation of HSCs is key target of reversing liver fibrosis. Yang
et al. [
32] performed experiments where in
in vitro study LX-2 human hepatic stellate cells were treated with GPS 1 h prior to transforming growth factor-β (TGF-β, 5 ng/mL, 24 h) stimulation, and a mouse hepatocytes cell line AML12 was pretreated by GPS 1 h prior to ethanol treatment. It was tested whether and how GPS could hold back the progression from liver steatosis to liver fibrosis. Also inhibitory capacity of HSC activation by GPS (25, 50 and 100 µM) in a human HSC cell line, LX-2, and the amelioration of lipid accumulation in a mouse hepatocytes cell line, AML12 was evaluated. It was found, that GPS pretreatment decreased collagen I and α-SMA was upregulated by TGF-β in LX-2 cells. This indicated that GPS could reverse the initiation of hepatic fibrosis during chronic alcoholic liver disease. GPS inhibited lipid accumulation by promoting lipid oxidation and inhibiting lipid synthesis.
It was also found by Xiao
et al. [
29] that GPS inhibited the production of inflammatory adhesion molecules (ICAM-1 and VCAM-1) and intercellular matrix (fibronectin and collagen IV) in liver, revealing a protective role for GPS in fatty liver fibrosis.
4.9. Miorelaxant Activity of GPS
Rojas
et al. [
33] have found that GPS (0.01 – 100 µg/mL), isolated from aerial parts of
Gentiana spathacea, inhibited, in a concentration dependent manner, the spontaneous contractions of isolated guinea pig ileum. GPS significantly reduced the contractions induced on the ileum by BaCl
2 and KCl (52.5 % and 32.2 % inhibition, respectively), and produced moderate inhibition of the contractions induced by acetylcholine and histamine (19.0 % and 22.6 %, respectively). These findings suggest that GPS may be blocking influx of the extracellular Ca
2+ to the smooth muscle cells. However, it was not excluded that GPS may also compete with Ca
2+ binding proteins such as calmodulin.
4.10. Antibacterial Potential of GPS
As was reported by Kumasaramy
et al. [
34] GPS from aerial parts of
Centaurium erythrea inhibited the growth of 12 from 17 pathogenic bacterial species (bacterial concentration of 5x10
5 cfu/mL was tested) using 96-well microplate-based broth dilution assay. The minimum inhibitory concentrations (MICs) were between 6.3x10
-3 and 1.0x10
-1 mg/mL. GPS was the most active against
Serratia marcescens (6.3x10
-3 mg/ml), and of weak activity against
Staphylococcus aureus (1.0x10
-1 mg/ml). GPS was not active against such pathogenic bacteria as
S. aureus (MRSA) and
S. epidermis, E. coli, or
Salmonella goldcoast.
4.10.1. Antibacterial Activity of Nanoparticles with GPS
Almukainzi
et al. [
35] prepared a nanoform of GPS. The compound was first isolated from
G. lutea roots and closed into appropriate nanocarriers (PLGA nanospheres, NSs) to enhance its solubility and improve its oral absorption. GPS-PLGA chemical and physical interactions have been analyzed using Fourier-transform IR spectroscopy (FTIR) and differential scanning calorimetry (DSC). The optimum GPS-PLGA NSs have been chosen for antimicrobial study to investigate its inhibitory action on
Staphylococcus aureus compared with the unloaded GPS NSs. The optimum GPS PLGA NSs (F5) with well-controlled particle size (250.10±07.86 nm), relative high entrapment efficiency (83.35±5.71), and the highest % cumulative release (85.79±8.74) have increased the antimicrobial activity as it exhibited a higher inhibitory effect on bacterial growth than free GPS.
4.11. In Vitro Antiviral Activity of Semi-Synthetic GPS Derivatives
Synthesis and pharmacological evaluation of a series of GPS derivatives as potential antiviral inhibitors was undertaken by Wu
et al. [
36]. Synthesized compounds were biologically evaluated for their inhibition of influenza virus and anti-HCV activity
in vitro. Some of the GPS derivatives, such as 11a (2’,3’,6’-Tri-O-benzoyl-4’-O-methylsulfonyl gentiopicroside), 13d (4’-fluoro-4’-deoxy gentiopicroside) and 16 (2’,3’,6’-Tri-O-benzoyl-4’,5’-olefin gentiopicroside) showed interesting anti-influenza virus activity with IC50 at 39.5 mM, 45.2 mM and 44.0 mM, respectively. No significant anti-HCV activity was found for all of GPS derivatives. The preliminary results indicate that modification of the sugar moiety of GPS was helpful for enhancing the anti-influenza activities.
4.12. Oxidative Stress Reducing Potential of GPS
Nonalcoholic fatty liver disease (NAFLD) is a multisystem disease. The abnormal lipid metabolism can produce lipotoxicity that induces oxidative stress and has influence on the development of cardiovascular and cardiac diseases, type 2 diabetes mellitus and chronic kidney disease. The nuclear erythroid 2-related factor 2 (Nrf2) is known as the “main regulator” of the antioxidant response that regulates the expression of hundreds of genes. Nrf2 can regulate the oxidant defense systems, including the expression of stress response proteins HO-1; synthesis of reducing factors, such as glutathione (GSH), promotion of catabolism of peroxides, and superoxide, such as superoxide dismutase (SOD). Nrf2 activation can be controlled by the phosphatidylinositol 3-kinase (PI3K)/AKT pathway. The protective effects of GPS on lipid accumulation and oxidative damage was investigated by Jin
et al. [
37] in free fatty acid- (FFA-) induced HepG2 cells of wild-type (WTHepG2) and Nrf2
-/-HepG2 to analyze if GPS could ameliorate lipid accumulation through Nff2 activation. WTHepG2 cells were exposed to 1mM FFA (oleate : palmitate = 2:1) for different time points from 0 h to 24 h. To demonstrate the cytotoxicity of GPS, HepG2 cells were incubated with different concentrations of GPS for 24 h. Because 500 μM GPS significantly decreased cell viability, the influence of GE was tested in the range from 0 – 500 µM (0, 6, 12, 18 and 24 h). Cell counting kit-8 assays, Oil Red O staining, Western blotting analysis, extraction of nuclear and cytosolic proteins, and biochemical index assay; measurement of triglyceride (TG), total cholesterol (TC), alanine transaminase (ALT), aspartate aminotransferase (AST) and malondialdehyde (MDA) in cell lysates, were employed to explore the mechanisms by which GPS exerts a protective effect on FFA-induced HepG2 cells. It was found, that GPS significantly regulated the activation of (PI3K)/AKT signaling pathway, Nrf2 antioxidant pathway and peroxisome proliferator-activated receptor α (PPARα). GPS also inhibited sterol regulatory element-binding protein-1c (SREBP-1c) expression in FFA-stimulated lipid accumulation of HepG2 cells. Through upregulation of the Nrf2 antioxidant pathway GPS can alleviate oxidative damage and lipid accumulation and strongly protected WTHepG2 cells NAFLD against oxidative damage and lipid accumulation.
4.13. Anti-Cancer Properties
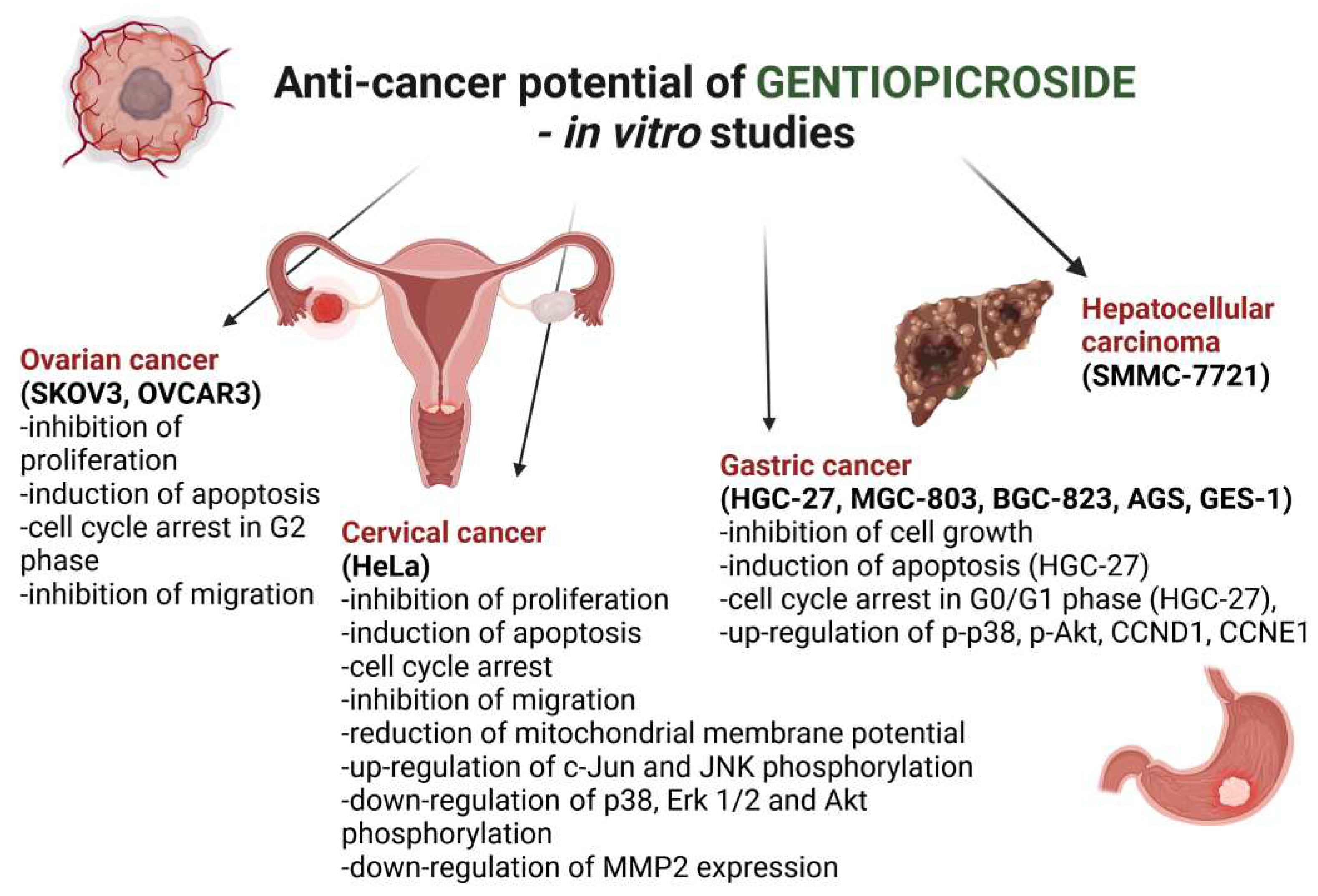
4.13.1. Ovarian Cancer
Ovarian cancer encompasses many types of neoplasms with distinct molecular and clinic-pathological features as well as prognoses [
38,
39]. Ovarian cancer is the seventh most common cancer with five-year survival rates below 45% and the eighth most common cause of cancer death in women worldwide. The number of cases diagnosed every year is increasing [
40]. It has been demonstrated that gentiopicroside exhibits significant anti-cancer activity against SKOV3 ovarian cancer cells. GPS (IC
50=20 μM) displays an anti-proliferative effect and inhibits the growth of SKOV3 cells in a dose-dependent manner. Moreover, GPS leads to apoptosis in a concentration-dependent fashion. The number of apoptotic cells increased from 2.6% in the control to 39.6% at 40 µM GPS. The expression of Bax protein was up-regulated and Bcl-2 protein was down-regulated in a dose-dependent manner (Bax/Bcl-2 ratio indicates that the mitochondrial pathway is involved in GPS-mediated apoptosis). It has been also detected that the number of cells in the G2 phase of the cell cycle increased after 40 µM GPS treatment in a dose-dependent manner leading to cell cycle arrest. Additionally, GPS (20 μM / 24 h) reduces the migration and motility of the SKOV3 ovarian cancer cells in a dose-dependent manner. Inhibition of SKOV3 cancer cells migration, suggests that GPS potentially restrains metastasis of cancer cells. Induction of mitochondrial apoptosis pathway, cell cycle arrest and inhibition of cancer cells migration and invasion indicates that GPS can be considered a promising molecule for the therapy of ovarian cancer [
41].
The other authors demonstrated that GPS can inhibit cell growth in OVCAR-3 ovarian cancer cells through the NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells) signaling pathway, mediation of apoptosis and altering the expression of several apoptosis-related proteins as well as disruption of mitochondrial transmembrane potential. GPS induced cytotoxic and anti-proliferative effects in OVCAR-3 cancer cells in a dose- and time-dependent manner. The IC
50 values were found at 22.4 and 8.2 μM after 24 and 48 hours of treatment, respectively. It has been also detected that GPS induces morphological features of apoptosis marked by reddish-orange fluorescence in ovarian cancer cells. OVCAR-3 cells treated with 0-100 μM of GPS for 48 h results in a significant decrease in cell count and induce morphological changes in OVCAR-3 cells observed under phase-contrasted microscopy. The number of ovarian cancer cells with a vacuolated cytoplasm was much higher after GPS treatment vs the control group. The occurrence of vacuoles in the cytoplasm is an indicator of early apoptosis. GPS can induce moderate as well as extensive apoptosis in OVCAR-3 cells at lower and higher doses, respectively. 20 and 40 μM doses of GPS caused mild apoptosis with morphological changes including membrane blebbing and nuclear fragmentation. In contrast, a higher dose of GPS (100 μM) led to extensive apoptosis characterized by reddish-orange fluorescence which arises due to the binding of acridine orange dye to the fragmented DNA. GPS also led to the loss of mitochondrial membrane potential in OVCAR-3 cancer cells. The percentage of cells with depolarized mitochondria increased from 5.2% in untreated cells to 16.7%, 44.5% and 67.3% in cells treated with 20, 40 and 100 μM doses of GPS, respectively. It was revealed that 0, 20, 40 and 100 μM doses of GPS treatment for 48 h led to up-regulation of cytochrome c, cleaved PARP-1 (poly (ADP-ribose) polymerase 1), caspase-3 and -9 as well as down-regulation of Bcl-2 (B-cell lymphoma 2) and NF-kB proteins expression in a dose-dependent manner. All these proteins play a pivotal role in the induction of apoptosis in cancer cells [
42].
4.14.2. Cervical Cancer
Despite the fact, that the screening programs are widespread, cervical cancer remains the third most common cancer in developing countries [
43]. It has been demonstrated that GPS exhibits anti-cancer activity against HeLa cervical cancer cells through the induction of apoptosis, inhibition of cell growth and migration and cell cycle arrest via the regulation of MAPK (mitogen-activated protein kinases)/AKT signaling pathway. GPS shows an anti-proliferation effect in a time- and dose-dependent manner on HeLa cancer cells, in contrast to normal human umbilical vein endothelial cells (HUVEC) at the same dosage of administration. It has been detected that GPS induces apoptosis in a dose- and time-dependent manner in comparison to untreated cells. Moreover, an increased number of cells with bright nuclear condensation and fragmentation of nuclei were observed in HeLa cells stained with Hoechst 33342 after GPS treatment. GPS also caused comet formation in HeLa cells. Furthermore, the tail area, tail DNA percentage, tail DNA, tail moment and tail length were significantly increased, which is correlated with apoptosis after GPS treatment. It has been shown that the mitochondrial pathway involves gentiopicroside-induced apoptosis in HeLa cells. GPS decreased JC-1 aggregates and significantly enhanced the creation of monomers, suggesting that the mitochondrial membrane potential decreased (which is a landmark event in the early phase of apoptosis). The level of cleaved-caspase-8 was decreased whereas the expression of cytochrome c, cleaved-caspase-9, and -3 and AIF (apoptosis-inducing factor) in the cytoplasm were increased. Dose- and time-dependent cell cycle arrest at the G2/M phase was observed in HeLa cells after GPS treatment. Moreover, protein expression of CDK1 (cyclin-dependent kinase 1) and cyclin-B1 were decreased, while p53 and p21 protein expression were increased after GPS treatment. Moreover, GPS strongly alters the AKT and MAPK-related proteins, which inhibits the phosphorylation of p38, Erk1/2 and AKT, while the phosphorylation levels of c-Jun and JNK (c-Jun N-terminal kinase) were significantly elevated. It has been revealed that GPS effectively inhibits the migration of cancer cells in a dose-dependent manner. The number of metastatic cells created after GPS treatment was significantly lower than in the control group. The protein expression of MMP-2 (matrix metalloproteinase-2) was significantly downregulated, while the level of MMP-9 (matrix metalloproteinase-9) was not altered. All these results suggest that GPS inhibits the migration and effectively suppresses the metastatic potential of HeLa cells through down-regulating the MMP-2 expression [
44].
4.14.3. Gastric Cancer
Gastric cancer is one of the most widespread malignant tumors in the digestive tract [
45]. Gastric adenocarcinoma is the fifth most common and the third most lethal type of cancer worldwide. Huang
et al. [
46] tried to find the molecular mechanisms and potential targets of GPS in gastric cancer using the computational methodology. They also used molecular docking and
in vitro assays to validate the effect of GPS in gastric cancer cells. It has been revealed that GPS can result in multiple effects in gastric cancer cells by regulating multiple pathways. Huang
et al. found 135 targets for GPS in gastric cancer. Docking analysis demonstrated that GPS has a good binding activity to cyclin-D1 (CCND1). The authors selected AKT, p38, CCND1 and CCNE1 as the potential targets of GPS. The level of expression of phosphoro-p38 (p-p38) protein was significantly lower after GPS treatment compared with the control group. However, the expression of CCND1, CCNE1 and p-AKT proteins was significantly higher. PI3K/AKT signaling pathway is one of the most frequently activated signal transduction pathways in cancer biology and is involved in cell proliferation, growth, migration, apoptosis, cell cycle and angiogenesis processes. GPS caused growth inhibition of HGC-27, MGC-803, BGC-823, AGS, and GES-1 human gastric cancer cells with IC
50 values around 62–205 μM for 48 hours. The HGC-27 cell line was the most sensitive to the cytotoxic effect of GPS with IC
50 value of 62.03±7.63 μM, whereas GES-1 cells were the less sensitive. It has been also reported that GPS was selectively active against human gastric cancer cells in contrast to normal gastric epithelial cells. The number of HGC-27 apoptotic cells was significantly higher after GPS treatment. Cell cycle distribution demonstrated, that the proportion of HGC-27 gastric cancer cells was increased in the G0/G1 phase and decreased in the S phase in the GPS-treated group [
47].
4.14.4. Hepatocellular Carcinoma
Hepatocellular carcinoma is one of the most common cancers worldwide, responsible for approximately one million deaths every year, mostly in sub-Saharan Africa and the Far East. Hepatocellular carcinoma presents very often at an advanced stage and has a very poor prognosis [
48]. GPS and five other kinds of traditional Chinese medicine extracts can lesion SMMC-7721 human hepatocellular carcinoma cells; however, the exact mechanism of action is not yet known [
49].
4.14.5 Other Types of Cancer
GPS was proved to exhibit anticancer effect when tested in other cancer models as well. In the manuscript by Antoniadi
et al. [
50] the antiproliferative action of GPS was reported for the following cancer cell lines: glioblastoma (T98G), lung (A549, NCI-H1299), rhabdomyosarcoma (TE671), breast (MCF7, MDA-MB-468), colon (HT-29, DLD-1) cancer cells and normal colon epithelial cells (CCD 841 CoTr). The strength of GPS action was dose dependent in the MTT assay and it was evident in the tested cancer cell lines, however, TE671 cells were the most sensitive (IC
50 = 78 µg/mL) to GPS, whereas the MDA-MB-468 cell line was the least sensitive (IC
50 = 208 µg/mL) among the tested ones to the GPS treatment.
Table 1 and
Figure 2 list the major points of the GPS anticancer activity proved in the
in vitro studies.
5.1. Possible Long-Term Adverse Effects of GPS as a Single Compound
The possible long-term adverse effects of GPS such as genotoxic, mutagenic, and clastogenic properties (5, 10, 25 and 50 µg/mL)were tested on the compound isolated from roots of
Cephalaria kotschyi (Dipsaceae) - an endemic plant from Caucasus region. For this purpose the
Salmonella typhimurium mutagenicity assay (Ames test) (TA97a, TA98, TA100, and TA102), the alkaline comet assay, and the
in vitro micronucleus assay on Chinese hamster ovary (CHO) cells, have been studied by Mustafayeva
et al. [
8]. The results have presented evidence that GPS exerts a genotoxic activity against both prokaryotic and eukaryotic cells. The specific positive response obtained with the TA102 tester strain suggested the involvement of oxidative DNA lesions, probably due to the presence of hydroxyl-groups that may produce oxygen singlets. When GPS was used as the one component of the plant extract no toxicity was observed, however, to gain more information long term study should be undertaken. However, the comparison between GPS molecular structure with predictive models in computational databases (Bioinformatics 2000, 2006) revealed a weak structural similarity to established genotoxic and mutagenic agents.
To analyse the toxicity of GPS isolated from
Gentiana lutea root Sobot
et al. [
52] conducted the experiment on peripheral blood mononuclear (PBMC) cells, often used model in toxicological studies. PBMCs were isolated from blood samples obtained from healthy donors 20-40 years. A part of PBMCs (T-lymphocytes) were transformed into blast forms with phytohemaglutinin (PHA). Cell culture was tested in viability (Tb and XTT viability assays) and genotoxicity (Alkaline comet assay) employing
Gentiana lutea root water extract (1:5, m/V ratio) and single GPS (20-130 µM), respectively. The other isolated secoiridoids, having similar structure, were also tested. Since PHA-stimulated PBMCs exhibit higher level of repair capacity compared to unstimulated, their parallel testing could reveal toxicity potential grade of structurally similar compounds. GPS at the concentration of 50 µM reduced the number of viable unstimulated PBMCs cells for 25% and significantly reduced the number of viable PHA-stimulated PBMCs compared to the control, ranging from 13 to 16%. Interestingly, GPS concentration in gentian root extract was 10 times higher than in GPS treatment exerting significant toxic effect, indicating that single isolated compound have different effect than when it is as one of the main components in crude extract.
6. Pharmacological Properties of Gentiopicroside In Vivo
According to the scientific databases recent years bring the development of
in vivo studies on gentiopicroside. The search performed in the Scopus database showed that the number of scientific articles that cover the topic of
in vivo testing of gentiopicroside reached 8 positions, whereas prior to 2014, there was 1 article released annually.
In vivo studies on GPS are conducted in various directions. The compound is researched for its antimicrobial, anti-inflammatory, central nervous system targeting, osteogenic, antidermatophyte, anti-diabetic or weight reducing properties. The detailed characteristics of the aforementioned properties are presented below and in the
Table 2 and
Figure 2.
In the study of Jia and co-investigators [
53] GPS was administered intragastrically at the concentrations of 20 and 40 mg/ kg b.w. for a period of 30 days to male mice C57BL/6J with arthritis induced by an intradermal injection of chicken type collagen II. A total of 30 animals were used in the study. As a consequence of GPS treatment an inhibition in paw edema was observed for both tested doses of GPS, however, therapeutic effects (reduced mechanical allodynia) of the dose of 40 mg/ kg appeared earlier – after 8 days of treatment in comparison to 22 days for 20 mg/ kg b.w. Furthermore, both doses reduced the inflammatory infiltration and joint destruction in comparison to the negative control group. Treatment with GPS reduced changes in the joints and prevented from bone damage as proved by micro-CT imaging. The described physiological impact of the compound on skeletomuscular system was explained by its molecular mechanism of action. GPS was proved to decrease serum cytokines levels (IL-1β, IL-6, and TNF-α), suppress CD147, p-p38, p-IκBα, MMP1, MMP2, and MMP3,
in vivo.
Proved anti-inflammatory potential of GPS encouraged the scientists to study the properties of its semi-synthetic derivatives. In the study of Zhang and collaborators [
19] 26 novel derivatives of the secoiridoid were assessed for their potential in xylene-induced mouse ear swelling model in KM mice. In their study GPS decreased the edema percentage by 34.17 % after 7 days administration of a dose of 0.28 mmol/ kg b.w.
Xu
et al. [
30] described the application of GPS in diabetic renal fibrosis – a symptom which is a consequence of neuropathy developed with the progression of diabetes mellitus and hyperglycemia that leads to an excessive deposition of extracellular matrix in the kidneys that disturbs their physiological functioning. The study was performed on 6-week-old male db/db mice that were administered intragastrically different doses of GPS: 50, 100 and 200 mg/ kg b.w. for 10 consecutive weeks. Valsartan (10 mg/kg b.w.) was used as a positive control and healthy male C57/BL6 mice – as a control group. Animals treated with GPS were found to have ameliorated metabolism of lipids and glucose that was confirmed by a decreased level of HbA1c, GSP, LDL-C, TG, body weight at all tested doses and FBG (after 3 weeks of treatment). Also, the compound supported renal functions. The levels of creatinine, urea nitrogen and albuminuria were significantly decreased in the treated animals. Staining tests showed an increased expansion in the lumen, glycogen accumulation in the renal cortex of the treated group and lowered degree of inflammation and fibrosis in the renal tubule. The latter effects were explained by an alleviated level of FN, α-SMA, and vimentin and increased expression of E-cadherin after GPS treatment. As a result of these studies the authors denoted that the secoiridoid suppresses the AT1R/CK2/NF-κB pathway.
According to Lu and collaborators [
54] GPS decreased hyperalgesia of Sprague Dawley rats stimulated with hot and cold and mechanical allodynia. The application of GPS in the treatment of diabetic peripheral neuropathy was suggested by the authors as the compound was capable of restoring nerve blood flow, improve moter nerve conduction velocity and sensory nerve conduction velocity parameters and regulate dyslipidemia. GPS influenced the expression of genes: of the PPAR-γ/AMPK/ACC signal pathway.
The anti-inflammatory properties of GPS were also tested in a gouty arthritis model triggered by the injection of monosodium urate into the paw of male C57BL/6 mice [
55]. The treatment was conducted 24 h long, with 50, 100, 200 mg/kg of GPS administered orally and colchicine (1 mg/kg) that was used for a separate group as a positive control. The secoiridoid at the doses 100 and 200 mg/ kg showed a reduction of swelling, exhibited analgesic properties and inhibited thermal hyperplasia. The same doses of GPS were directly engaged in the reduction of inflammatory neutrophil infiltration, alleviation of the levels of interleukins: IL-1β, IL-6, IL-18, TNF-α, caspase-1, NOD-like receptor protein 3, and ACS.
Another mechanism of anti-inflammatory action was proposed by Wang
et al. [
22]. According to the authors GPS alleviates the action of ROS-NF-kB-NLRP3 axis in synoviocytes and also NF-kB pathway. The study on male Sprague-Dawley rats with induced arthritis were administered 100 and 200 mg/ kg GPS for 14 days. As a consequence of the proposed therapy 200 mg/kg dose led to the reduction of paw swelling and decrease in arthritis index value. Histopathological analysis of joints showed a reduced inflammation, bone destruction, synovial hyperplasia and pannus formation, in comparison to the untreated group, with no impact on body weight or spleen index, but a slight reduction in thymus index value. The analysis of animal synovial tissue confirmed an inhibition of IkBa degradation and a reduction in p-IkBa and p-p65 expression levels by GPS treatment, which suggest joint protective role of this natural product and confirm its action is related to NFkB signaling.
The secoiridoid administered orally at a dose of 50 mg/ kg b.w. for 12 weeks to mice fed with high fat diet was proved to inhibit all key adipogenic transcription factors, like PPARγ, C/EBPα, SREBP-1c, to down-regulate the expression of genes related to the transport of fatty acids, like lipid uptake gene, fatty acid (FABP4) and triglycerides (TG) transport-related gene, but also synthesis related genes (DGAT2, FAS, SDC1) [
56]. Also, the confirmed anti-inflammatory properties of GPS were expressed as regulatory towards the inflammatory genes, leading to a decreased cytokines release helped to reduce the weight of animals together with visceral fat mass decreasing the size of adipocytes.
GPS was also tested for its potential application in the treatment of alcoholic hepatosteatosis. The study of Li
et al. [
57] that was performed on male C57BL/6 mice that were treated with GPS and fed with ethanol in acute (3 days) and chronic (10 days) experiments showed several protective properties exhibited by the secoiridoid. Already a lower dose of 40 mg/ kg b.w. in the chronic administration prevented from the increase of ALT and AST levels as well as TG levels in serum and liver. The formed lipid droplets in the liver were smaller and less abundant. Also, increased LKB1 and AMPK phosphorylation levels were noted, SREBP1 protein expression was alleviated, phosphorylation of ACC was restored, PPARα regulatory genes were down-regulated. A protective role of GPS may be based on the inhibition of lipogenesis that occurs upon the influence of ethanol and through the elevation of lipid oxidation. GPS decreased the alcohol-induced expression of the receptors NLRP3 and P2X7, but only as a consequence of alcohol toxicity.
Anti-inflammatory properties of GPS could find application in the treatment of pulmonary fibrosis [
58]. The compound administered to male SPF mice Kunming strain intraperitoneally for 28 days decreased the concentration of hydroxyproline in the lung tissue in a dose dependent manner and ameliorated both fibrotic parameters (alveolar space narrowing, alveolar wall thickening, deposition of collagen) and inflammatory responses. The formation of degenerated typelalveolar epithelial cells and also capillary basement membranes was ameliorated in the animal group with GPS treatment. The described properties occurred together with the decreased levels of TNF-α, IL-1β and TGF-β1 that were elevated in bleomycine-induced fibrosis group. The dose of 10 mg/ kg b.w. was found to act more significantly. GPS at a dose of 30 µM was found to exert anti-inflammatory properties in a zebrafish model of COX-2 enzyme inhibition [
59] in fish that were treated with 12-Otetradecanoylphorbol 13-acetate (TPA) to induce inflammation. GPS was found to inhibit the enzyme at 65 ± 5 %, whereas the antiinflamamtory drug – indometacin at a concentration of 0.9 µM was prone to inhibiti the enzyme at 56 ± 1 %..
According to Jiang
et al., GPS promotes osteogenesis [
23]. In a 3-month long study on ovariectomized female C57BL/6 mice the secoiridoid at a daily dose of 50 mg/ kg b.w. (oral gavage) promoted alkaline phosphatase activity, increased the level of osteogenic factors like Runx2, OCN, BMP2 and thickness and number of trabeculae after the performed therapy what was visualized in micro-CT test. These observations provide scientific evidence on the possibility of GPS application in regulating bone metabolism.
GPS was proved to cross the blood-brain barrier and exhibit central functions. Several scientific papers describe its antidepressant activity. In the study of Liu
et al. [
60] who used a mouse model of reserpine-induced depression GPS was administered intragastrically to male animals twice daily for 3 days and once on the fourth day before behavioral testing in three doses: 50, 100 and 200 mg/kg b.w. GPS at two highest doses decreased mechanical allodynia, immobility time in forced swimming test and tail suspension test, increased the traveled distance and the time in the center area in the open-field test. The observed behavioral changes in the studied animals were possibly triggered by the changes in thneuromodulator’s levels. GPS reversed the activity of reserpine that decreased the levels of norepinephrine, dopamine and serotonin. However, when administered alone at a dose of 100 mg/kg, it did not cause any changes in the neurotransmitter levels. Also, GPS reduced the intracellular levels of MDA production in basolateral amygdala (BLA) of treated mice, elevated the activity of catalase in their tissue, Bcl-2 expression and inhibited the activity of caspase-3 in a dose dependent manner. Administration of reserpine led to an increased expression of AMPA and NMDA subunits of BLA homogenates. In this study GPS was not affecting the expression of GluA1 and GluN2A subunits, but decreased the expression of GluN2B in reserpinized mice, which – according to the authors – may contribute to the attenuation of depression dyad in tested mice.
In the corticosterone-induced model of depression applied by Yao and co-investigators [
61] on rats led to the recognition of following molecular mechanisms of GPS’s action that was administered by gastric instillation at a dose of 100 mg/kg b.w. 2 h prior corticosterone for 21 days. The authors proved a protective role of the compound against the steroid: the ability to inhibit apoptosis in hippocampus that was caused by the steroid, to reduce the loss of Niss bodies, to increase the level of serotonin in the brain tissue, to elevate the expression of BDNF and alleviate caspase 3 and bax expression in brain. HPLC-MS based chemometric analysis of GPS-treated and untreated groups showed marked differences in the metabolite profiles between the groups. The former group was characterized with alleviated levels of sphinganine, steroylethanolamide and guanosine, and decreased level of arachidonic acid, oxoadipic acid, L-phenylalanine and thiamine. Pathway analysis provided evidence on the influence of GPS on sphingolipids, glycine/serine/threonine and pyrimidine metabolism.
Deng
et al. [
62] in their study on BALB/C mice with lipopolysaccharide-induced depression that were administered with GPS (50 mg/ kg b.w. i.p.) once a day for 3 days observed an alleviated activation of tryptophan metabolic pathways, a reduction in TNF-α and IL-1β levels in brain (BLA) and plasma, and confirmed the expression of GluN2B subunit of NMDA receptor. Also, down-regulation of indoleamine 2,3-oxygenase by GPS was noted. According to the authors the antidepressant effect that was cisualized in behavioral studies in forced swimming test and tail suspension test and protection against the injected lipopolysaccharide may be at least partially influenced by the ability of GPS to block different steps of tryptophan-degrading pathway.
As mentioned above GPS shows an antimicrobial potential. In the study published by Almukainzi and co-investigators [
63] its antimicrobial properties were used in diabetic rats with infected wounds. GPS together with thymoquinone that were released from the constructed nanofibers led to a speedy recovery of the tissue and triggered the healing process. Another study of these authors [
35] that was performed on streptozotocin-induced diabetic rats intended to analyze histologically the state of would incisions at different day intervals after the application of PLGA nanoparticles loaded GPS. Interestingly, the tested formulation administering orally 300 mg/kg b.w. GPS, by increasing the bioavailability of GPS poorly dissolved in water, enhanced the antimicrobial effect in model animals (Sprague-Dawley rats) in comparison with free GPS. The proposed treatment led to the complete wound healing and wound closure within 12 days. Free GPS was found to induce wound healing properties; too, however the recovery time was longer and might have needed more often administration. The regenerated tissue was normally organized with developed mature collagen fibers, minimal number of inflammatory cells and physiological vasculatures.
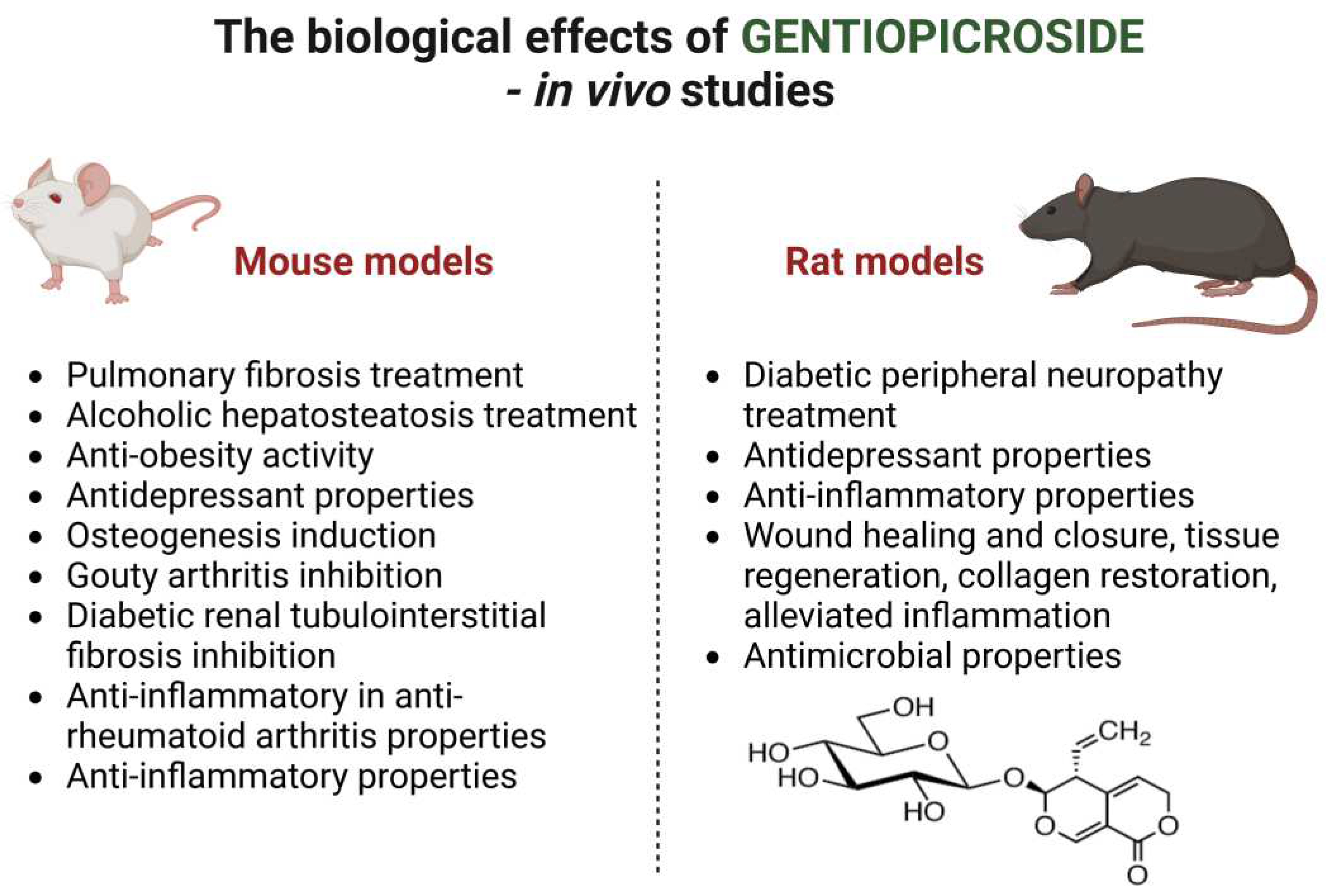
The
Table 2 and
Figure 3 summarize the biological effects of gentiopicroside in
in vivo studies conducted on animals; mice and rats.
5. Conclusions and Future Perspectives
Cosuming food products, dietary supplements or medicines we deliver majority of various non-nutrients, including bioactive or toxic compounds [
65]. Gentiopicroside is a multifunctional metabolite which exerts various pharmacological effects proved in the
in vitro and
in vivo models. It interacts with different receptors present in various tissues and organs, however, the detailed mechanisms still need to be extensively studied. It is also still challenging to understand the role of T2Rs polymorphism in the body, and its’ correlation with such disease as asthma, cardiovascular disease, glucose and insulin homeostasis, Parkinson disease, colorectal cancer and thyroid problems [
2]. All of these may be the target of the GPS, and being the area of the future research. If in the future some new models will be designed and accesible, the detailed studies of the interaction of GPS in various, tissue specific bitter taste receptors may be possible, and it is definitelly worth of trying, as it could be beneficial from the therapeutic points of view. The new solutions in
in vitro studies as recently introduced 3D cell cultures or organoids could be interesting, being a next step in the evaluating of the biological activity of GPS. Taking into account the various possible effects of GPS in the body, and the fact that the barriers to the action of this compound on some tissues still seem to be unresolved, new therapeutic approaches taking into account the possible better bioavaiability of GPS could be beneficial. The health benefits of GPS cannot be overestimated if it would be possible to introduce new drug formulations (such as microencapsulation, nanoparticles, liposomes or micelles) to increase the bioavailability of this compound and its distribution to the target organs and tissues [
1,
35]. It enables to conduct the clinical studies of GPS (until this time not so many) to establish bioactivity, efficacy and potential toxicity/safety of GPS as an active natural molecule with great therapeutic potential, especially in the neuroinflammatory and metabolic diseases.
Author Contributions
Conceptualization, W-K-K, MB, A.A., M.H., L.A.; methodology, all authors.; formal analysis, L.A, W-K-K, MB; data curation, W-K-K, MB, L.A, AA; writing—original draft preparation, all authors, writing—review and editing, W.K-K, MB, A.W., M.H., A.A.; supervision, W.K-K, A.A., L.A.S., M.H..; project administration, W-K-K, L.A.S., M.H.; funding acquisition, A.W., L.A.S.. All authors have read and agreed to the published version of the manuscript.