1. Introduction
The ubiquity of cardiovascular ailments, particularly Acute Coronary Syndrome (ACS), in contemporary healthcare landscapes is a stark reality. With alarming mortality rates and propensity for rapid deterioration, ACS has emerged as a focal concern in emergency medical care, necessitating innovative and precise diagnostic measures [
1]. Consequently, the demand for accessible and efficacious evaluation methods in the emergency department (ED) has never been high. In recent years, the Perfusion Index (PI), a non-invasive parameter, has attracted the attention of medical researchers and practitioners [
2]. The PI, derived from infrared signals through a finger probe, computes the ratio of pulsatile to non-pulsatile components of peripheral blood flow. It serves as a nuanced monitor of a patient’s cardiac output and overall hemodynamics, transcending the boundaries of traditional vital sign measurements [
1]. Acute Coronary syndrome, with its capricious clinical trajectory and potential to abruptly escalate into life-threatening scenarios, necessitates an urgent, precise, and comprehensive diagnostic strategy. As a formidable global health adversary, ACS demands not merely a timely but also an astutely accurate intervention to thwart its potentially devastating consequences. While conventional diagnostic approaches, such as electrocardiograms (ECGs) and cardiac biomarker analysis, have remained pivotal, their utility can be substantially amplified when synchronized with innovative tools like the Peripheral Perfusion Index (PI).
The merit of PI emerges vividly in its capacity to instantaneously unravel the complexities of hemodynamic parameters, shedding light on physiological realms that are ordinarily less palpable through traditional monitoring [
3,
4,
5,
6]. With its potential to deftly differentiate diagnostic conundrums, adeptly assess risk, and proficiently navigate the intricate management maze of ACS, PI promises not only an enhanced diagnostic acumen but also a more nuanced patient-centric care strategy in the emergency department [
4].
Technological advancements in the realm of cardiovascular diagnostics, such as PI, digital pulse amplitude tonometry, and flow-mediated brachial artery dilation, underscore a vibrant evolution in emergency care, where precise, timely, and patient-friendly diagnostic tools are progressively altering the medical landscape. While the innovative potential of these methodologies has been demonstrated through improved patient assessments and outcomes, their widescale implementation is often tempered by challenges encompassing financial investments and the requisition of specialized training [
7,
8].
Conversely, PI, with its inherently non-invasive methodology and the ability to indirectly assess microvascular peripheral perfusion, emerges as a notably accessible and versatile diagnostic adjunct. The preliminary indications of PI’s efficacy in evaluating cardiac function, particularly in critical, high-stakes contexts such as ACS, is emblematic of a broader movement towards incorporating noninvasive diagnostic strategies into the emergency care matrix [
3,
4,
6].
As ACS continues to assert its presence in emergency care, the integration of PI may pave the way for a more nuanced and agile response, aligning with the broader mission of enhancing the accuracy, timeliness, and efficiency of emergency care for cardiovascular patients.
Leveraging insights from foundational research that explored the relationship between the PI and coronary artery diseases, the present study aims to meticulously examine the comparative efficacy of peripheral perfusion indices and blood gas lactate values in patients diagnosed with ACS in the ED. Karaman et al. [
2] probed into the role of the Peripheral Perfusion Index in differentiating diagnoses between cardiac and pulmonary-origin dyspnea within the emergency service domain. Conversely, Chequer et al. [
9] embarked on an investigative journey into the noninvasive assessment of ST-segment alterations during exercise testing in coronary artery disease through the lens of PI, while Menezes et al. [
1] directed their investigative focus towards the exploration of endothelial functions in atherosclerosis employing PI as a critical tool in their research methodology [
19].
ACS, characterized by its unpredictable trajectory and potential severity, commands a swift and accurate diagnosis. The urgency for timely intervention cannot be overstated in the face of the global menace that the ACS poses. Conventional diagnostic pathways, while indispensable, are complemented by novel tools, such as PI. This alignment with noninvasive technologies such as PI can potentially sharpen diagnostic precision and expedite crucial treatments. The virtue of PI lies in its ability to provide instantaneous insights into hemodynamic parameters. Its role in differentiating diagnoses, assessing risks, and navigating the complex landscape of ACS management is promising. The integration of PI within the ED, akin to emerging technologies in other circulatory disease contexts, symbolizes an exciting frontier in patient care.
Newer methodologies such as PI, akin to digital pulse amplitude tonometry, and flow-mediated brachial artery dilation in the assessment of peripheral arteries highlight the evolving arsenal in emergency care diagnostics. While these techniques have shown valuable results in patient assessment, their implementation often faces barriers, such as high costs and the need for specialized training. In contrast, PI’s non-invasive nature and indirect measurement of microvascular peripheral perfusion make it a potentially versatile and accessible tool. Although preliminary, the use of PI to evaluate cardiac function in critical scenarios, such as ACS, aligns with the trend of maximizing noninvasive evaluations.
As ACS continues to assert its presence in emergency care, the integration of PI may pave the way for a more nuanced and agile response, aligning with the broader mission of enhancing the accuracy, timeliness, and efficiency of emergency care for cardiovascular patients. Building on prior foundational studies, such as those by Karaman [
2], Gürmen [
3], and others [
1,
4], this research aimed to delve into the comparative efficacy of peripheral perfusion indices and blood gas lactate values in patients diagnosed with ACS in the ED. By synergizing novel and traditional diagnostic methods, we aimed to elucidate the potential of PI in enhancing patient management protocols in ACS scenarios.
As emergency medicine continues to evolve in response to new diagnostic techniques and the ever-increasing demands of acute care, the integration of noninvasive tools such as PI may offer a transformative approach to patient care. This study seeks to shed light on the potential of PI, hoping to contribute meaningfully to the broader discourse on optimizing ACS management in emergency settings.
2. Materials and Methods
2.1. Study Design and Population
Our team embarked on a meticulous, prospective observational study unfolding from January 1 to July 1, 2023, set within the bustling environment of our emergency department. The focal point of this investigation was a distinctive population: patients greeted with the ominous diagnosis of Acute Coronary Syndrome (ACS) upon their unforeseen arrival at the department. Our investigation peered into the intricate nuances of ACS, aiming to shed light on the myriad facets of its occurrence, diagnosis, and subsequent management, thereby contributing to the ever-expanding reservoir of clinical knowledge pertaining to cardiac emergencies.
2.2. Inclusion Criteria
In a bid to uphold the integrity and specificity of our study, a well-defined eligibility criterion was established. Only individuals diagnosed with ACS during the specified study window were brought under the investigative lens. Conversely, exclusion criteria were judiciously identified to mitigate confounding variables, these encompassed patients below the tender age of 18, those who faced the calamity of cardiopulmonary arrest, and individuals with a history mired by prolonged cardiopulmonary resuscitation (CPR) during their presentation, thereby ensuring a homogenous and relevant study population.
2.3. Procedure
Upon the precarious entry of patients into the emergency department, an emergency service assistant physician, seasoned with three years of unbridled exposure and experience in emergency service, meticulously validated the ACS diagnosis. Subsequent to this critical verification, an exploration into the Peripheral Perfusion Index (PI) was embarked upon, utilizing a specific device dedicated to such measurements. Ensuring a systematic and error-free documentation, data for each patient were scrupulously logged using a pre-structured case form, designed to harness vital clinical information efficiently and accurately.
2.4. Data Collection
The data, an amalgamation of quantifiable clinical snapshots, was conscientiously recorded by the emergency service assistant. This encompassed a rich tapestry of patient demographics, traversing age, sex, along with indispensable clinical metrics like blood gas parameters and pivotal cardiac biomarker readings. This data, not merely numbers and values but a story of each patient’s physiological state, was assimilated with the utmost precision to enable a holistic understanding of their clinical picture.
2.5. Consultation and Diagnosis Confirmation
Ensuring robustness in diagnostic validity, all patients shrouded with the suspicion of ACS underwent a thorough consultation with the esteemed cardiology department. The seasoned experts within the cardiology department lent their profound insights and recommendations, serving as a bedrock for diagnostic crystallization. This collaborative multidisciplinary approach enhanced the accuracy and reliability of the finalized diagnoses, intertwining clinical acumen with holistic patient care.
2.6. Statistical Analysis
The Kolmogorov–Smirnov test was employed to determine the normality of the data distribution. Pearson’s chi-squared test was used to discern relationships between categorical variables, while the Mann-Whitney U test was used to assess relationships between numerical variables. Continuous variables are presented as mean ± standard deviation (SD). Statistical significance was set at P < 0.05. The ANOVA test further bolsters the analytical approach. All statistical computations were conducted using the Statistical Program for the Social Sciences (SPSS), version 18.0.
2.7. Ethical Considerations
This study was conducted in accordance with ethical guidelines and was approved by the non-interventional clinical research ethics committee at the Tekirdag Namik Kemal University on 27.12.2022, with Approval Number 2022.225.12.03. Informed consent was obtained from all participants, and confidentiality was maintained throughout the research process.
3. Results
The study cohort consisted of 90 individuals subdivided into two groups: patients diagnosed with the condition under investigation (n=60, 66.7%) and control subjects (n=30, 33.3%). The sex distribution was equally balanced across both groups, with 45 males (50%) and 45 females (50%). Among the patients, prevalent comorbidities were identified, including diabetes mellitus (DM) in 29 (32.2%), hypertension (HT) in 45 (50%), chronic heart failure (CHF) in 10 (11.1%), chronic kidney disease (CKD) in 6 (6.7%), coronary artery disease (CAD) in 40 (44.4%), and chronic obstructive pulmonary disease (COPD) in 7 (7.8%).
Table 1 presents the general characteristics of the study cohort in detail.
A cohort of 90 individuals was further delineated into specific diagnostic categories within the emergency department. The control group comprised of 30 participants (33.3%). Among the patients, 15 were diagnosed with Unstable Angina Pectoris (USAP), constituting 16.7% of the cases, 31 with Non-ST Elevation Myocardial Infarction (NON-ST MI), representing 34.4%, and 14 with ST-Elevation Myocardial Infarction (ST MI), comprising 15.6%. Following the emergency department evaluation, the disposition of patients varied. Thirty control cases (33.3%) were recorded, along with 16 transfers to the coronary angiography unit (17.8%), 43 admissions to the coronary intensive care unit (47.8%), and one death (EX), representing 1.1% of the cases.
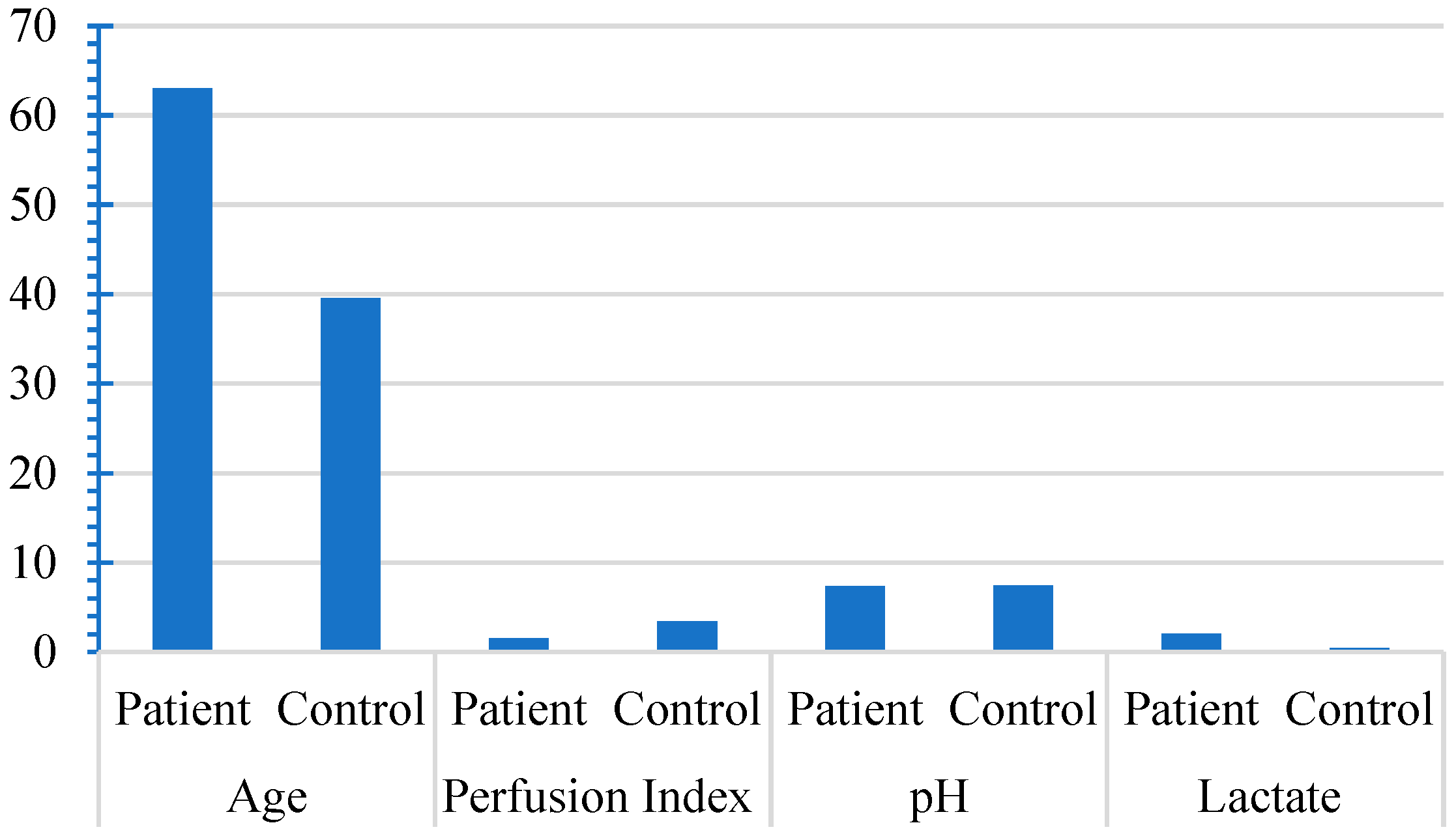
Upon conducting a detailed comparative evaluation of the patient and control cohorts, salient variations in specific clinical parameters were evident. The patient group exhibited an older mean age of 63.03 compared with 39.57 in the control group (p<0.001), indicating a significant age difference. Additionally, the perfusion index in the patient group was notably lower, with a mean value of 1.55, compared to 3.39 in the control group (p<0.001). However, pH levels did not differ significantly between the two groups. Lactate levels were significantly higher in the patient group (mean of 2.02, compared than 0.41 in the control group (p<0.001). These findings highlight the distinctive profiles of the two groups, with significant differences in age, perfusion index, and lactate levels (
Figure 1). These differences are listed in
Table 2.
Table 1.
Demographic and clinical characteristics of acute coronary syndrome patients and controls.
Table 1.
Demographic and clinical characteristics of acute coronary syndrome patients and controls.
| Variable |
Subcategory |
Count (n) |
Percentage (%) |
| Cohort |
Patient |
60 |
66.7 |
| Control |
30 |
33.3 |
| Gender |
Male |
45 |
50 |
| Female |
45 |
50 |
| Diabetes Mellitus (DM) |
Yes |
29 |
32.2 |
| No |
61 |
67.8 |
| Hypertension (HT) |
Yes |
45 |
50 |
| No |
45 |
50 |
| Congestive Heart Failure (CHF) |
Yes |
10 |
11.1 |
| No |
80 |
88.9 |
| Chronic Kidney Disease (CKD) |
Yes |
6 |
6.7 |
| No |
84 |
93.3 |
| Coronary Artery Disease (CAD) |
Yes |
40 |
44.4 |
| No |
50 |
55.6 |
| Chronic Obstructive Pulmonary Disease (COPD) |
Yes |
7 |
7.8 |
| No |
83 |
92.2 |
| Reason for Emergency Department Visit |
Control |
30 |
33.3 |
| Unstable Angina (USAP) |
15 |
16.7 |
| Non-ST Elevation Myocardial Infarction (NON-ST MI) |
31 |
34.4 |
| ST-Elevation Myocardial Infarction (ST-ELEVE MI) |
14 |
15.6 |
| Outcome |
Discharged with Control |
30 |
33.3 |
| Referred to Angiography Unit |
16 |
17.8 |
| Admitted to Coronary Care Unit (CCU) |
43 |
47.8 |
| |
Expired (EX) |
1 |
1.1 |
| Total |
- |
90 |
100 |
Table 2.
Comparative analysis of Age, Perfusion Index, pH, and Lactate levels between patient and control groups.
Table 2.
Comparative analysis of Age, Perfusion Index, pH, and Lactate levels between patient and control groups.
| Observed Parameters |
Group |
N |
Mean |
Standard Deviation |
Median |
P-value |
| Age |
Patient |
60 |
63.03 |
10.84 |
63.00 |
0.001* |
| Control |
30 |
39.57 |
10.79 |
39.00 |
|
| Perfusion Index |
Patient |
60 |
1.55 |
0.86 |
1.400 |
0.001** |
| Control |
30 |
3.39 |
0.83 |
3.300 |
|
| pH |
Patient |
60 |
7.38 |
0.07 |
7.390 |
0.177* |
| Control |
30 |
7.40 |
0.03 |
7.390 |
|
| Lactate |
Patient |
60 |
2.02 |
2.07 |
1.500 |
0.001** |
| Control |
30 |
0.41 |
0.20 |
0.355 |
|
Figure 1.
The means of perfusion index, pH, and lactate between patient and control groups.
Figure 1.
The means of perfusion index, pH, and lactate between patient and control groups.
The dataset was meticulously analyzed to explore the relationships between various continuous variables, with particular attention given to the correlation between arterial blood gas pH and lactate values. Nuanced and multifaceted interconnections were discerned among the assessed variables. A prominent negative correlation emerged between age and PI (r = -0.677, p<0.001), along with a substantial positive correlation between age and lactate levels (r = 0.426, p<0.001).
The core investigation of the analysis, focusing on the relationship between arterial blood gas pH and lactate values, revealed a significant negative correlation (r = -0.301, p=0.004). This association implies that decreased pH values tend to coincide with increased lactate levels in the study population. The intricacies of these correlations are detailed and synthesized in
Table 3, providing valuable insights into the underlying dynamics of the examined parameters.
Table 3.
Interrelationships among Age, Perfusion Index, arterial blood gas pH, and Lactate levels in the study population.
Table 3.
Interrelationships among Age, Perfusion Index, arterial blood gas pH, and Lactate levels in the study population.
| Observed Parameters |
|
Age |
Perfusion Index |
pH |
| Perfusion Index |
r |
-0.677 |
|
|
| p |
0.000 |
|
|
| pH |
r |
-0.199 |
0.174 |
|
| p |
0.060 |
0.101 |
|
| Lactate |
r |
0.426 |
-0.461 |
-0.301 |
| p |
0.000 |
0.000 |
0.004 |
4. Discussion
In the present study of 90 participants, we investigated how well the peripheral perfusion index and blood gas lactate values could diagnose acute coronary syndrome. Neither PPI nor lactate values were very reliable in predicting who would be hospitalized or exactly determining the type of ACS. This is different from what some other studies suggested. As far as we know, ours is the first study to closely link PPI and lactate values to ACS diagnosis. Our participants, generally older with specific PPI and lactate trends, reflected what is commonly seen in ACS patients. Yet, our results highlight the need to better understand the use of PPI and lactate in diagnosing ACS and perhaps to explore other methods or indicators.
This investigation of a cohort of 90 individuals, focusing on the diagnosis and differentiation of ACS using PPI and blood gas lactate values, has revealed significant insights into the complex clinical scenario. The patient pool reflected a diverse spectrum of prevalent comorbidities and ACS presentations commonly encountered in emergency departments.
The cohort included patients of both sexes with various common comorbidities, such as diabetes, hypertension, and heart disease. It is divided into specific diagnostic categories, such as Unstable Angina and different types of Myocardial Infarction. This diversity reflects the complexity of real-world patients in the ED, supporting the validity of this study and enabling a more nuanced analysis of the ACS spectrum.
The central finding of our study was that neither PPI nor blood gas lactate values were effective in predicting hospitalization and discriminating the ACS form. Contrary to expectations, PPI failed to serve as an effective marker of peripheral perfusion, hospitalization, or mortality in this study. Despite the demonstrated use of PPI as an objective indicator of peripheral circulatory disorders, our findings, consistent with previous reports [
9,
10,
11], showed that PI was an insignificant parameter for deciding hospitalization or diagnosing outcomes of ACS in the ED. PPI has become recognized as a straightforward and noninvasive method for indicating compromised peripheral perfusion in critically ill patients [
11,
12]. Initially, Lima et al. [
13] established that in intensive care settings, a PPI value of 1.4 or lower served as a strong sign of reduced perfusion [
13]. Pirneskoski et al. [
12] suggested that measuring the pulse photoplethysmography wave amplitude might function as an assessment tool in the ED [
14]. This could imply that PPI might act as a determinant of hospitalization and fatality rates in ED patients. Our findings align more closely with Oskay et al. [
15]’s report that PPI use was insignificant in predicting ED outcomes [
15].
In our research, the study most closely aligned with ours was conducted by Compagnoni et al. [
4]. In contrast to our approach, they evaluated the diagnostic value of Perfusion Index (PI) in coronary artery patients who experienced medically-induced arrests. Specifically, Compagnoni et al. [
3] explored the diagnostic utility of PI in patients who experienced medical out-of-hospital cardiac arrests, particularly for diagnosing ST-Elevation Myocardial Infarction (STEMI). They discovered a correlation between PI values and the percentage of false-positive Electrocardiograms (ECG). This implies that an ECG may reveal transmural myocardial ischemia without the presence of a significant obstructive coronary artery disease that warrants percutaneous transluminal coronary angioplasty (PTCA), especially in patients with low peripheral perfusion following resuscitation. Intriguingly, patients who experienced efficient perfusion post-resuscitation were often those with a lower rate of false-positive ECGs, thereby yielding more dependable diagnostic ECG results. This pivotal finding from their study invites discussion, both from pathophysiological and practical viewpoints. From a pathophysiological perspective, it’s notable that the profound ischemia, induced by the reduced perfusion during the no-flow or low-flow phases of cardiac arrest, is likely to influence post-Return of Spontaneous Circulation (ROSC) ECG findings. Consequently, a decrease in PI values following resuscitation can be anticipated.
The role of lactate levels has emerged as a point of notable interest in our study, despite the findings not substantiating blood gas lactate values as effective in diagnosing ACS. Contrarily, existing literature underscores lactate’s role in prognosticating ACS outcomes, inclusive of ST-Elevation Myocardial Infarction (STEMI) and non-ST-segment elevation myocardial infarction [
1,
2]. Lazzeri et al. [
14] articulate that temporal serial lactate measurements, or lactate clearance, tend to exhibit more clinical reliability than absolute lactate values, even within acute cardiac patient populations [
1]. This is corroborated across various study designs, temporal lactate measurement intervals, and acute cardiac conditions, such as cardiogenic shock and cardiac arrest. A pervasive theme in existing studies denotes that elevated lactate levels upon admission and superior lactate clearance are congruent with non-survival and improved outcomes, respectively.
Furthermore, the presence of hyperlactatemia upon admission has been correlated with suboptimal outcomes in STEMI patients and has demonstrated a surge in 30-day mortality rates in ACS patients according to a large retrospective cohort study by Liang et al. [
17]. Lactate not only emerged as an independent predictor of mortality and complications but also, the zenith of blood lactate within the initial 24 hours has been identified as a predictor of mortality in ACS patients undergoing extracorporeal membrane oxygenation [
3,
4].
Interestingly, lactate levels have drawn significant attention in our study. While our results did not find blood gas lactate values effective in diagnosing ACS, the literature supports the role of lactate in the prognosis of ACS, including STEMI and non-ST-segment elevation myocardial infarction [
10,
11]. For example, hyperlactatemia at arrival has been associated with worse outcomes in patients with STEMI, increased 30-day mortality, and intra-aortic balloon pump use [
11]. In some studies, lactate served as an independent predictor of mortality and complications, such as acute pulmonary edema and arrhythmias [
12,
13].
The patient group in our study, characterized by a higher mean age, lower PPI, and increased lactate levels, mirrors the general trends often noted in patients with ACS. These significant correlations, along with the negative correlation observed between arterial blood gas pH and lactate values, accentuate the multifaceted metabolic and physiological interactions intrinsic to ACS [
2,
20]. They may even hint at underlying compensatory mechanisms at play, as indicated by the absence of significant differences in pH levels.
Additionally, the correlations between age and PPI could be indicative of age-related changes in the peripheral circulation, adding another layer to the complex network of interactions within ACS. The intricate interrelationships among these parameters not only affirm the consistency and generalizability of our study but also elucidate the complexity of the physiological and metabolic aspects of ACS. This compelling evidence calls for further research to unravel these complex interactions and their potential implications in clinical practice.
4.1. Clinical Implications
These results, being the first of their kind in patients with ACS, contribute significantly to our understanding of the role of PPI and blood gas lactate values in this context. The fact that these measures were not effective in diagnosing ACS may guide clinicians in seeking alternative markers or methodologies.
4.2. Limitations
This study should be interpreted in light of its limitations, including the sample size, which may affect the power to detect subtle differences. Furthermore, given that both PI and Blood Gas Lactate Values can be affected by sympathetic activity [
9,
10], a thorough examination of other relevant parameters could enhance interpretation.
5. Conclusions
While our study found that PPI and blood gas lactate values were ineffective in diagnosing ACS in the ED, it adds to the growing body of evidence surrounding the complex relationships between these parameters, age, and various comorbidities. The relevance of lactate as an independent prognostic marker in ACS emphasizes the need for further research to explore its role in diagnostic settings. Our findings also prompt the reconsideration of PPI as an assessment tool in the ED, encouraging more extensive studies to confirm or challenge its applicability in different patient populations and clinical contexts.
Author Contributions
Conceptualization, H.Ş. and M.E.C.K.; methodology, H.Ş. and M.E.C.K; formal analysis, H.Ş. and M.E.C.K.; investigation, H.Ş. and M.E.C.K.; data curation, H.Ş. and M.E.C.K.; writing—original draft preparation, H.Ş.; writing—review and editing, H.Ş. and M.E.C.K.; project administration, H.Ş. and M.E.C.K. All authors have read and agreed to the published version of the man.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Non-interventional Clinical Research Ethics Committee of the Tekirdağ Namık Kemal University (protocol code: 2022.225.12.03, date of approval: 27.12.2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Menezes, I.A.C.d.; Santos, M.R.V.; Cunha, C.L.P.d. Evaluation of endothelial function on atherosclerosis using perfusion index from pulse oximeter. Arquivos Brasileiros de Cardiologia 2014, 102, 237–44. [Google Scholar] [CrossRef] [PubMed]
- Karaman, K.; Sağlam, G.E. The role of peripheral perfusion index in differential diagnosis of cardiac and pulmonary-origin dyspnea in emergency service. Hong Kong Journal of Emergency Medicine 2023, 30, 30–4. [Google Scholar] [CrossRef]
- Sivaprasath P, Mookka Gounder R, Mythili B. Prediction of shock by peripheral perfusion index. Indian J Pediatr 2019, 86, 903–8. [CrossRef] [PubMed]
- Compagnoni S, Gentile FR, Baldi E, Contri E, Palo A, Primi R, et al. Peripheral perfusion index and diagnostic accuracy of the post-ROSC electrocardiogram in patients with medical out-of-hospital cardiac arrest. Resuscitation 2021, 168, 19–26. [CrossRef] [PubMed]
- Daş M, Bardakci O, Siddikoglu D, Akdur G, Yilmaz MC, Akdur O, et al. Prognostic performance of peripheral perfusion index and shock index combined with ESI to predict hospital outcome. The American Journal of Emergency Medicine 2020, 38, 2055–9. [CrossRef] [PubMed]
- Savastano S, Baldi E, Contri E, De Pirro A, Sciutti F, Compagnoni S, et al. Post-ROSC peripheral perfusion index discriminates 30-day survival after out-of-hospital cardiac arrest. Int. Emerg. Med 2021, 16, 455–62. [CrossRef] [PubMed]
- Broch O, Bein B, Gruenewald M, Höcker J, Schöttler J, Meybohm P, et al. Accuracy of the pleth variability index to predict fluid responsiveness depends on the perfusion index. Acta Anaesthesiologica Scandinavica 2011, 55, 686–93. [CrossRef] [PubMed]
- Hasanin A, Mukhtar A, Nassar H. Perfusion indices revisited. Journal of Intensive Care 2017, 5, 1–8. [CrossRef]
- Chequer, G.; Navarro, T.; Nascimento, B.; Falqueto, E.; Nascimento, D.; Alencar, M.; Saad, A.; Fonseca, C.P.; Ribeiro, A.L. Noninvasive assessment of endothelial function and ST segment changes during exercise testing in coronary artery disease. Brazilian Journal of Medical and Biological Research 2009, 42, 413–9. [Google Scholar] [CrossRef] [PubMed]
- Durali, G.; Armağan, H.; Karaman, K. Diagnostic Efficacy of Perfusion Index and Pleth Variability Index in Patients Admitted to the Emergency Department with Chest Pain. CBU-SBED 2022, 9, 38–41. [Google Scholar] [CrossRef]
- Mostafa H.; Shaban M.; Hasanin A.; Mohamed H.; Fathy S.; Abdelreheem H.M.; Ahmed L.; Ayman A.; Ahmed M.; Akram El-A. Evaluation of peripheral perfusion index and heart rate variability as early predictors for intradialytic hypotension in critically ill patients. BMC Anesthesiology 2019, 19, 1–5. [CrossRef]
- Granelli AdW.; Östman-Smith I. Noninvasive peripheral perfusion index as a possible tool for screening for critical left heart obstruction. Acta Paediatrica 2007, 96, 1455–9. [CrossRef] [PubMed]
- Lima, A.P.; Beelen, P.; Bakker, J. Use of a peripheral perfusion index derived from the pulse oximetry signal as a noninvasive indicator of perfusion. Critical Care Medicine 2002, 30, 1210–3. [Google Scholar] [CrossRef] [PubMed]
- Pirneskoski, J.; Harjola, V.P.; Jeskanen, P.; Linnamurto, L.; Saikko, S.; Nurmi, J. Critically ill patients in emergency department may be characterized by low amplitude and high variability of amplitude of pulse photoplethysmography. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 2013, 21, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Oskay, A.; Eray, O.; Dinç, S.E.; Aydın, A.G.; Eken, C. Prognosis of critically ill patients in the ED and value of perfusion index measurement: a cross-sectional study. The American Journal of Emergency Medicine 2015, 33, 1042–4. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, C.; Valente, S.; Chiostri, M.; Gensini, G.F. Clinical significance of lactate in acute cardiac patients. World Journal of Cardiology 2015, 7, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Zhou, X.; Hong, X.; Feng, X.; Shan, P.; Xie, Q.; Xu, T.; Cai, M.; Zhou, J.; Wang, S.; Huang, W. Association between admission lactate levels and mortality in patients with acute coronary syndrome: a retrospective cohort study. Coronary Artery Disease. 2019, 30, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti F, Montecucco F, Boroli F, Rey F, Gencer B, Cikirikcioglu M, et al. The peak of blood lactate during the first 24 h predicts mortality in acute coronary syndrome patients under extracorporeal membrane oxygenation. International Journal of Cardiology 2016, 221, 741. [CrossRef] [PubMed]
- Jouffroy, R.; Lamhaut, L.; Guyard, A.; Phillipe, P.; Deluze, T.; Jaffry, M.; Dagron, C.; Bourgoin, W.; Orsini, J.P.; An, K.; Jouven, X.; Spaulding, C.; Carli, P. Base excess and lactate as prognostic indicators for patients treated by extra corporeal life support after out hospital cardiac arrest due to acute coronary syndrome. Resuscitation 2014, 85, 1764–1768. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.U.; Han, K.H.; Ryu, S.; Yoo, I.S. Availability of the peripheral perfusion index for monitoring of hemodynamic stability in the Emergency Department. Journal of the Korean Society of Emergency Medicine 2011, 22, 59–64. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).