Submitted:
22 October 2023
Posted:
23 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
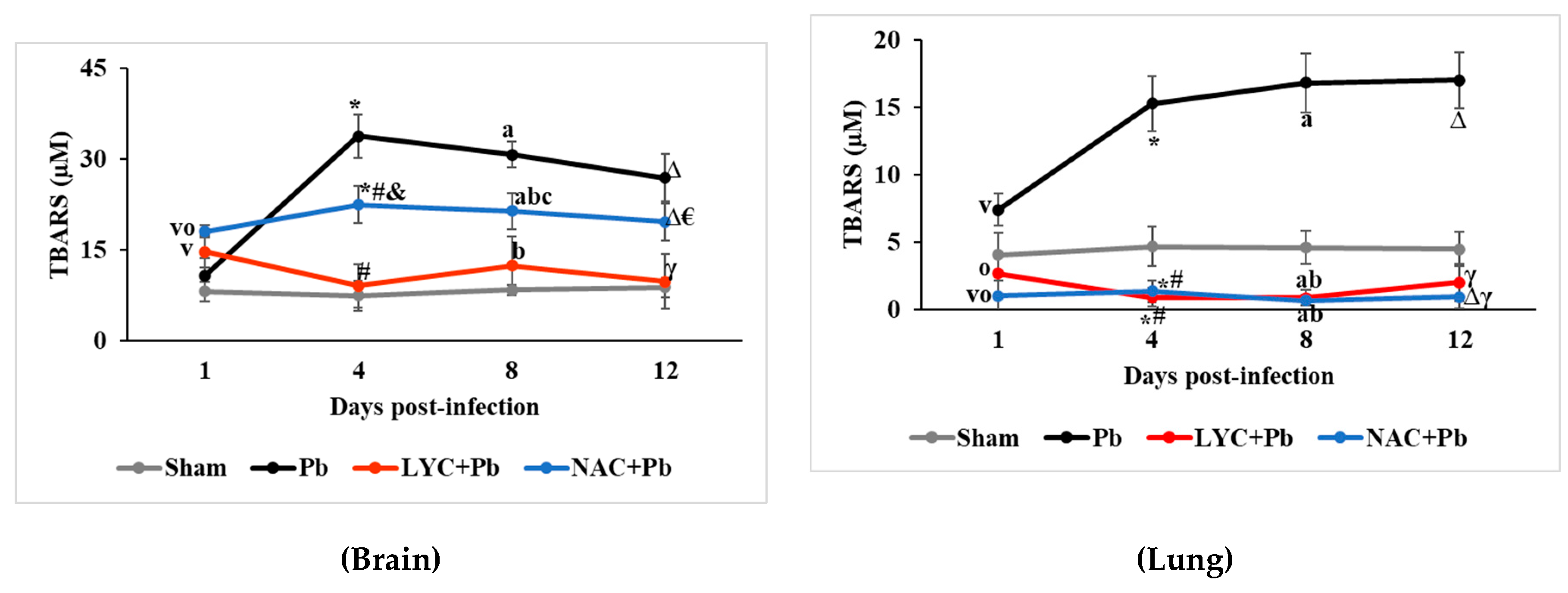
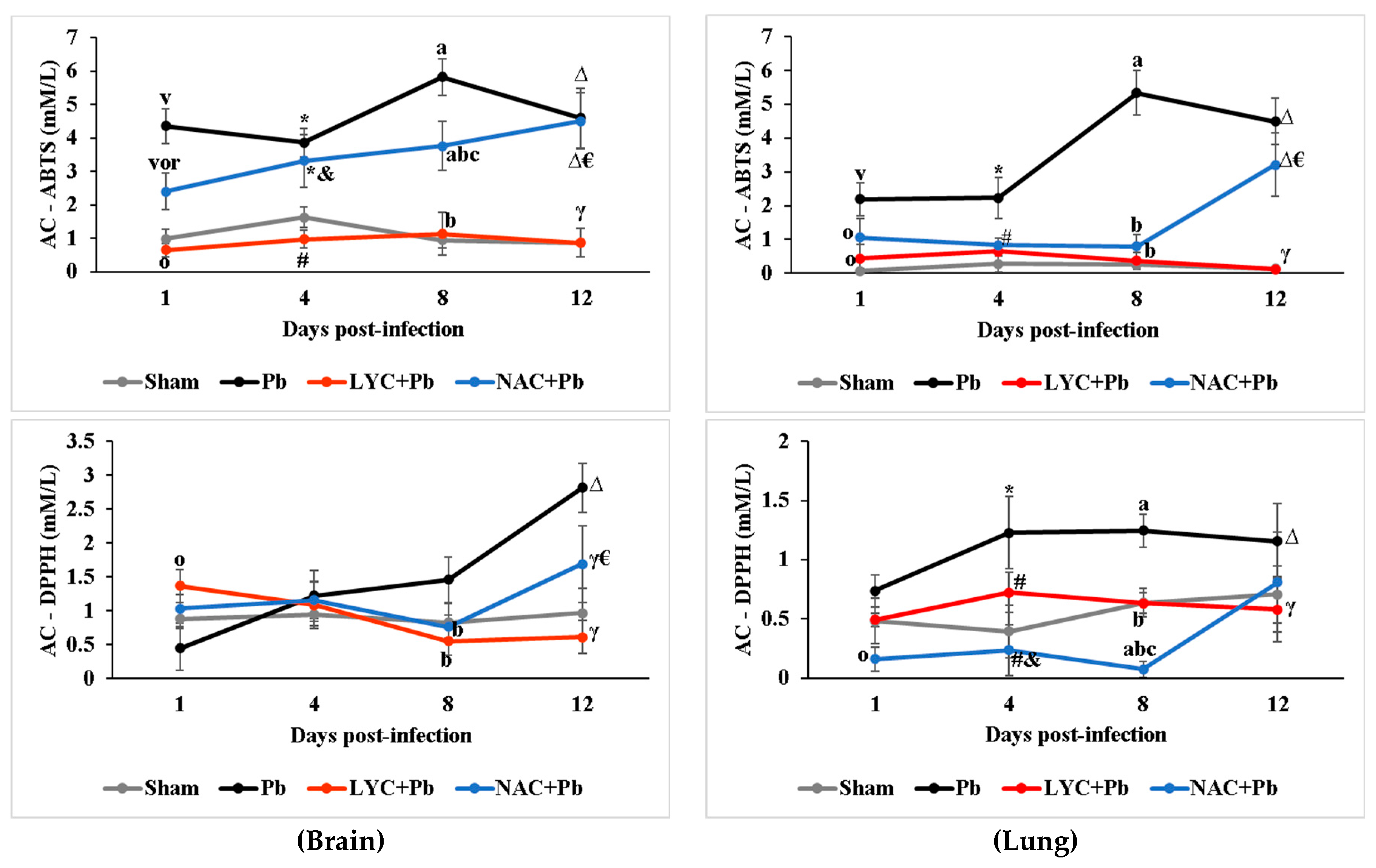
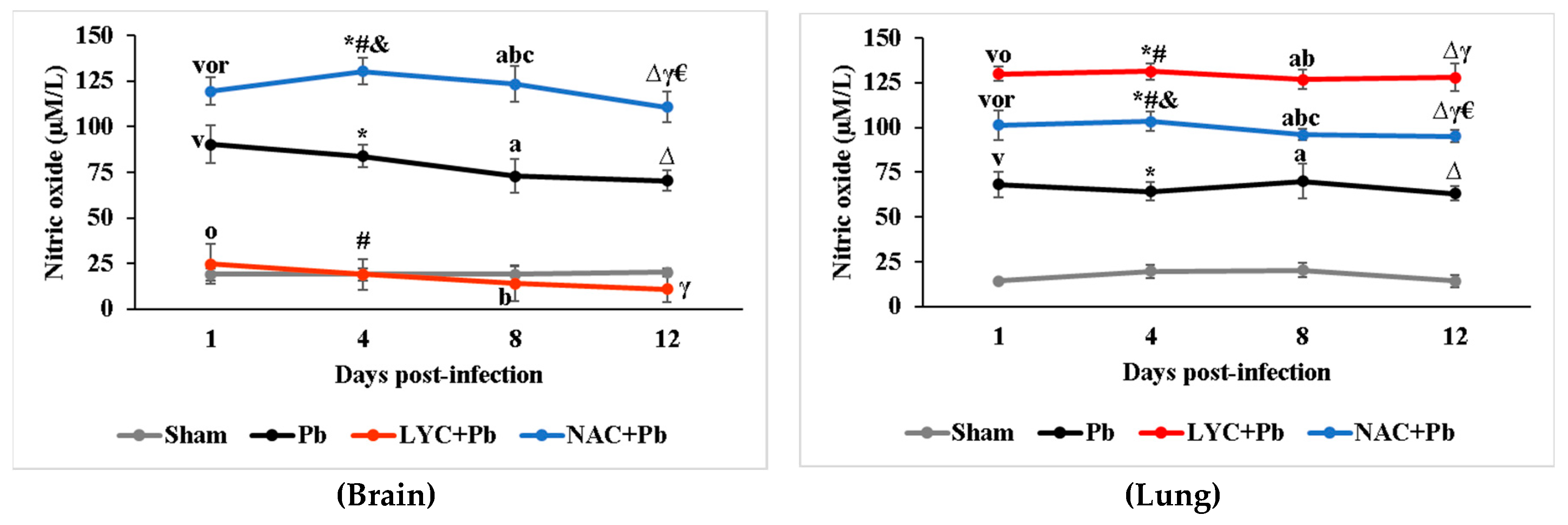
2. Results
3. Discussion
4. Materials and Methods
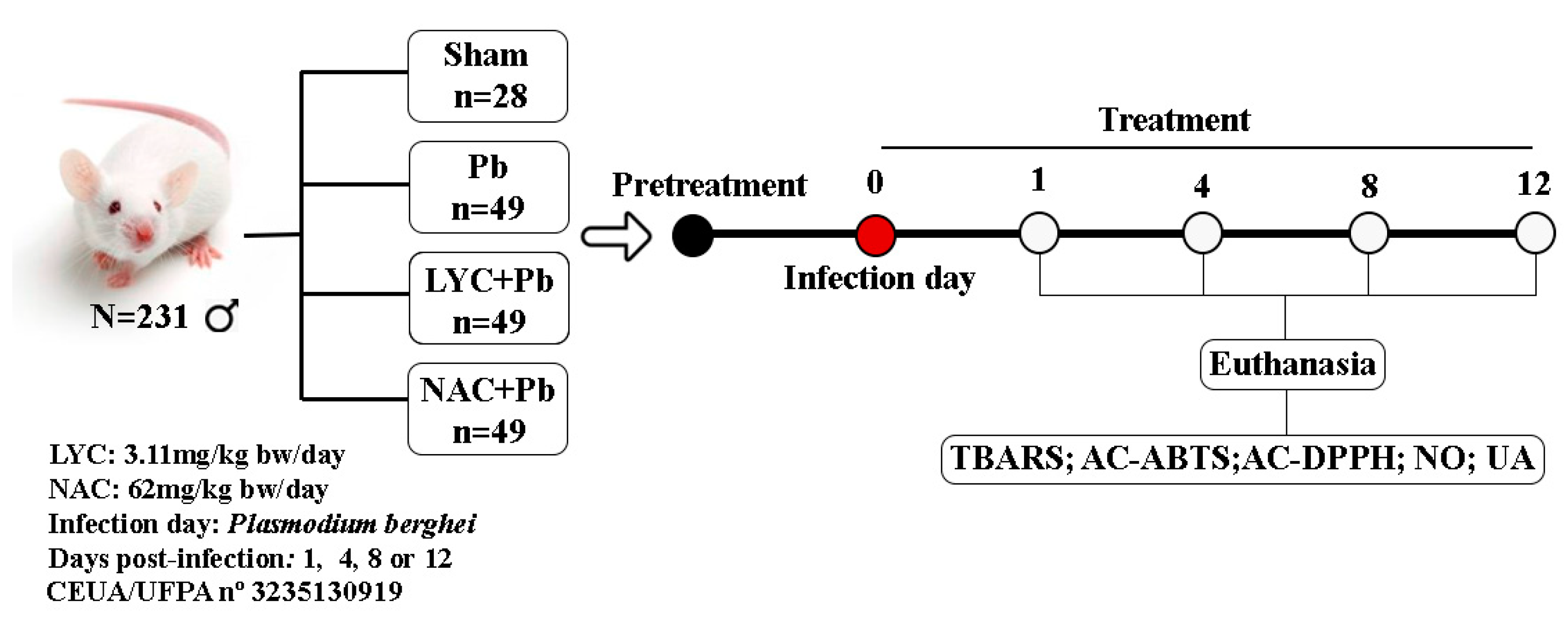
2.1. Protocol for the preparation and administration of lycopene and N-acetylcysteine
2.2. Plasmodium berghei ANKA -infection protocol
2.3. Protocol for subdivision of the experimental groups
2.4. Euthanasia Protocol and Sample Preparation
2.5. Biochemical Measurements Protocol
2.5.1. Thiobarbituric acid reactive substances (TBARS)
2.5.2. Antioxidant capacity by radical ABTS•+ inhibition (AC-ABTS)
2.5.3. Antioxidant capacity by radical DPPH• inhibition (AC-DPPH)
2.5.4. Nitric oxide (NO)
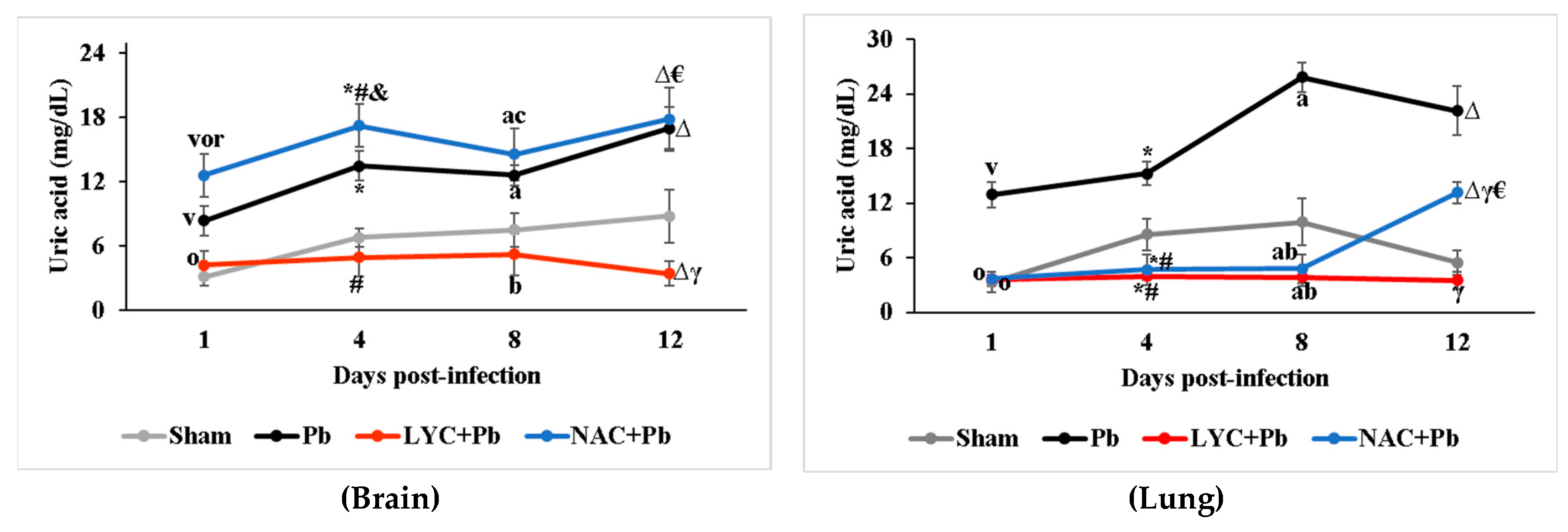
2.5.5. Uric acid (UA)
2.6. Statistical analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzen, S.; Gurer-Orhan, H.; Saso, L. Detection of Reactive Oxygen and Nitrogen Species by Electron Paramagnetic Resonance (EPR) Technique. Molecules 2017, 22, 181. [Google Scholar] [CrossRef]
- Hardy, M.; Zielonka, J.; Karoui, H.; Sikora, A.; Michalski, R.; Podsiadły, R.; Lopez, M.; Vasquez-Vivar, J.; Kalyanaraman, B.; Ouari, O. Detection and Characterization of Reactive Oxygen and Nitrogen Species in Biological Systems by Monitoring Species-Specific Products. Antioxid. Redox Signal. 2018, 28, 1416–1432. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; Riveros-Rosas, H.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Glutathione Participation in the Prevention of Cardiovascular Diseases. Antioxidants 2021, 10, 1220. [Google Scholar] [CrossRef]
- Nyui, M.; Nakanishi, I.; Anzai, K.; Ozawa, T.; Matsumoto, K. Reactivity of Redox Sensitive Paramagnetic Nitroxyl Contrast Agents with Reactive Oxygen Species. J. Clin. Biochem. Nutr. 2019, 64, 13–19. [Google Scholar] [CrossRef]
- Gomes, A.R.Q.; Cunha, N.; Varela, E.L.P.; Brígido, H.P.C.; Vale, V.V.; Dolabela, M.F.; de Carvalho, E.P.; Percário, S. Oxidative Stress in Malaria: Potential Benefits of Antioxidant Therapy. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Varela, E.L.P.; Gomes, A.R.Q.; da Silva Barbosa dos Santos, A.; de Carvalho, E.P.; Vale, V.V.; Percário, S. Potential Benefits of Lycopene Consumption: Rationale for Using It as an Adjuvant Treatment for Malaria Patients and in Several Diseases. Nutrients 2022, 14, 5303. [Google Scholar] [CrossRef]
- Nie, L.; Nusantara, A.C.; Damle, V.G.; Baranov, M. V.; Chipaux, M.; Reyes-San-Martin, C.; Hamoh, T.; Epperla, C.P.; Guricova, M.; Cigler, P.; et al. Quantum Sensing of Free Radicals in Primary Human Dendritic Cells. Nano Lett. 2022, 22, 1818–1825. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Herbas, M.S.; Ueta, Y.Y.; Ichikawa, C.; Chiba, M.; Ishibashi, K.; Shichiri, M.; Fukumoto, S.; Yokoyama, N.; Takeya, M.; Xuan, X.; et al. Alpha-Tocopherol Transfer Protein Disruption Confers Resistance to Malarial Infection in Mice. Malar. J. 2010, 9, 101. [Google Scholar] [CrossRef]
- Wu, R.L.; Idris, A.H.; Berkowitz, N.M.; Happe, M.; Gaudinski, M.R.; Buettner, C.; Strom, L.; Awan, S.F.; Holman, L.A.; Mendoza, F.; et al. Low-Dose Subcutaneous or Intravenous Monoclonal Antibody to Prevent Malaria. N. Engl. J. Med. 2022, 387, 397–407. [Google Scholar] [CrossRef]
- Becker, K.; Tilley, L.; Vennerstrom, J.L.; Roberts, D.; Rogerson, S.; Ginsburg, H. Oxidative Stress in Malaria Parasite-Infected Erythrocytes: Host–Parasite Interactions. Int. J. Parasitol. 2004, 34, 163–189. [Google Scholar] [CrossRef]
- Percário, S.; Moreira, D.R.; Gomes, B.A.Q.; Ferreira, M.E.S.; Gonçalves, A.C.M.; Laurindo, P.S.O.C.; Vilhena, T.C.; Dolabela, M.F.; Green, M.D. Oxidative Stress in Malaria. Int. J. Mol. Sci. 2012, 13, 16346. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.P.; Bhatt, R.; Singh, V. Genetic Profiling of the Plasmodium Falciparum Parasite Population in Uncomplicated Malaria from India. Malar. J. 2019, 18, 1–11. [Google Scholar] [CrossRef]
- Suzuki, H.; Kume, A.; Herbas, M. Potential of Vitamin E Deficiency, Induced by Inhibition of α-Tocopherol Efflux, in Murine Malaria Infection. Int. J. Mol. Sci. 2018, 20, 64. [Google Scholar] [CrossRef]
- Schmidt, H.M.; Kelley, E.E.; Straub, A.C. The Impact of Xanthine Oxidase (XO) on Hemolytic Diseases. Redox Biol. 2019, 21, 101072. [Google Scholar] [CrossRef]
- Jortzik, E.; Becker, K. Thioredoxin and Glutathione Systems in Plasmodium Falciparum. Int. J. Med. Microbiol. 2012, 302, 187–194. [Google Scholar] [CrossRef]
- Gupta, M.; Kumar, S.; Kumar, R.; Kumar, A.; Verma, R.; Darokar, M.P.; Rout, P.; Pal, A. Inhibition of Heme Detoxification Pathway in Malaria Parasite by 3-Hydroxy-11-Keto-β-Boswellic Acid Isolated from Boswellia Serrata. Biomed. Pharmacother. 2021, 144, 112302. [Google Scholar] [CrossRef]
- Ty, M.C.; Zuniga, M.; Götz, A.; Kayal, S.; Sahu, P.K.; Mohanty, A.; Mohanty, S.; Wassmer, S.C.; Rodriguez, A. Malaria Inflammation by Xanthine Oxidase-produced Reactive Oxygen Species. EMBO Mol. Med. 2019, 11, e9903. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, K.P.; Bali, P.; Anwar, S.; Kaul, A.; Singh, O.P.; Gupta, B.K.; Kumari, N.; Noor Alam, M.; Raziuddin, M.; et al. INOS Polymorphism Modulates INOS/NO Expression via Impaired Antioxidant and ROS Content in P. Vivax and P. Falciparum Infection. Redox Biol. 2018, 15, 192–206. [Google Scholar] [CrossRef]
- Moreira, A.S.; Estato, V.; Malvar, D.C.; Sanches, G.S.; Gomes, F.; Tibirica, E.; Daniel-Ribeiro, C.T.; Carvalho, L.J.M. L-Arginine Supplementation and Thromboxane Synthase Inhibition Increases Cerebral Blood Flow in Experimental Cerebral Malaria. Sci. Reports 2019 91 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dhangadamajhi, G.; Mohapatra, B.N.; Kar, S.K.; Ranjit, M. Genetic Variation in Neuronal Nitric Oxide Synthase (NNOS) Gene and Susceptibility to Cerebral Malaria in Indian Adults. Infect. Genet. Evol. 2009, 9, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Kondrikov, D.; Elms, S.; Fulton, D.; Su, Y. ENOS-β-Actin Interaction Contributes to Increased Peroxynitrite Formation during Hyperoxia in Pulmonary Artery Endothelial Cells and Mouse Lungs. J. Biol. Chem. 2010, 285, 35479–35487. [Google Scholar] [CrossRef]
- Knackstedt, S.L.; Georgiadou, A.; Apel, F.; Abu-Abed, U.; Moxon, C.A.; Cunnington, A.J.; Raupach, B.; Cunningham, D.; Langhorne, J.; Krüger, R.; et al. Neutrophil Extracellular Traps Drive Inflammatory Pathogenesis in Malaria. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Wedgwood, S.; Lakshminrusimha, S.; Farrow, K.N.; Czech, L.; Gugino, S.F.; Soares, F.; Russell, J.A.; Steinhorn, R.H. Apocynin Improves Oxygenation and Increases ENOS in Persistent Pulmonary Hypertension of the Newborn. Am. J. Physiol. Cell. Mol. Physiol. 2012, 302, L616–L626. [Google Scholar] [CrossRef]
- Kang, D.-M.; Kwon, J.-M.; Jeong, W.-J.; Jung, Y.J.; Kang, K.K.; Ahn, M.-J. Antioxidant Constituents and Activities of the Pulp with Skin of Korean Tomato Cultivars. Molecules 2022, 27, 8741. [Google Scholar] [CrossRef]
- Liu, D.; Shi, J.; Colina Ibarra, A.; Kakuda, Y.; Jun Xue, S. The Scavenging Capacity and Synergistic Effects of Lycopene, Vitamin E, Vitamin C, and β-Carotene Mixtures on the DPPH Free Radical. LWT - Food Sci. Technol. 2008, 41, 1344–1349. [Google Scholar] [CrossRef]
- Carvalho, G.C.; Marena, G.D.; Leonardi, G.R.; Sábio, R.M.; Corrêa, I.; Chorilli, M.; Bauab, T.M. Lycopene, Mesoporous Silica Nanoparticles and Their Association: A Possible Alternative against Vulvovaginal Candidiasis? Molecules 2022, 27, 8558. [Google Scholar] [CrossRef]
- Mannino, F.; D’Angelo, T.; Pallio, G.; Ieni, A.; Pirrotta, I.; Giorgi, D.A.; Scarfone, A.; Mazziotti, S.; Booz, C.; Bitto, A.; et al. The Nutraceutical Genistein-Lycopene Combination Improves Bone Damage Induced by Glucocorticoids by Stimulating the Osteoblast Formation Process. Nutrients 2022, 14, 4296. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, H.; Wang, J.; Liu, P.; Tan, X.; Ren, B.; Liu, Z.; Liu, X. Lycopene Supplementation Attenuates Oxidative Stress, Neuroinflammation, and Cognitive Impairment in Aged CD-1 Mice. J. Agric. Food Chem. 2018, 66, 3127–3136. [Google Scholar] [CrossRef]
- Liu, S.; Yang, D.; Yu, L.; Aluo, Z.; Zhang, Z.; Qi, Y.; Li, Y.; Song, Z.; Xu, G.; Zhou, L. Effects of Lycopene on Skeletal Muscle-Fiber Type and High-Fat Diet-Induced Oxidative Stress. J. Nutr. Biochem. 2021, 87, 108523. [Google Scholar] [CrossRef]
- Ni, Y.; Zhuge, F.; Nagashimada, M.; Nagata, N.; Xu, L.; Yamamoto, S.; Fuke, N.; Ushida, Y.; Suganuma, H.; Kaneko, S.; et al. Lycopene Prevents the Progression of Lipotoxicity-Induced Nonalcoholic Steatohepatitis by Decreasing Oxidative Stress in Mice. Free Radic. Biol. Med. 2020, 152, 571–582. [Google Scholar] [CrossRef]
- Cheng, J.; Miller, B.; Balbuena, E.; Eroglu, A. Lycopene Protects against Smoking-Induced Lung Cancer by Inducing Base Excision Repair. Antioxidants 2020, 9, 643. [Google Scholar] [CrossRef]
- LEI, X.; LEI, L.; ZHANG, Z.; CHENG, Y. Neuroprotective Effects of Lycopene Pretreatment on Transient Global Cerebral Ischemia-Reperfusion in Rats: The Role of the Nrf2/HO-1 Signaling Pathway. Mol. Med. Rep. 2016, 13, 412–418. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Aparicio, R.; Carrón, R.; Sevilla, M.Á.; Monroy-Ruiz, J.; Montero, M.J. Lycopene-Supplemented Diet Ameliorates Cardiovascular Remodeling and Oxidative Stress in Rats with Hypertension Induced by Angiotensin II. J. Funct. Foods 2018, 47, 279–287. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Liu, Z.; Wang, Q.; Liu, J.; Feng, D.; Zou, J. Lycopene Reduces Cholesterol Absorption and Prevents Atherosclerosis in ApoE –/– Mice by Downregulating HNF-1α and NPC1L1 Expression. J. Agric. Food Chem. 2021, 69, 10114–10120. [Google Scholar] [CrossRef]
- Mortensen, A.; Skibsted, L.H.; Truscott, T.G. The Interaction of Dietary Carotenoids with Radical Species. Arch. Biochem. Biophys. 2001, 385, 13–19. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Pillai, K.R.; Kumar, P.R.; Sudhakaran, P.R.; Menon, V.P. Lycopene as a Natural Protector against γ-Radiation Induced DNA Damage, Lipid Peroxidation and Antioxidant Status in Primary Culture of Isolated Rat Hepatocytes in Vitro. Biochim. Biophys. Acta - Gen. Subj. 2007, 1770, 659–665. [Google Scholar] [CrossRef]
- Kujawska, M.; Ewertowska, M.; Adamska, T.; Sadowski, C.; Ignatowicz, E.; Jodynis-Liebert, J. Antioxidant Effect of Lycopene-Enriched Tomato Paste on N-Nitrosodiethylamine-Induced Oxidative Stress in Rats. J. Physiol. Biochem. 2014, 70, 981–990. [Google Scholar] [CrossRef]
- Renju, G.L.; Kurup, G.M.; Saritha Kumari, C.H. Effect of Lycopene from Chlorella Marina on High Cholesterol-Induced Oxidative Damage and Inflammation in Rats. Inflammopharmacology 2014, 22, 45–54. [Google Scholar] [CrossRef]
- da Silva Brito, A.K.; de Morais Lima, G.; de Farias, L.M.; Rodrigues, L.A.R.L.; de Carvalho, V.B.L.; de Carvalho Pereira, C.F.; de Macedo Gonçalves Frota, K.; Conde-Júnior, A.M.; Moura, A.M.O.; dos Santos Rizzo, M.; et al. Lycopene-Rich Extract from Red Guava (Psidium Guajava L.) Decreases Plasma Triglycerides and Improves Oxidative Stress Biomarkers on Experimentally-Induced Dyslipidemia in Hamsters. Nutrients 2019, 11, 393. [Google Scholar] [CrossRef]
- Pan, X.; Niu, X.; Li, Y.; Yao, Y.; Han, L. Preventive Mechanism of Lycopene on Intestinal Toxicity Caused by Cyclophosphamide Chemotherapy in Mice by Regulating TLR4-MyD88/TRIF-TRAF6 Signaling Pathway and Gut-Liver Axis. Nutrients 2022, 14, 4467. [Google Scholar] [CrossRef]
- Torre, S.; Langlais, D.; Gros, P. Genetic Analysis of Cerebral Malaria in the Mouse Model Infected with Plasmodium Berghei. Mamm. Genome 2018, 29, 488–506. [Google Scholar] [CrossRef]
- Vandermosten, L.; Pham, T.-T.; Possemiers, H.; Knoops, S.; Van Herck, E.; Deckers, J.; Franke-Fayard, B.; Lamb, T.J.; Janse, C.J.; Opdenakker, G.; et al. Experimental Malaria-Associated Acute Respiratory Distress Syndrome Is Dependent on the Parasite-Host Combination and Coincides with Normocyte Invasion. Malar. J. 2018, 17, 102. [Google Scholar] [CrossRef]
- Aitio, M. N-acetylcysteine – Passe-partout or Much Ado about Nothing? Br. J. Clin. Pharmacol. 2006, 61, 5–15. [Google Scholar] [CrossRef]
- Atkuri, K.R.; Mantovani, J.J.; Herzenberg, L.A.; Herzenberg, L.A. N-Acetylcysteine—a Safe Antidote for Cysteine/Glutathione Deficiency. Curr. Opin. Pharmacol. 2007, 7, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.A.; Comim, C.M.; Hermani, F.; Silva, B.; Barichello, T.; Portella, A.C.; Gomes, F.C.A.; Sab, I.M.; Frutuoso, V.S.; Oliveira, M.F.; et al. Cognitive Dysfunction Is Sustained after Rescue Therapy in Experimental Cerebral Malaria, and Is Reduced by Additive Antioxidant Therapy. PLoS Pathog. 2010, 6. [Google Scholar] [CrossRef]
- Quadros Gomes, B.A.; da Silva, L.F.D.; Quadros Gomes, A.R.; Moreira, D.R.; Dolabela, M.F.; Santos, R.S.; Green, M.D.; Carvalho, E.P.; Percário, S. N-Acetyl Cysteine and Mushroom Agaricus Sylvaticus Supplementation Decreased Parasitaemia and Pulmonary Oxidative Stress in a Mice Model of Malaria. Malar. J. 2015, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Böhm, V.; Puspitasari-Nienaber, N.L.; Ferruzzi, M.G.; Schwartz, S.J. Trolox Equivalent Antioxidant Capacity of Different Geometrical Isomers of α-Carotene, β-Carotene, Lycopene, and Zeaxanthin. J. Agric. Food Chem. 2002, 50, 221–226. [Google Scholar] [CrossRef]
- Agarwal, S.; Sharma, V.; Kaul, T.; Abdin, M.Z.; Singh, S. Cytotoxic Effect of Carotenoid Phytonutrient Lycopene on P. Falciparum Infected Erythrocytes. Mol. Biochem. Parasitol. 2014, 197, 15–20. [Google Scholar] [CrossRef]
- Hempel, C.; Combes, V.; Hunt, N.H.; Kurtzhals, J.A.L.; Grau, G.E.R. CNS Hypoxia Is More Pronounced in Murine Cerebral than Noncerebral Malaria and Is Reversed by Erythropoietin. Am. J. Pathol. 2011, 179, 1939–1950. [Google Scholar] [CrossRef]
- Sharma, L.; Kaur, J.; Rishi, P.; Shukla, G. Plasmodium Berghei: Influence of Infection on the Oxidant and Antioxidants Levels in Pregnant BALB/c Mice. Exp. Parasitol. 2012, 131, 215–222. [Google Scholar] [CrossRef]
- Zhao, S.; Duan, H.; Yang, Y.; Yan, X.; Fan, K. Fenozyme Protects the Integrity of the Blood–Brain Barrier against Experimental Cerebral Malaria. Nano Lett. 2019, 19, 8887–8895. [Google Scholar] [CrossRef]
- Baptista, F.G.; Pamplona, A.; Pena, A.C.; Mota, M.M.; Pied, S.; Vigário, A.M. Accumulation of Plasmodium Berghei -Infected Red Blood Cells in the Brain Is Crucial for the Development of Cerebral Malaria in Mice. Infect. Immun. 2010, 78, 4033–4039. [Google Scholar] [CrossRef]
- Kurutas, E.B. The Importance of Antioxidants Which Play the Role in Cellular Response against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2015, 15, 71. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Brito, C.X.L.; Teixeira, S.A.; Barboza, R.; Dos Reis, A.S.; Azevedo-Santos, A.P.S.; Muscará, M.; Costa, S.K.P.; Marinho, C.R.F.; Brain, S.D.; et al. TRPV1 Antagonism by Capsazepine Modulates Innate Immune Response in Mice Infected with Plasmodium Berghei ANKA. Mediators Inflamm. 2014, 2014. [Google Scholar] [CrossRef]
- Scaccabarozzi, D.; Deroost, K.; Corbett, Y.; Lays, N.; Corsetto, P.; Salè, F.O.; Van den Steen, P.E.; Taramelli, D. Differential Induction of Malaria Liver Pathology in Mice Infected with Plasmodium Chabaudi AS or Plasmodium Berghei NK65. Malar. J. 2018, 17, 18. [Google Scholar] [CrossRef]
- Chuljerm, H.; Maneekesorn, S.; Somsak, V.; Ma, Y.; Srichairatanakool, S.; Koonyosying, P. Anti-Malarial and Anti-Lipid Peroxidation Activities of Deferiprone-Resveratrol Hybrid in Plasmodium Berghei-Infected Mice. Biology (Basel). 2021, 10, 911. [Google Scholar] [CrossRef]
- Elsayed, A.; Elkomy, A.; Elkammar, R.; Youssef, G.; Abdelhiee, E.Y.; Abdo, W.; Fadl, S.E.; Soliman, A.; Aboubakr, M. Synergistic Protective Effects of Lycopene and N-Acetylcysteine against Cisplatin-Induced Hepatorenal Toxicity in Rats. Sci. Rep. 2021, 11, 13979. [Google Scholar] [CrossRef]
- Ezeriņa, D.; Takano, Y.; Hanaoka, K.; Urano, Y.; Dick, T.P. N-Acetyl Cysteine Functions as a Fast-Acting Antioxidant by Triggering Intracellular H2S and Sulfane Sulfur Production. Cell Chem. Biol. 2018, 25, 447–459. [Google Scholar] [CrossRef]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The Chemistry and Biological Activities of N-Acetylcysteine. Biochim. Biophys. Acta - Gen. Subj. 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, P.; Xu, F.; Huang, W.; Ji, Q.; Han, Y.; Shao, B.; Li, Y. Protective Effects of Lycopene against AFB1-Induced Erythrocyte Dysfunction and Oxidative Stress in Mice. Res. Vet. Sci. 2020, 129, 103–108. [Google Scholar] [CrossRef]
- Coles, L.D.; Tuite, P.J.; Öz, G.; Mishra, U.R.; Kartha, R. V.; Sullivan, K.M.; Cloyd, J.C.; Terpstra, M. Repeated-Dose Oral N-Acetylcysteine in Parkinson’s Disease: Pharmacokinetics and Effect on Brain Glutathione and Oxidative Stress. J. Clin. Pharmacol. 2018, 58, 158–167. [Google Scholar] [CrossRef]
- Aqeel, S.; Naheda, A.; Raza, A.; Khan, K.; Khan, W. Differential Status and Significance of Non-Enzymatic Antioxidants (Reactive Oxygen Species Scavengers) in Malaria and Dengue Patients. Acta Trop. 2019, 195, 127–134. [Google Scholar] [CrossRef]
- Sánchez-Villamil, J.P.; Bautista-Niño, P.K.; Serrano, N.C.; Rincon, M.Y.; Garg, N.J. Potential Role of Antioxidants as Adjunctive Therapy in Chagas Disease. Oxid. Med. Cell. Longev. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Ton, A.M.M.; Campagnaro, B.P.; Alves, G.A.; Aires, R.; Côco, L.Z.; Arpini, C.M.; Guerra e Oliveira, T.; Campos-Toimil, M.; Meyrelles, S.S.; Pereira, T.M.C.; et al. Oxidative Stress and Dementia in Alzheimer’s Patients: Effects of Synbiotic Supplementation. Oxid. Med. Cell. Longev. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Velmurugan, B.; Bhuvaneswari, V.; Nagini, S. Antiperoxidative Effects of Lycopene during N-Methyl-N′-Nitro-N-Nitrosoguanidine-Induced Gastric Carcinogenesis. Fitoterapia 2002, 73, 604–611. [Google Scholar] [CrossRef]
- Hsiao, G.; Fong, T.H.; Tzu, N.H.; Lin, K.H.; Chou, D.S.; Sheu, J.R. A Potent Antioxidant, Lycopene, Affords Neuroprotection against Microglia Activation and Focal Cerebral Ischemia in Rats. In Vivo 2004, 18, 351–356. [Google Scholar]
- Neyestani, T.R.; Shariatzadeh, N.; Gharavi, A.; Kalayi, A.; Khalaji, N. Physiological Dose of Lycopene Suppressed Oxidative Stress and Enhanced Serum Levels of Immunoglobulin M in Patients with Type 2 Diabetes Mellitus: A Possible Role in the Prevention of Long-Term Complications. J. Endocrinol. Invest. 2007, 30, 833–838. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Dieye, Y.; Mbengue, B.; Dagamajalu, S.; Fall, M.M.; Loke, M.F.; Nguer, C.M.; Thiam, A.; Vadivelu, J.; Dieye, A. Cytokine Response during Non-Cerebral and Cerebral Malaria: Evidence of a Failure to Control Inflammation as a Cause of Death in African Adults. PeerJ 2016, 4, e1965. [Google Scholar] [CrossRef]
- Techarang, T.; Jariyapong, P.; Viriyavejakul, P.; Punsawad, C. High Mobility Group Box-1 (HMGB-1) and Its Receptors in the Pathogenesis of Malaria-Associated Acute Lung Injury/Acute Respiratory Distress Syndrome in a Mouse Model. Heliyon 2021, 7, e08589. [Google Scholar] [CrossRef] [PubMed]
- Gramaglia, I.; Sobolewski, P.; Meays, D.; Contreras, R.; Nolan, J.P.; Frangos, J.A.; Intaglietta, M.; van der Heyde, H.C. Low Nitric Oxide Bioavailability Contributes to the Genesis of Experimental Cerebral Malaria. Nat. Med. 2006, 12, 1417–1422. [Google Scholar] [CrossRef]
- Filogonio, R.; Sartori, M.R.; Morgensen, S.; Tavares, D.; Campos, R.; Abe, A.S.; Taylor, E.W.; Rodrigues, G.J.; De Nucci, G.; Simonsen, U.; et al. Cholinergic Regulation along the Pulmonary Arterial Tree of the South American Rattlesnake: Vascular Reactivity, Muscarinic Receptors, and Vagal Innervation. Am. J. Physiol. Integr. Comp. Physiol. 2020, 319, R156–R170. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Mao, X.; Zhang, J.; Sun, P.; Wang, H.; Zhang, Y.; Ma, Y.; Xu, S.; Lv, R.; Liu, X. Oral Delivery of Lycopene-Loaded Microemulsion for Brain-Targeting: Preparation, Characterization, Pharmacokinetic Evaluation and Tissue Distribution. Drug Deliv. 2019, 26, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Goyal, S.; Sharma, C.; Arora, S.; Kumari, S.; Arya, D. Cardioprotective Effect of Lycopene against Isoproterenol-Induced Myocardial Infarction in Rats. Hum. Exp. Toxicol. 2013, 32, 492–503. [Google Scholar] [CrossRef]
- Yonar, M.E.; Sakin, F. Ameliorative Effect of Lycopene on Antioxidant Status in Cyprinus Carpio during Pyrethroid Deltamethrin Exposure. Pestic. Biochem. Physiol. 2011, 99, 226–231. [Google Scholar] [CrossRef]
- Dobrzyńska, M.M.; Gajowik, A.; Radzikowska, J. The Effect of Lycopene Supplementation on Radiation-Induced Micronuclei in Mice Reticulocytes in Vivo. Radiat. Environ. Biophys. 2019, 58, 425–432. [Google Scholar] [CrossRef]
- Cabrales, P.; Zanini, G.M.; Meays, D.; Frangos, J.A.; Carvalho, L.J.M. Nitric Oxide Protection Against Murine Cerebral Malaria Is Associated With Improved Cerebral Microcirculatory Physiology. J. Infect. Dis. 2011, 203, 1454–1463. [Google Scholar] [CrossRef]
- Ong, P.K.; Melchior, B.; Martins, Y.C.; Hofer, A.; Orjuela-Sánchez, P.; Cabrales, P.; Zanini, G.M.; Frangos, J.A.; Carvalho, L.J.M. Nitric Oxide Synthase Dysfunction Contributes to Impaired Cerebroarteriolar Reactivity in Experimental Cerebral Malaria. PLoS Pathog. 2013, 9, e1003444. [Google Scholar] [CrossRef]
- Kondrikov, D.; Gross, C.; Black, S.M.; Su, Y. Novel Peptide for Attenuation of Hyperoxia-Induced Disruption of Lung Endothelial Barrier and Pulmonary Edema via Modulating Peroxynitrite Formation. J. Biol. Chem. 2014, 289, 33355–33363. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.F.; Granger, D.N. Blood Cells and Endothelial Barrier Function. Tissue Barriers 2015, 3, e978720. [Google Scholar] [CrossRef]
- Cantu-Medellin, N.; Kelley, E.E. Xanthine Oxidoreductase-Catalyzed Reduction of Nitrite to Nitric Oxide: Insights Regarding Where, When and How. Nitric Oxide 2013, 34, 19–26. [Google Scholar] [CrossRef]
- Das, D.; Guha, S.; Talukdar, A.; Sau, T.; Sanghai, R.; Pant, N.; Jena, A. A Study of Uric Acid Level as a Marker of Severity in Malaria. Int. J. Med. Sci. Diagnosis Res. 2022, 6. [Google Scholar] [CrossRef]
- Devaraj, S.; Mathur, S.; Basu, A.; Aung, H.H.; Vasu, V.T.; Meyers, S.; Jialal, I. A Dose-Response Study on the Effects of Purified Lycopene Supplementation on Biomarkers of Oxidative Stress. J. Am. Coll. Nutr. 2008, 27, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-P.; Wen, F.-Q.; Bai, C.-X.; Wan, H.-Y.; Kang, J.; Chen, P.; Yao, W.-Z.; Ma, L.-J.; Li, X.; Raiteri, L.; et al. Twice Daily N-Acetylcysteine 600 Mg for Exacerbations of Chronic Obstructive Pulmonary Disease (PANTHEON): A Randomised, Double-Blind Placebo-Controlled Trial. Lancet Respir. Med. 2014, 2, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Kohn, H.I.; Liversedge, M. On a New Aerobic Metabolite Whose Production by Brain Is Inhibited by Apomorphine, Emetine, Ergotamine, Epinephrine, and Menadione. J. Pharmacol. Exp. Ther. 1944, 82, 292–300. [Google Scholar]
- Percário, S.; Vital, A.; Jablonka, F. Dosagem Do Malondialdeido. Newslab 1994, 2, 46–50. [Google Scholar]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A Novel Method for Measuring Antioxidant Capacity and Its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




