Submitted:
16 October 2023
Posted:
23 October 2023
You are already at the latest version
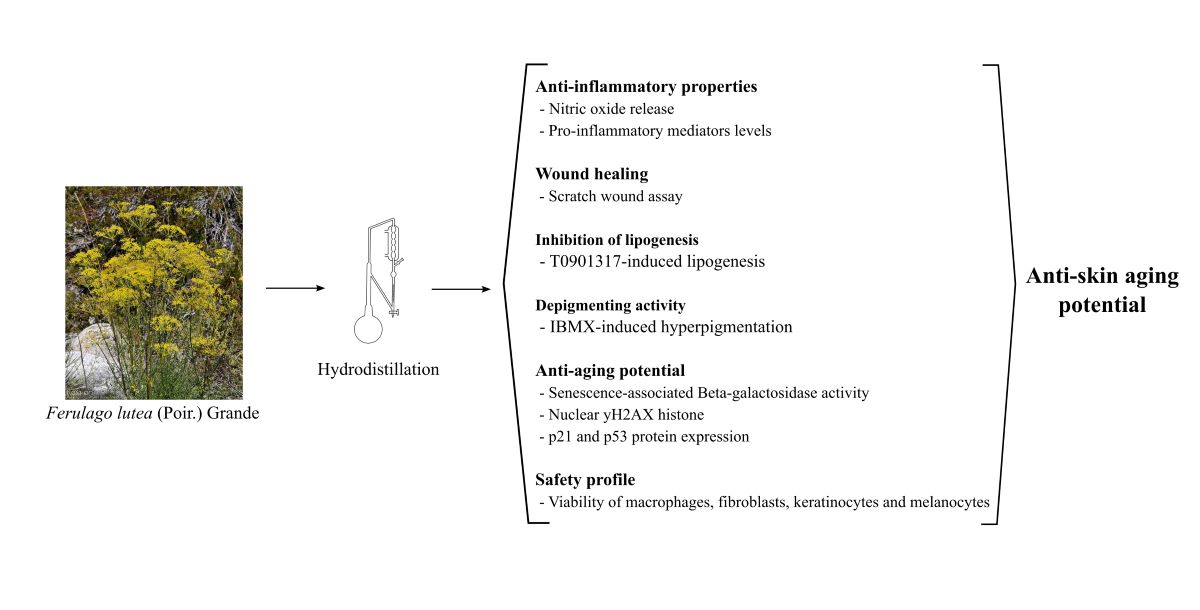
Abstract
Keywords:
1. Introduction
2. Results
2.1. Chemical composition
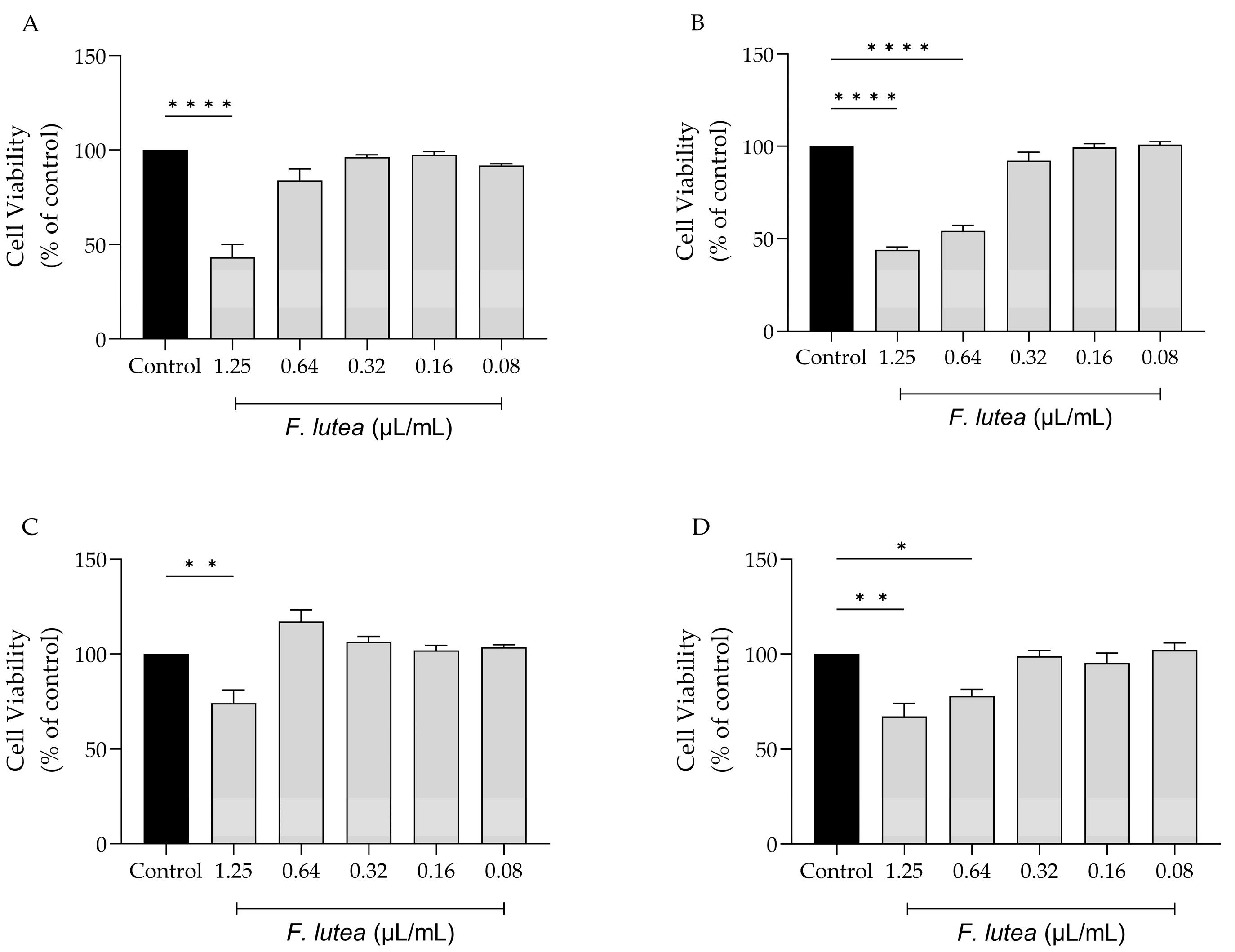
2.2. Effect of F. lutea on cell viability
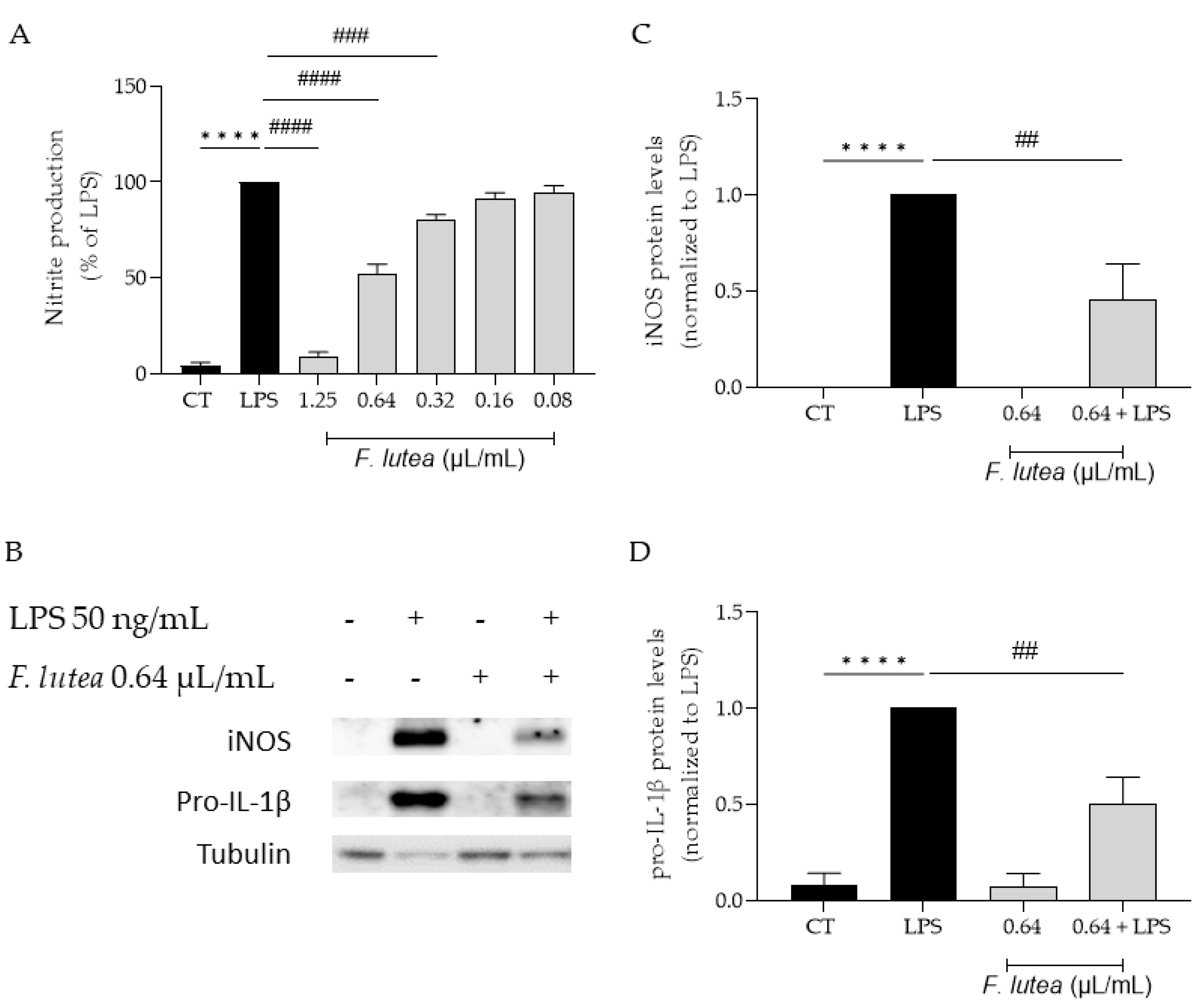
2.2. The essential oil of F. lutea exerts anti-inflammatory effect via inhibition of NF-κB pathway
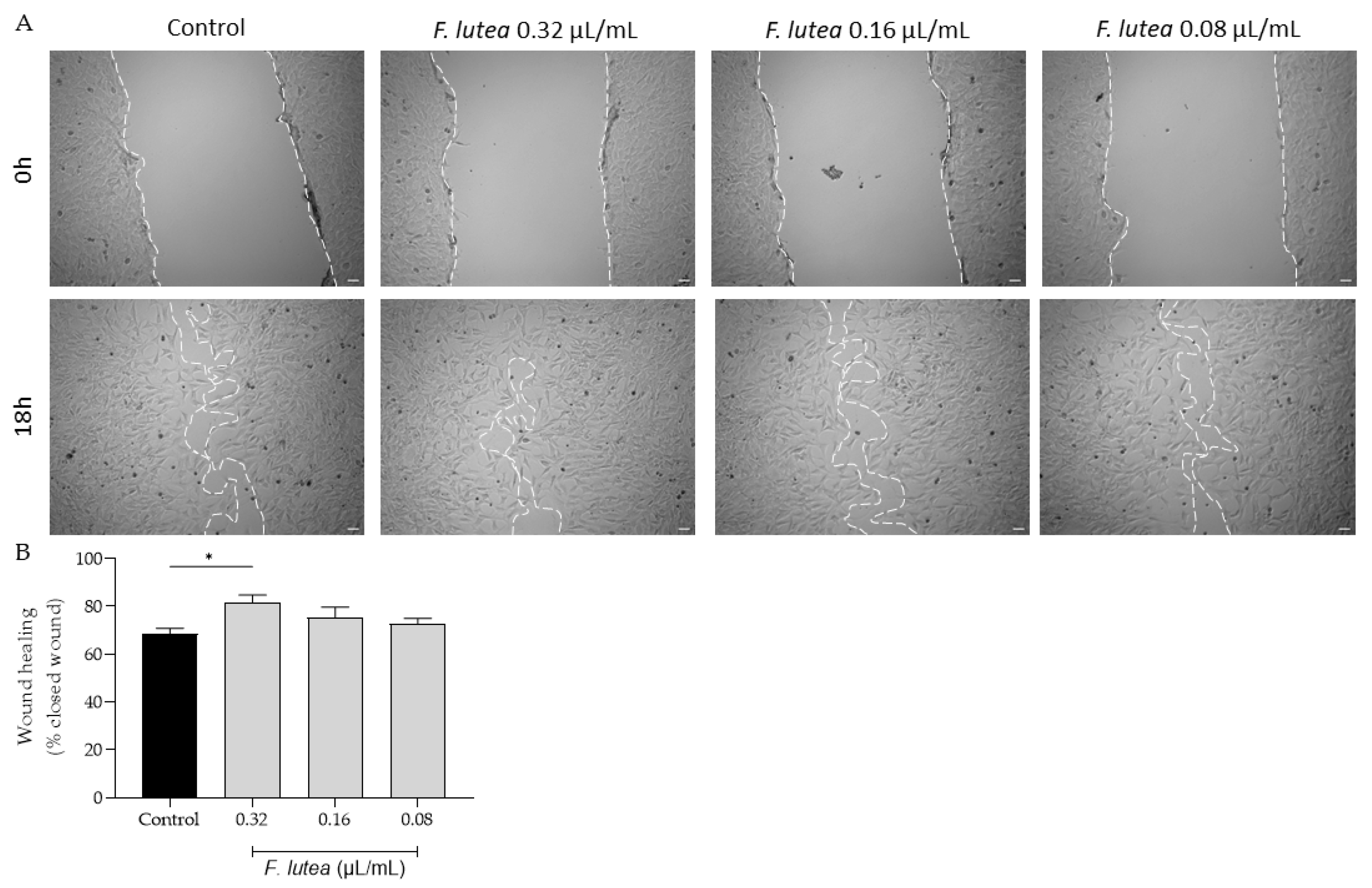
2.3. Ferulago lutea essential oil promotes cell migration
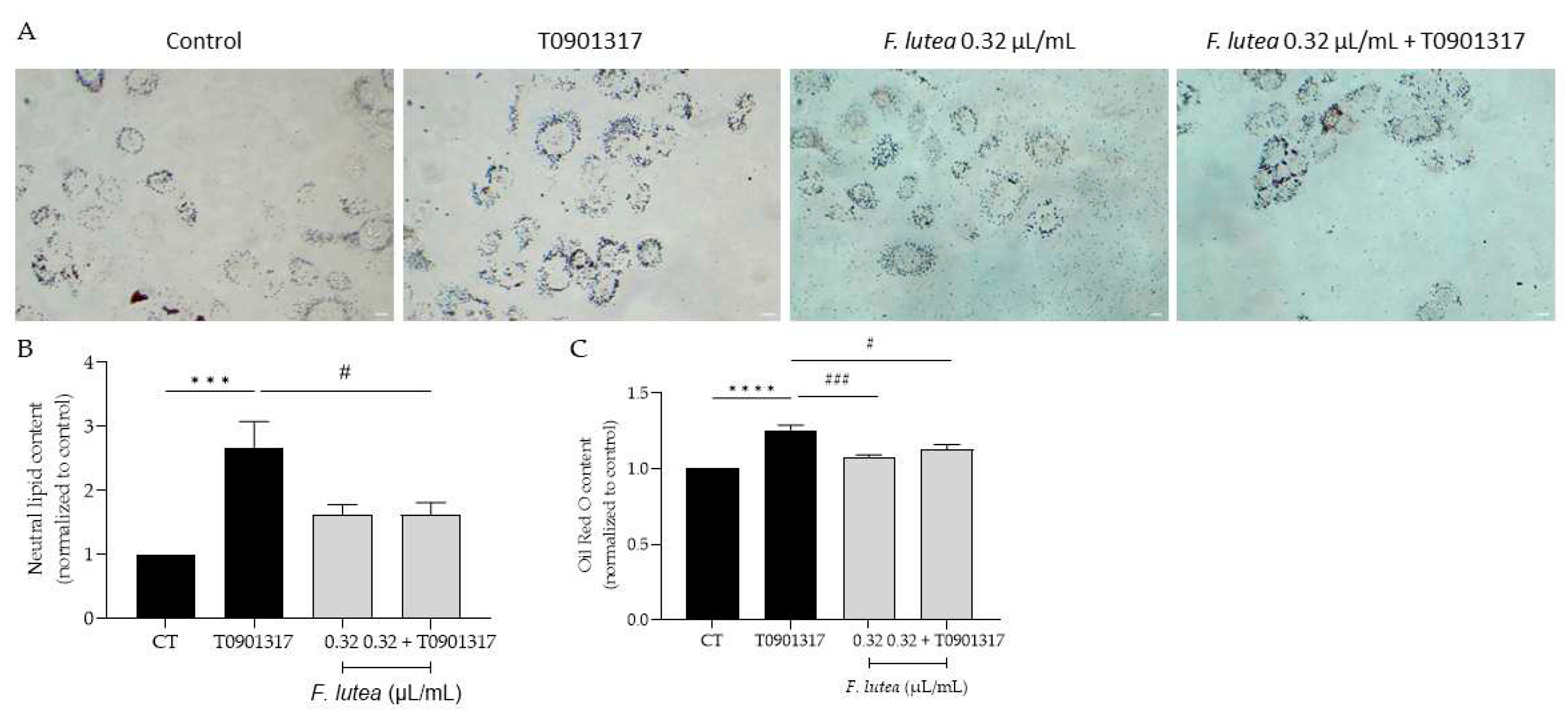
2.4. Ferulago lutea essential oil affects lipogenesis differentially
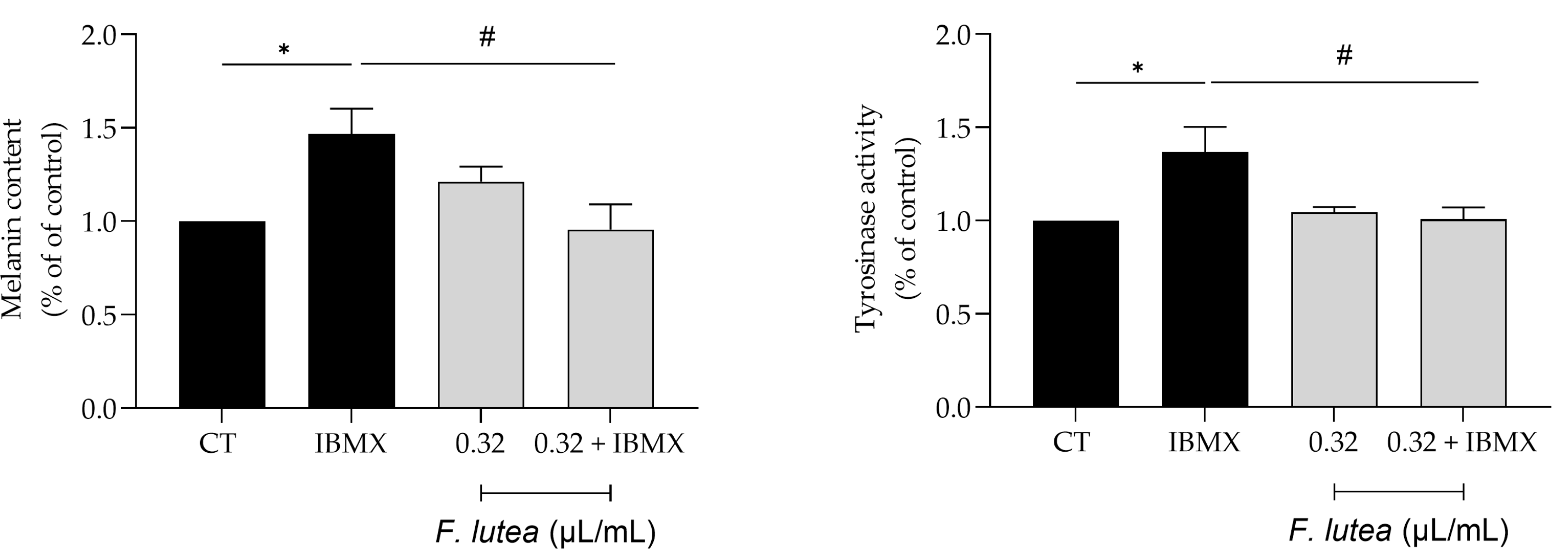
2.5. Ferulago lutea essential oil exerts depigmenting properties
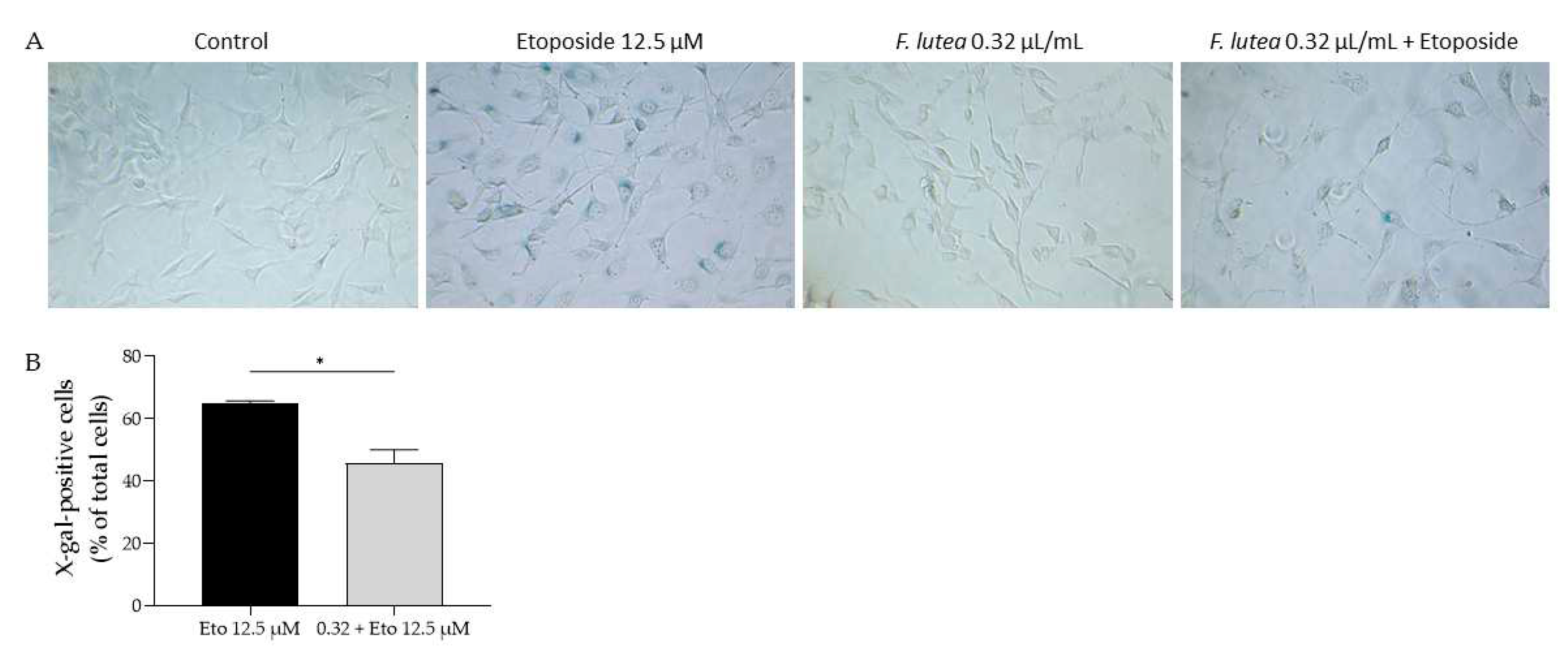
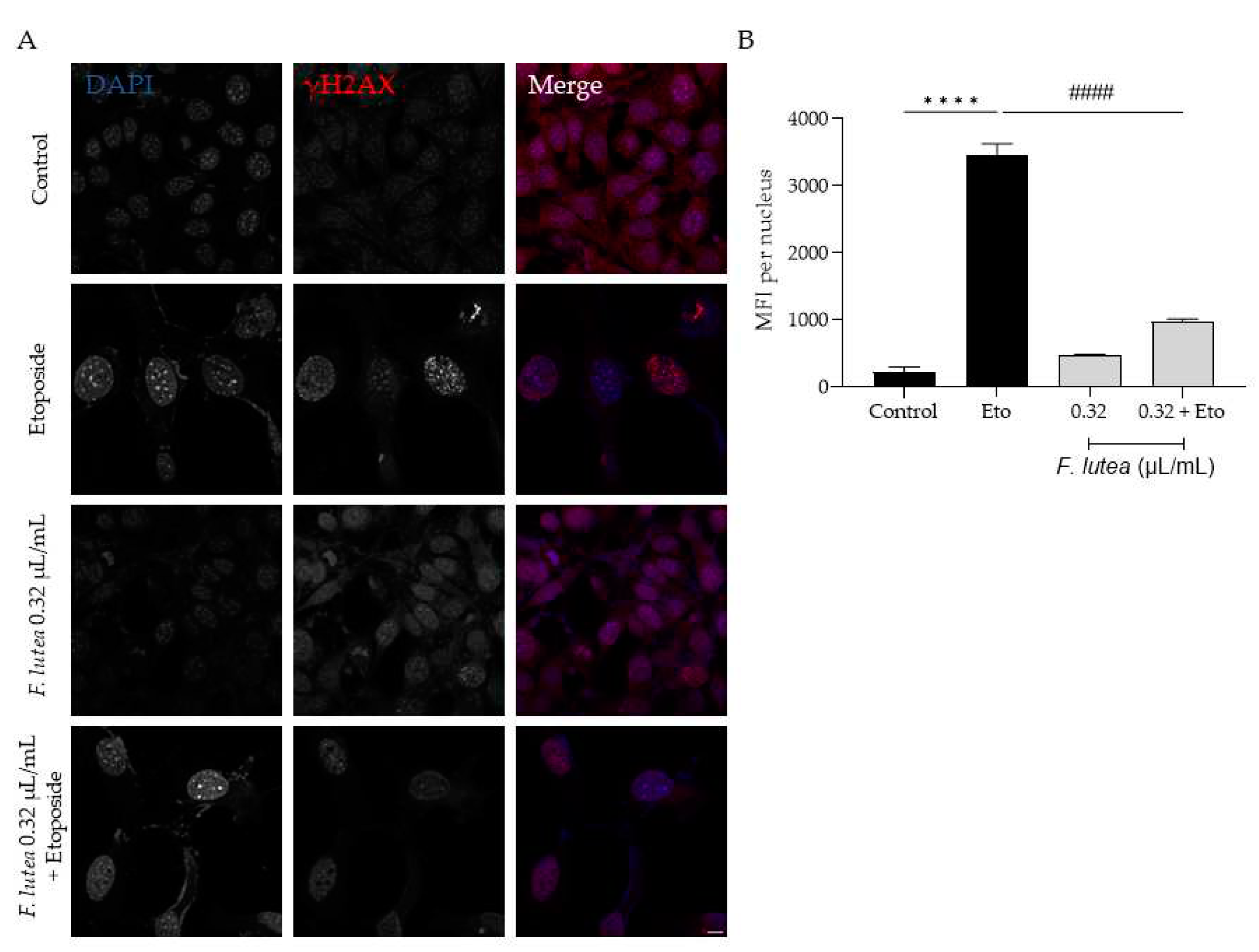
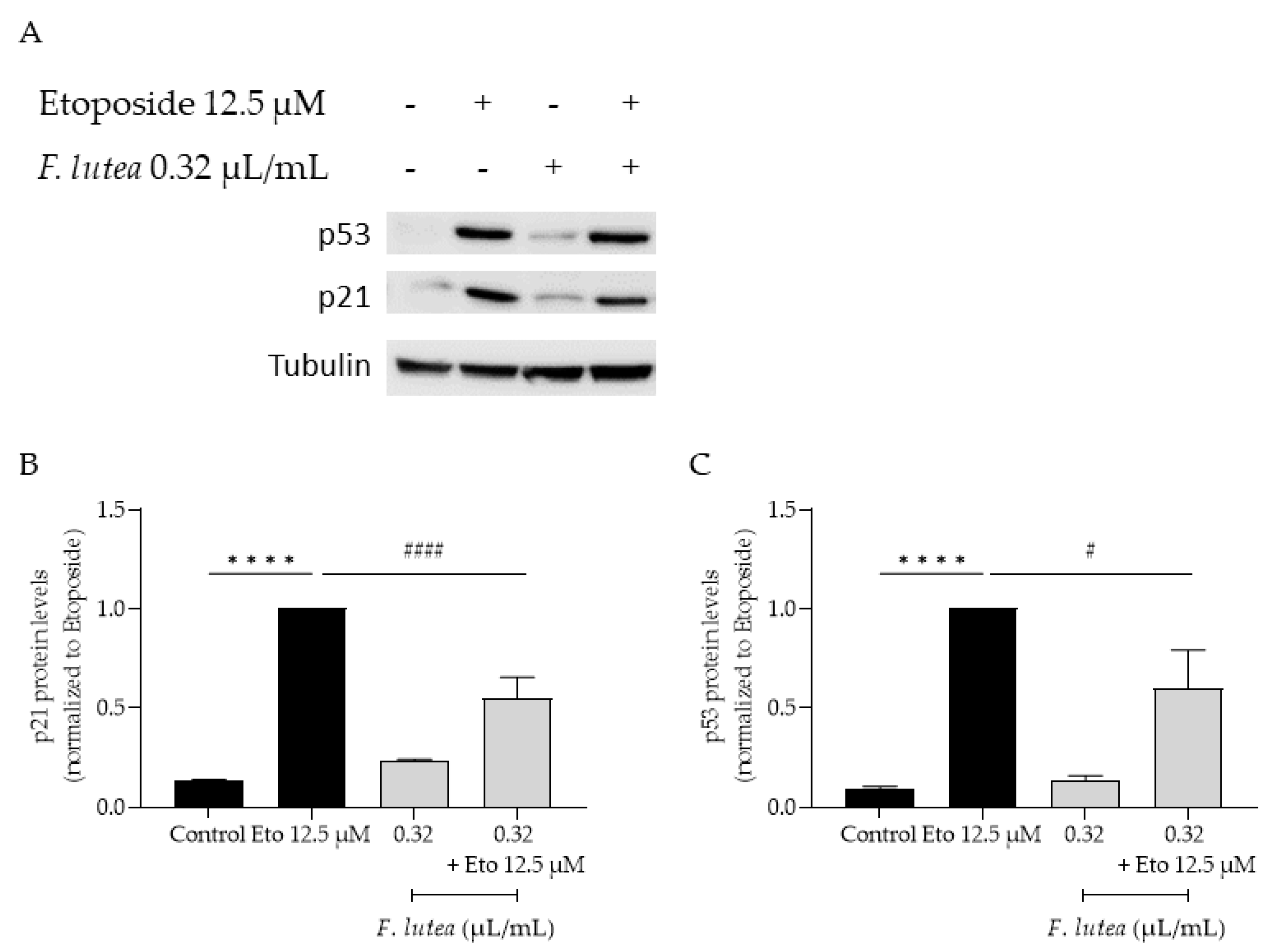
2.6. Ferulago lutea essential oil has anti-senescence properties
3. Discussion
4. Materials and Methods
4.1. Plant material and essential hydrodistillation
4.2. Chemical characterization of the essential oil
4.3. Cell culture
4.4. Effect on cell viability
4.5. Anti-inflammatory potential
4.5.1. Nitric oxide production
4.5.2. Western blot analysis of pro-inflammatory mediators
4.6. Cell migration
4.7. Inhibition of lipogenesis
4.7.1. Lipogenesis induction
4.7.2. Oil Red-O fluorescent staining
4.7.3. Lipid accumulation quantification by Oil Red O staining
4.8. Depigmenting effect
4.9. Anti-senescence potential
4.9.1. Senescence-associated β-galactosidase activity
4.9.2. yH2AX staining
4.9.3. p21 and p53 protein levels
4.10. Statistical analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization World Report on Ageing and Health; ISBN 9789241565042.
- Pilkington, S.M.; Bulfone-Paus, S.; Griffiths, C.E.M.; Watson, R.E.B. Inflammaging and the Skin. Journal of Investigative Dermatology 2021, 141, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Park, T.J.; Kang, H.Y. Skin-Aging Pigmentation: Who Is the Real Enemy? Cells 2022, 11, 2541. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Dürr, P. Plant Extracts and Natural Compounds Used against UVB-Induced Photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Estanqueiro, M.; Oliveira, M.; Sousa Lobo, J. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- WFO Ferulago W. D., J. Koch. Available online: https://www.worldfloraonline.org/taxon/wfo-4000014699 (accessed on 19 September 2023).
- Badalamenti, N.; Ilardi, V.; Rosselli, S.; Bruno, M. The Ethnobotany, Phytochemistry and Biological Properties of Genus Ferulago – A Review. J Ethnopharmacol 2021, 274, 114050. [Google Scholar] [CrossRef]
- Pinto, E.; Hrimpeng, K.; Lopes, G.; Vaz, S.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L. Antifungal Activity of Ferulago Capillaris Essential Oil against Candida, Cryptococcus, Aspergillus and Dermatophyte Species. European Journal of Clinical Microbiology & Infectious Diseases 2013, 32, 1311–1320. [Google Scholar] [CrossRef]
- Znati, M.; Jabrane, A.; Hajlaoui, H.; Harzallah-Skhiri, F.; Bouajila, J.; Casanova, J.; Jannet, H. Ben Chemical Composition and in Vitro Evaluation of Antimicrobial and Anti-Acetylcholinesterase Properties of the Flower Oil of Ferula Lutea. Nat Prod Commun 2012, 7, 1934578X1200700. [Google Scholar] [CrossRef]
- Ben Salem, S.; Znati, M.; Jabrane, A.; Casanova, J.; Ben Jannet, H. Chemical Composition, Antimicrobial, Anti-Acetylcholinesterase and Cytotoxic Activities of the Root Essential Oil from the Tunisian Ferula Lutea (Poir.) Maire (Apiaceae). Journal of Essential Oil Bearing Plants 2016, 19, 897–906. [Google Scholar] [CrossRef]
- Rahmouni, M.; Laouer, H.; Dahamna, S.; Gali, L.; Bensouici, C.; Flamini, G.; Akkal, S. Biological Activities and Phytochemical Content of Essential Oil and Methanol Extracts of Ferula Lutea (Poir.) Maire Growing in Algeria. Biocatal Agric Biotechnol 2021, 34, 102017. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct Target Ther 2017, 2, 17023. [Google Scholar] [CrossRef]
- Vu, R.; Jin, S.; Sun, P.; Haensel, D.; Nguyen, Q.H.; Dragan, M.; Kessenbrock, K.; Nie, Q.; Dai, X. Wound Healing in Aged Skin Exhibits Systems-Level Alterations in Cellular Composition and Cell-Cell Communication. Cell Rep 2022, 40, 111155. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Zouboulis, C.C. Skin Alterations and Diseases in Advanced Age. Drug Discov Today Dis Mech 2008, 5, e153–e162. [Google Scholar] [CrossRef]
- Esler, W.P.; Tesz, G.J.; Hellerstein, M.K.; Beysen, C.; Sivamani, R.; Turner, S.M.; Watkins, S.M.; Amor, P.A.; Carvajal-Gonzalez, S.; Geoly, F.J.; et al. Human Sebum Requires de Novo Lipogenesis, Which Is Increased in Acne Vulgaris and Suppressed by Acetyl-CoA Carboxylase Inhibition. Sci Transl Med 2019, 11. [Google Scholar] [CrossRef]
- Mitro, N.; Vargas, L.; Romeo, R.; Koder, A.; Saez, E. T0901317 Is a Potent PXR Ligand: Implications for the Biology Ascribed to LXR. FEBS Lett 2007, 581, 1721–1726. [Google Scholar] [CrossRef]
- Kudlova, N.; De Sanctis, J.B.; Hajduch, M. Cellular Senescence: Molecular Targets, Biomarkers, and Senolytic Drugs. Int J Mol Sci 2022, 23, 4168. [Google Scholar] [CrossRef]
- Sá, D.C. de; Festa Neto, C. Inflammasomes and Dermatology. An Bras Dermatol 2016, 91, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, Y.; Zhou, Y.; Follansbee, T.; Hwang, S.T. Immune Mediators and Therapies for Pruritus in Atopic Dermatitis and Psoriasis. Journal of Cutaneous Immunology and Allergy 2019, 2, 4–14. [Google Scholar] [CrossRef]
- Schwingen, J.; Kaplan, M.; Kurschus, F.C. Review—Current Concepts in Inflammatory Skin Diseases Evolved by Transcriptome Analysis: In-Depth Analysis of Atopic Dermatitis and Psoriasis. Int J Mol Sci 2020, 21, 699. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Lee, S.E.; Kim, J.H. Immunopathology and Immunotherapy of Inflammatory Skin Diseases. Immune Netw 2022, 22. [Google Scholar] [CrossRef]
- Goh, B.H.; Mocan, A.; Xiao, J.; Mah, S.H.; Yap, W.H. Editorial: Targeting Human Inflammatory Skin Diseases With Natural Products: Exploring Potential Mechanisms and Regulatory Pathways. Front Pharmacol 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Papa, F.; Shi, J.; Maggi, F.; Chen, X. The Chemical Constituents and the Hepato-Protective Effect of the Essential Oil of Ferulago Campestris (Besser) Grecescu (Apiaceae). Journal of Essential Oil Bearing Plants 2016, 19, 1701–1708. [Google Scholar] [CrossRef]
- Bantal, V.; Biradar, S. Screening of Natural Antioxidants by Using L-Arginine Induced Acute Pancreatitis Model; 2012; Vol. 4.
- Yu, L.; Yan, J.; Sun, Z. D-Limonene Exhibits Anti-Inflammatory and Antioxidant Properties in an Ulcerative Colitis Rat Model via Regulation of INOS, COX-2, PGE2 and ERK Signaling Pathways. Mol Med Rep 2017, 15, 2339–2346. [Google Scholar] [CrossRef]
- Santana, H.S.R.; de Carvalho, F.O.; Silva, E.R.; Santos, N.G.L.; Shanmugam, S.; Santos, D.N.; Wisniewski, J.O.; Junior, J.S.C.; Nunes, P.S.; Araujo, A.A.S.; et al. Anti-Inflammatory Activity of Limonene in the Prevention and Control of Injuries in the Respiratory System: A Systematic Review. Curr Pharm Des 2020, 26, 2182–2191. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-Inflammatory and Chondroprotective Activity of (+)-α-Pinene: Structural and Enantiomeric Selectivity. J Nat Prod 2014, 77, 264–269. [Google Scholar] [CrossRef]
- Li, X.-J.; Yang, Y.-J.; Li, Y.-S.; Zhang, W.K.; Tang, H.-B. α-Pinene, Linalool, and 1-Octanol Contribute to the Topical Anti-Inflammatory and Analgesic Activities of Frankincense by Inhibiting COX-2. J Ethnopharmacol 2016, 179, 22–26. [Google Scholar] [CrossRef]
- Rahbar, I.; Abbasnejad, M.; Haghani, J.; Raoof, M.; Kooshki, R.; Esmaeili-Mahani, S. The Effect of Central Administration of Alpha-pinene on Capsaicin-induced Dental Pulp Nociception. Int Endod J 2019, 52, 307–317. [Google Scholar] [CrossRef]
- Khalid, K.A.; Nawi, A.F.M.; Zulkifli, N.; Barkat, Md.A.; Hadi, H. Aging and Wound Healing of the Skin: A Review of Clinical and Pathophysiological Hallmarks. Life 2022, 12, 2142. [Google Scholar] [CrossRef]
- Salas-Oropeza, J.; Jimenez-Estrada, M.; Perez-Torres, A.; Castell-Rodriguez, A.E.; Becerril-Millan, R.; Rodriguez-Monroy, M.A.; Jarquin-Yañez, K.; Canales-Martinez, M.M. Wound Healing Activity of α-Pinene and α-Phellandrene. Molecules 2021, 26, 2488. [Google Scholar] [CrossRef]
- d’Alessio, P.; Mirshahi, M.; Bisson, J.-F.; Bene, M. Skin Repair Properties of D-Limonene and Perillyl Alcohol in Murine Models. Antiinflamm Antiallergy Agents Med Chem 2014, 13, 29–35. [Google Scholar] [CrossRef]
- Ahmad, M.; Khan, T.H.; Ansari, M.N.; Ahmad, S.F. Enhanced Wound Healing by Topical Administration of D-Limonene in Alloxan Induced Diabetic Mice through Reduction of pro-Inflammatory Markers and Chemokine Expression. BMC Genomics 2014, 15, P29. [Google Scholar] [CrossRef]
- Keskin, I.; Gunal, Y.; Ayla, S.; Kolbasi, B.; Sakul, A.; Kilic, U.; Gok, O.; Koroglu, K.; Ozbek, H. Effects of Foeniculum Vulgare Essential Oil Compounds, Fenchone and Limonene, on Experimental Wound Healing. Biotechnic & Histochemistry 2017, 92, 274–282. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Boschnakow, A. Chronological Ageing and Photoageing of the Human Sebaceous Gland. Clin Exp Dermatol 2001, 26, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Soundharrajan, I.; Kim, D.H.; Srisesharam, S.; Kuppusamy, P.; Choi, K.C. R -Limonene Enhances Differentiation and 2-Deoxy-D-Glucose Uptake in 3T3-L1 Preadipocytes by Activating the Akt Signaling Pathway. Evidence-Based Complementary and Alternative Medicine 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Liao, J.-T.; Huang, Y.-W.; Hou, C.-Y.; Wang, J.-J.; Wu, C.-C.; Hsieh, S.-L. D-Limonene Promotes Anti-Obesity in 3T3-L1 Adipocytes and High-Calorie Diet-Induced Obese Rats by Activating the AMPK Signaling Pathway. Nutrients 2023, 15, 267. [Google Scholar] [CrossRef]
- Hakozaki, T.; Swanson, C.L.; Bissett, D.L. Hyperpigmentation in Aging Skin. In Textbook of Aging Skin; Springer Berlin Heidelberg: Berlin, Heidelberg, 2015; pp. 1–10. [Google Scholar]
- Zolghadri, S.; Beygi, M.; Mohammad, T.F.; Alijanianzadeh, M.; Pillaiyar, T.; Garcia-Molina, P.; Garcia-Canovas, F.; Munoz-Munoz, J.; Saboury, A.A. Targeting Tyrosinase in Hyperpigmentation: Current Status, Limitations and Future Promises. Biochem Pharmacol 2023, 212, 115574. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.J.S.; Vani, M.G.; Wang, S. Limonene Protects Human Skin Keratinocytes against <scp>UVB</Scp> -induced Photodamage and Photoaging by Activating the Nrf2-dependent Antioxidant Defense System. Environ Toxicol 2022, 37, 2897–2909. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, A.; Liyanage, G.; Sirimanna, G.; Schürer, N. Comparative Study on Depigmenting Agents in Skin of Color. J Clin Aesthet Dermatol 2022, 15, 12–17. [Google Scholar] [PubMed]
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int J Mol Sci 2021, 22, 12641. [Google Scholar] [CrossRef]
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin Senescence: Mechanisms and Impact on Whole-Body Aging. Trends Mol Med 2022, 28, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Passos, J.F.; Nelson, G.; Wang, C.; Richter, T.; Simillion, C.; Proctor, C.J.; Miwa, S.; Olijslagers, S.; Hallinan, J.; Wipat, A.; et al. Feedback between P21 and Reactive Oxygen Production Is Necessary for Cell Senescence. Mol Syst Biol 2010, 6. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; François, M.; Fenech, M.F.; Leifert, W.R. Persistent ΓH2AX: A Promising Molecular Marker of DNA Damage and Aging. Mutation Research/Reviews in Mutation Research 2015, 766, 1–19. [Google Scholar] [CrossRef]
- Secerli, J.; Erdem, O.; Bacanlı, M. Antiaging Effects of Limonene in Ageing-Induced HaCaT Cells. Genetics & Applications 2023, 7. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Kanimozhi, G.; Madahavan, N.R.; Agilan, B.; Ganesan, M.; Prasad, N.R.; Rathinaraj, P. Alpha-Pinene Attenuates UVA-Induced Photoaging through Inhibition of Matrix Metalloproteinases Expression in Mouse Skin. Life Sci 2019, 217, 110–118. [Google Scholar] [CrossRef]
- Council of Europe European Pharmacopoeia 7th Edition; Directorate for the Quality of Medicines & HealthCare of the Council of Europe: Strasbourg, France, 2010; ISBN 978-92-871-6700-2.
- Alves-Silva, J.M.; Zuzarte, M.; Gonçalves, M.J.; Cruz, M.T.; Cavaleiro, C.; Salgueiro, L. Unveiling the Bioactive Potential of the Essential Oil of a Portuguese Endemism, Santolina Impressa. J Ethnopharmacol 2019, 244, 112–120. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; 4th ed.; Allured Publishing Corporation, Carol Stream: IL, 2007.
- Stein, S.E. Retention Indices” by NIST Mass Spec Data Center. In NIST Chemistry WebBook, NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.J., Eds.; National Institute of Standards and Technology: Gaithersburg MD, 20899, 2017. [Google Scholar]
- Joulain, D.; König, W.A. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; E.B.-Verlag: Hamburg, 1998; ISBN 9783930826483. [Google Scholar]
- El-Sayed, A. The Pherobase: Database of Insect Pheromones and Semiochemicals; 2007.
- McLafferty, F.W. Wiley Registry of Mass Spectral Data/NIST08; 9th ed.; John Wiley and Sons Ltd.: Hoboken, USA, 2009; ISBN 0470520361. [Google Scholar]
- Joulain, D.; König, W.A. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; E.B.-Verlag: Hamburg, 1998; ISBN 9783930826483. [Google Scholar]
- Alves-Silva, J.M.; Guerra, I.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Figueirinha, A.; Salgueiro, L. Chemical Composition of Crithmum Maritimum L. Essential Oil and Hydrodistillation Residual Water by GC-MS and HPLC-DAD-MS/MS, and Their Biological Activities. Ind Crops Prod 2020, 149. [Google Scholar] [CrossRef]
- Moreira, P.; Sousa, F.J.; Matos, P.; Brites, G.S.; Gonçalves, M.J.; Cavaleiro, C.; Figueirinha, A.; Salgueiro, L.; Batista, M.T.; Branco, P.C.; et al. Chemical Composition and Effect against Skin Alterations of Bioactive Extracts Obtained by the Hydrodistillation of Eucalyptus Globulus Leaves. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Maccioni, A.; Falconieri, D.; Porcedda, S.; Gonçalves, M.J.; Alves-Silva, J.M.; Silva, A.; Cruz, M.T.; Salgueiro, L.; Maxia, A. Chemical Composition and Biological Activity of Essential Oil of Teucrium Scordium L. Subsp. Scordioides (Schreb.) Arcang. (Lamiaceae) from Sardinia Island (Italy). Nat Prod Res 2022, 36. [Google Scholar] [CrossRef]
- Cruz, M.T.; Duarte, C.B.; Gonçalo, M.; Figueiredo, A.; Carvalho, A.P.; Lopes, M.C. Granulocyte-Macrophage Colony-Stimulating Factor Activates the Transcription of Nuclear Factor Kappa B and Induces the Expression of Nitric Oxide Synthase in a Skin Dendritic Cell Line. Immunol Cell Biol 2001, 79, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Alves-Silva, J.M.; Alves, M.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. New Insights on the Anti-Inflammatory Potential and Safety Profile of Thymus Carnosus and Thymus Camphoratus Essential Oils and Their Main Compounds. J Ethnopharmacol 2018, 225, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, J.M.; Pedreiro, S.; Cavaleiro, C.; Cruz, M.T.; Figueirinha, A.; Salgueiro, L. Effect of Thymbra Capitata (L.) Cav. on Inflammation, Senescence and Cell Migration. Nutrients 2023, 15, 1930. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Scratch Wound Healing Assay. In Methods in Molecular Biology; Humana Press Inc., 2020; Vol. 2109, pp. 225–229.
- Hong, I.; Rho, H.S.; Kim, D.H.; Lee, M.O. Activation of LXRα Induces Lipogenesis in HaCaT Cells. Arch Pharm Res 2010, 33, 1443–1449. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Pedreiro, S.; Cruz, M.T.; Salgueiro, L.; Figueirinha, A. Exploring the Traditional Uses of Thymbra Capitata Infusion in Algarve (Portugal): Anti-Inflammatory, Wound Healing, and Anti-Aging. Pharmaceuticals 2023, 16, 1202. [Google Scholar] [CrossRef]
| Compounds * | RI SPB-1a |
RI SW 10b |
% Peak area |
|---|---|---|---|
| α-Pinene | 930 | 1030 | 36.5 |
| Camphene | 943 | 1077 | 1.5 |
| Sabinene | 964 | 1128 | 0.5 |
| β-Pinene | 970 | 1118 | 1.5 |
| Myrcene | 980 | 1161 | 5.0 |
| p-Cymene | 1009 | 1271 | 1.5 |
| Limonene | 1020 | 1206 | 31.2 |
| β-Phellandrene | 1020 | 1215 | 5.5 |
| Z-β-Ocimene | 1025 | 1235 | 3.5 |
| E-β-Ocimene | 1035 | 1253 | 1.0 |
| γ-Terpinene | 1046 | 1249 | 0.5 |
| Z-Linalool oxyde | 1055 | 1439 | 1.0 |
| Terpinolene | 1076 | 1288 | 1.0 |
| p-Cymenene-8-ol | 1160 | 1621 | 0.5 |
| α-Copaene | 1364 | 1487 | 0.5 |
| E-Caryophyllene | 1408 | 1590 | 0.5 |
| Germacrene-D | 1466 | 1699 | 0.5 |
| δ-Cadinene | 1508 | 1751 | 0.6 |
| Total identified | 92.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





