Submitted:
19 October 2023
Posted:
23 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Specimen preparation
2.2. Immersion tests
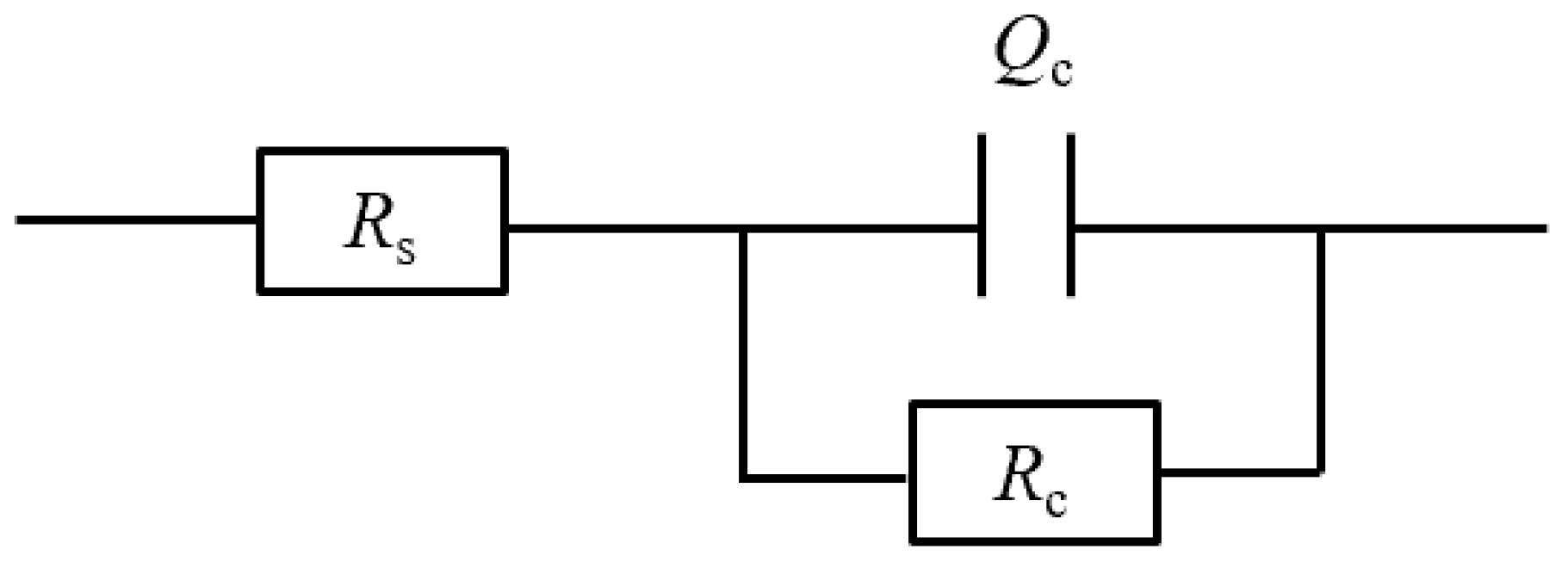
2.3. EIS measurement
2.4. AC-DC-AC tests at atmospheric and hydrostatic pressures
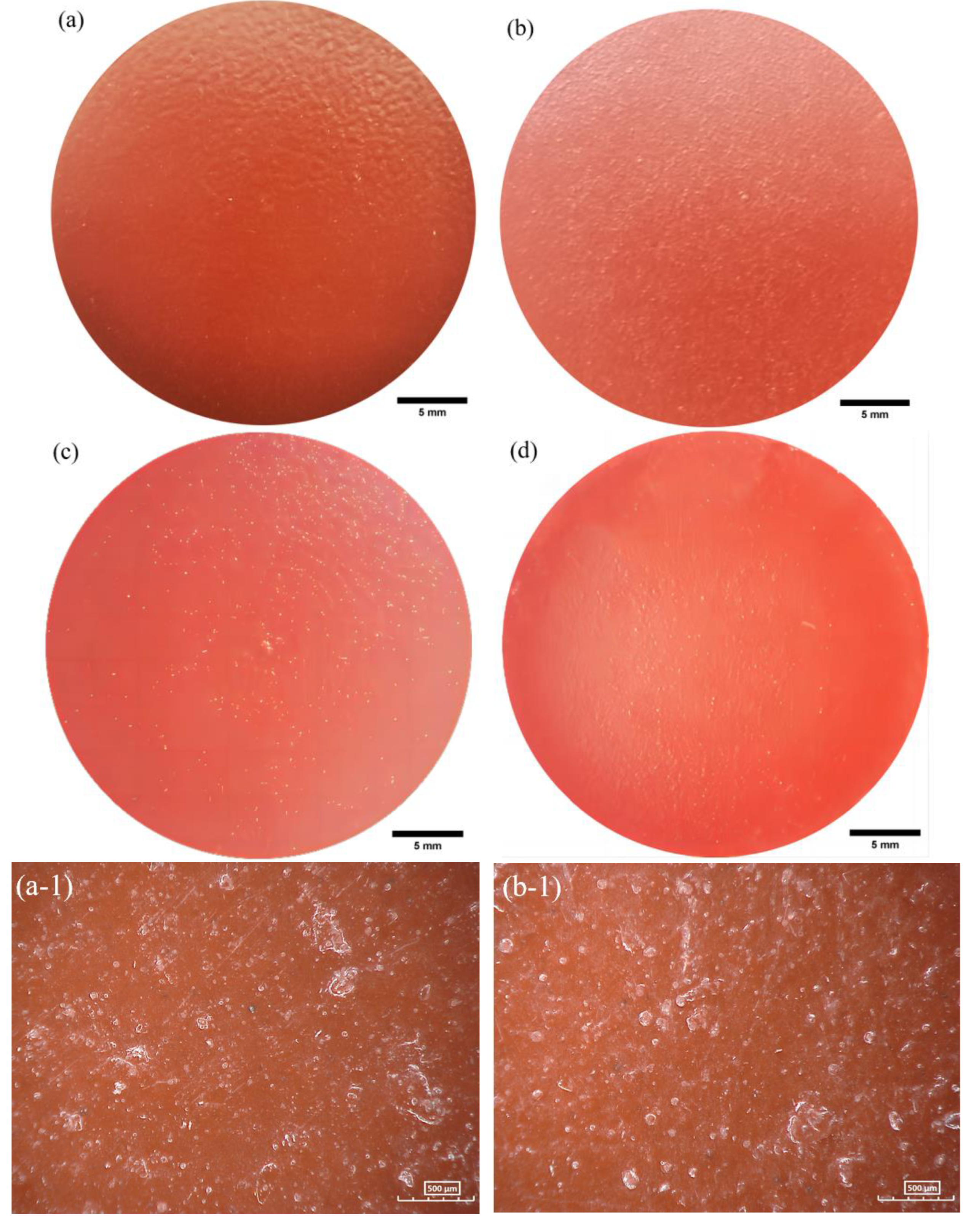
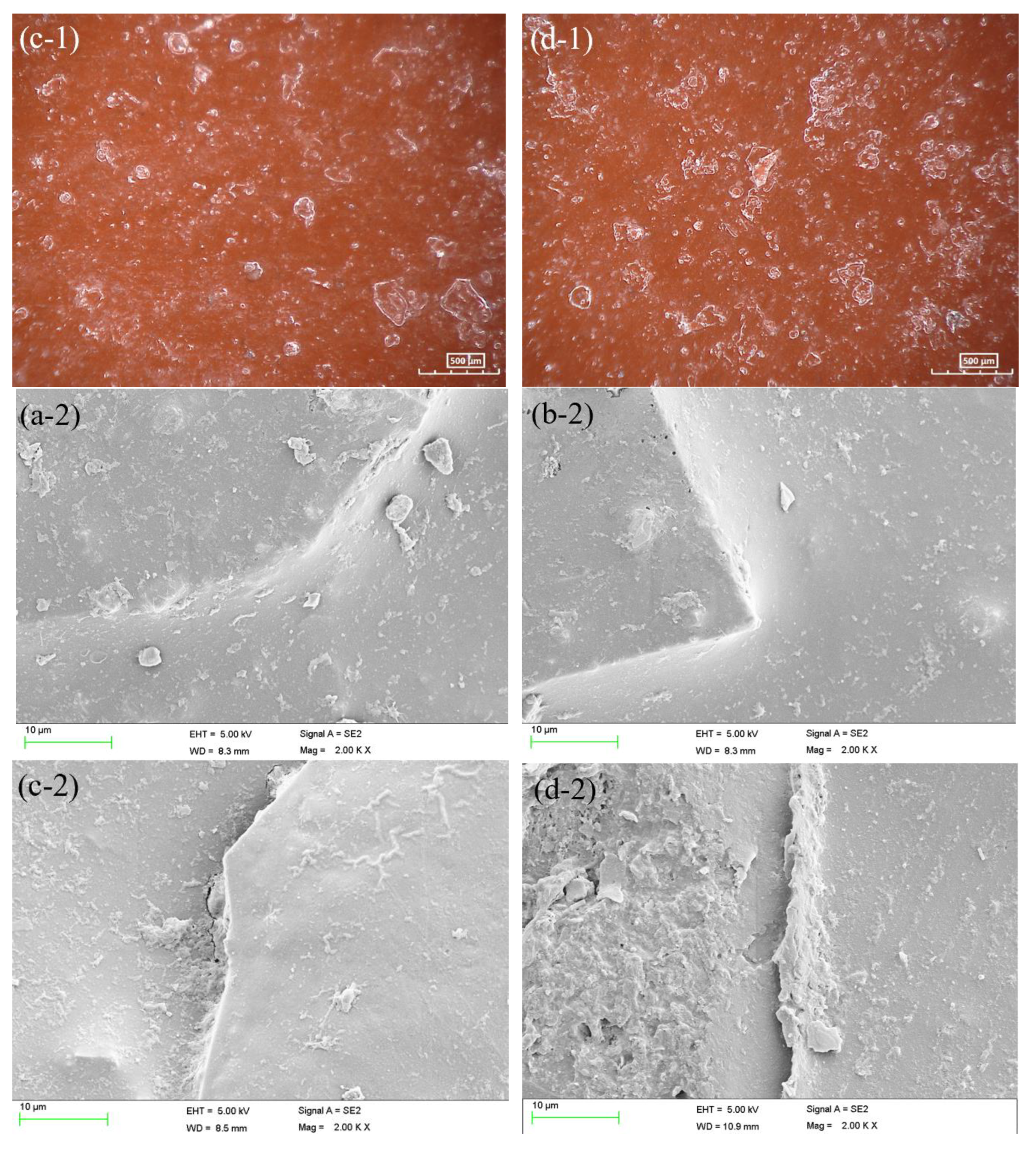
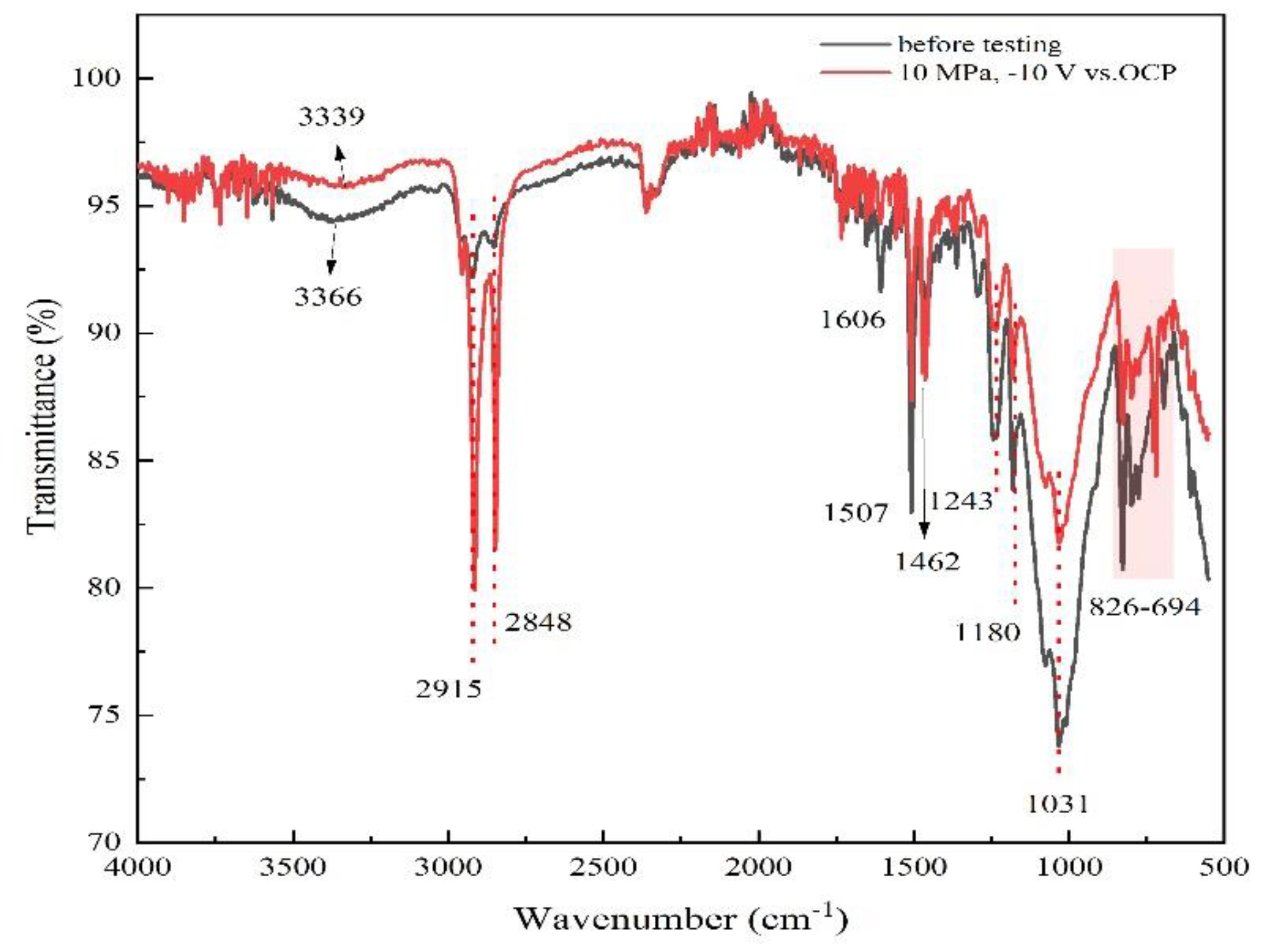
2.5. Surface characterization
3. Results and discussion
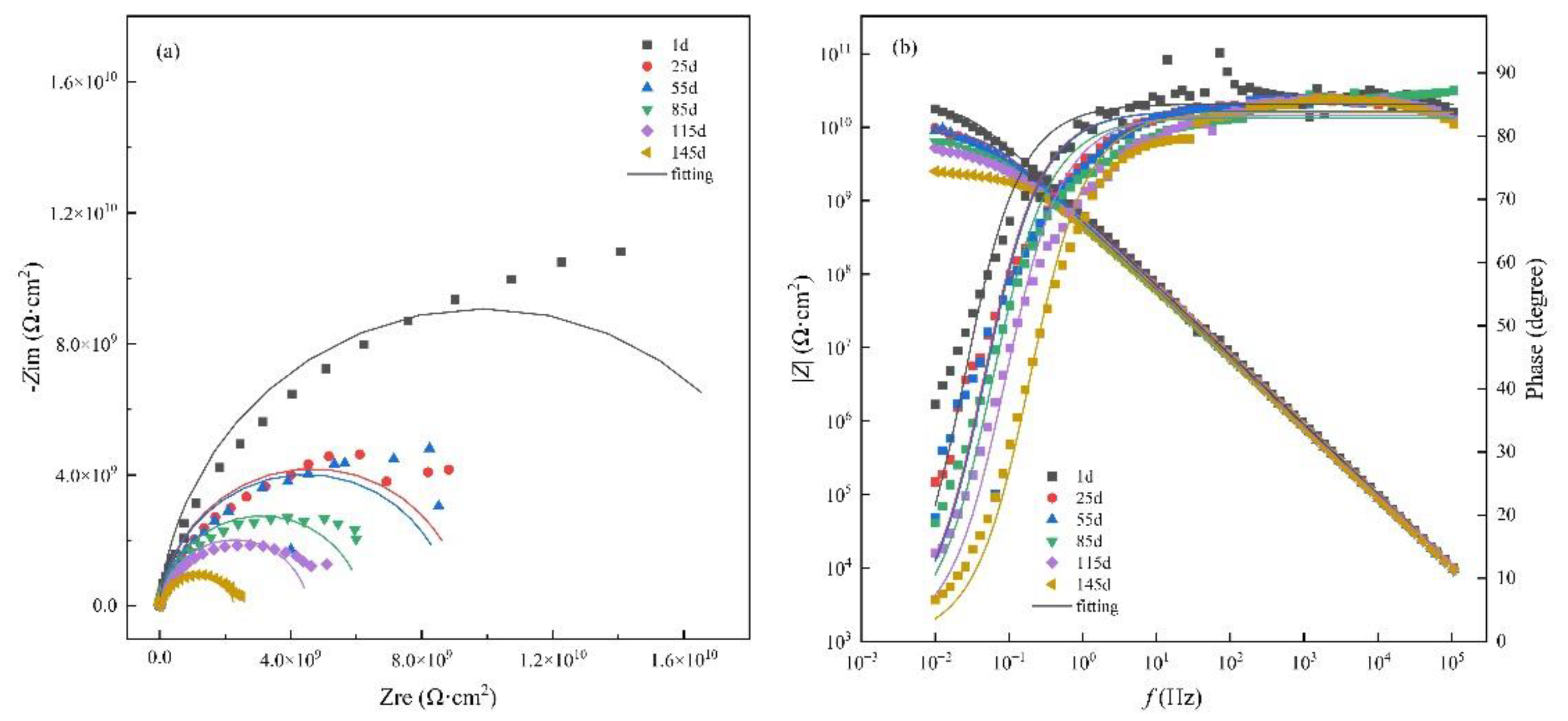
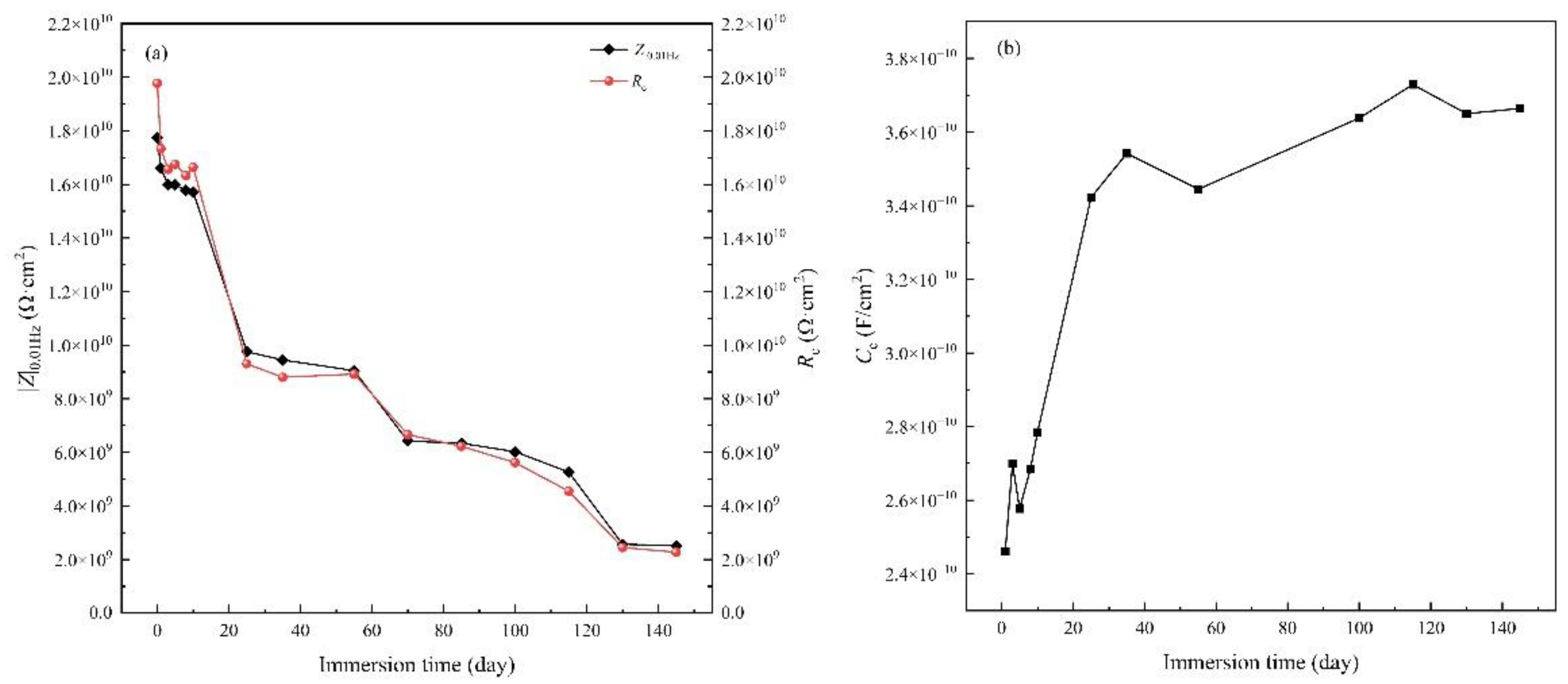
3.1. Immersion test under atmospheric pressure
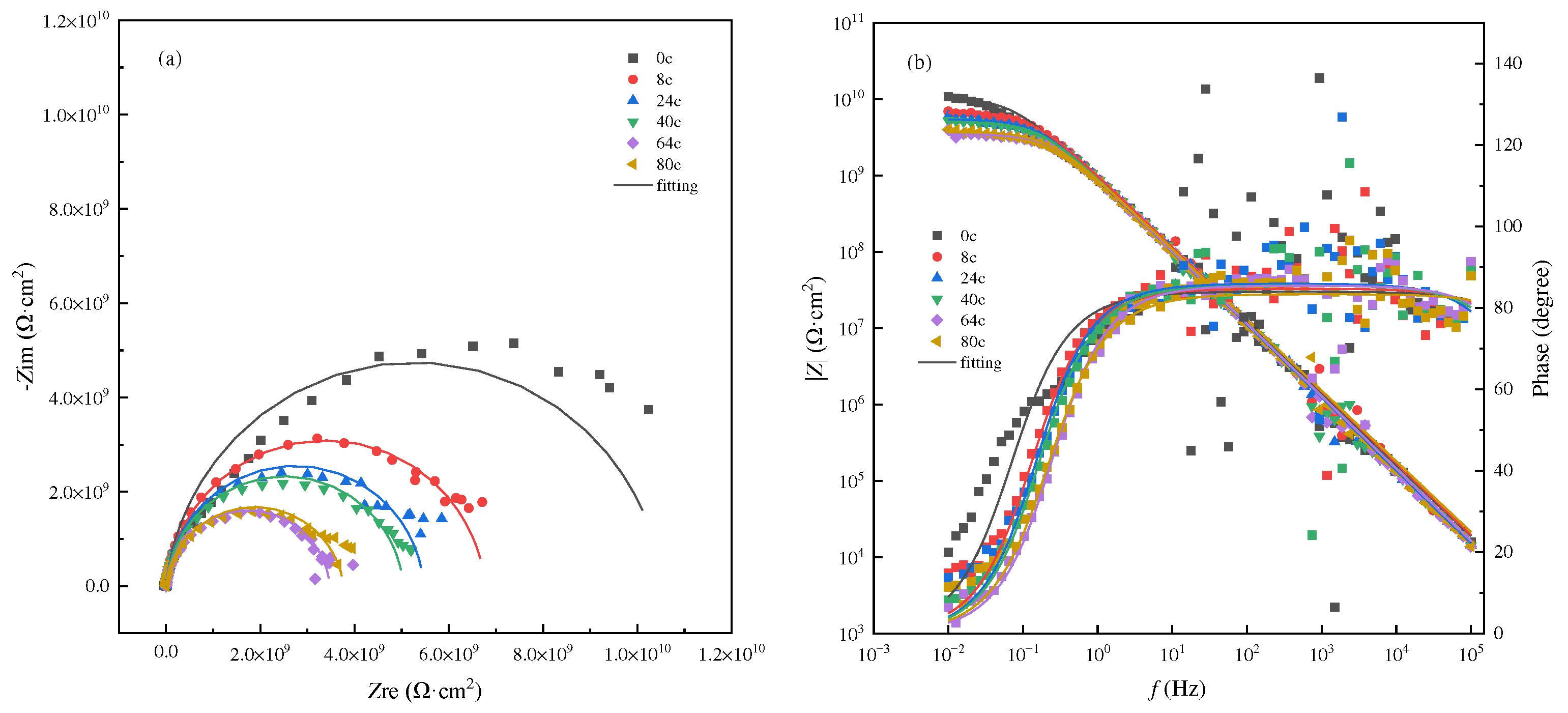
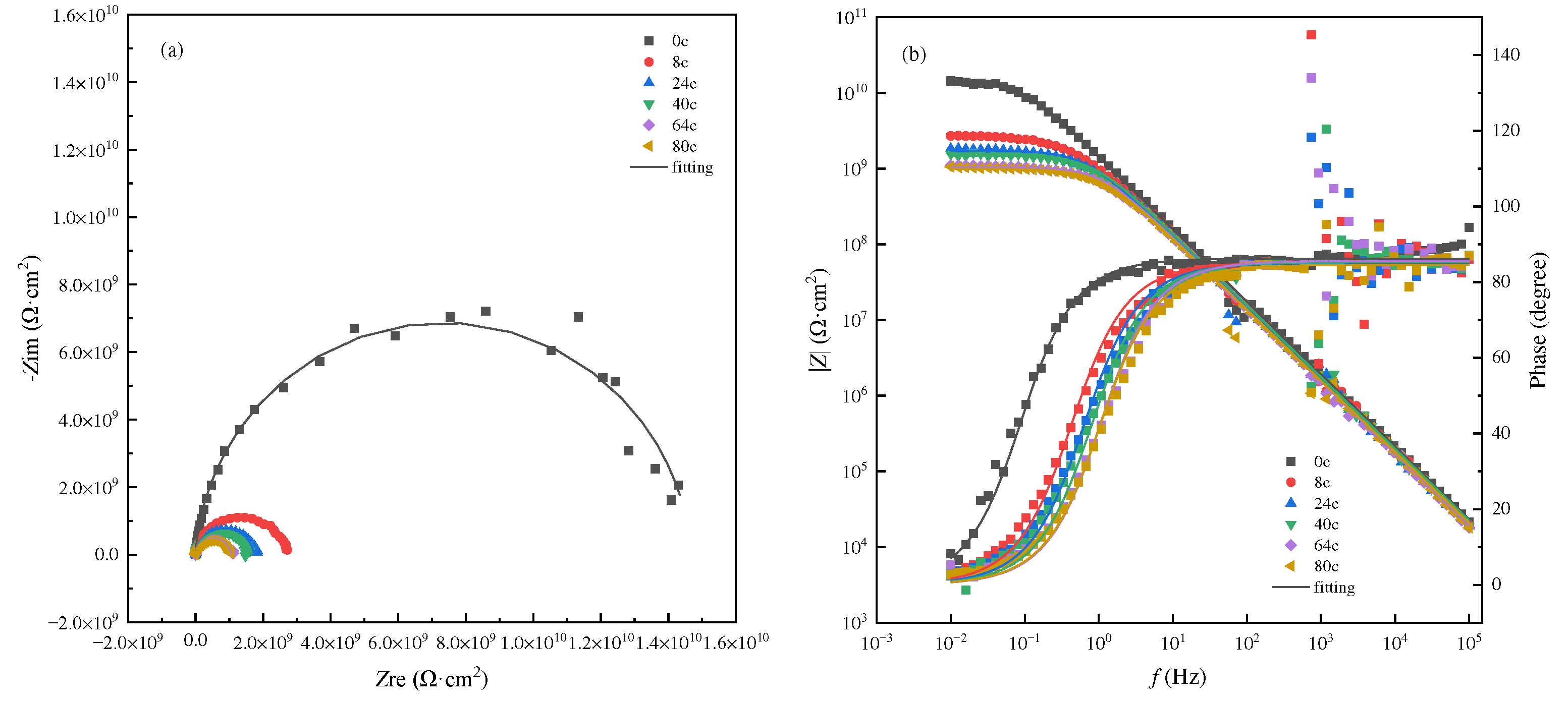
3.2. AC-DC-AC test under atmospheric pressure
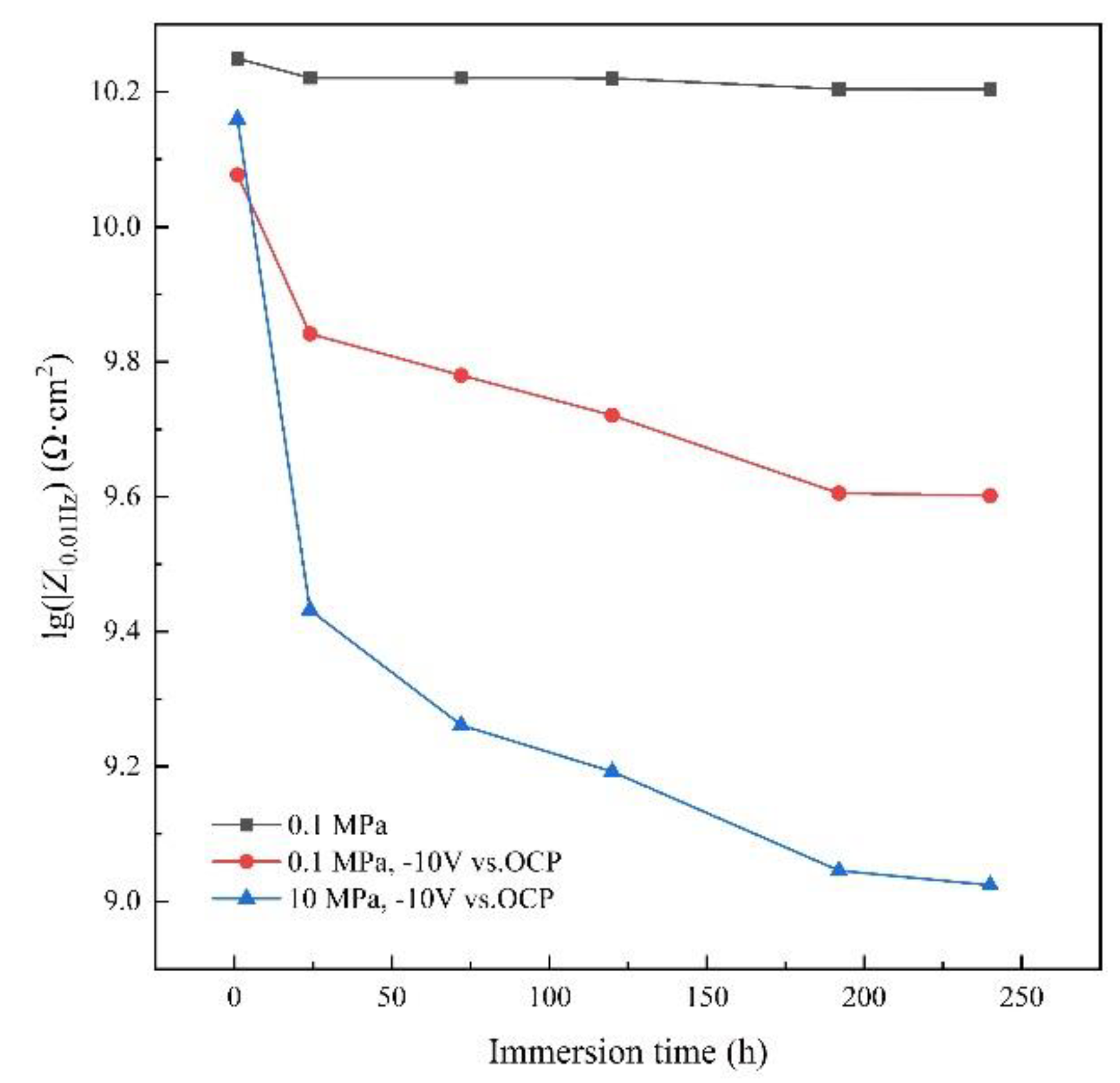
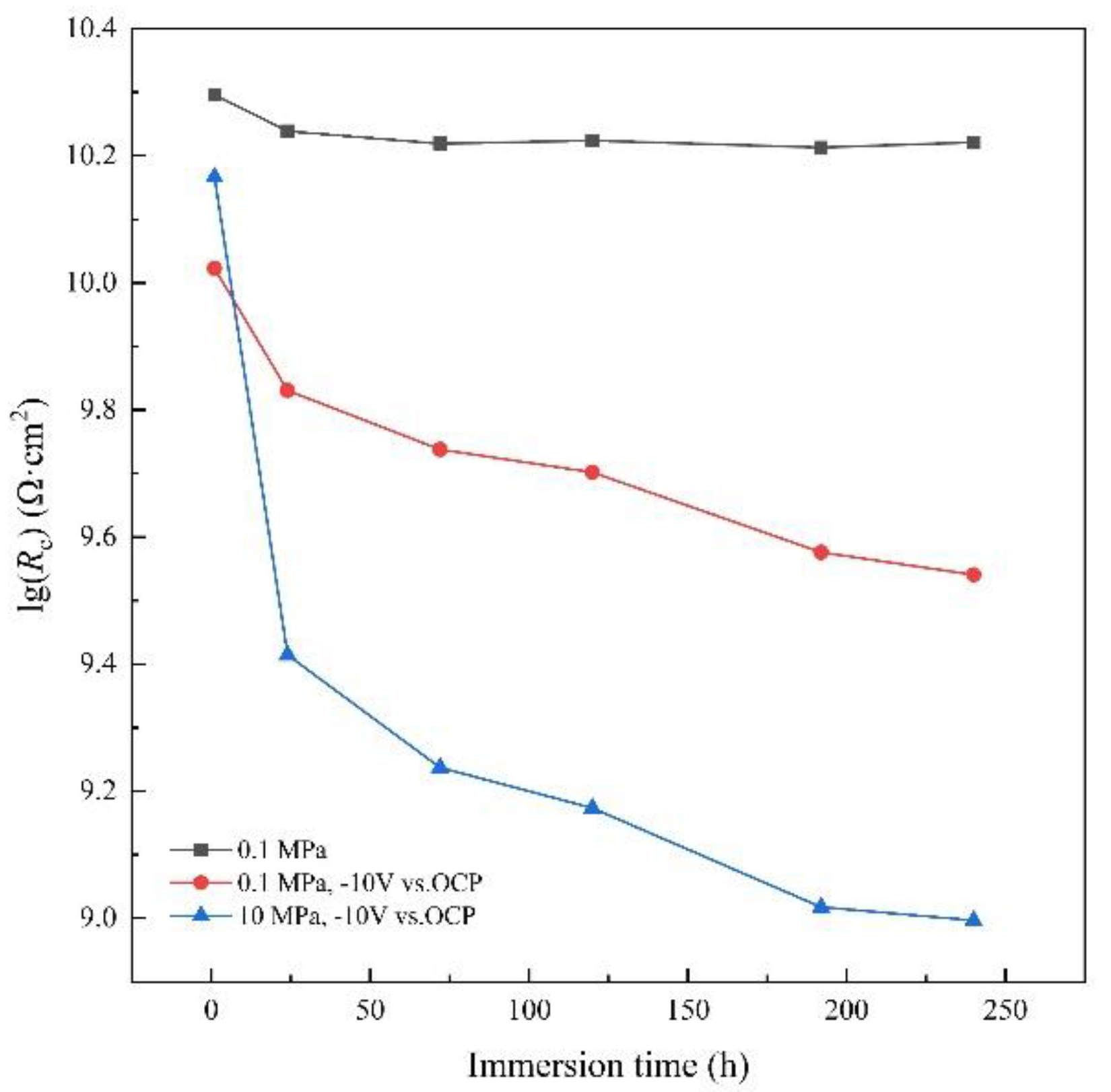
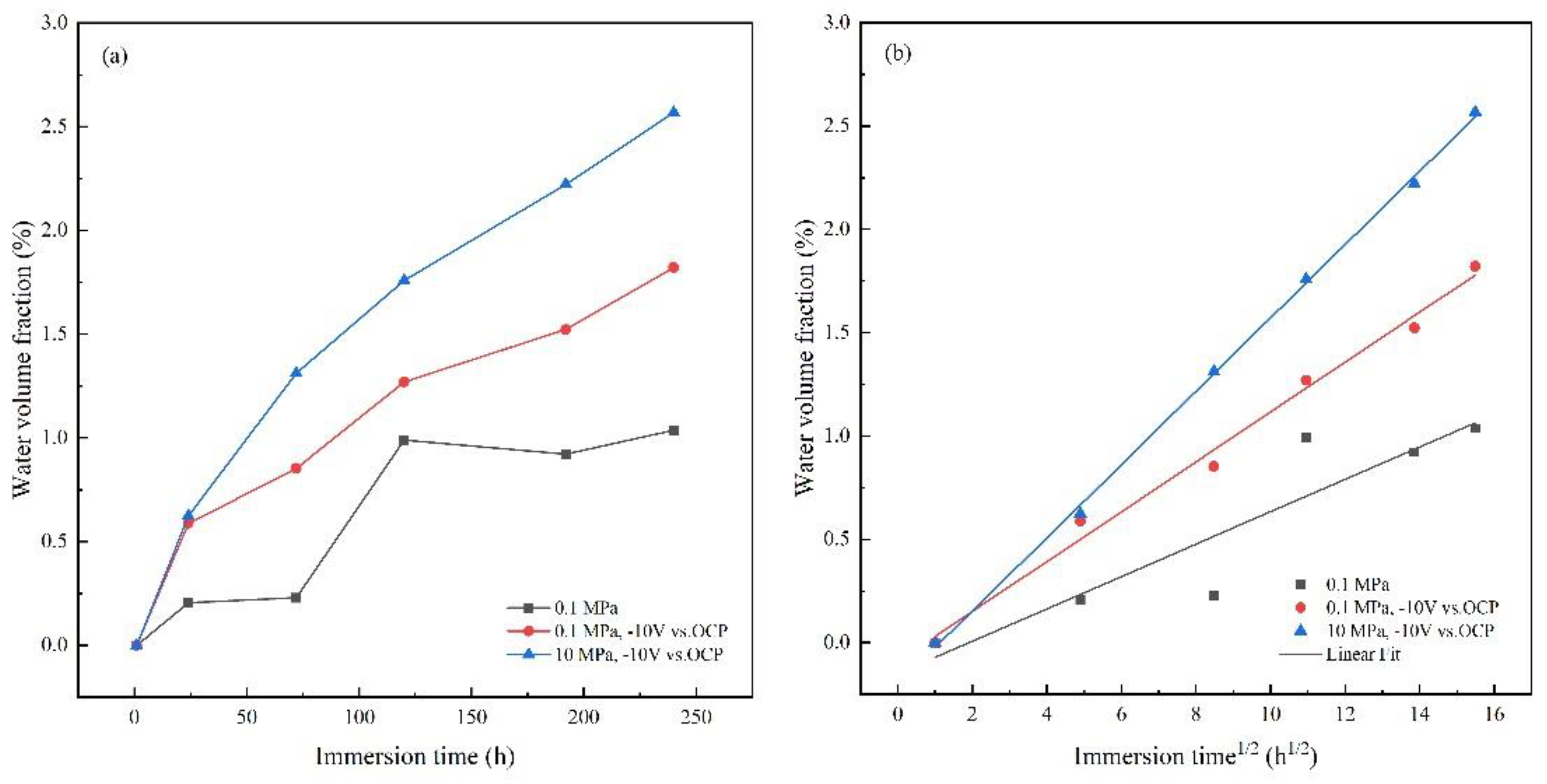
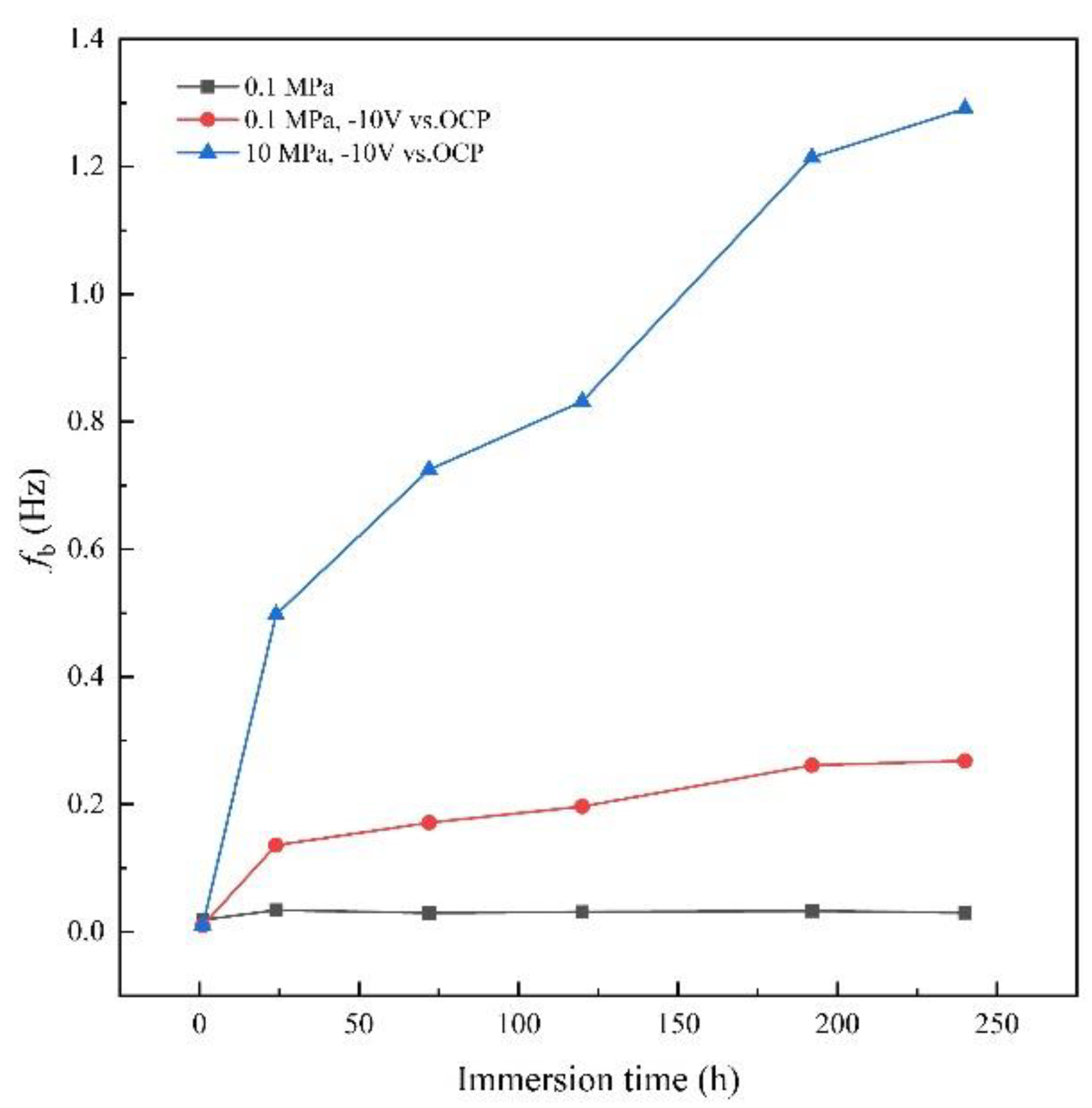
3.3. AC-DC-AC test under hydrostatic pressure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, L.; Xin, Y.; Ma, L.; Zhang, H.; Lin, Z.; Li, X. Challenges and Solutions of Cathodic Protection for Marine Ships. Corros. Commun. 2021, 2, 33–40. [CrossRef]
- Woloszyk, K.; Garbatov, Y.; Kowalski, J. Experimental Ultimate Strength Assessment of Stiffened Plates Subjected to Marine Immersed Corrosion. Appl. Ocean Res. 2023, 138, 103679. [CrossRef]
- Bhandari, J.; Khan, F.; Abbassi, R.; Garaniya, V.; Ojeda, R. Modelling of Pitting Corrosion in Marine and Offshore Steel Structures – A Technical Review. J. Loss Prev. Process Ind. 2015, 37, 39–62. [CrossRef]
- Olajire, A.A. Recent Advances on Organic Coating System Technologies for Corrosion Protection of Offshore Metallic Structures. J. Mol. Liq. 2018, 269, 572–606. [CrossRef]
- Lyon, S.B.; Bingham, R.; Mills, D.J. Advances in Corrosion Protection by Organic Coatings: What We Know and What We Would like to Know. Prog. Org. Coat. 2017, 102, 2–7. [CrossRef]
- Buchheit, R.G. Chapter 18 - Corrosion Resistant Coatings and Paints. In Handbook of Environmental Degradation of Materials; Kutz, M., Ed.; William Andrew Publishing: Norwich, NY, 2005; pp. 367–385 ISBN 978-0-8155-1500-5.
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-Healing Mechanisms in Smart Protective Coatings: A Review. Corros. Sci. 2018, 144, 74–88. [CrossRef]
- Resolution, M.S.C. 215 (82),“Performance Standard for Protective Coatings for Dedicated Seawater Ballast Tanks in All Types of Ships and Double-Side Skin Spaces of Bulk Carriers.” Int. Marit. Organ. 2006.
- Wei, C.; Wang, G.; Cridland, M.; Olson, D.L.; Liu, S. Chapter 25 - Corrosion Protection of Ships. In Handbook of Environmental Degradation of Materials (Third Edition); Kutz, M., Ed.; William Andrew Publishing, 2018; pp. 533–557 ISBN 978-0-323-52472-8.
- Iannarelli, P.; Beaumont, D.; Liu, Y.; Zhou, X.; Burnett, T.L.; Curioni, M.; Lyon, S.B.; Gibbon, S.R.; Morsh, S.; Emad, S.; et al. The Degradation Mechanism of a Marine Coating under Service Conditions of Water Ballast Tank. Prog. Org. Coat. 2022, 162, 106588. [CrossRef]
- Oriaifo, E.; Perera, N.; Guy, A.; Leung, P.S.; Tan, K. A Review of Test Protocols for Assessing Coating Performance of Water Ballast Tank Coatings.; Istanbul, Turkey, 2014; pp. 1610–1615.
- ISO 20340:2009, Paints and Varnishes — Performance Requirements for Protective Paint Systems for Offshore and Related Structures 2009.
- ISO 12944-9:2018, Paints and varnishes - Corrosion protection of steel structures by protective paint systems - Part 9: Protective paint systems and laboratory performance test methods for offshore and related structures 2018.
- Lajevardi Esfahani, S.; Ranjbar, Z.; Rastegar, S. Evaluation of Anticorrosion Behavior of Automotive Electrocoating Primers by the AC-DC-AC Accelerated Test Method. Prog. Color Color. Coat. 2013, 7, 187–199. [CrossRef]
- de Vooys, A.C.A.; Boelen, B.; van der Weijde, D.H. Screening of Coated Metal Packaging Cans Using EIS. Prog. Org. Coat. 2012, 73, 202–210. [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive Coatings: A Review. J. Coat. Technol. Res. 2009, 6, 135–176. [CrossRef]
- Kotnarowska, D. Influence of Ultraviolet Radiation and Aggressive Media on Epoxy Coating Degradation. Prog. Org. Coat. 1999, 37, 149–159. [CrossRef]
- García, S.J.; Suay, J. Optimization of Deposition Voltage of Cataphoretic Automotive Primers Assessed by EIS and AC/DC/AC. Prog. Org. Coat. 2009, 66, 306–313. [CrossRef]
- Deflorian, F.; Rossi, S.; Fedel, M. Organic Coatings Degradation: Comparison between Natural and Artificial Weathering. Corros. Sci. 2008, 50, 2360–2366. [CrossRef]
- Yu, M.; Fan, C.; Ge, F.; Lu, Q.; Wang, X.; Cui, Z. Anticorrosion Behavior of Organic Offshore Coating Systems in UV, Salt Spray and Low Temperature Alternation Simulated Arctic Offshore Environment. Prog. Org. Coat. 2021, 28, 102545. [CrossRef]
- ISO 17463:2022, Paints and Varnishes — Guidelines for the Determination of Anticorrosive Properties of Organic Coatings by Accelerated Cyclic Electrochemical Technique 2022.
- Hollaender, J. Rapid Assessment of Food/Package Interactions by Electrochemical Impedance Spectroscopy (EIS). Food Addit. Contam. 1997, 14, 617–626. [CrossRef]
- Bierwagen, G.; Tallman, D.; Li, J.; He, L.; Jeffcoate, C. EIS Studies of Coated Metals in Accelerated Exposure. Prog. Org. Coat. 2003, 46, 149–158. [CrossRef]
- Liu, X.; Xiong, J.; Lv, Y.; Zuo, Y. Study on Corrosion Electrochemical Behavior of Several Different Coating Systems by EIS. Prog. Org. Coat. 2009, 64, 497–503. [CrossRef]
- García, S.J.; Suay, J. A Comparative Study between the Results of Different Electrochemical Techniques (EIS and AC/DC/AC). Prog. Org. Coat. 2007, 59, 251–258. [CrossRef]
- Usman, B.J.; Scenini, F.; Curioni, M. Exploring the Use of an AC-DC-AC Technique for the Accelerated Evaluation of Anticorrosion Performance of Anodic Films on Aluminium Alloys. Prog. Org. Coat. 2020, 144, 105648. [CrossRef]
- Gimeno, M.J.; Chamorro, S.; March, R.; Oró, E.; Pérez, P.; Gracenea, J.; Suay, J. Anticorrosive Properties Enhancement by Means of Phosphate Pigments in an Epoxy 2k Coating. Assessment by NSS and ACET. Prog. Org. Coat. 2014, 77, 2024–2030. [CrossRef]
- Puig, M.; Gimeno, M.J.; Gracenea, J.J.; Suay, J.J. Anticorrosive Properties Enhancement in Powder Coating Duplex Systems by Means of ZMP Anticorrosive Pigment. Assessment by Electrochemical Techniques. Prog. Org. Coat. 2014, 77, 1993–1999. [CrossRef]
- Abdolah Zadeh, M.; van der Zwaag, S.; García, S.J. Assessment of Healed Scratches in Intrinsic Healing Coatings by AC/DC/AC Accelerated Electrochemical Procedure. Surf. Coat. Technol. 2016, 303, 396–405. [CrossRef]
- Gimeno, M.J.; Puig, M.; Chamorro, S.; Molina, J.; March, R.; Oró, E.; Pérez, P.; Gracenea, J.J.; Suay, J.J. Improvement of the Anticorrosive Properties of an Alkyd Coating with Zinc Phosphate Pigments Assessed by NSS and ACET. Prog. Org. Coat. 2016, 95, 46–53. [CrossRef]
- Bethencourt, M.; Botana, F.J.; Cano, M.J.; Osuna, R.M.; Marcos, M. Lifetime Prediction of Waterborne Acrylic Paints with the AC–DC–AC Method. Prog. Org. Coat. 2004, 49, 275–281. [CrossRef]
- da Silva Lopes, T.; Lopes, T.; Martins, D.; Carneiro, C.; Machado, J.; Mendes, A. Accelerated Aging of Anticorrosive Coatings: Two-Stage Approach to the AC/DC/AC Electrochemical Method. Prog. Org. Coat. 2020, 138, 105365. [CrossRef]
- Zheng, D.; Gui, Q.; Xu, Y.; Song, G.-L. Modified AC-DC-AC Method for Evaluation of Corrosion Damage of Acrylic Varnish Paint Coating/Q215 Steel System. Prog. Org. Coat. 2021, 159, 106401. [CrossRef]
- Xu, Y.; Song, G.-L.; Zheng, D.; Feng, Z. The Corrosion Damage of an Organic Coating Accelerated by Different AC-DC-AC Tests. Eng. Fail. Anal. 2021, 126, 105461. [CrossRef]
- Esfahani, S.L.; Ranjbar, Z.; Rastegar, S. An Electrochemical and Mechanical Approach to the Corrosion Resistance of Cathodic Electrocoatings under Combined Cyclic and DC Polarization Conditions. Prog. Org. Coat. 2014, 77, 1264–1270. [CrossRef]
- Allahar, K.N.; Bierwagen, G.P.; Gelling, V.J. Understanding Ac–Dc–Ac Accelerated Test Results. Corros. Sci. 2010, 52, 1106–1114. [CrossRef]
- Kan, B.; Wu, W.; Yang, Z.; Zhang, X.; Li, J. Effects of Hydrostatic Pressure and pH on the Corrosion Behavior of 2205 Duplex Stainless Steel. J. Electroanal. Chem. 2021, 886, 115134. [CrossRef]
- Yang, Z.X.; Kan, B.; Li, J.X.; Su, Y.J.; Qiao, L.J. Hydrostatic Pressure Effects on Stress Corrosion Cracking of X70 Pipeline Steel in a Simulated Deep-Sea Environment. Int. J. Hydrog. Energy 2017, 42, 27446–27457. [CrossRef]
- Liu, R.; Cui, Y.; Liu, L.; Zhang, B.; Wang, F. A Primary Study of the Effect of Hydrostatic Pressure on Stress Corrosion Cracking of Ti-6Al-4V Alloy in 3.5% NaCl Solution. Corros. Sci. 2020, 165, 108402. [CrossRef]
- Liu, R.; Liu, L.; Wang, F. The Role of Hydrostatic Pressure on the Metal Corrosion in Simulated Deep-Sea Environments — a Review. J. Mater. Sci. Technol. 2022, 112, 230–238. [CrossRef]
- Meng, F.; Liu, L.; Liu, E.; Zheng, H.; Liu, R.; Cui, Y.; Wang, F. Synergistic Effects of Fluid Flow and Hydrostatic Pressure on the Degradation of Epoxy Coating in the Simulated Deep-Sea Environment. Prog. Org. Coat. 2021, 159, 106449. [CrossRef]
- Peng, W.; Duan, T.; Hou, J.; Guo, X.; Zhang, Y.; Ma, L.; Yu, M.; Xin, Y.; Xing, S.; Zhang, H. Electrochemical Corrosion Behavior of High Strength Steel in Simulated Deep-Sea Environment under Different Hydrostatic Pressure. J. Mater. Res. Technol. 2023, 23, 2301–2316. [CrossRef]
- Meng, F.; Zhang, T.; Liu, L.; Cui, Y.; Wang, F. Failure Behaviour of an Epoxy Coating with Polyaniline Modified Graphene Oxide under Marine Alternating Hydrostatic Pressure. Surf. Coat. Technol. 2019, 361, 188–195. [CrossRef]
- Shao, Z.; Ren, P.; Jia, T.; Lei, B.; Feng, Z.; Guo, H.; Chen, S.; Zhang, P.; Meng, G. High-Pressure Induced Acceleration Pathways for Water Diffusion in Heavy Duty Anticorrosion Coatings under Deep Ocean Environment: (I) The Samples Subjected to High-Pressure Pre-Processing. Prog. Org. Coat. 2022, 170, 106948. [CrossRef]
- Liu, Y.; Wang, J.; Liu, L.; Li, Y.; Wang, F. Study of the Failure Mechanism of an Epoxy Coating System under High Hydrostatic Pressure. Corros. Sci. 2013, 74, 59–70. [CrossRef]
- Dong, J.-J.; Fan, L.; Zhang, H.-B.; Xu, L.-K.; Xue, L.-L. Electrochemical Performance of Passive Film Formed on Ti–Al–Nb–Zr Alloy in Simulated Deep Sea Environments. Acta Metall. Sin. Engl. Lett. 2020, 33, 595–604. [CrossRef]
- ISO-5668:Corrosion of Metals and Alloys Guidelines and Requirements for Corrosion Testing in Simulated Environment of Deep-Sea Water 2023.
- Corfias, C.; Pébère, N.; Lacabanne, C. Characterization of Protective Coatings by Electrochemical Impedance Spectroscopy and a Thermostimulated Current Method: Influence of the Polymer Binder. Corros. Sci. 2000, 42, 1337–1350. [CrossRef]
- Liu, J.; Lu, Z.; Zhang, L.; Li, C.; Ding, R.; Zhao, X.; Zhang, P.; Wang, B.; Cui, H. Studies of Corrosion Behaviors of a Carbon Steel/Copper-Nickel Alloy Couple under Epoxy Coating with Artificial Defect in 3.5 Wt.% NaCl Solution Using the WBE and EIS Techniques. Prog. Org. Coat. 2020, 148, 105909. [CrossRef]
- Trentin, A.; Pakseresht, A.; Duran, A.; Castro, Y.; Galusek, D. Electrochemical Characterization of Polymeric Coatings for Corrosion Protection: A Review of Advances and Perspectives. Polymers 2022, 14, 2306. [CrossRef]
- Shi, C.; Shao, Y.; Wang, Y.; Meng, G.; Liu, B. Influence of Submicro-Sheet Zinc Phosphate Modified by Urea-Formaldehyde on the Corrosion Protection of Epoxy Coating. Surf. Interfaces 2020, 18, 100403. [CrossRef]
- Zhang, Y.; Shao, Y.; Liu, X.; Shi, C.; Wang, Y.; Meng, G.; Zeng, X.; Yang, Y. A Study on Corrosion Protection of Different Polyaniline Coatings for Mild Steel. Prog. Org. Coat. 2017, 111, 240–247. [CrossRef]
- Ganborena, L.; Vega, J.M.; Özkaya, B.; Grande, H.-J.; García-Lecina, E. AN SKP and EIS Study of Microporous Nickel-Chromium Coatings in Copper Containing Electrolytes. Electrochimica Acta 2019, 318, 683–694. [CrossRef]
- Monetta, T.; Bellucci, F.; Nicodemo, L.; Nicolais, L. Protective Properties of Epoxy-Based Organic Coatings on Mild Steel. Prog. Org. Coat. 1993, 21, 353–369. [CrossRef]
- Del Grosso Destreri, M.; Vogelsang, J.; Fedrizzi, L.; Deflorian, F. Water Up-Take Evaluation of New Waterborne and High Solid Epoxy Coatings. Part II: Electrochemical Impedance Spectroscopy. Prog. Org. Coat. 1999, 37, 69–81. [CrossRef]
- Del Grosso Destreri, M.; Vogelsang, J.; Fedrizzi, L. Water Up-Take Evaluation of New Waterborne and High Solid Epoxy Coatings. Prog. Org. Coat. 1999, 37, 57–67. [CrossRef]
- Loveday, D.; Peterson, P.; Rodgers, B. Evaluation of Organic Coatings with Electrochemical Impedance Spectroscopy. JCT Coat. Tech 2004, 8, 46–52.
- Lashgari, S.M.; Yari, H.; Mahdavian, M.; Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Application of Nanoporous Cobalt-Based ZIF-67 Metal-Organic Framework (MOF) for Construction of an Epoxy-Composite Coating with Superior Anti-Corrosion Properties. Corros. Sci. 2021, 178, 109099. [CrossRef]
- Brasher, D.M.; Kingsbury, A.H. Electrical Measurements in the Study of Immersed Paint Coatings on Metal. I. Comparison between Capacitance and Gravimetric Methods of Estimating Water-Uptake. J. Appl. Chem. 1954, 4, 62–72. [CrossRef]
- Miszczyk, A.; Darowicki, K. Water Uptake in Protective Organic Coatings and Its Reflection in Measured Coating Impedance. Prog. Org. Coat. 2018, 124, 296–302. [CrossRef]
- Fan, C.; Shi, J.; Dilger, K. Water Uptake and Interfacial Delamination of an Epoxy-Coated Galvanized Steel: An Electrochemical Impedance Spectroscopic Study. Prog. Org. Coat. 2019, 137, 105333. [CrossRef]
- Liu, R.; Liu, L.; Meng, F.; Tian, W.; Liu, Y.; Li, Y.; Wang, F. Finite Element Analysis of the Water Diffusion Behaviour in Pigmented Epoxy Coatings under Alternating Hydrostatic Pressure. Prog. Org. Coat. 2018, 123, 168–175. [CrossRef]
- Mansfeld, F. Use of Electrochemical Impedance Spectroscopy for the Study of Corrosion Protection by Polymer Coatings. J. Appl. Electrochem. 1995, 25, 187–202. [CrossRef]
- Liu, J.; Xiang-Bo, L.; Jia, W.; Tian-Yuan, L.; Xiao-Ming, W. Studies of Impedance Models and Water Transport Behaviours of Epoxy Coating at Hydrostatic Pressure of Seawater. Prog. Org. Coat. 2013, 76, 1075–1081. [CrossRef]
- Fadl, A.M.; Abdou, M.I.; Hamza, M.A.; Sadeek, S.A. Corrosion-Inhibiting, Self-Healing, Mechanical-Resistant, Chemically and UV Stable PDMAS/TiO2 Epoxy Hybrid Nanocomposite Coating for Steel Petroleum Tanker Trucks. Prog. Org. Coat. 2020, 146, 105715. [CrossRef]
- Contu, F.; Fenzy, L.; Taylor, S.R. An FT-IR Investigation of Epoxy Coatings as a Function of Electrolyte Composition. Prog. Org. Coat. 2012, 75, 92–96. [CrossRef]
- Bajat, J.B.; Milošev, I.; Jovanović, Ž.; Mišković-Stanković, V.B. Studies on Adhesion Characteristics and Corrosion Behaviour of Vinyltriethoxysilane/Epoxy Coating Protective System on Aluminium. Appl. Surf. Sci. 2010, 256, 3508–3517. [CrossRef]
- Jaisai, M.; Baruah, S.; Dutta, J. Paper Modified with ZnO Nanorods – Antimicrobial Studies. Beilstein J. Nanotechnol. 2012, 3, 684–691. [CrossRef]
- Meng, F.; Liu, L.; Tian, W.; Wu, H.; Li, Y.; Zhang, T.; Wang, F. The Influence of the Chemically Bonded Interface between Fillers and Binder on the Failure Behaviour of an Epoxy Coating under Marine Alternating Hydrostatic Pressure. Corros. Sci. 2015, 101, 139–154. [CrossRef]
- Tian, W.; Meng, F.; Liu, L.; Li, Y.; Wang, F. The Failure Behaviour of a Commercial Highly Pigmented Epoxy Coating under Marine Alternating Hydrostatic Pressure. Prog. Org. Coat. 2015, 82, 101–112. [CrossRef]
- Tian, W.; Liu, L.; Meng, F.; Liu, Y.; Li, Y.; Wang, F. The Failure Behaviour of an Epoxy Glass Flake Coating/Steel System under Marine Alternating Hydrostatic Pressure. Corros. Sci. 2014, 86, 81–92. [CrossRef]
- Hongyang GAO, W.W. Degradation Behavior of a Modified Epoxy Coating in Simulated Deep-Sea Environment. J. Chin. Soc. Corros. Prot. 2017, 37, 247–263. [CrossRef]
- Liu, L.; Cui, Y.; Li, Y.; Zhang, T.; Wang, F. Failure Behavior of Nano-SiO2 Fillers Epoxy Coating under Hydrostatic Pressure. Electrochimica Acta 2012, 62, 42–50. [CrossRef]
- Zhang, X.; Wu, W.; Li, Y.; Li, J.; Qiao, L. Corrosion Form Transition of Mooring Chain in Simulated Deep-Sea Environments: Remarkable Roles of Dissolved Oxygen and Hydrostatic Pressure. J. Mater. Sci. Technol. 2023, 162, 118–130. [CrossRef]
| Testing conditions | Ad (cm2) | α |
|---|---|---|
| Immersion in seawater at atmospheric pressure | 2.981×10-6 | 4.217×10-7 |
| AC-DC-AC cycling at atmospheric pressure | 2.642×10-5 | 3.738×10-6 |
| AC-DC-AC coupled with hydrostatic pressure | 1.272×10-4 | 1.800×10-5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





