1. Introduction
The origin of the universe is still a great mystery for humanity, but it is possible to advance in understanding the origin of the universe, only with observations of facts, so it will be possible to obtain information about the origin of the universe.
From the observation of the facts, it is possible to reach the conclusion that the first things arose out of obligatory necessity, developing the “theory of obligatory necessity” that explains the existence of the first things. Thus, the big bang appears giving rise to other things.
Currently, elements and facts still depend on physical spaces.
There are countless needs in the universe, such as:
The characteristics of physical spaces offer conditions or not for the existence of elements and facts. The numerous observations of spaces confirm the theory.
2. The Existence and Interactions or Actions
The existence of elements or facts compared to other existences tend to have different characteristics, reinforcing the idea that there is not only a single case of the theory. In terms of interactions or actions of elements or facts that give rise to new elements or facts, they tend to have different physical concepts, all influenced by space. Examples serve to understand the existence and interactions or actions [
5,
6]:
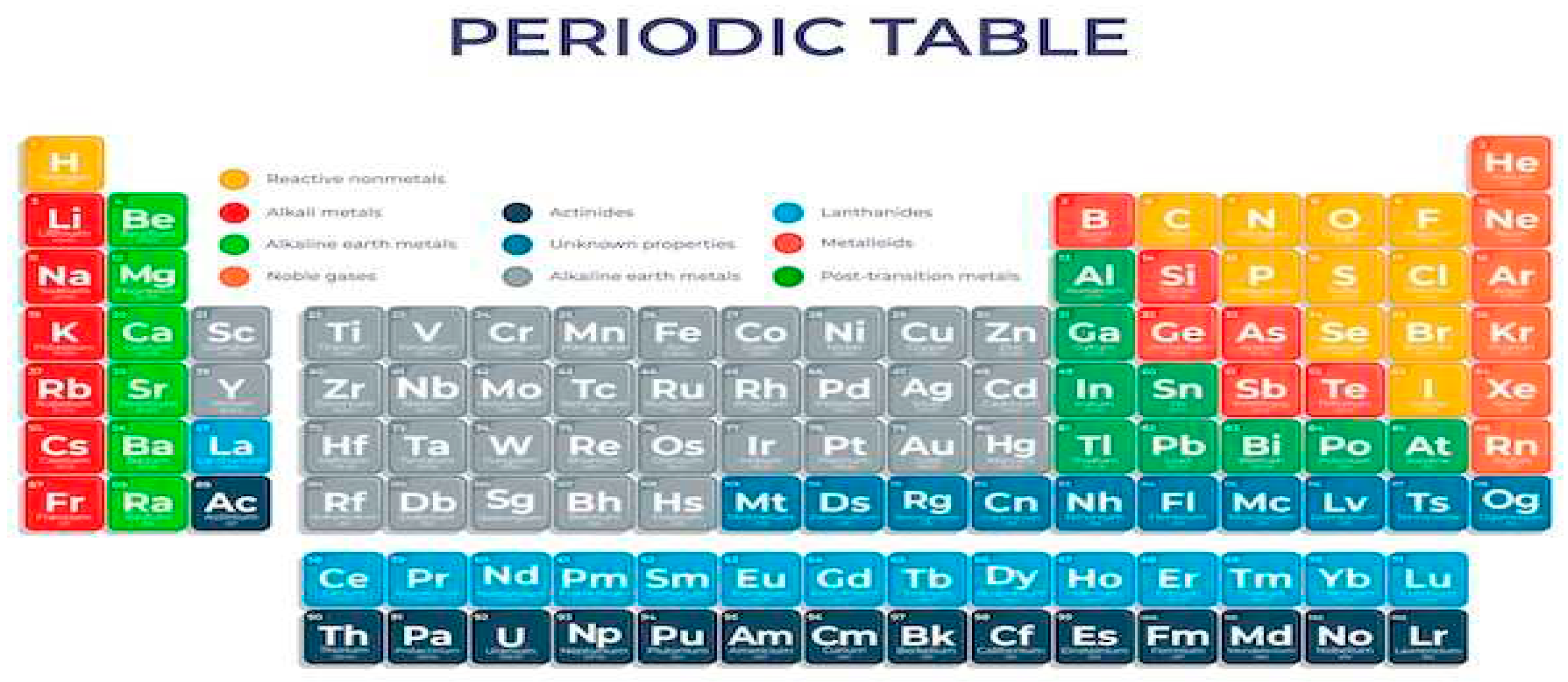
An example of existence that usually has different characteristics are the chemical elements that are a set of atoms with the same atomic number, that is, they have the same amount of protons in the nucleus. This characteristic defines a chemical element, differentiates it from other elements and determines its properties. In this way, it is worth reflecting that there were countless influences of spaces to reach the environment of countless chemical elements. To try to organize the countless elements according to their characteristics, the periodic table was developed to meet this need.
Figure 1.
Periodic table, Source:Freepik.
Figure 1.
Periodic table, Source:Freepik.
The periodic table is composed of 118 chemical elements, arranged by atomic number, in ascending order from left to right. The families or groups of the periodic tableare the vertical lines, which are numbered from 1 to 18. The chemical elements of the same family have similar chemical properties, they are the following groups or families:
-
Metals:Metals make up most of the elements on the Periodic Table. Some examples are gold, silver, copper, zinc, iron, platinum, aluminum, sodium, potassium, among others. Elements belonging to this group have the following main properties:
- -
To possessshine
- -
They are solid
- -
Conducts electric current
- -
Conducts heat
- -
Are malleable
- -
Are ductile
-
Non-metals:They are composed of 11 elements carbon, nitrogen, phosphorus, oxygen, sulfur, selenium, fluorine, chlorine, bromine, iodine and astatine that have different properties than metals:
- -
Does not glow
- -
Does not conduct electricity
- -
Does not conduct heat
- -
Fragmentation occurs
-
Semimetals: They are composed of seven elements boron, silicon, germanium, arsenic, antimony, tellurium and polonium that have intermediate properties to metals and non-metals:
- -
Has a metallic sheen
- -
Poor conduction of electricity
- -
Fragmentation occurs
Noble gases:They are the elements of family 18 of the periodic table. They are helium, neon, argon, krypton, xenonandradon.
Hydrogen: Hydrogen is different from any other chemical element, as it does not fit into any of the groups presented.
In the periodic table, the horizontal lines are the periods that have elements in order of increasing atomic number.
Any natural element of any physical states in comparison with other elements has distinctive characteristics. As per the following examples:
- A)
Mercury (hg) and bromine (br): both are liquid at room temperature, but have different characteristics. Mercury has characteristics that allow its use in the manufacture of mirrors and thermometers. Bromine has characteristics that allow its use in firefighting.
- B)
Carbon (c), phosphorus (p), sulfur (s): all are solid, but have different characteristics. Carbon has characteristics that allow its use in the production of energy and in the manufacture of jewelry. Phosphorus has characteristics that make it used in the manufacture of matchboxes. Sulfur has characteristics that allow its use in the production of fertilizers and paper.
- C)
Oxygen (o), nitrogen (n): both are gases, but with different characteristics. Oxygen is used in the respiration of many living things. Nitrogen has characteristics that make it used in dyes and explosives.
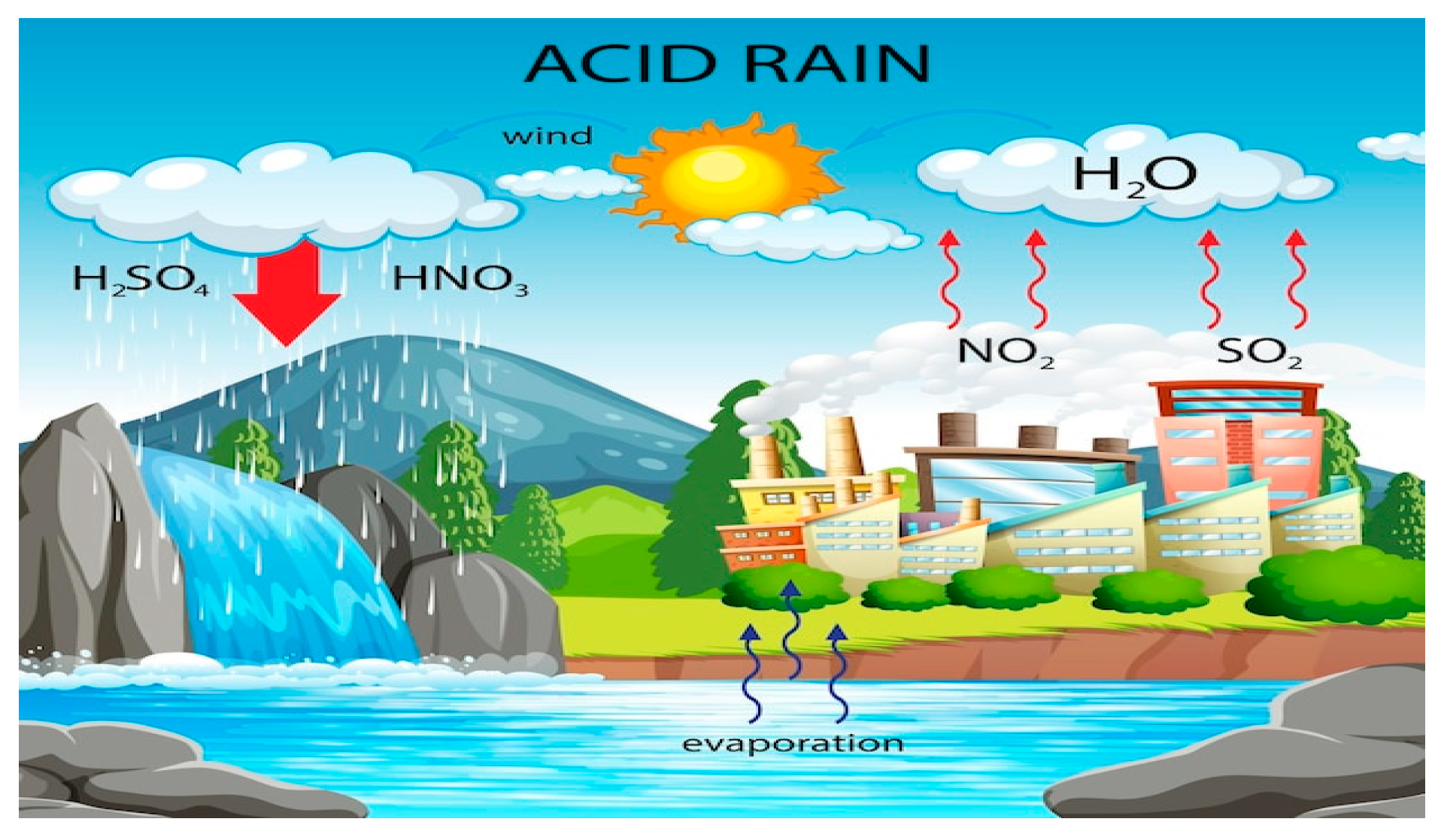
An example of interactions or actions are chemical reactions, which is when the material undergoes a transformation in which its constitution changes; that is, forming new substances provided by the conditions or influence of spaces. Chemical reactions are starting substances that are called reactants and end products, and reactions represented by chemical equations, acid rain and rust formation are ideal examples to understand chemical reactions:
Figure 2.
Formation of acid rain.Source: Freepik.
Figure 2.
Formation of acid rain.Source: Freepik.
Equation SO3 + H2O → H2SO4 is the reaction of sulfur dioxide with water giving rise to sulfuric acid, known as acid rain. Water (H2O) has some characteristics, such as: regulating body temperature, detoxifying the body,and, aiding in the absorption of nutrients. But when water interacts with sulfur dioxide (SO3) the characteristics change; that is, the new element sulfuric acid (H2SO4) has different characteristics from water, promoted by the reaction due to the conditions and influences of the spaces.
-
B.
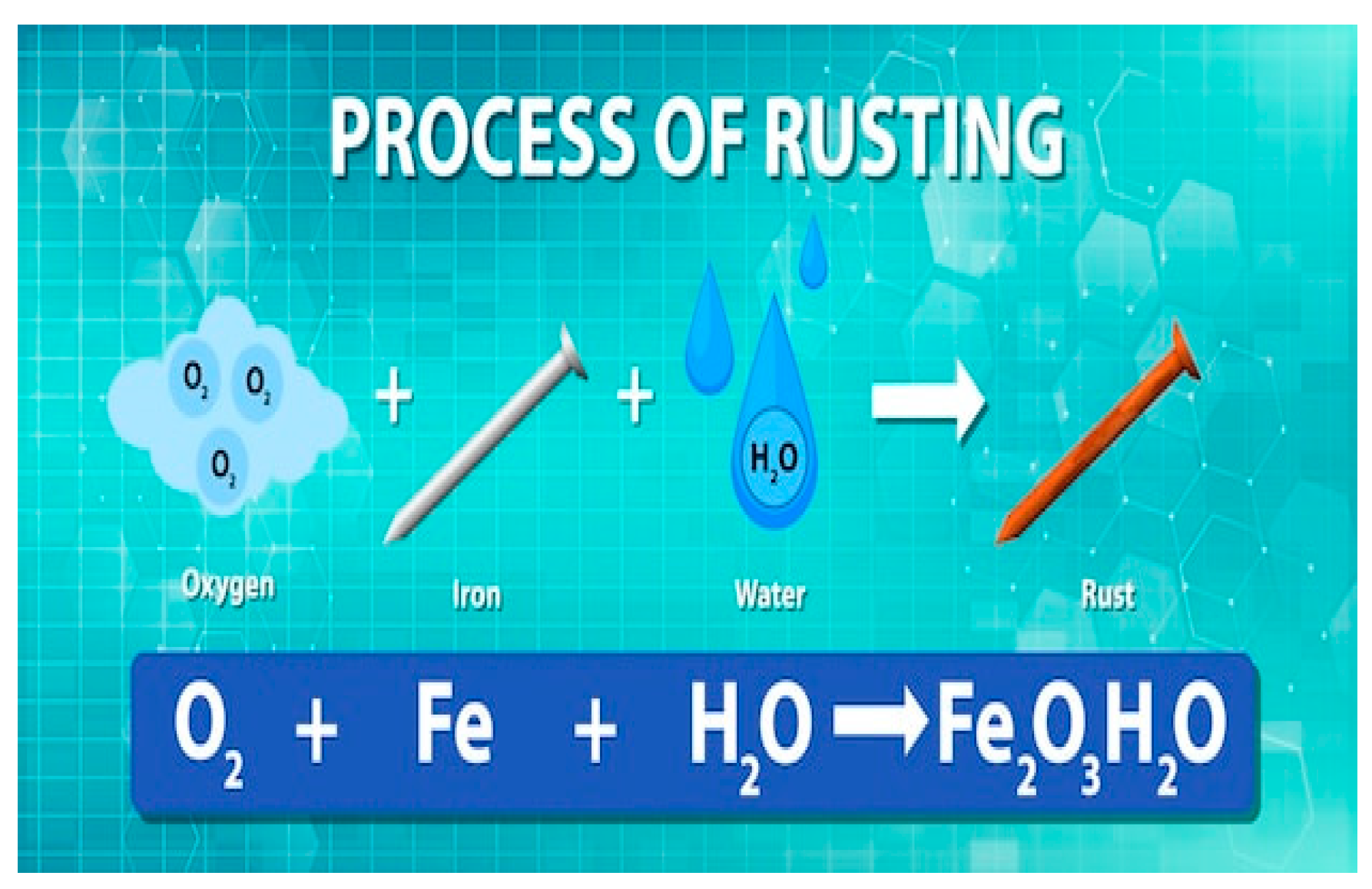
Example of rust
Figure 3.
Rust formation. Source: Freepik.
Figure 3.
Rust formation. Source: Freepik.
The general Equation 2 Fe + O2 + 2 H2O → 2 Fe(OH)2 describes rust, which occurs when iron (Fe) comes into contact with water (H2O) or oxygen (O2). In the beginning, iron had characteristics that allowed its use in the formation of metallic alloys, in the production of automobiles and metallic structures of buildings. But when iron comes into contact with water or oxygen, it does not retain its characteristics as the developed rust promotes damage to iron; thus, the emergence of new elements occurs due to the conditions and influence of spaces.
3. Theory of Everything
Numerous scientists are attempting to unify the phenomena of quantum physics and the theory of relativity into a single scientific theory. However, quantum physics and the general theory of relativity describe different phenomena. Nevertheless, there is a compelling need for a theory that can explain elements and facts within a unified framework. This is because specific elements or facts are influenced by space but exhibit different phenomena due to the requirements of existence, interaction, action, and adaptation, which are dependent on the influence of space according to the theory of mandatory necessity[
1],[
2].
4. List of Examples that Reinforce the Theory
1) Viruses enter the cell to maintain their existence, once inside the cell, they multiply and manage to survive. Viral mutations help viruses survive as viruses adapt through viral mutations to continue to exist.
2) Animals have numerous needs, such as growing in size to continue existing, then decreasing in size to oppose existence. In addition, animals need to eat to obtain energy to carry out their daily activities. Animal bones are needed to protect internal organs such as the lungs, heart and brain. Body hair helps animals adapt to cold weather and solar radiation. The absence of body hair causes infections and irritations, so body hair is essential. In all organs of animals, its existence is essential, as each organ performs functions such as the heart that brings blood to the body, the digestive system helps in obtaining food for energy and the brain that helps in the actions of the body.
3)The universe is expanding to sustain its existence, but there is a possibility that it may contract in the future to neutralize its existence. Similarly, the sun, with an age of approximately 10 billion years, is not permanent. Many entities that exist are not eternal, including the universe and the sun.
4) Eukaryotic cells have a nucleus, while prokaryotic cells do not have a nucleus. All this, for needs.
5) Numerous chemical elements are essential for its usefulness, such as: oxygen, which is responsible for the respiration of many living beings, carbon is used in power plants, nitrogen plays a role in plant growth, sodium regulates blood volume and acts in nerve impulses, calcium acts on bones and teeth, potassium helps in the proper functioning of cells, aluminum is used in airplanes, automobiles and armored vehicles. Therefore, the elements are needed.
6)Many objects emerged out of necessity, such as: the light bulb that illuminates, the telephone to communicate, the television to inform the population, the means of transport to move around.
7) Studies help to make life easier, such as: medicine that developed vaccines to save lives, mathematics that describes the world in a rigorous way that even helps in construction, physics to understand natural phenomena, and history to understand the past and the present.
8)In water, certain elements and phenomena are favored that do not exist outside of water.
9) The climate of different regions favors different elements and phenomena.
10)The characteristics of a volcano do not provide conditions for the existence of certain elements and phenomena.
11)Countless planets offer various conditions, or lack thereof, for the existence of certain phenomena and elements.
5. Example of Physical Spaces and Elements to Reinforce the Theory
Let us imagine that squares 1,2,3,4, etc. Represent some spaces that make up the universe, space 1 is the city of Rio de Janeiro, space 2 is the volcano, space 3 is the city of Belém and space 4 is the sun.
Figure 4.
Imagination of physical spaces and elements, Source: Author.
Figure 4.
Imagination of physical spaces and elements, Source: Author.
- →
the elements that are part of space 1 (Rio de Janeiro), are not part of space 2 and 4 due to their characteristics, I do not allow the elements of space 1
- →
space 1 (Rio de Janeiro) may have the same elements as space 3 (Belém), as they have almost the same characteristics[
3],[
4].
6. A Practical Example of the Theory
Placing a glass of water at a specific temperature encourages water evaporation, whereas placing a pellet with a relevant temperature inside the glass of water causes a change in the pellet’s temperature.
7. No Luck
In a dice game, the outcome is also influenced by physical factors such as height, position, force, gravity, among others. If the die is positioned at number three, at a negligible height, without any force or significant changes, and is immediately released, the die will remain in position three.
8. The Different Universe
If the universe underwent changes in its characteristics, it is possible that the composition of elements would also be different or certain elements may be absent. The presence or absence of specific characteristics can significantly alter the reality of the elements within the universe.
9. Before the Big Bang
To get an idea of what happened before the Big Bang, it is necessary to have information about the initial elements and factsat the exact moment the Big Bang occurred.These initial elements or factscan help explain what happened before the Big Bang, after all, what influenced the existence of these initial elements and facts of the Big Bang.
Example: Assuming that the initial element or fact of the moment in which the Big Bang occurred is influenced by the element or factorX; then, what influenced this element or factorX? If it was influenced by elements or factor Y, what influenced these elements or factor Y? It can be inferred that by observing the causes of influence on these elements or facts, we can gain insight into what came before the Big Bang.
Note: Being aware of what happened before the Big Bangby looking at surrounding influences works best in cases where the universe has almost no elemental interaction.
10. Later Elements
When elements interact, changes occur in their characteristics, leading to the formation of new elements. Therefore, in the origin of the universe, elements had specific characteristics, but through interactions, they gave rise to other elements. However, these changes are always influenced by the physical spaces in which they occur. For example, the element X and the element Y inside space Z can result in different realities. It is important to consider that at some point, the element X or Y was influenced by a specific space, either before or after the interaction took place.
11. Representation and Its Relations
IF X→ EF X ∨ RE X ∨ AD X ∨ Ø EF X ∴ ∃ IF U
IF X = INFLUENCE OF SPACE X
EF X = ELEMENT OR FACT X
RE X = REACTION OF THE X ELEMENTS
AD X = ADAPTATION OF ELEMENT X
Ø EF X= ABSENCE OF ELEMENT OR FACT X.
IF X= PE . IC
IF X = EF X
IF X = INFLUENCE OF SPACE X
Pe = PERMANENCE
IC= PHYSICAL CONCEPT INTENSITY
12. Conclusion
The theory of obligatory necessity aligns with observations of facts, as physical spaces play a significant role in influencing the existence of elements and phenomena. This influence can be attributed to the necessity of existence, the necessity of opposition to existence, the necessity for adaptation, or the necessity for interactions and actions. These factors highlight the crucial relationship between physical spaces and the manifestation of elements and phenomena.
According to the theory of obligatory necessity, the emergence of the first elements and phenomena in the universe can be attributed to the concept of mandatory necessity. This concept is particularly relevant when considering the Big Bang theory, which describes the initial expansion and development of the universe.
In addition, the theory states that the universe did not arise by chance, that is, the universe was not an accident, as it emerged by determination that is influenced by what surrounds it; that is, physical space influences the existence of facts and elements.
The study helps to explain the elements and facts of the universe from its origin to the present moment.
References
- Hawking, Stephen. A Brief History of Time. Intrinsic, rio de janeiro, 256 p. January 2015.
- Hawking, Stephen. The Universe in a nutshell. Arx, Sao Paulo, 216 p. April 2004.
- TAHAN, M. The Man Who Counted. Rio de Janeiro, Record, 2010.
- 02 - Numbers of our day-to-day life - Mathematics - Ens. Bottom. – Telecurso, new telecurso, youtube, 2013, 11 minutes and 45 seconds. Available online: https://www.youtube.com/watch?v=6HNOg12ExEI&t=224s.
- HARARI, Yuval Noah. Sapiens: A Brief History of Humankind.
- LAZKOZ, Ruth.How much time has passed since the Big Bang and how is it measured.Science - BBC News Brazil. June 20, 2022.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).