Submitted:
21 October 2023
Posted:
24 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
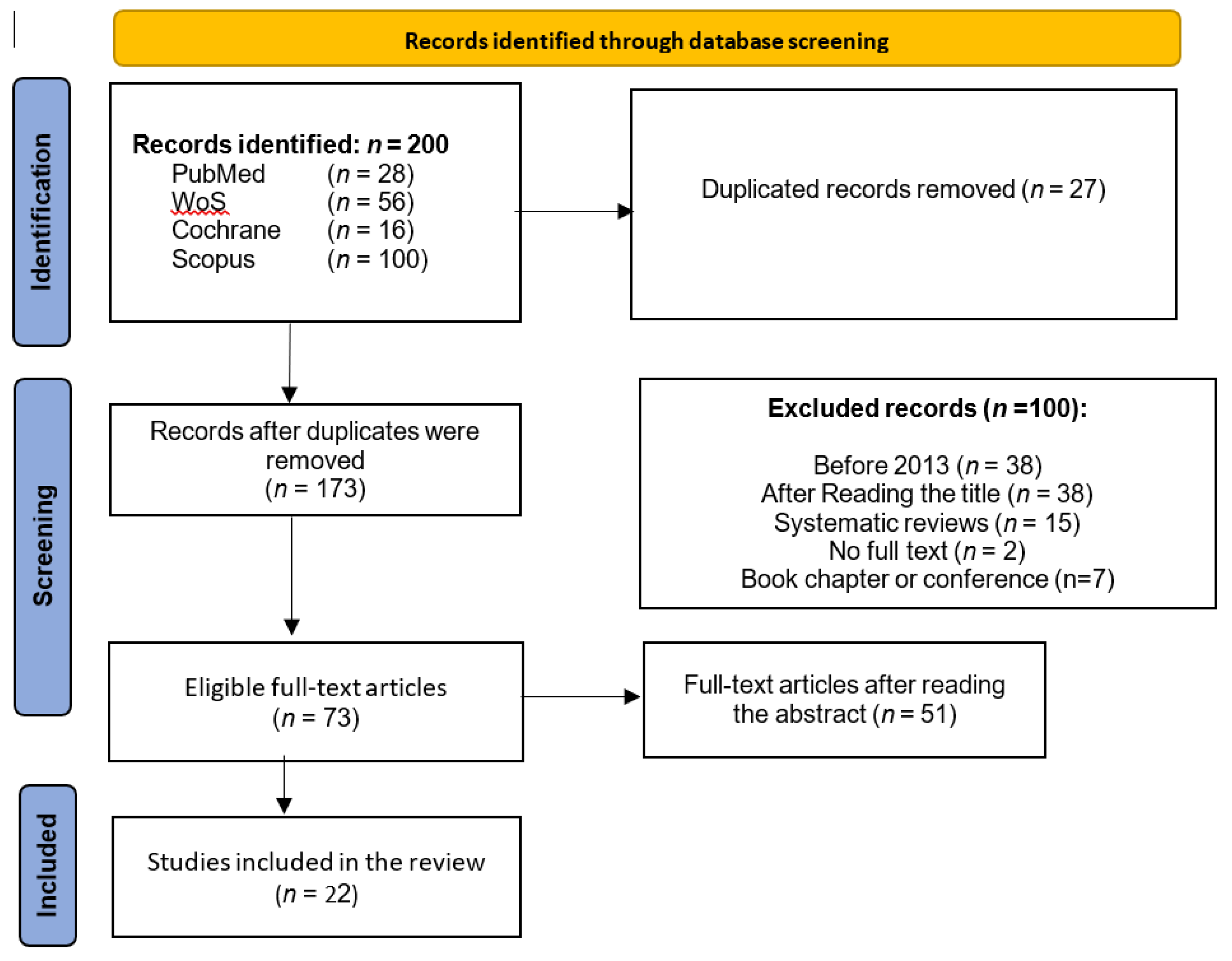
2. Materials and Methods
3. Results
| Inclusion and source | Random assign | Hidden assign | Baseline comparability | Blinded subjects | Blinded therapists | Blinded raters | Results above 85% | Analysis by “intention to treat” | Statiscal comparisons between groups | Measurement and variability data | SCORE | |
| Machin G et al. (2019) | ✓ | ✕ | ✕ | ✓ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | 5 |
| Petrova NL el al. (2018) | ✓ | ✕ | ✕ | ✓ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | 5 |
| Mory T et al. (2013) | ✓ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | 4 |
| Petrova NL et al. (2020) | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | 8 |
| Van Netten JJ et al. (2013) | ✓ | ✕ | ✓ | ✓ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | 6 |
| Van Dorelamen RFM et al. (2019) | ✓ | ✕ | ✕ | ✓ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | 5 |
| Aliahmad B et al. (2019) | ✓ | ✓ | ✕ | ✓ | ✕ | ✓ | ✕ | ✓ | ✓ | ✓ | ✓ | 7 |
| Kanazawa T et al. (2016) | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| McDonald A et al. (2019) | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | 8 |
| Gatt A et al. (2018) | ✓ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | 4 |
| Astasio-Picado A et al. (2018) | ✓ | ✕ | ✕ | ✓ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | 5 |
| Liu C et al. (2015) | ✓ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | 4 |
| Zhou Q et al. (2021) | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | 8 |
| Ashok BH et al. (2020) | ✓ | ✕ | ✕ | ✓ | ✓ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | 6 |
| Rai M et al. (2022) | ✓ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | 4 |
| Arteaga-Marrero N et al. (2021) | ✓ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | 3 |
| Hernandez-Contreras DA et al. (2019) | ✓ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | 3 |
| Carabott M et al. (2021) | ✓ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | 3 |
| Van Dorelamen RFM et al. (2020) | ✓ | ✕ | ✓ | ✕ | ✓ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | 5 |
| Ilo et al. (2019) | ✓ | ✕ | ✕ | ✓ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | 5 |
| Astasio-picado A (2019) | ✓ | ✕ | ✕ | ✓ | ✕ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | 4 |
| McDonald A et al. (2017) | ✓ | ✕ | ✕ | ✓ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | 5 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Enciso Rojas ÁD. Risk factors associated with diabetic foot. Rev Virtual la Soc Paraguaya Med Interna 2016, 3(2):58–70. [CrossRef]
- Rincón, Y.; Gil, V.; Pacheco, J.; Benítez, I.; Sánchez, M. Evaluación y tratamiento del pie diabético. Rev Venez Endocrinol y Metab 2012, 10, 176–87. [Google Scholar]
- Liu C, van Netten JJ, van Baal JG, Bus SA, van der Heijden F. Automatic detection of diabetic foot complications with infrared thermography by asymmetric analysis. J Biomed Opt 2015, Feb 11;20(2):026003. [CrossRef]
- Ramírez-Arbeláez, L.M.; Jiménez-Díaz, K.T.; Correa-Castañeda, A.C.; Giraldo-Restrepo, J.A.; Fandiño-Toro, H.A. Protocolo de adquisición de imágenes diagnósticas por termografía infrarroja. Med y Lab 2015, 21, 161–78. [Google Scholar] [CrossRef]
- Ilo A, Romsi P, Mäkelä J. Infrared Thermography and Vascular Disorders in Diabetic Feet. J Diabetes Sci Technol 2019, 14(1):28–36. [CrossRef]
- Hidalgo Salvador, E.; Álvarez González, F.; Salvador Luna, A. Application of Infrared thermography in legal medicine. Is it a valid test for an objective assessm. Cuad Med Forense 2014, 20, 77–84. [Google Scholar] [CrossRef]
- Franciele C da S, Beatriz Angélica VA, Rodrigo da RI, Paulo Jose BGF, Rudney da S. Escalas y listas de evaluación de la calidad de estudios científicos. Rev Cuba Inf en Ciencias la Salud [Internet] 2013, 295–312. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S2307-21132013000300007&lang=pt. /.
- Albanese E, Bütikofer L, Armijo-Olivo S, Ha C, Egger M. Construct validity of the Physiotherapy Evidence Database (PEDro) quality scale for randomized trials: Item response theory and factor analyses. Res Synth Methods 2020, 11(2):227–36. [CrossRef]
- Escala PEDro - PEDro [Internet]. Available from: https://pedro.org.au/spanish/resources/pedro-scale/.
- Machin, G., Whittam, A., Ainarkar, S., Allen, J., Bevans, J., Edmonds, M., Kluwe, B. M, A., Petrova, N., Plassmann, P., Ring, F., Rogers, L., Simpson R. A medical thermal imaging device for the prevention of diabetic foot ulceration. iopscience 2019, 6(12):125337. [CrossRef]
- Petrova NL, Whittam A, MacDonald A, Ainarkar S, Donaldson AN, Bevans J, et al. Reliability of a novel thermal imaging system for temperature assessment of healthy feet. J Foot Ankle Res 2018, 11. [CrossRef]
- Macdonald A, Petrova N, Ainarkar S, Allen J, Plassmann P, Whittam A, et al. Reproducibility of Thermal Images: Some Healthy Examples. In: Ng EYK, Etehadtavakol M, editors. APPLICATION OF INFRARED TO BIOMEDICAL SCIENCES. 2017. p. 265–76.
- Mori, Taketoshi., Nagase, Takashi., Takehara, K., Oe, Makoto., Ohashi, Y., Amemiya, Ayumi., Noguchi, Hiroshi., Ueki, Kohijiro., Kadowaki, Takashi., Sanada H. Morphological pattern classification system for plantar thermography of patients with diabetes. J Diabetes Sci Technol 2013, 7(5):1102–12. [CrossRef]
- Petrova NL, Donaldson NK, Tang W, MacDonald A, Allen J, Lomas C, et al. Infrared thermography and ulcer prevention in the high-risk diabetic foot: data from a single-blind multicentre controlled clinical trial. Diabet Med 2020, 37(1):95–104. [CrossRef]
- Van Netten JJ, Van Baal JG, Liu C, Van Der Heijden F, Bus SA. Infrared thermal imaging for automated detection of diabetic foot complications. J Diabetes Sci Technol 2013, 7(5):1122–9. [CrossRef]
- Van Doremalen RFM, van Netten JJ, van Baal JG, Vollenbroek-Hutten MMR, van der Heijden F. Infrared 3D Thermography for Inflammation Detection in Diabetic Foot Disease: A Proof of Concept. J Diabetes Sci Technol 2020, 14(1):46–54. [CrossRef]
- Aliahmad B, Tint AN, Poosapadi Arjunan S, Rani P, Kumar DK, Miller J, et al. Is Thermal Imaging a Useful Predictor of the Healing Status of Diabetes-Related Foot Ulcers? A Pilot Study. J Diabetes Sci Technol 2019, 13(3):561–7. [CrossRef]
- Kanazawa, T., Nakagami, G., Goto, T., Noguchi, H., Oe, M., Miyagaki, T., Hayashi, A., Sasaki, S., & Sanada H. Use of smartphone attached mobile thermography assessing subclinical inflammation: a pilot study. J Wound Care [Internet] 2016, 25(4):177–82. [CrossRef]
- Macdonald, A., Petrova, N., Ainarker, S., Allen, John., Lomas, C., Tang, W., Plassmann, P., Ehittam, A., Bevans, J., Ring, F., Kluwe, B., Simpson, R., Rogers, L., Machin, G., & Edmonds M. Between visit variability of thermal imaging of feet in people attending podiatric clinics with diabetic neuropathy at high risk of developing foot ulcers. Physiol Meas 2019, 40(8): 0–68. [CrossRef]
- Gatt A, Falzon O, Cassar K, Camilleri KP, Gauci J, Ellul C, et al. The Application of Medical Thermography to Discriminate Neuroischemic Toe Ulceration in the Diabetic Foot. Int J Low Extrem Wounds 2018, 17(2):102–5. [CrossRef]
- Astasio-Picado Á, Martínez EE, Gómez-Martín B. Comparison of Thermal Foot Maps between Diabetic Patients with Neuropathic, Vascular, Neurovascular, and No Complications. Curr Diabetes Rev 2019, 15(6):503–9. [CrossRef]
- Astasio-Picado A, Martinez EE, Nova AM, Rodriguez RS, Gomez-Martin B. Thermal map of the diabetic foot using infrared thermography. INFRARED Phys Technol 2018, 93:59–62. [CrossRef]
- Zhou Q, Qian Z, Wu J, Liu J, Ren L, Ren L. Early diagnosis of diabetic peripheral neuropathy based on infrared thermal imaging technology. Diabetes Metab Res Rev 2021, Oct 1;37(7). [CrossRef]
- Ashok BH, Karnam Anantha S, Janarthan K. Plantar temperature and vibration perception in patients with diabetes: A cross-sectional study. Biocybern Biomed Eng 2020, 40(4):1600–10. [CrossRef]
- Rai M, Maity T, Sharma R, Yadav RK. Early detection of foot ulceration in type II diabetic patient using registration method in infrared images and descriptive comparison with deep learning methods. J Supercomput [Internet] 2022, (0123456789). [CrossRef]
- Arteaga-Marrero N, Bodson LC, Hernandez A, Villa E, Ruiz-Alzola J. Morphological Foot Model for Temperature Pattern Analysis Proposed for Diabetic Foot Disorders. Appl Sci 2021, 11(16). [CrossRef]
- Hernandez-Contreras DA, Peregrina-Barreto H, Rangel-Magdaleno JD, Renero-Carrillo FJ. Plantar Thermogram Database for the Study of Diabetic Foot Complications. IEEE ACCESS 2019, 7:161296–307. [CrossRef]
- Carabott M, Formosa C, Mizzi A, Papanas N, Gatt A. Thermographic Characteristics of the Diabetic Foot with Peripheral Arterial Disease Using the Angiosome Concept. Exp Clin Endocrinol Diabetes 2021, Feb 1;129(2):93–8. [CrossRef]
- Van Doremalen RFM, van Netten JJ, van Baal JG, Vollenbroek-Hutten MMR, van der Heijden F. Validation of low-cost smartphone-based thermal camera for diabetic foot assessment. Diabetes Res Clin Pract 2019, Mar 1;149:132–9. [CrossRef]
| P | Patient | Healthy or diabetic patients with or without ulcers. |
| I | Intervention | Diagnosis of diabetic foot complications. |
| C | Comparison | Use of thermography as a diagnostic tool for complications versus not using this technology. |
| O | Outcomes | Early detection of complications. |
| Database | Search strategies | Data | Results | Selected |
|---|---|---|---|---|
| PubMed | ((((“Diabetic Foot”[Title/Abstract]) OR (diabetic foot[MeSH Terms])) OR ((“Diabetic Neuropathies”[Title/Abstract]) OR (diabetic neuropathies[MeSH Terms])))) AND (((“thermal imaging”[Title/Abstract]) OR (differential thermal analysis[MeSH Terms])) OR (analyses, differential thermal[MeSH Terms])) | Sept 2023 | 29 | 11 |
| WoS | (“Diabetic Foot” OR “Diabetic Neuropathies”) AND (“thermal imaging” OR “differential thermal analysis”) | Sept 2023 | 58 | 73 |
| Cochrane | ((diabetic foot) OR (neuropathy)) and ((thermal imaging) OR (infrared thermography) OR (temperature monitoring) or (infrared image) OR (skin temperature) OR (thermal imaging) OR (infrared sensor technology)) | Sept 2023 | 19 | 0 |
| SCOPUS | (((diabetic AND foot) OR (neuropathy)) AND ((“thermal imaging”) OR (“infrared thermography”) OR (“temperature monitoring”) OR (“infrared image”) OR (“skin temperature”) OR (“thermal imaging”) OR (“infrared sensor technology”))) | Sept 2023 | 165 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





