1. Introduction
The Theory helps to understand the differences in the physical concepts of the elements that make up the Universe, so it is necessary to be aware that there are many differences between elements and their physical concepts in the Universe, such as the differences between quantum physics and the Theory of general relativity, the observations show that the reaction and composition of the elements change the reality of the element; therefore, the laws of quantum physics are different from the Theory of general relativity, in addition, the general similarities of physical concepts between different elements are difficult. In this way, the study, in order to be understood, needs to be attentive to three ideas:
The reactions of the elements make the final element gain or lose characteristics, changing the reality of the elements, the composition of the elements presents characteristics that differ from the other elements. Therefore, the laws of quantum physics have numerous differences with the general Theory of relativity.
2. Reactions between Elements
A chemical reaction is when the material undergoes a transformation in which its constitution changes, that is, forming new substances. Chemical reactions are initial substances that are called reactants and final products, and reactions are represented by chemical equations, which follow the following general structure:
The chemical reaction is present in numerous situations, such as: Digestion process, Food preparation, Combustion of vehicles, Appearance of rust, Manufacture of medicines, Photographic record, Fire extinguisher, Explosion and acid rain. To understand how the reactions occur, it is worth mentioning two examples, such as the formation of acid rain and the formation of rust.
A. example of acid rain
Figure 1.
Formation of acid rain. Source: freepik.
Figure 1.
Formation of acid rain. Source: freepik.
so3 + h2o → h2so4 is the reaction of sulfur dioxide with water giving rise to sulfuric acid, known as acid rain. Water (H2O) has some characteristics, such as: regulating body temperature, detoxifying the body, and aiding in the absorption of nutrients. But when water interacts with sulfur dioxide (so3), the characteristics change; that is, the new element sulfuric acid (h2so4) has different characteristics from water, promoted by the reaction.
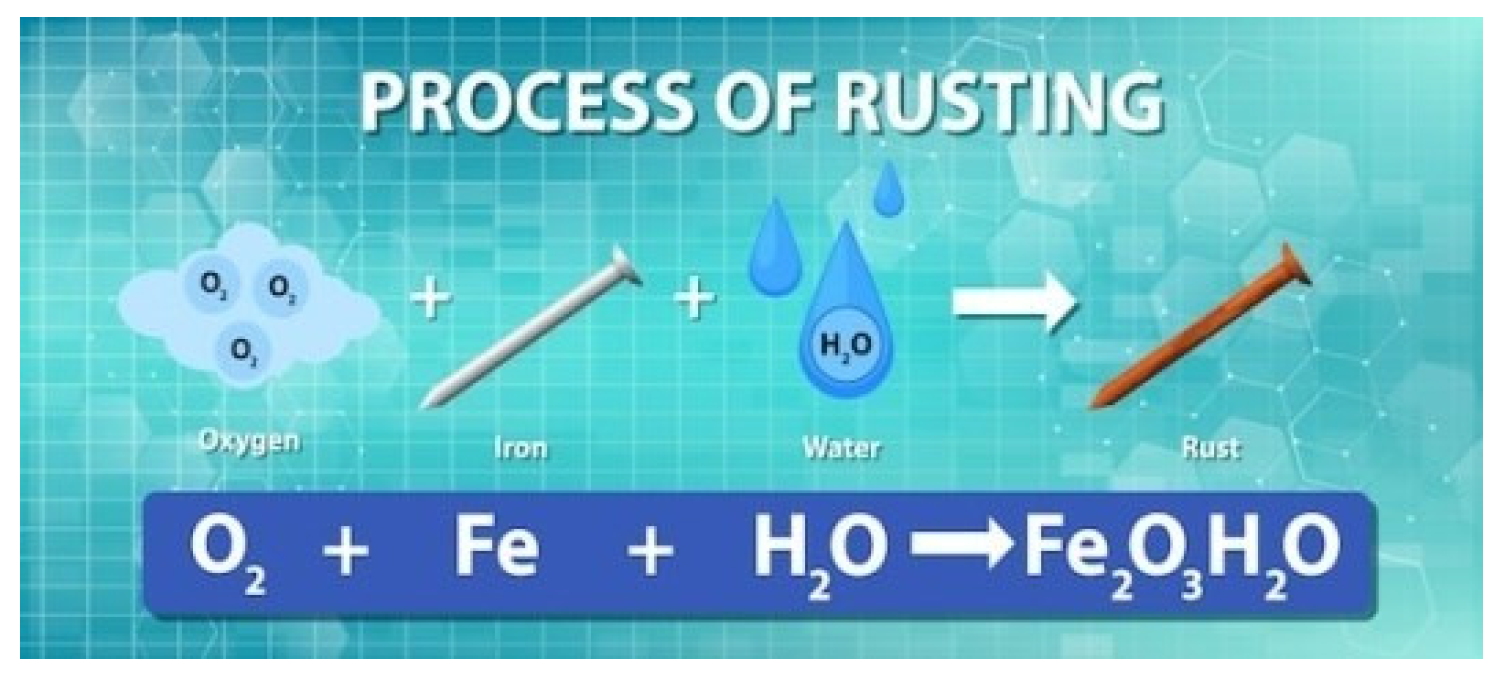
B. example of rust
Figure 2.
Formation of rust. Source: freepik.
Figure 2.
Formation of rust. Source: freepik.
2 Fe + o2 + 2 h2o → 2 Fe(oH)2 is the general equation that describes rust, which occurs when iron (Fe) comes into contact with water (h2o) or oxygen (o2). In the beginning, iron had characteristics that allowed its use in the formation of metallic alloys, in the production of automobiles and metallic structures of buildings. But when iron comes into contact with water or oxygen, the iron does not retain its characteristics, as the developed rust promotes damage to the iron.
c. example of water
2 H2 + O2 → 2 H2O It is an occurrence of hydrogen and oxygen reacting to form water. The hydrogen and oxygen reactants have different physical concepts than the product (water), since the reactants are in a gaseous physical state after entering the liquid state, which is water. Furthermore, other losses and gains occur in the physical concept of reactant and product.
Several types of chemical reactions follow the same line of reasoning to give rise to another element with different characteristics, such as:
synthesis reactionA + B → AB is when the reactants combine to form the single element
analysis reaction AB → A + B is when the reagents decompose giving rise to simple elements
The single exchange reaction A + BC → AC + B is when a simple substance reacts with a compound forming a new simple substance and another compound.
double substitution reaction AB + CD → AD + CB is when compound substances react with another compound substance giving rise to two compound substances.
The conditions for chemical reaction are contact between reactants and chemical affinity.
In this sense, chemical reactions are capable of altering physical concepts by comparing the before and after of the chemical reaction. It is worth mentioning that it is possible to observe this pattern within chemistry.
3. Influence of Spaces on Elements
Influences on chemical elements are motivated by temperature, electricity and light (and other intensity of the specific physical concept). The following examples demonstrate influences on chemical reactions:
- A)
Pyrolysisis a reaction that occurs by the action of high temperatures in an environment with little or no oxygen, an example is calcinationcaco3 → cao + co2 which is a reaction influenced by temperature.
- B)
Photolysis is another reaction that occurs by influence, in this case photolysis is influenced by light, an example is the photosynthesis 6co2 +12h2o + light → c6 h12o6 + 6 o2 + 6 h2o which needs light, another example is hydrogen peroxide which can be influenced by coming into contact with light decomposing into water and oxygen.
Furthermore, other intensities of specific physical concepts assist in the new element or fact, that is, the variety of specific physical concept intensity can imply a variety of elements or facts that express different physical concepts.
4. Composition of Elements
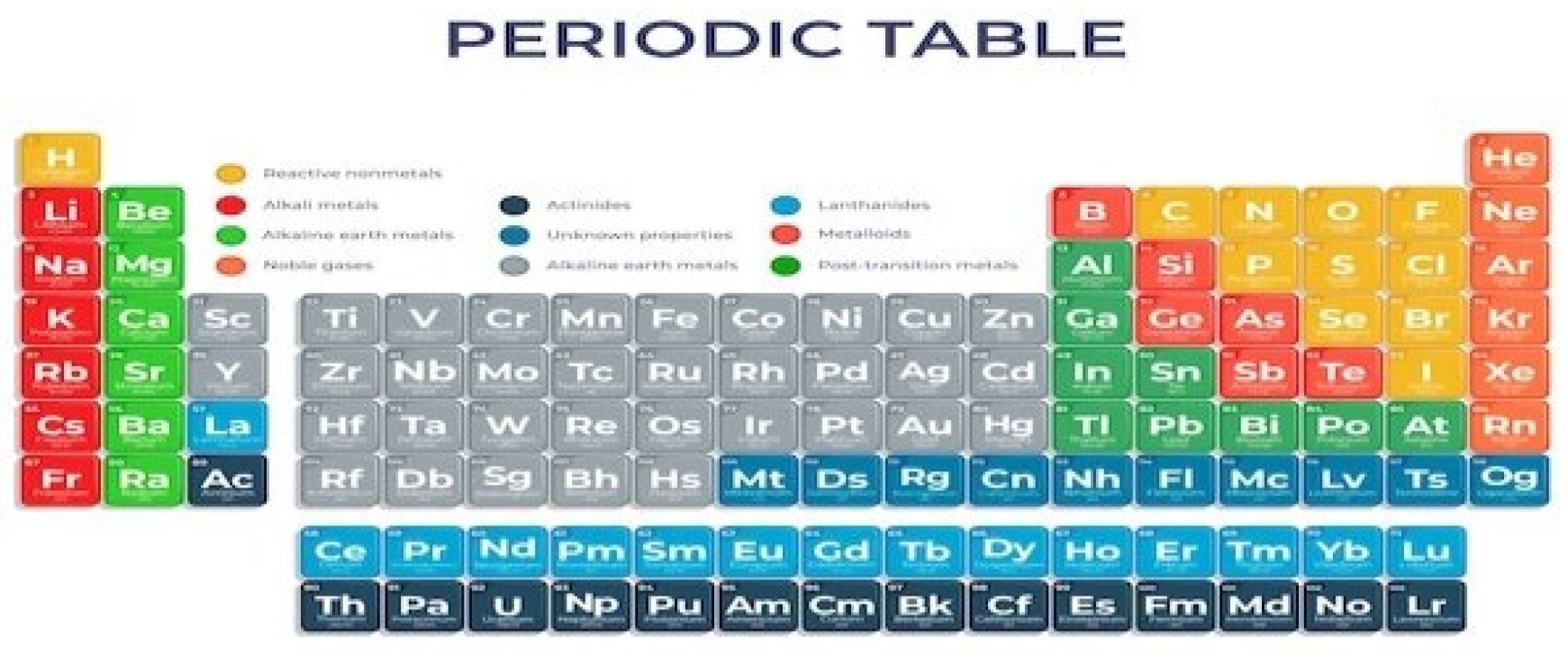
Chemical elements are a group of atoms with the same atomic number, that is, they have the same number of protons in the nucleus. This characteristic defines a chemical element, differentiates it from other elements and determines its properties. To try to organize the countless elements according to their characteristics, the periodic table was developed to meet this need.
Figure 3.
Periodic table. Source: freepik.
Figure 3.
Periodic table. Source: freepik.
The periodic table is composed of 118 chemical elements, arranged by atomic number, in ascending order from left to right. The families or groups of the periodic tableare the vertical lines, which are numbered from 1 to 18. The chemical elements of the same family have similar chemical properties, they are the following groups or families:
Metals: Metals make up most of the elements on the Periodic Table. Some examples are gold, silver, copper, zinc, iron, platinum, aluminum, sodium, potassium, among others. Elements belonging to this group have the following main properties:
- -
to have shine
- -
They are solid
- -
conducts electric current
- -
conducts heat
- -
They are malleable
- -
They are ductile
Non-metals: They are composed of 11 elements carbon, nitrogen, phosphorus, oxygen, sulfur, selenium, fluorine, chlorine, bromine, iodine and astatine that have different properties than metals:
- -
don't shine
- -
Does not conduct electricity
- -
Does not conduct heat
- -
fragmentation occurs
Semimetals: They are composed of 7 elements boron, silicon, germanium, arsenic, antimony, tellurium and polonium that have intermediate properties to metals and non-metals:
- -
They have shine
- -
poor conduction of electricity
- Separation occurs.
Noble gases: They are the elements of family 18 of the Periodic Table. They are helium, neon, argon, krypton, xenon and radon.
hydrogen: Hydrogen is different from any other chemical element, as it does not fit into any of the groups presented.
In the periodic table, the horizontal lines are the periods that have elements in order of increasing atomic number.
Any natural element of any physical states in comparison with other elements has distinctive characteristics. As per the following examples:
- A)
Mercury (hg) and bromine (br): both are liquid at room temperature, but have different characteristics. Mercury has characteristics that allow its use in the manufacture of mirrors and thermometers. Bromine has characteristics that allow its use in firefighting.
- B)
Carbon (c), phosphorus (p), sulfur (s).....: both are solid, but have different characteristics. Carbon has characteristics that allow its use in the production of energy and in the manufacture of jewelry. Phosphorus has characteristics that make it used in the manufacture of matchboxes. Sulfur has characteristics that allow its use in the production of fertilizers and paper.
- C)
Oxygen (o), nitrogen (n) both gases, but with different characteristics. Oxygen is used in the respiration of many living things. Nitrogen has characteristics that make it used in dyes and explosives.
Thus, it is possible to observe from the periodic table that the variety of elementary composition implies a variety of elements that express different physical concepts.
5. Relationship of uses of elements with physical concepts
If the x elements have different uses than the y element, it is because the physical concepts are different, in this way, the reactions between the elements, the composition of the elements and the influence of the spaces on the elements alter the uses of the elements, that is, they change the physical concepts.
6. The physics between different elements
The atomic radius number is a characteristic that varies between elements, considering that the atomic radius is half the distance between two nuclei of neighboring atoms and the greater the number of energy levels of the atom, the greater the radius.
Density is a characteristic that occurs differently among elements, as density is the ratio of their mass to their volume.
Boiling is another characteristic that differs from the elements, as it is characterized as a transformation from liquid to gas.
Other physics concepts is the same idea of differences between elements. [
1]
I. LEVELS OF ORGANIZATION AND THEIR COMPARISONS
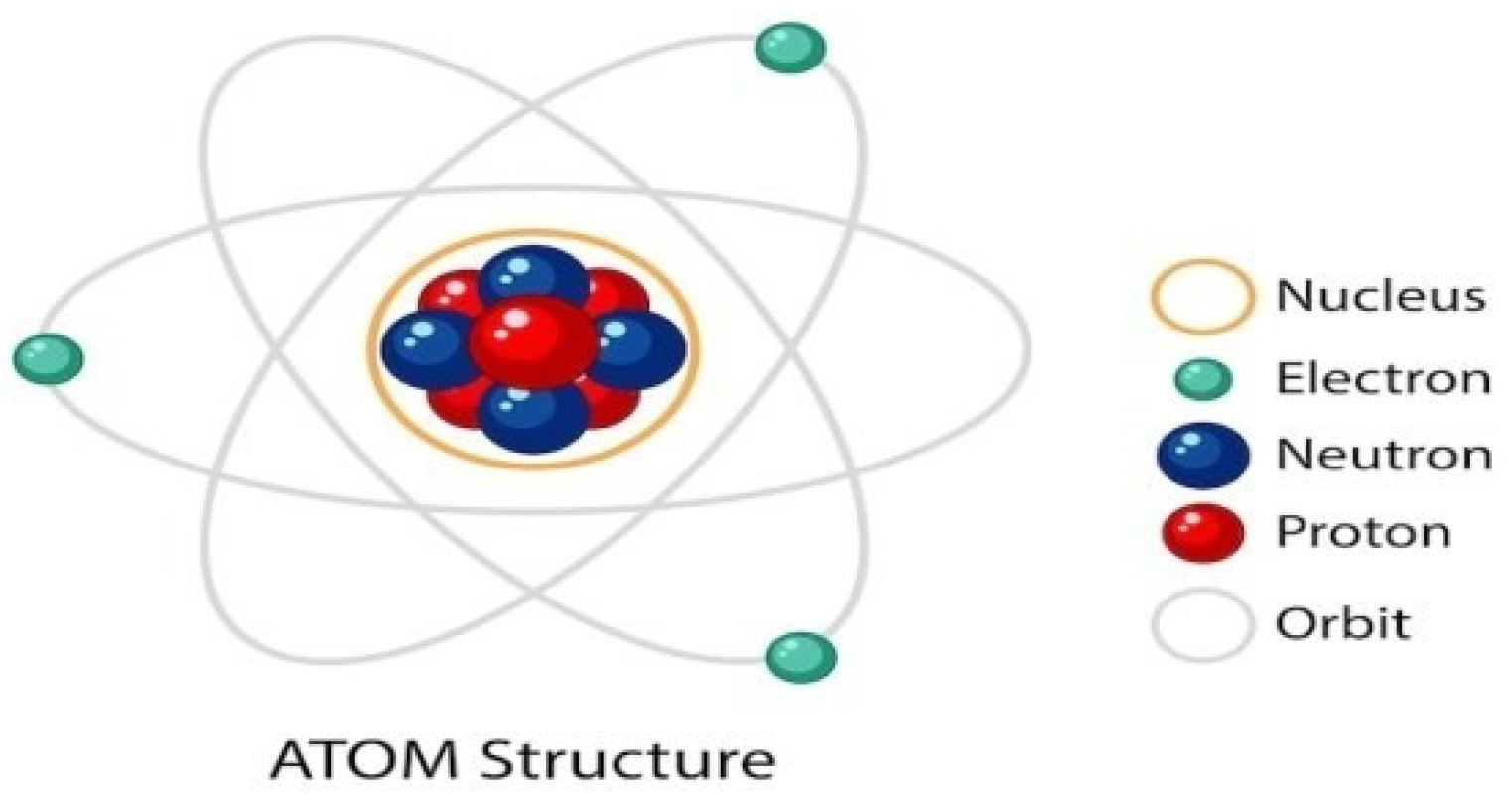
Figure 4.
Set of particles that give rise to the atom. Source: freepik.
Figure 4.
Set of particles that give rise to the atom. Source: freepik.
A group of particles forms an atom, and a group of atoms forms a molecule, and a group of molecules forms a substance has certain characteristics, which a group of atoms that forms a molecule does not have.
In this way, the interaction of particles with other particles gives rise to the element with characteristics different from the particles, that is, on a scale that goes from quantum physics to the Theory of general relativity, there are losses and gains of characteristics, in this way, there is no way quantum physics and the Theory of relativity have similar general characteristics. An example is the levels of organization of living beings [
5]:
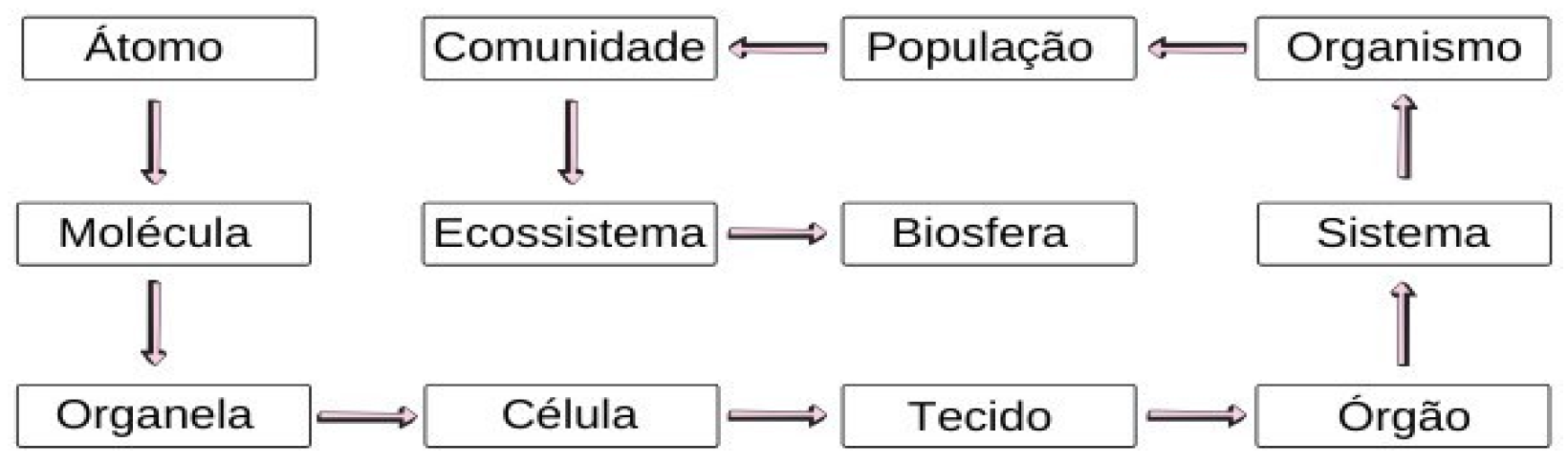
Figure 5.
Levels of organization of living beings. Source: BrasilEscola.
Figure 5.
Levels of organization of living beings. Source: BrasilEscola.
Observing the levels of organization of beings, it is understood that the levels of each one have different characteristics from the previous and subsequent ones, for example the tissue has different characteristics from the cell and the organ, in addition, within each level there are differences between them, as is the case of organs where there are differences between them, for example the heart has different characteristics from the kidney. Having thus, different types of cells, organelles, organs, tissues and among others.
7. Examples that reinforce the Theory
- A)
Soccer balls and basketballs with the same shape are made of different elements and tend to move differently.
- B)
Styrofoam and iron of the same shape and size have different masses.
- C)
an increase in the same chemical element causes changes in physical concepts, such as the amount of carbon. an example is the differences between methane (CH4) and pentane (C5H12), which are the same chemical elements in different quantities. Methane can be found in its physical gaseous state, while pentane can be found in its liquid state. Furthermore, the melting and boiling points are different between methane and pentane.
8. Knowing Planet Earth
Faced with so many different elements on planet earth, it is possible to deduce that there were numerous chemical reactions and influences from space to originate the current reality of planet earth. After all, it is necessary to question the reasons why elements vary between planets, it is not coherent to consider this variety without a plausible explanation. [
2]
9. Quantum Physics
It is the area of study that analyzes and describes the behavior of physical systems of reduced measurements, close to the sizes of particles, atoms and molecules.
Quantum Physics has no specific laws, but it does have its principles:
Heisenberg uncertainty: it is not possible to determine the energy of a quantum particle and the moment when a certain energy occurs. Furthermore, it is not possible to determine the position and momentum of the particles.
Planck's Theory: energy is absorbed or emitted in the form of packets of energy called quanta. Duality of light behavior: light behaves both as a wave and as a particle [
6].
10. General Theory of Relativity
The general Theory was put forward by Einstein, the Theory says that the presence of matter warps space-time. Thus, the greater the mass of the body, the more it will curve space time around it. time is influenced by gravitational fields. The more intense the field, the slower time would pass [
7].
11. Quantum Physics and General Theory of Relativity
It is understood, therefore, from the information presented in the text that quantum physics and the Theory of general relativity present different phenomena. A general similarity of all physical concepts is impossible [
4].
12. Theory of Everything
There is an attempt by many scientists to unify the phenomena of quantum physics and the Theory of relativity in a single scientific theory, however, quantum physics and the general Theory of relativity present descriptions of different phenomena.
13. Representation and Its Relations
RE X→ EF X ∨ CF X→ EF X ∨ CO X → EFX ∨ RE X+CF X+CO X → EF X ≠ RE Y→ EF Y ∨ CF Y→
EF Y ∨ CO Y → EF Y ∨ RE Y+CF Y+CO Y → EF Y ∴ EF X ≠ EF Y
RE X = REACTION OF THE X
ELEMENTS CF X = PHYSICAL
CONCEPT X
CO X= COMPOSITION OF
ELEMENT X EF X = ELEMENT OR
FACT X
RE Y = REACTION OF Y
ELEMENTS CF Y = PHYSICAL
CONCEPT Y
CO F = COMPOSITION OF
ELEMENT Y EF Y = ELEMENT OR
FACT Y
According to the information presented, it is possible to conclude that the differences between the elements are motivated by elemental composition, chemical reaction and influence. If there is a change in the elementary composition, chemical reaction and influence (intensity of specific physical concepts) it is possible to develop an element or fact that expresses new physical concepts. It is worth mentioning that the small particles that provide different physical concepts such as strong nuclear force, weak nuclear force and electromagnetic force are influenced by the theory of differences between elements, that is, at the microscopic or macroscopic level of any variety is explained by the theory
14. Using the Example of Reaction, Composition and Influence
Quantum physics and the theory of general relativity are questions at the microscopic and macroscopic level, therefore, for quantum physics to move from the microscopic level to the theory of general relativity, reactions are necessary: a set of atoms forms molecules, a set of molecules forms substances. Thus, involve reactions or actions. Furthermore, as composition (example of the periodic table) and influence (example of temperature) are relevant factors of change, it is possible to have a relationship with microscopic and macroscopic physical concepts.
15. Elements in the Universe and Their Physical Concepts
If the elements of planet earth are different outside the earth, the facts tend to be different, that is, when the elements are different, the physical concepts change. An example is gravity which tends to be different on each planet, since planets are elements of different masses. In addition, it is necessary to observe the factors that interfere with the elements, such as the influence of spaces, which is the case of the sun where there are planets near and far from the sun. An ideal example of elements in the Universe is the Milky Way. In addition, it is possible to reflect on the beginning of the Universe until the present moment, that is, if at the beginning of the Universe there were elements, reactions between elements and influences of space different from the present moment, there is a possibility that the physical concepts present at the beginning of the Universe have disappeared, arise new, continue, therefore, the early and current universe tend not to have a general resemblance of physical concepts. [
3]
16. Conclusion
The Theory of differences between elements, which promotes an understanding of the differences in physical concepts between different elements as a prime example quantum physics and the Theory of relativity, is a coherent theory, as observations confirm that elementary reactions, elemental composition and the influence of elements Spaces physically change concepts and therefore elements tend to be different.
In addition, the Theory helps in understanding the formation of the elements that make up the planets, such as planet Earth, which possibly had numerous chemical reactions and influences of spaces on the elements, which, in this way, explains the richness of elements on planet Earth.
References
- Stephen Hawking. A Brief History of Time. Intrinsic, rio de janeiro, 256 p. January 2015.
- Hawking, Stephen. The Universe in a nutshell. Arx, Sao Paulo, 216 p. April 2004.
- LAZKOZ, Ruth.How much time has passed since the Big Bang and how is it measured.Science - BBC News Brazil.June 20, 2022.
- String Theory: How to Understand the Universe with the Mathematics of Pythagoras' Music.Science - BBC News Brazil.October 26, 2021.
- SANTOS, Vanessa Sardinha dos. "Levels of Organization in Biology"; Brazil School. Available at: https://brasilescola.uol.com.br/biologia/niveis-organizacao-biologia.htm. Accessed on February 9, 2023.
- What is quantum physics and what is it for?Science - BBC News Brazil.June 30, 2020.
- Costa, Camilla, Pais, Ana. What is Einstein's Theory of general relativity?.Science - BBC News Brazil.May 24, 2019.
- Wilson, Alastair.What was the Universe like before the Big Bang?Science - BBC News Brazil.January 6, 2022.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).