1. Introduction
The phenomenon of ageing represents one of the most significant social transformations of the twenty-first century, being a major issue of public health, because closely linked to increased prevalence and incidence of a large array of chronic inflammatory diseases and conditions, such as acute/chronic kidney diseases (CKD) and failure [
1,
2]. Accordingly, it is imperative to identify biomarkers, able to facilitate their prevention and management [
3]. KD organizations and societies recommend the use of the estimated glomerular filtration rate (eGFR) [
4,
5] for their diagnosis. eGFR index is considered the best overall index of kidney function in health and disease. Its GFR values cannot be quantified directly and require the assessment of the clearance of either exogenous or endogenous filtration markers. Precisely, GFR is obtained through a mathematic equation, which needs to detect the serum creatinine or cystatin C levels and incorporate demographic factors, i.e., age, sex, and ethnicity [
5,
6]. Values of estimated GFR (eGFR) < of 60mL/min/1.73m
2 identify CKD in adults [
6]. However, this fixed threshold shows a low appropriateness in the CKD diagnosis, since it does not consider the physiological decrease of eGFR with the advancing of age, as mostly debated in the last years [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15]. It may, indeed, lead to underdiagnosis in young individuals and overdiagnosis in elderly individuals. Accordingly, eGFR physiologically decreases with kidney aging [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15]. It reports an eGFR annual decline having a variability ranging from 0.3 to 2.6 ml/min per 1.73m
2. This is related to increased nephrosclerosis associated with the nephron loss during ageing.
16 Accordingly, healthy adults aged 18-29 years to 70-75 years show a nephron loss of about 48%, and this knowledge is crucial because associated with increased mortality [
16].
Furthermore, eGFR values appear to be influenced not only by age, but also by sex/gender. Higher baseline eGFR values associated with a faster eGFR decline with age were detected in men than women (0.92 vs. 0.75), in a large population of 12062 participants having the 58.7% of women, from the Rotterdam study conducted by Chaker and coworkers in 2021 [
1]. This implies also amending the fixed threshold for gender, since the well-recognized sexual dimorphism of kidney ageing [
17].
Based on current evidence, it is possible to affirm that age and gender appear among the major determinants of kidney function decline, and this implies a revised definition of KD accompanied with revised values of eGFR. Some advances have been achieved with a new CKD definition adapted with age and based on new eGFR thresholds of 75, 60, and 45 mL/min/ 1.73 m2 across the age classes ranging from 40, 40-64, to 65 years or older, respectively [
12,
17]. However, it is not yet clear which of the definitions allows us to identify true cases of CKD from those without, and, therefore, whether the revised cutoffs really allow us to distinguish age-related functional decline from true CKD or from failure, and whether sex/gender should be included in a future more standardized definition relating to the new thresholds [
12,
18]. This is also leading to consider new biomarkers. A recent study, based on a modest number of CKD patients has interestingly proposed to use in CKD management, the inflammation-based modified Glasgow prognostic score (mGPS) [
19], given by the combination of C reactive protein (CRP) values with albumin levels. mGPS represents, however, the most used risk score in oncology, proving ulterior evidence on the fundamental role of inflammation and nutritional decline in cancer, even if it is well recognized in other diseases, such as CKD [
20].
Based on the observations above described, we wanted to test and confirm the variability of eGFR values according to age classes and sex in a very large Western Sicilian population, represented by 57449 adult participants (age ≥18 years). Moreover, we evaluated the association between eGFR classes and mGPS, and the relationship between age-classes and gender with the mGPS categories.
2. Methods
2.1. Study Design, Sources, and Population
We enrolled a sample of individuals selected by using the linked laboratory and administrative dataset from the A.R.N.A.S. Civico Di Cristina Benfratelli Hospitals. Precisely, 57449 adult participants (age≥18 years; See
Table 1) were encompassed and admitted for medical examinations between the second half of 2021 and December 31, 2022. No upper limit of age for inclusion criteria was used. However, we included in the analysis individuals whose serum creatinine levels were measured and who were followed up at least once during the observation period. eGFR was calculated using the CKD-EPI 2021 equation without a race coefficient [
6]. The institutional ethics review boards at the Universities of Palermo and the A.R.N.A.S. Civico Di Cristina Benfratelli Hospitals approved this study with a waiver of participant consent because of the retrospective study design and secondary use of routinely collected administrative data.
2.2. Detection of Circulating Biomarkers
Blood samples were obtained from all the individuals enrolled in the study for detecting Creatinine (CRE) levels, as well as some inflammatory variables, including White Blood Cells (WBC), Neutrophils, Basophils, Eosinophils, Lymphocytes, Monocytes, Monocyte Distribution Width (MDW), Red Cell Distribution Width (RDW), Procalcitonin (PCT) and CRP. Moreover, we also assessed and considered other two parameters related to inflammation: Neutrophils to Lymphocytes ratio (NLR) and the mGPS [
19,
21]. Furthermore, Albumin (ALB), Creatine Phosphokinase (CPK), Alkaline Phosphatase (ALP) and Albumin to Creatinine ratio for kidney injury evaluation were also evaluated. For their detection routine methods and failures of Complex Operative Unit of Clinical Pathology, ARNAS Civico Di Cristina e Benfratelli Hospitals, were used. The are described in detail in the eMethods of the Supplement.
2.3. Statistical Analysis
Continuous variables were reported as means ± standard deviation (SD), and categorical variables were presented as absolute frequency (%). Missing values are also reported. Pearson chi-square test was used to investigate the association among categorical variables. Pearson r correlation coefficients were estimated to evaluate the relationship between eGFR and the other quantitative variables. Multinomial multivariate logistic regression model was used to predict eGFR Classes and mGPS classes using Age in class and Gender as fixed factors. Odds ratios (ORs), 95% Confidence interval (CI), and p-value are reported. A p-value < 0.05 was considered statistically significant. Statistical analysis was performed using the R environment for statistical computing and graphics version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Baseline Characteristics
We collected 57449 samples (Female=29329 (51.1%) Male=28120 (48.9%), who were stratified by following age classes: (18-40) n=14789, (40-60) n=16595, (60-80) n=19747, (80-100) n=6318 (see
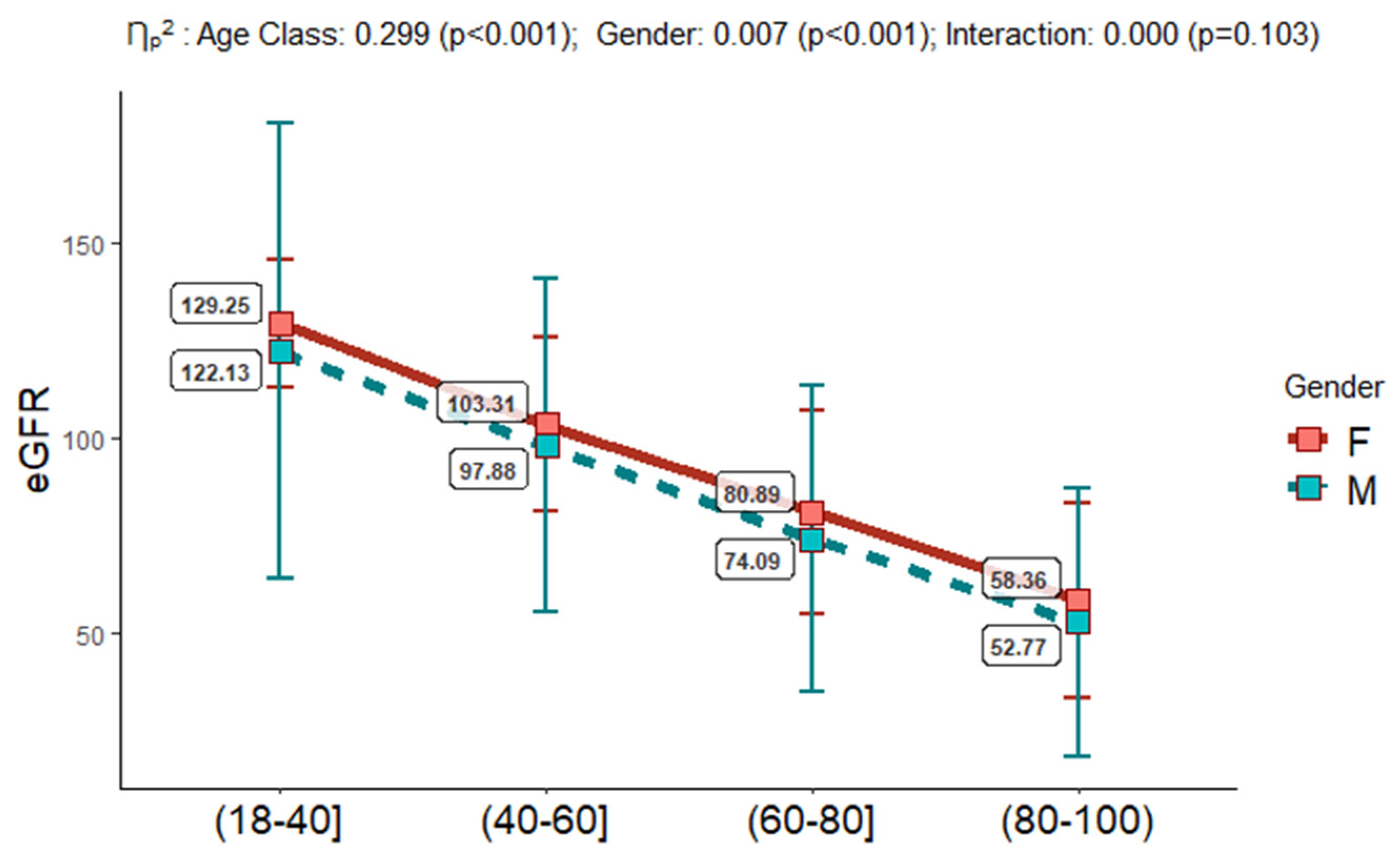
Table 1). eGFR values were assessed as above described and eGFR classes were detected. Interestingly, we observed that only 2.94% of the study population belonged to the G5/eGFR class, and the 18.9% was of mG2/mGPS category. The eGFR estimation, into the four age groups (18-40, 40-60, 60-80, 80-100), and in females vs males, confirmed its documented reduction across the age classes, with higher mean values in females respect to males (see
Figure 1). According to our overall data, we detected an increase of inflammatory biomarkers, such as CRP, PCT and the NLR, with higher values in the oldest people class (see
Table 1). The NLR was validated in numerous studies as a very sensitive indicator of infection, inflammation, sepsis and may be considered an independent risk factor for KD progression in CKD patients [
22,
23].
3.2. Association of eGFR with the Inflammatory and Damage Circulating Biomarkers
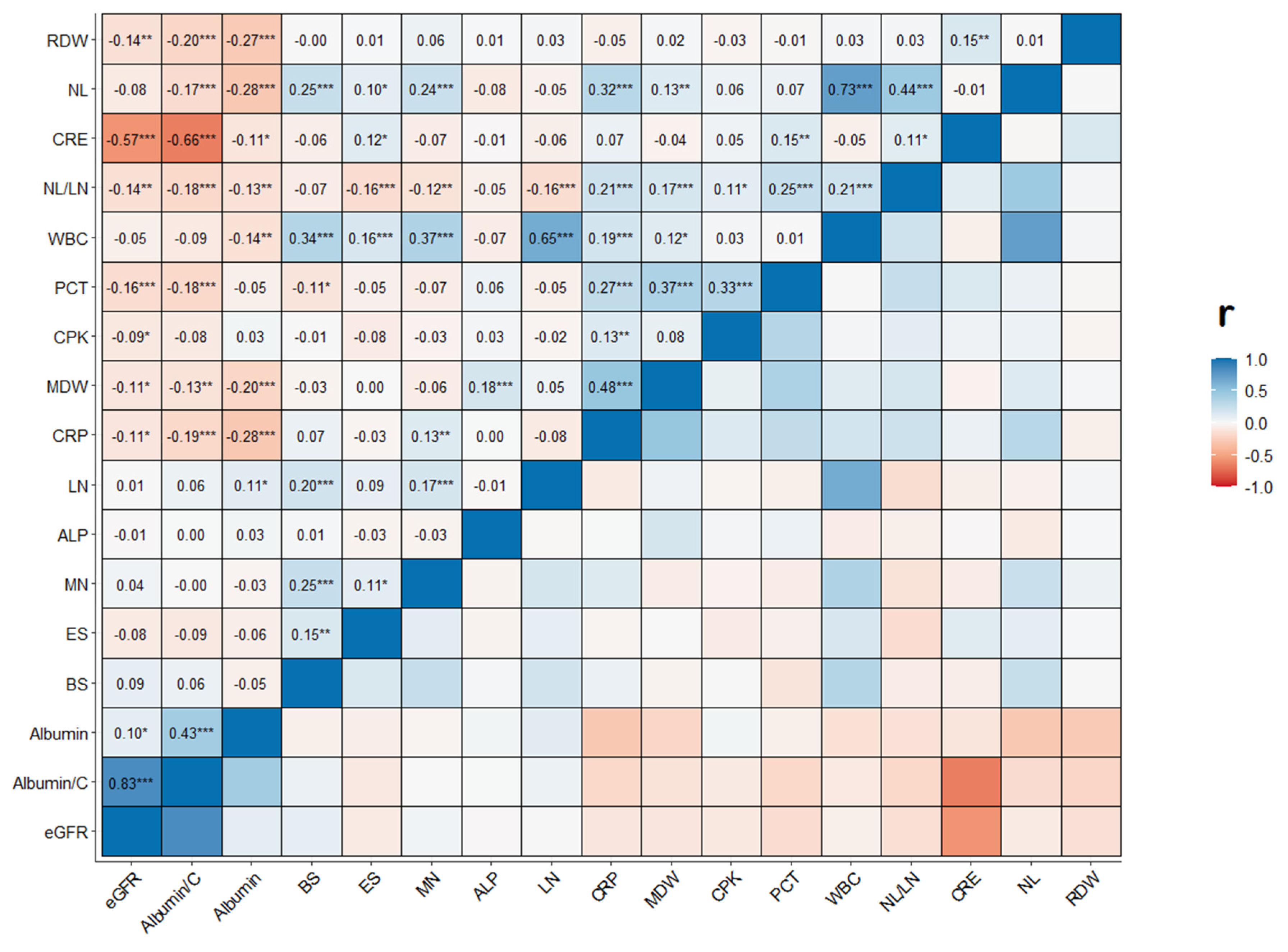
Using eGFR in its continuous nature, we performed correlation analysis to assess the relationship with the inflammatory and damage circulating biomarkers. As shown in
Figure 2, eGFR presented a weak statistically significant positive correlation with Albumin (r=0.10 and p<0.05). Conversely, it showed a weak statistically significant negative correlation with CRP (r=-0.11, p<0.05), MDW (r=-0.11, p<0.05), PTC (r=-0.16, p<0.001), CPK (r=-0.09, p<0.05), RDW (r=-0.14, p<0.01) and NLR (r=-0.14, <0.01). Creatinine and Albumin to Creatinine ratio presented a strong correlation with the eGFR parameter (r=-0.57 and r=0.83, p<0.001, respectively).
3.3. eGFR-Based CKD Risk Definition
To better stratify risk in the population studied we used the eGFR in the categorical form. The six eGFR categories are: G1= 90 or higher mL/min/ 1.73 m
2, the normal range; G2= 60–89 mL/min/ 1.73 m
2 : may mean early kidney disease; G3a= 45 to 59 mL/min/ 1.73 m
2: mild to moderate loss of kidney function; G3b= 30 to 44 mL/min/ 1.73 m
2, G4= 15 to 29 mL/min/ 1.73 m
2, and G5= less than 15 mL/min/ 1.73 m
2: kidney failure). The association of age classes and gender with different combinations of kidney dysfunction traits was investigated by multivariate multinomial logistic regression analysis (see
Table 2). The subjects aged between 18 and 40 years had statistically significant lower OR values to develop G2-G5 pathological conditions respect to the oldest people (age-class = 80-100 years), given that the other variables in the model are held constant. For age-classes, there was evidence of a consistently lower risk of one for all eGFR classes respect to G1. To be female also resulted to reduce the risk to be G2-G5 with respect to G1 (p<0.001), given that the other variables in the model are held constant.
3.4. mGPS Categories and Risk for CKD in Different Age-Classes
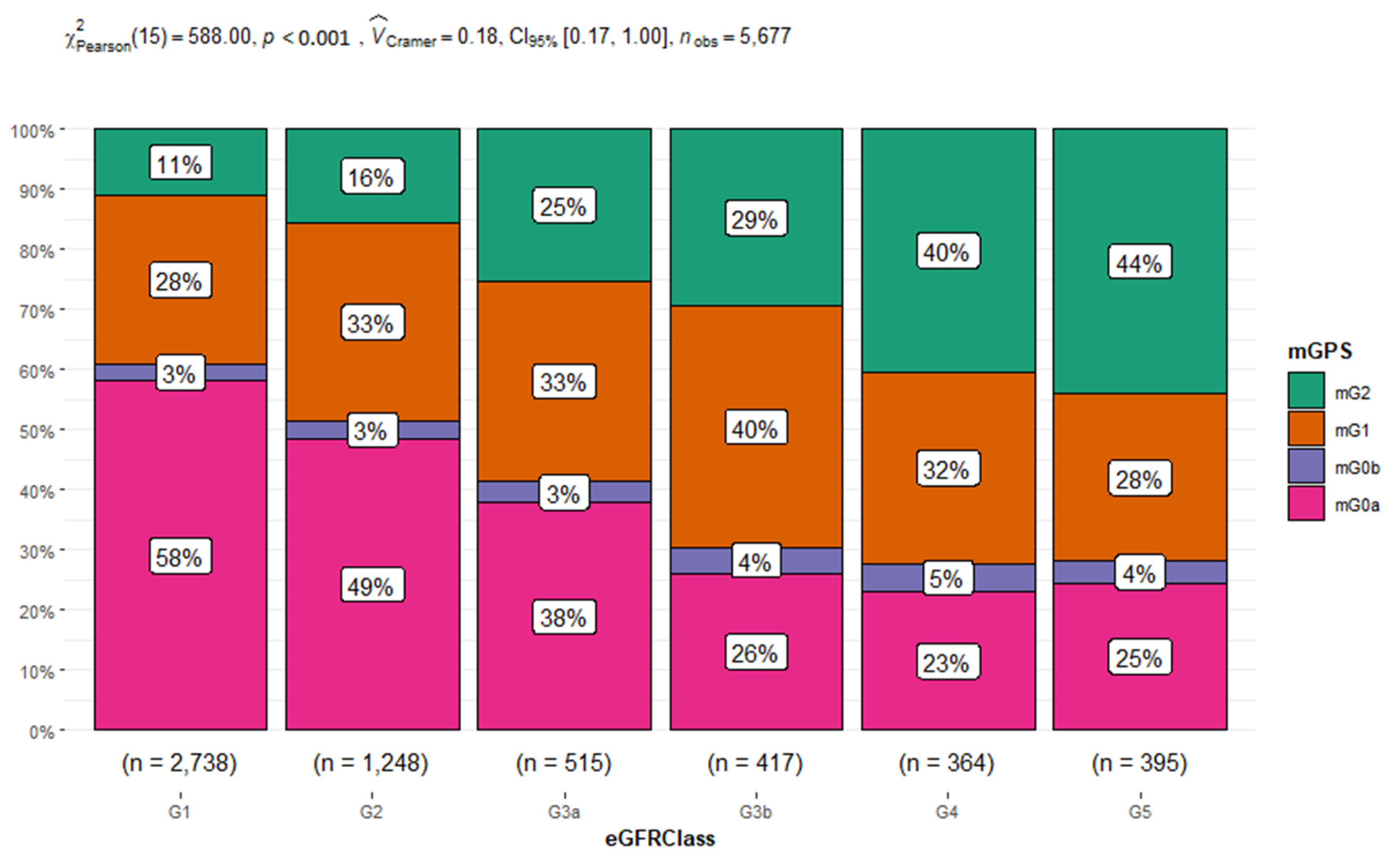
We assessed the association between the mGPS and eGFR. The Chi-squared test showed a statistically significant association between the variables with a p<0.001 (see
Figure 3). Moreover, we found that the 61% of eGFR class=G1 presented a mGPS score of 0 and only the 11% belonging to eGFR class=G1 a mGPS score of 2. While the 29% of people belonging to eGFR class=G5 presented a mGPS score of 0 and the 44% a mGPS score of 2. In addition, we studied the relationship between age-classes and gender with mGPS (see
Table 3), by using a multinomial multivariate logistic regression model for mGPS and age-classes and gender as fixed factors. The results obtained demonstrated that the subjects aged between 18 and 40 years had higher risk for mGPS0a, mGPS0b and 1 respect to the oldest people (age class = 80-100 years) with a p<0.001. To be female also appeared to increase the risk to have a mGPS0 score rather than mGPS2 with an OR of 1.22 and a p<0.009.
4. Discussion
The phenomenon of ageing represents one of the most significant social transformations of the twenty-first century, representing the major cause of dangerous health challenges in the current era [
21]. As with any other age-related chronic disease, CKD shows an increasing prevalence with the advancing age, as well as an increase of kidney failure cases accompanied by an augmented demand for dialysis and/or kidney transplantation in old population [
21,
24,
25]. This implies the imperative request to identify more appropriate criteria and biomarkers for an accurate kidney function evaluation, which possibly varies in relation of various age-classes. Accordingly, it is under discussion the use of eGFR fixed thresholds, which appear to show diverse caveats, including the overestimation of the CKD burden in old population, overdiagnosis, and unnecessary interventions in many elderly having only an age-related loss of eGFR. Consequently, eGFR as first-line tool to estimate kidney function in real-world practice requires some revaluations, which first consider both the effect of age-related changes on the eGFR, and its sex/gender related variability. CKD presents sex disparities in the pathophysiology, as well as in the epidemiology, clinical manifestations, and disease progression [
25,
26,
27].
The current eGFR is detected by using creatinine values and equations derived from younger populations, and precisely under 65 years of age, and the application of other equations can lead to different eGFR results, affecting the CKD categories and severity ‘s classification [
6,
7]. This has led some researchers to suggest that these limitations can in part be reduced using equations based on cystatin C levels, even if non-GFR related factors, such as obesity, inflammation, and a history of smoking can affect circulating cystatin c values [
28]. To the light of this evidence, other research groups emphasise the estimation of the GFR using both creatinine and cystatin C as biomarkers, since the use of this combined equation is more accurate than the use of either creatinine or cystatin C alone in both older and younger patients [
27,
28,
29,
30].
Based on such evidence, in our study, including 57449 adult participants (age ≥ 18 years), we first evaluated the variability of eGFR with age and gender, by estimating its values in four age-classes (18-40, 40-60, 60-80, 80-100) and for gender (females vs. males). In parallel, we also assessed the circulating levels of molecular/cellular inflammatory and damage biomarkers, currently documented to be related to CKD, including among these not only the albumin levels, but also the recent NLR [
18,
22,
23] and mGPS [
19].
First, the data obtained confirmed the significant decrease of eGFR across the four age-classes, with values starting from 129.25-122.13 in females vs. males in the 18-40 age-class (corresponding to G1 category with the fixed eGFR threshold), which slowly decrease in the 40-60 age-class, maintaining higher levels in females than males (103.31 vs. 97.68 ml/min per 1.73 m2). In the 60-80 age-class the values of eGFR were of 80.89-74.09 ml/min per 1.73 m2 (corresponding to G2 category with the fixed eGFR threshold), for arriving to values of 58.38 - 52.77 ml/min per 1.73 m2 in females vs. males, respectively, in the oldest people belonging to 80-100 age class and corresponding to G3a category of fixed threshold. Our analysis evidenced that the age-classes from 18 to 80 had OR values to develop from G2 to G5 pathological conditions respect to G1 significantly lower than the oldest age-class of 80-100 years (p<0.001).
Since our correlation analysis showed a weak association of eGFR with all the inflammatory biomarkers, eGFR appears to be not influenced by the inflammatory state. However, recent evidence demonstrates the key role of inflammation in CKD pathogenesis and progression [
31]. Several mechanisms have been shown to contribute to an increased inflammatory response in patients with CKD, such as decreased renal clearance of proinflammatory cytokines, oxidative and carbonyl stress, volume overload with endotoxemia, decreased levels of antioxidants and increased presence of comorbid conditions [
31,
32]. This is in accordance with current evidence on the promising role of other biomarkers related to inflammation in the progression of CKD, including those of tubule cell injury, e.g., KIM-1, MCP-1, and those of mark tubule cell dysfunction (e.g., α1M, UMOD) [
32], as well as mGPS[
19]. The mGPS, encompassing both CRP and serum albumin, can capture not only the presence of the systemic inflammatory response, but the nutritional status in pre-dialysis CKD patients [
19]. We observed a statistically significant association between the mGPS and eGFR. We found that patients belonging to G1 presented higher percentage of mGPS0, while patients belonging to G5 displayed higher percentage of mGPS2. In addition, we analysed the relationship between age classes and gender with the mGPS categories. We observed that patients belonging to 18-40, 40-60 and 60-80 age classes had higher risk for mGPS0a and 1 rather than mGPS2 respect to the oldest people (age-class = 80-100) with a p<0.001. This statistical data was also presented by an OR>1 but not statistically significant for mGPS0b score. Only subjects aged between 18 and 40 years had lower risk for mGPS2 respect to the oldest people (age-class = 80-100) with a p<0.001. For the other age classes, this data was not statistically significant, possibly because mGPS0b and mGPS2 were both characterized by albumin level <3.5 g/dl. Moreover, we must consider that elderly individuals with low skeletal muscle mass have been demonstrated to have an increased risk of albuminuria [
28]. The reason for this relationship is not fully evident, even if age-related endothelial dysfunction characterized by an alteration in nitric oxide (NO) generation and an increased oxidative stress is also suggested as possible synergistic explanations, as well as hypertension [
29].
Our data add another relevant evidence about the evolving concept of sex differences in CKD. We observed that the female sex reduces the risk of having highest eGFR levels and increase the risk of having mGPS0a score respect mGPS2. These sex differences could be explained by the direct effects of sex hormones on the kidney or sex differences in lifestyle factors [
26].
5. Strengths and limitations
This study has some strengths. First, we used population-based data from a geographically defined area served by a universal health care system. Thus, we analyzed a homogeneous population. Second, we examined a very large sample size. Third, we used validated algorithms to ascertain the presence or absence of comorbidity, and we applied recommended methods in the setting of competing risks. Fourth, we observed for the first time that the combination of mGPS with eGFR could represent the best parameter to differentiate age-related renal decline from KD and better identify KD severity grades.
This study has several limitations. First, it relied on routinely collected data from people who accessed medical services. Second, we used the Inker et al. equation [
6] to estimate kidney function. Because this equation has not been as well validated in older people as other equations, it could have led to inaccurate eGFR for some people, although this inaccuracy should not have affected the findings regarding the relative risk of kidney failure in the study population. Third, we had insufficient information to assess the clinical data of the population studied, even if they were in health. Although the findings require validation in other settings, we do not believe that these limitations pose a serious threat to the validity of our conclusions.
6. Conclusions
Based on the necessity to identify kidneys affected by CKD from aging kidneys [
29] and the important role of inflammation in CKD pathogenesis and progression [
30], we propose mGPS as a parameter to use in association with eGFR for improving stratification of CKD patients. Other studies, likely multicenter, might help to better define eGFR and mGPS relationship in CKD staging, and propose new CKD definition and guidelines.
Author Contributions
Drs Carella, Porreca and Aronica had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Balistreri, Carella and Aronica. Acquisition, analysis, or interpretation of data: Balistreri, Carella, Porreca, Magro, Venezia, Piazza, Gervasi, Lo Verso, Vitale, Agnello. Drafting of the manuscript: Balistreri, Carella, and Porreca. Critical revision of the manuscript for important intellectual content: Balistreri and Aronica. Statistical analysis: Porreca. Obtained funding: Balistreri. Administrative, technical, or material support: Carella, Porreca, Magro, Venezia, Piazza, Gervasi, Lo Verso, Vitale, Agnello, Aronica. Supervision: Balistreri.
Funding
This research was supported by the grants from Italian Ministerial Institute of Research, CUP PJ_RIC_FFABR_2017_161446 project with C.R.B. as PI.
Data Availability Statement
Data sharing not applicable; no new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflict of Interest Disclosures
the authors have not conflict of interests. No other disclosures were reported.
References
- van der Burgh AC, Rizopoulos D, Ikram MA, Hoorn EJ, Chaker L. Determinants of the Evolution of Kidney Function With Age. Kidney Int Rep. 2021;6(12):3054-3063. [CrossRef]
- Dybiec J, Szlagor M, Młynarska E, Rysz J, Franczyk B. Structural and Functional Changes in Aging Kidneys. Int J Mol Sci. 2022;23(23):15435. [CrossRef]
- Balistreri CR. Anti-Inflamm-Ageing and/or Anti-Age-Related Disease Emerging Treatments: A Historical Alchemy or Revolutionary Effective Procedures? Mediators Inflamm. 2018; 2018:3705389. [CrossRef]
- Levey AS, Coresh J, Tighiouart H, Greene T, Inker LA. Measured and estimated glomerular filtration rate: Current status and future directions. Nat Rev Nephrol. 2020;16(1):51-64. [CrossRef]
- Inker LA, Collier W, Greene T, Miao S, Chaudhari J, Appel GB, Badve SV, Caravaca-Fontán F, Del Vecchio L, Floege J, Goicoechea M, Haaland B, Herrington WG, Imai E, Jafar TH, Lewis JB, Li PKT, Maes BD, Neuen BL, Perrone RD, Remuzzi G, Schena FP, Wanner C, Wetzels JFM, Woodward M, Heerspink HJL; CKD-EPI Clinical Trials Consortium. A meta-analysis of GFR slope as a surrogate endpoint for kidney failure. Nat Med. 2023. [CrossRef]
- Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, Grams ME, Greene T, Grubb A, Gudnason V, Gutiérrez OM, Kalil R, Karger AB, Mauer M, Navis G, Nelson RG, Poggio ED, Rodby R, Rossing P, Rule AD, Selvin E, Seegmiller JC, Shlipak MG, Torres VE, Yang W, Ballew SH, Couture SJ, Powe NR, Levey AS; Chronic Kidney Disease Epidemiology Collaboration. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med. 2021;385(19):1737-1749. [CrossRef]
- Levey AS, Grams ME, Inker LA. Use of eGFR in Older Adults with Kidney Disease. Reply. N Engl J Med. 2022;387(6):575. [CrossRef]
- Carnevale V, Tinti MG. Use of eGFR in Older Adults with Kidney Disease. N Engl J Med. 2022;387(6):574-575. [CrossRef]
- Toyama T, Kitagawa K, Oshima M, Kitajima S, Hara A, Iwata Y, Sakai N, Shimizu M, Hashiba A, Furuichi K, Wada T. Age differences in the relationships between risk factors and loss of kidney function: A general population cohort study. BMC Nephrol. 2020;21(1):477. [CrossRef]
- Minutolo R, Gabbai FB, Chiodini P, Provenzano M, Borrelli S, Garofalo C, Bellizzi V, Russo D, Conte G, De Nicola L; Collaborative Study Group on the Conservative Treatment of CKD of the Italian Society of Nephrology. Sex Differences in the Progression of CKD Among Older Patients: Pooled Analysis of 4 Cohort Studies. Am J Kidney Dis. 2020;75(1):30-38. [CrossRef]
- Ravani P, Quinn R, Fiocco M, Liu P, Al-Wahsh H, Lam N, Hemmelgarn BR, Manns BJ, James MT, Joanette Y, Tonelli M. Association of Age With Risk of Kidney Failure in Adults With Stage IV Chronic Kidney Disease in Canada. JAMA Netw Open. 2020;3(9):e2017150. [CrossRef]
- Liu P, Quinn RR, Lam NN, Elliott MJ, Xu Y, James MT, Manns B, Ravani P. Accounting for Age in the Definition of Chronic Kidney Disease. JAMA Intern Med. 2021;181(10):1359-1366. [CrossRef]
- Noronha IL, Santa-Catharina GP, Andrade L, Coelho VA, Jacob-Filho W, Elias RM. Glomerular filtration in the aging population. Front Med (Lausanne). 2022;9:769329. [CrossRef]
- Jaques DA, Vollenweider P, Bochud M, Ponte B. Aging and hypertension in kidney function decline: A 10 year population-based study. Front Cardiovasc Med. 2022;9:1035313. [CrossRef]
- Singh-Manoux A, Oumarou-Ibrahim A, Machado-Fragua MD, Dumurgier J, Brunner EJ, Kivimaki M, Fayosse A, Sabia S. Association between kidney function and incidence of dementia: 10-year follow-up of the Whitehall II cohort study. Age Ageing. 2022;51(1):afab259. [CrossRef]
- Baylis C. Sexual dimorphism in the aging kidney: Differences in the nitric oxide system. Nat Rev Nephrol. 2009;5(7):384-96. [CrossRef]
- Delanaye P, Jager KJ, Bökenkamp A; et al. CKD: A call for an age-adapted definition. J AmSoc Nephrol. 2019;30(10):1785-1805. [CrossRef]
- Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy. 2021;122(7):474-488. [CrossRef]
- Stefan G, Stancu S, Zugravu A, Capusa C. Inflammation-based modified Glasgow prognostic score and renal outcome in chronic kidney disease patients: Is there a relationship? Intern Med J. 2022;52(6):968-974. [CrossRef]
- Wu TH, Tsai YT, Chen KY, Yap WK, Luan CW. Utility of High-Sensitivity Modified Glasgow Prognostic Score in Cancer Prognosis: A Systemic Review and Meta-Analysis. Int J Mol Sci. 2023;24(2):1318. Published 2023 Jan 10. [CrossRef]
- Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571(7764):183-192. [CrossRef]
- Yoshitomi R, Nakayama M, Sakoh T, Fukui A, Katafuchi E, Seki M, Tsuda S, Nakano T, Tsuruya K, Kitazono T. High neutrophil/lymphocyte ratio is associated with poor renal outcomes in Japanese patients with chronic kidney disease. Ren Fail. 2019;41(1):238-243. [CrossRef]
- Mureșan, A.V.; Russu, E.; Arbănași, E.M.; Kaller, R.; Hosu, I.; Arbănași, E.M.; Voidăzan, S.T. The Predictive Value of NLR, MLR, and PLR in the Outcome of End-Stage Kidney Disease Patients. Biomedicines 2022, 10, 1272. [CrossRef]
- Bowe B, Xie Y, Li T, Mokdad AH, Xian H, Yan Y, Maddukuri G, Al-Aly Z. Changes in the US Burden of Chronic Kidney Disease From 2002 to 2016: An Analysis of the Global Burden of Disease Study. JAMA Netw Open. 2018 Nov 2;1(7):e184412. [CrossRef]
- Ke C, Liang J, Liu M, Liu S, Wang C. Burden of chronic kidney disease and its risk-attributable burden in 137 low-and middle-income countries, 1990-2019: Results from the global burden of disease study 2019. BMC Nephrol. 2022 Jan 5;23(1):17. [CrossRef]
- Conte C, Antonelli G, Melica ME, Tarocchi M, Romagnani P, Peired AJ. Role of Sex Hormones in Prevalent Kidney Diseases. Int J Mol Sci. 2023 May 4;24(9):8244. [CrossRef]
- Thurlow JS, Joshi M, Yan G, Norris KC, Agodoa LY, Yuan CM, Nee R. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. Am J Nephrol. 2021;52(2):98-107. [CrossRef]
- Yoo JH, Kim G, Park SW; et al. Effects of low skeletal muscle mass and sarcopenic obesity on albuminuria: A 7-year longitudinal study. Sci Rep. 2020;10:5774. [CrossRef]
- Balistreri CR. Promising Strategies for Preserving Adult Endothelium Health and Reversing Its Dysfunction: From Liquid Biopsy to New Omics Technologies and Noninvasive Circulating Biomarkers. Int J Mol Sci. 2022 Jul 7;23(14):7548. [CrossRef]
- Delanaye P, Jager KJ, Bökenkamp A, Christensson A, Dubourg L, Eriksen BO, Gaillard F, Gambaro G, van der Giet M, Glassock RJ, Indridason OS, van Londen M, Mariat C, Melsom T, Moranne O, Nordin G, Palsson R, Pottel H, Rule AD, Schaeffner E, Taal MW, White C, Grubb A, van den Brand JAJG. CKD: A Call for an Age-Adapted Definition. J Am Soc Nephrol. 2019 Oct;30(10):1785-1805. [CrossRef]
- Mihai S, Codrici E, Popescu ID, Enciu AM, Albulescu L, Necula LG; et al. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J Immunol Res. 2018;2018:2180373. [CrossRef]
- Ix JH, Shlipak MG. The Promise of Tubule Biomarkers in Kidney Disease: A Review. Am J Kidney Dis. 2021;78(5):719-727. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).