Submitted:
23 October 2023
Posted:
25 October 2023
You are already at the latest version
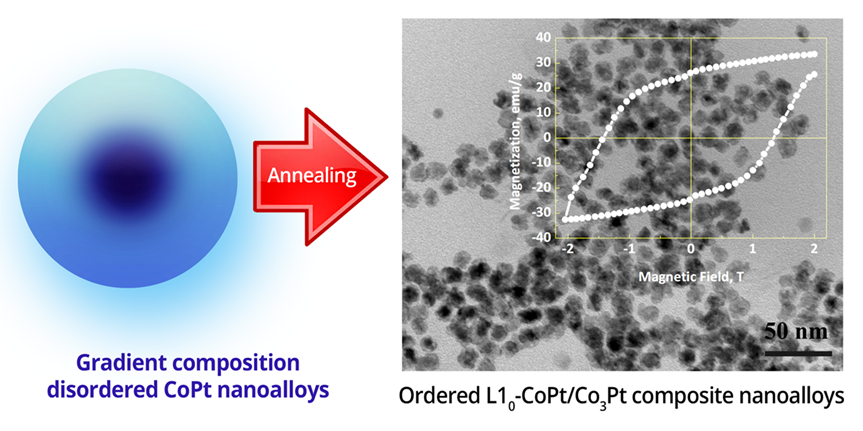
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
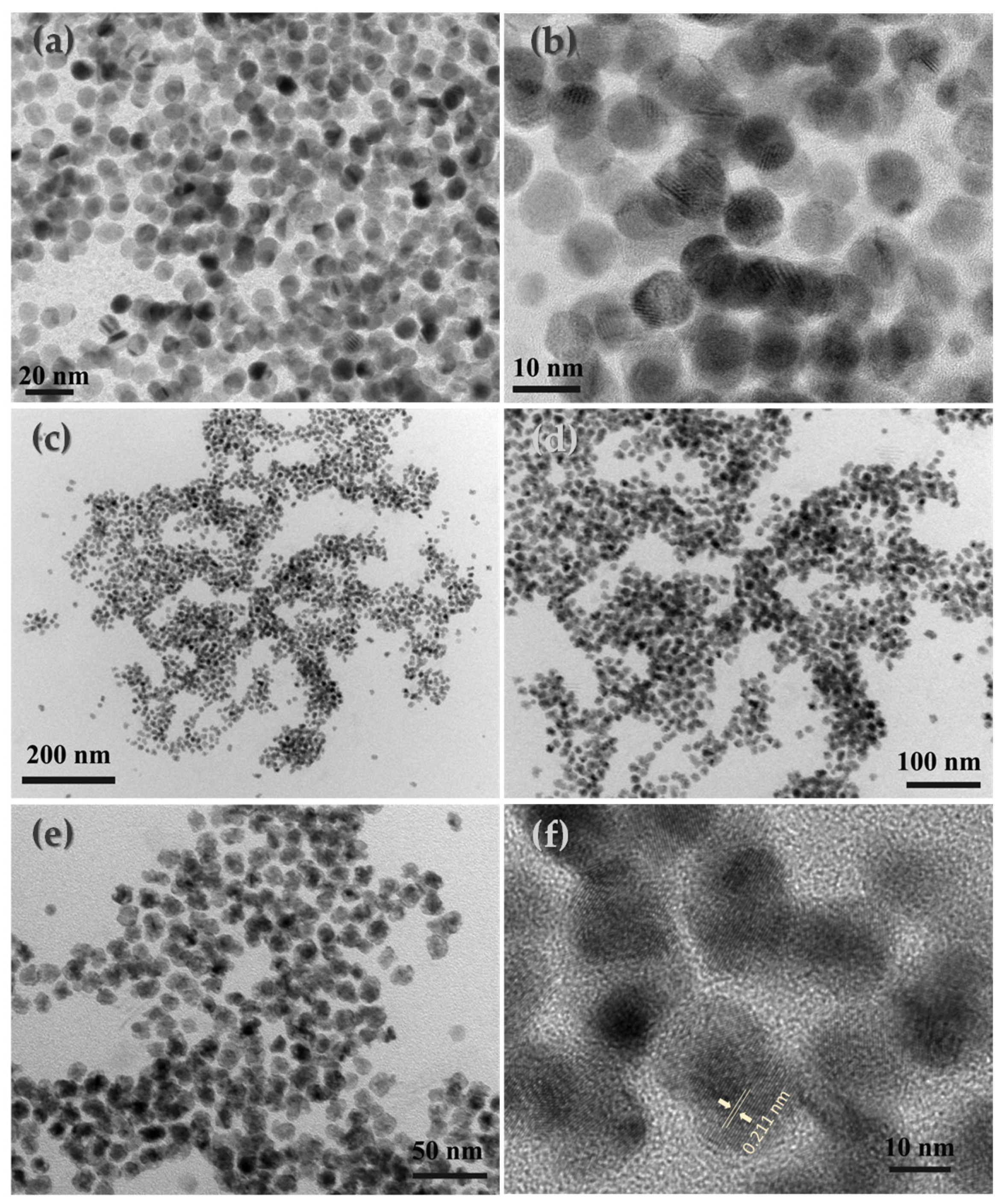
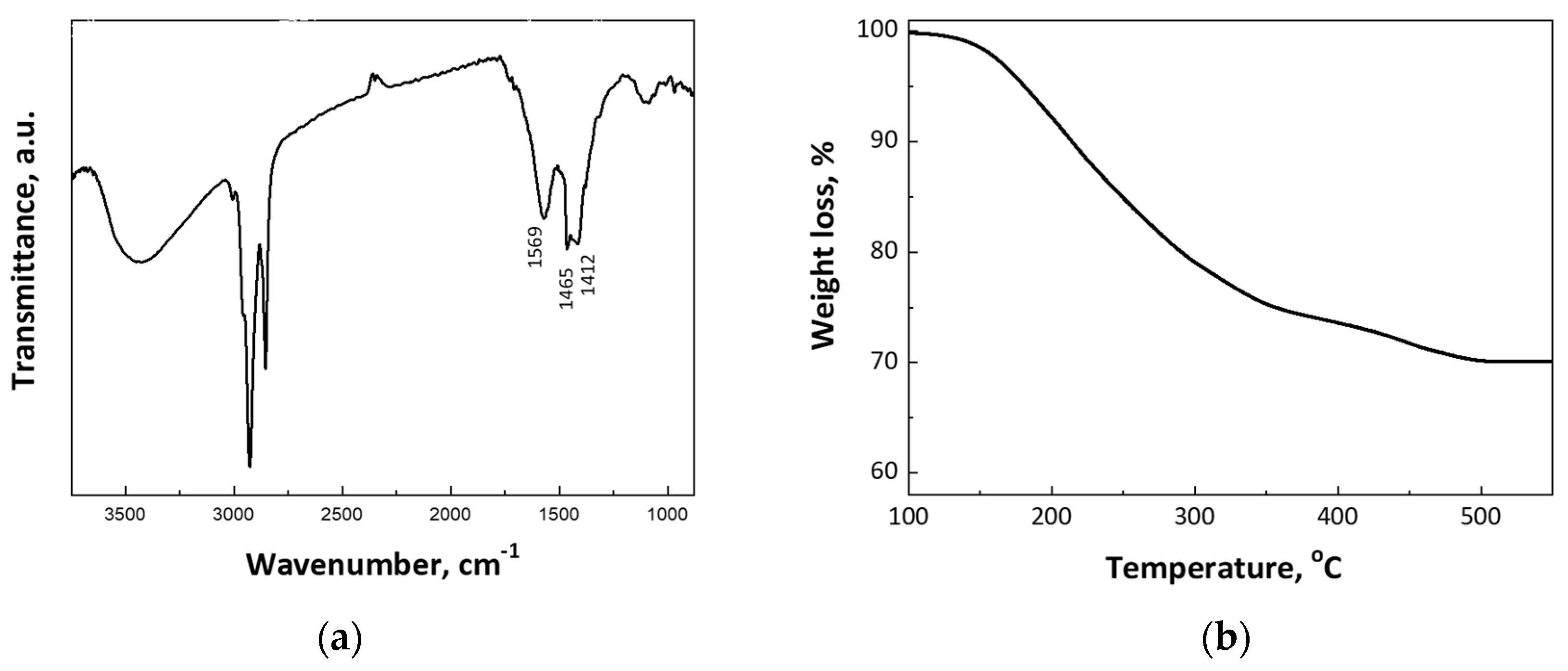
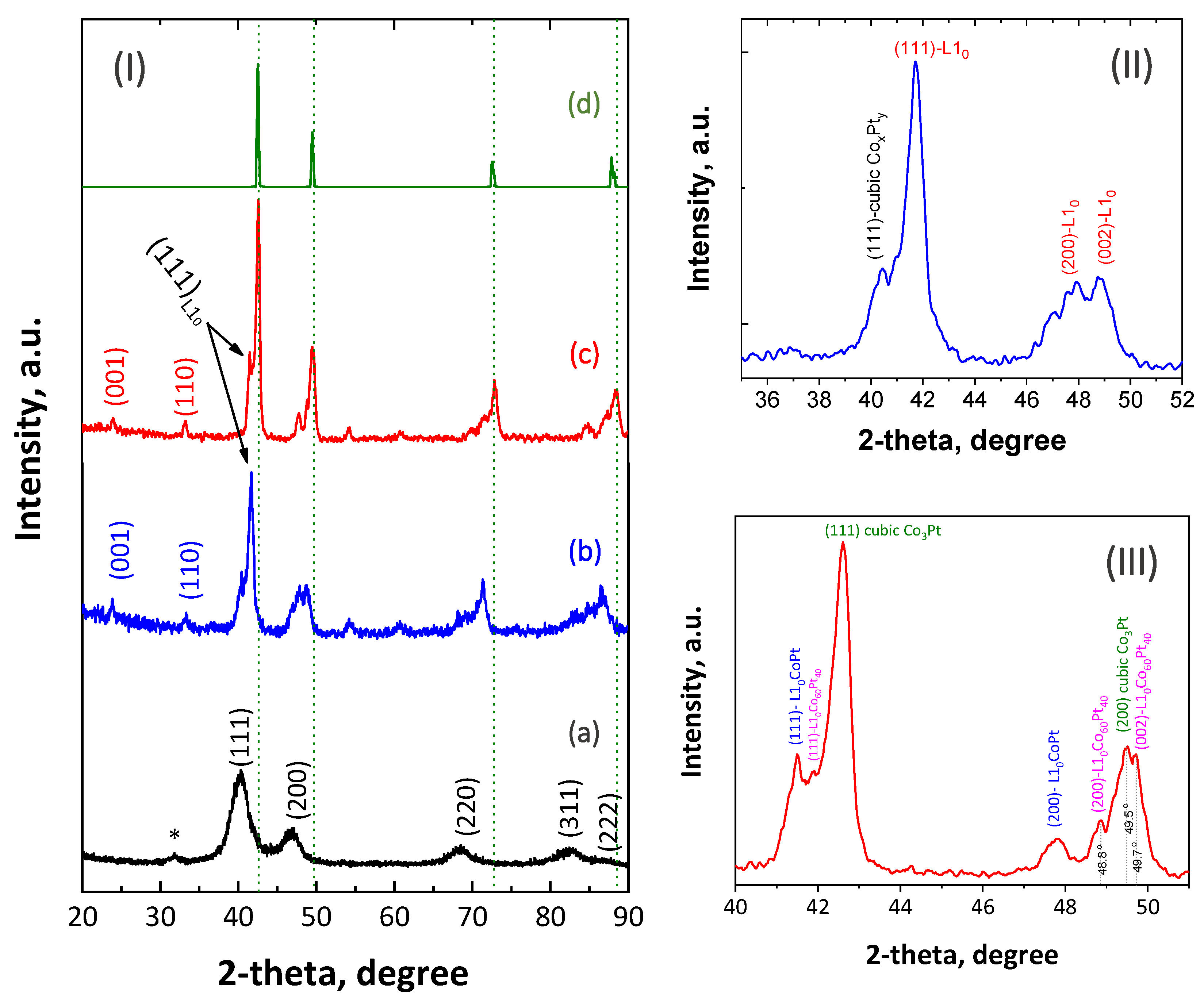
2.2. Materials Characterization
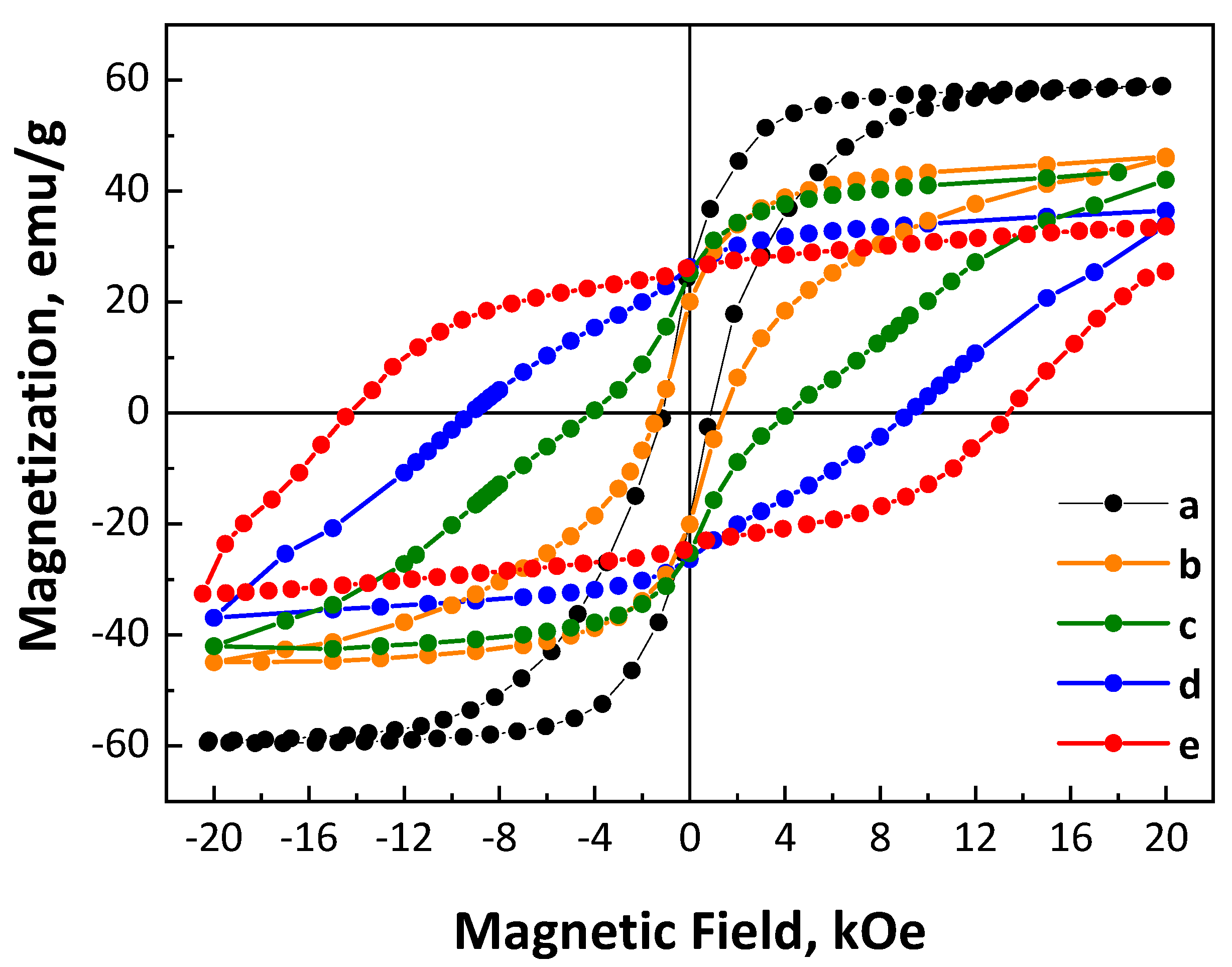
3. Results and Discussion
4. Conclusions
Supplementary Material
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Krishnan, K.M.; Pakhomov, A.B.; Bao, Y.; Blomqvist, P.; Chun, Y.; Gonzales, M.; Griffin, K.; Ji, X.; Roberts, B.K. Nanomagnetism and spin electronics: materials, microstructure and novel properties. Journal of Materials Science 2006, 41, 793–815. [Google Scholar] [CrossRef]
- Binns, C. Nanomagnetism: fundamentals and applications; Elsevier: Oxford, England, 2014. [Google Scholar]
- Fernández-Pacheco, A.; Streubel, R.; Fruchart, O.; Hertel, R.; Fischer, P.; Cowburn, R.P. Three-dimensional nanomagnetism. Nature Communications 2017, 8, 15756. [Google Scholar] [CrossRef] [PubMed]
- Abel, F.M.; Tzitzios, V.; Devlin, E.; Alhassan, S.; Sellmyer, D.J.; Hadjipanayis, G.C. Enhancing the Ordering and Coercivity of L10 FePt Nanostructures with Bismuth Additives for Applications Ranging from Permanent Magnets to Catalysts. ACS Applied Nano Materials 2019, 2, 3146–3153. [Google Scholar] [CrossRef]
- Liang, J.; Ma, F.; Hwang, S.; Wang, X.; Sokolowski, J.; Li, Q.; Wu, G.; Su, D. Atomic Arrangement Engineering of Metallic Nanocrystals for Energy-Conversion Electrocatalysis. Joule 2019, 3, 956–991. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Frontiers in Chemistry 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Singamaneni, S.; Bliznyuk, V.N.; Binek, C.; Tsymbal, E.Y. Magnetic nanoparticles: recent advances in synthesis, self-assembly and applications. Journal of Materials Chemistry 2011, 21, 16819–16845. [Google Scholar] [CrossRef]
- Wu, L.; Mendoza-Garcia, A.; Li, Q.; Sun, S. Organic Phase Syntheses of Magnetic Nanoparticles and Their Applications. Chemical Reviews 2016, 116, 10473–10512. [Google Scholar] [CrossRef] [PubMed]
- Andreazza, P.; Pierron-Bohnes, V.; Tournus, F.; Andreazza-Vignolle, C.; Dupuis, V. Structure and order in cobalt/platinum-type nanoalloys: from thin films to supported clusters. Surface Science Reports 2015, 70, 188–258. [Google Scholar] [CrossRef]
- Zhao, Z.; Fisher, A.; Shen, Y.; Cheng, D. Magnetic Properties of Pt-Based Nanoalloys: A Critical Review. Journal of Cluster Science 2016, 27, 817–843. [Google Scholar] [CrossRef]
- Okamoto, H. Supplemental Literature Review of Binary Phase Diagrams: Au-La, Ce-Pt, Co-Pt, Cr-S, Cu-Sb, Fe-Ni, Lu-Pd, Ni-S, Pd-Ti, Si-Te, Ta-V, and V-Zn. Journal of Phase Equilibria and Diffusion 2019, 40, 743–756. [Google Scholar] [CrossRef]
- Kim, D.; Saal, J.E.; Zhou, L.; Shang, S.; Du, Y.; Liu, Z.-K. Thermodynamic modeling of fcc order/disorder transformations in the Co–Pt system. Calphad 2011, 35, 323–330. [Google Scholar] [CrossRef]
- Lu, X.; Laughlin, D.E.; Zhu, J.-G. On the conditions for ordered hexagonal mm2 Co3Pt. Journal of Magnetism and Magnetic Materials 2019, 491, 165570. [Google Scholar] [CrossRef]
- Karoui, S.; Amara, H.; Legrand, B.; Ducastelle, F. Magnetism: the driving force of order in CoPt, a first-principles study. Journal of Physics: Condensed Matter 2013, 25, 056005. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Suzuki, T.; Kanazawa, H.; Österman, J.C. The origin of the large perpendicular magnetic anisotropy in Co3Pt alloy thin films. Journal of Applied Physics 1999, 85, 5094–5096. [Google Scholar] [CrossRef]
- Tokushige, M.; Matsuura, A.; Nishikiori, T.; Ito, Y. Formation of Co–Pt Intermetallic Compound Nanoparticles by Plasma-Induced Cathodic Discharge Electrolysis in a Chloride Melt. Journal of The Electrochemical Society 2011, 158, E21. [Google Scholar] [CrossRef]
- Skumryev, V.; Stoyanov, S.; Zhang, Y.; Hadjipanayis, G.; Givord, D.; Nogués, J. Beating the superparamagnetic limit with exchange bias. Nature 2003, 423, 850–853. [Google Scholar] [CrossRef]
- Hasegawa, T.; Long, L.D.; Nakamura, Y. MFM Observation of High Coercivity in Nanostructured Tetragonally Distorted FeCo Films. IEEE Transactions on Magnetics 2021, 57, 1–5. [Google Scholar] [CrossRef]
- Tzitzios, V.; Basina, G.; Tzitzios, N.; Alexandrakis, V.; Hu, X.; Hadjipanayis, G. Direct liquid phase synthesis of ordered L10 FePt colloidal particles with high coercivity using an Au nanoparticle seeding approach. New Journal of Chemistry 2016, 40, 10294–10299. [Google Scholar] [CrossRef]
- Miyashita, E.; Funabashi, N.; Taguchi, R.; Tamaki, T.; Nakamura, S. Dependence of thermal decay on the magnetic cluster size of perpendicular magnetic recording media. Journal of Magnetism and Magnetic Materials 2005, 287, 96–101. [Google Scholar] [CrossRef]
- Tzitzios, V.; Basina, G.; Gjoka, M.; Boukos, N.; Niarchos, D.; Devlin, E.; Petridis, D. The effect of Mn doping in FePt nanoparticles on the magnetic properties of the L10 phase. Nanotechnology 2006, 17, 4270. [Google Scholar] [CrossRef]
- Maat, S.; Marley, A.C. Physics and Design of Hard Disk Drive Magnetic Recording Read Heads. In Handbook of Spintronics, Xu, Y., Awschalom, D.D., Nitta, J., Eds.; Springer Netherlands: Dordrecht, 2016; pp. 977–1028. [Google Scholar]
- Christodoulides, J.A.; Huang, Y.; Zhang, Y.; Hadjipanayis, G.C.; Panagiotopoulos, I.; Niarchos, D. CoPt and FePt thin films for high density recording media. Journal of Applied Physics 2000, 87, 6938–6940. [Google Scholar] [CrossRef]
- Coey, J.M.D. Hard Magnetic Materials: A Perspective. IEEE Transactions on Magnetics 2011, 47, 4671–4681. [Google Scholar] [CrossRef]
- Advanced Magnetic Nanostructures; Sellmyer, D.; Skomski, R.; Springer US: Boston, MA, 2006.
- Wang, P.; Shao, Q.; Huang, X. Updating Pt-Based Electrocatalysts for Practical Fuel Cells. Joule 2018, 2, 2514–2516. [Google Scholar] [CrossRef]
- Wang, S.; Luo, Q.; Zhu, Y.; Tang, S.; Du, Y. Facile Synthesis of Quaternary Structurally Ordered L12-Pt(Fe, Co, Ni)3 Nanoparticles with Low Content of Platinum as Efficient Oxygen Reduction Reaction Electrocatalysts. ACS Omega 2019, 4, 17894–17902. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, N.; Arruda, T.M.; Wen, W.; Hakim, N.; Saha, M.; Gullá, A.; Mukerjee, S. Enhanced activity and interfacial durability study of ultra low Pt based electrocatalysts prepared by ion beam assisted deposition (IBAD) method. Electrochimica Acta 2009, 54, 6756–6766. [Google Scholar] [CrossRef]
- Huang, S.; Shan, A.; Wang, R. Low Pt Alloyed Nanostructures for Fuel Cells Catalysts. Catalysts 2018, 8, 538. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, X.; Kang, Y.; Miao, L.; Xia, D.; Gan, L. Structurally Ordered Low-Pt Intermetallic Electrocatalysts toward Durably High Oxygen Reduction Reaction Activity. Advanced Functional Materials 2019, 29, 1902987. [Google Scholar] [CrossRef]
- Li, J.; Xi, Z.; Pan, Y.-T.; Spendelow, J.S.; Duchesne, P.N.; Su, D.; Li, Q.; Yu, C.; Yin, Z.; Shen, B.; et al. Fe Stabilization by Intermetallic L10-FePt and Pt Catalysis Enhancement in L10-FePt/Pt Nanoparticles for Efficient Oxygen Reduction Reaction in Fuel Cells. Journal of the American Chemical Society 2018, 140, 2926–2932. [Google Scholar] [CrossRef] [PubMed]
- Kongkanand, A.; Gu, W.; Mathias, M.F. Proton-Exchange Membrane Fuel Cells with Low-Pt Content. In Encyclopedia of Sustainability Science and Technology, Meyers, R.A., Ed.; Springer New York: New York, NY, 2017; pp. 1–20. [Google Scholar]
- Li, M.; Lei, Y.; Sheng, N.; Ohtsuka, T. Preparation of low-platinum-content platinum–nickel, platinum–cobalt binary alloy and platinum–nickel–cobalt ternary alloy catalysts for oxygen reduction reaction in polymer electrolyte fuel cells. Journal of Power Sources 2015, 294, 420–429. [Google Scholar] [CrossRef]
- Cao, Y.-Q.; Zi, T.-Q.; Liu, C.; Cui, D.-P.; Wu, D.; Li, A.-D. Co–Pt bimetallic nanoparticles with tunable magnetic and electrocatalytic properties prepared by atomic layer deposition. Chemical Communications 2020, 56, 8675–8678. [Google Scholar] [CrossRef]
- Demortière, A.; Petit, C. First Synthesis by Liquid−Liquid Phase Transfer of Magnetic CoxPt100-x Nanoalloys. Langmuir 2007, 23, 8575–8584. [Google Scholar] [CrossRef]
- Park, J.-I.; Cheon, J. Synthesis of “Solid Solution” and “Core-Shell” Type Cobalt−Platinum Magnetic Nanoparticles via Transmetalation Reactions. Journal of the American Chemical Society 2001, 123, 5743–5746. [Google Scholar] [CrossRef] [PubMed]
- Ely, T.O.; Pan, C.; Amiens, C.; Chaudret, B.; Dassenoy, F.; Lecante, P.; Casanove, M.J.; Mosset, A.; Respaud, M.; Broto, J.M. Nanoscale Bimetallic CoxPt1-x Particles Dispersed in Poly(vinylpyrrolidone): Synthesis from Organometallic Precursors and Characterization. The Journal of Physical Chemistry B 2000, 104, 695–702. [Google Scholar] [CrossRef]
- Chen, M.; Nikles, D.E. Synthesis, Self-Assembly, and Magnetic Properties of FexCoyPt100-x-y Nanoparticles. Nano Letters 2002, 2, 211–214. [Google Scholar] [CrossRef]
- Bian, B.; He, J.; Du, J.; Xia, W.; Zhang, J.; Liu, J.P.; Li, W.; Hu, C.; Yan, A. Growth mechanism and magnetic properties of monodisperse L10-Co(Fe)Pt@C core–shell nanoparticles by one-step solid-phase synthesis. Nanoscale 2015, 7, 975–980. [Google Scholar] [CrossRef]
- Li, J.; Sharma, S.; Wei, K.; Chen, Z.; Morris, D.; Lin, H.; Zeng, C.; Chi, M.; Yin, Z.; Muzzio, M.; et al. Anisotropic Strain Tuning of L10 Ternary Nanoparticles for Oxygen Reduction. Journal of the American Chemical Society 2020, 142, 19209–19216. [Google Scholar] [CrossRef]
- Karmaoui, M.; Amaral, J.S.; Lajaunie, L.; Puliyalil, H.; Tobaldi, D.M.; Pullar, R.C.; Labrincha, J.A.; Arenal, R.; Cvelbar, U. Smallest Bimetallic CoPt3 Superparamagnetic Nanoparticles. The Journal of Physical Chemistry Letters 2016, 7, 4039–4046. [Google Scholar] [CrossRef] [PubMed]
- Abel, F.M.; Basina, G.; Tzitzios, V.; Alhassan, S.M.; Sellmyer, D.J.; Hadjipanayis, G.C. Ferromagnetic L10-Structured CoPt Nanoparticles for Permanent Magnets and Low Pt-Based Catalysts. ACS Applied Nano Materials 2021, 4, 9231–9240. [Google Scholar] [CrossRef]
- Le Bouar, Y.; Loiseau, A.; Finel, A. Origin of the complex wetting behavior in Co-Pt alloys. Physical Review B 2003, 68, 224203. [Google Scholar] [CrossRef]
- Tzitzios, V.; Niarchos, D.; Margariti, G.; Fidler, J.; Petridis, D. Synthesis of CoPt nanoparticles by a modified polyol method: characterization and magnetic properties. Nanotechnology 2005, 16, 287. [Google Scholar] [CrossRef]
- Wellons, M.S.; Gai, Z.; Shen, J.; Bentley, J.; Wittig, J.E.; Lukehart, C.M. Synthesis of L10 ferromagnetic CoPt nanopowders using a single-source molecular precursor and water-soluble support. Journal of Materials Chemistry C 2013, 1, 5976–5980. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Liu, Y.; Jiang, Y.; Zhang, Y.; Wang, J.; Liu, Y.; Liu, H.; Sun, Y.; S D Beach, G.; et al. Fabrication, structure and magnetic properties of CoPt3, CoPt and Co3Pt nanoparticles. Journal of Physics D: Applied Physics 2012, 45, 485001. [Google Scholar] [CrossRef]
- Min, J.H.; Wu, J.H.; Cho, J.U.; Lee, J.H.; Ko, Y.-D.; Liu, H.-L.; Chung, J.-S.; Kim, Y.K. Electrochemical preparation of Co3Pt nanowires. physica status solidi (a) 2007, 204, 4158–4161. [Google Scholar] [CrossRef]
- Chen, H.M.; Hsin, C.F.; Chen, P.Y.; Liu, R.-S.; Hu, S.-F.; Huang, C.-Y.; Lee, J.-F.; Jang, L.-Y. Ferromagnetic CoPt3 Nanowires: Structural Evolution from fcc to Ordered L12. Journal of the American Chemical Society 2009, 131, 15794–15801. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, V.; Cavallotti, P.L.; Rognoni, R.; Xu, X.; Zangari, G.; Fratesi, G.; Trioni, M.I.; Bernasconi, M. Unusually Large Magnetic Anisotropy in Electrochemically Deposited Co-Rich Co–Pt Films. ACS Applied Materials & Interfaces 2011, 3, 1800–1803. [Google Scholar]
- Maret, M.; Cadeville, M.C.; Staiger, W.; Beaurepaire, E.; Poinsot, R.; Herr, A. Perpendicular magnetic anisotropy in CoxPt1 − x alloy films. Thin Solid Films 1996, 275, 224–227. [Google Scholar] [CrossRef]
- Maret, M.; Cadeville, M.C.; Herr, A.; Poinsot, R.; Beaurepaire, E.; Lefebvre, S.; Bessière, M. Enhanced perpendicular magnetic anisotropy in chemically long-range ordered (0001) CoxPt1−x films. Journal of Magnetism and Magnetic Materials 1999, 191, 61–71. [Google Scholar] [CrossRef]
- Radychev, N.; Lokteva, I.; Witt, F.; Kolny-Olesiak, J.; Borchert, H.; Parisi, J. Physical Origin of the Impact of Different Nanocrystal Surface Modifications on the Performance of CdSe/P3HT Hybrid Solar Cells. The Journal of Physical Chemistry C 2011, 115, 14111–14122. [Google Scholar] [CrossRef]
- He, T.; Chen, D.; Jiao, X. Controlled Synthesis of Co3O4 Nanoparticles through Oriented Aggregation. Chemistry of Materials 2004, 16, 737–743. [Google Scholar] [CrossRef]
- Tzitzios, V.; Niarchos, D.; Gjoka, M.; Boukos, N.; Petridis, D. Synthesis and Characterization of 3D CoPt Nanostructures. Journal of the American Chemical Society 2005, 127, 13756–13757. [Google Scholar] [CrossRef]
- Panagiotopoulos, I.; Alexandrakis, V.; Basina, G.; Pal, S.; Srikanth, H.; Niarchos, D.; Hadjipanayis, G.; Tzitzios, V. Synthesis and Magnetic Properties of Pure Cubic CoO Nanocrystals and Nanoaggregates. Crystal Growth & Design 2009, 9, 3353–3358. [Google Scholar]
- Henglein, A. Small-particle research: physicochemical properties of extremely small colloidal metal and semiconductor particles. Chemical Reviews 1989, 89, 1861–1873. [Google Scholar] [CrossRef]
- Xiao, Q.F.; Brück, E.; Zhang, Z.D.; de Boer, F.R.; Buschow, K.H.J. Phase transformation and magnetic properties of bulk CoPt alloy. Journal of Alloys and Compounds 2004, 364, 64–71. [Google Scholar] [CrossRef]
- Xia, G.; Wang, S.; Jeong, S.-J. A universal approach for template-directed assembly of ultrahigh density magnetic nanodot arrays. Nanotechnology 2010, 21, 485302. [Google Scholar] [CrossRef]
- Ohtake, M.; Suzuki, D.; Futamoto, M. Characterization of metastable crystal structure for Co-Pt alloy thin film by x-ray diffraction. Journal of Applied Physics 2014, 115. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Simeonidis, K.; Gloystein, K.; Angelakeris, M.; Dendrinou-Samara, C.; Tsiaoussis, I.; Kalogirou, O. Tailoring the morphology of CoxPt1−x magnetic nanostructures. Journal of Magnetism and Magnetic Materials 2009, 321, 3120–3125. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Mora-Loṕez, J.L.; Leroux, C.; Cadeville, M.C. CHEMICAL AND MAGNETIC ORDERING IN CoPt. J. Phys. Colloques 1988, 49, C8-107-C108-108. [Google Scholar] [CrossRef]
- Leroux, C.; Cadeville, M.C.; Pierron-Bohnes, V.; Inden, G.; Hinz, F. Comparative investigation of structural and transport properties of L10 NiPt and CoPt phases; the role of magnetism. Journal of Physics F: Metal Physics 1988, 18, 2033. [Google Scholar] [CrossRef]
- Hadjipanayis, G.; Gaunt, P. An electron microscope study of the structure and morphology of a magnetically hard PtCo alloy. Journal of Applied Physics 2008, 50, 2358–2360. [Google Scholar] [CrossRef]
- Skomski, R.; Coey, J.M.D. Giant energy product in nanostructured two-phase magnets. Physical Review B 1993, 48, 15812–15816. [Google Scholar] [CrossRef]
- Zeng, H.; Li, J.; Liu, J.P.; Wang, Z.L.; Sun, S. Exchange-coupled nanocomposite magnets by nanoparticle self-assembly. Nature 2002, 420, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Chakka, V.M.; Shan, Z.S.; Liu, J.P. Effect of coupling strength on magnetic properties of exchange spring magnets. Journal of Applied Physics 2003, 94, 6673–6677. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




