Submitted:
25 October 2023
Posted:
25 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
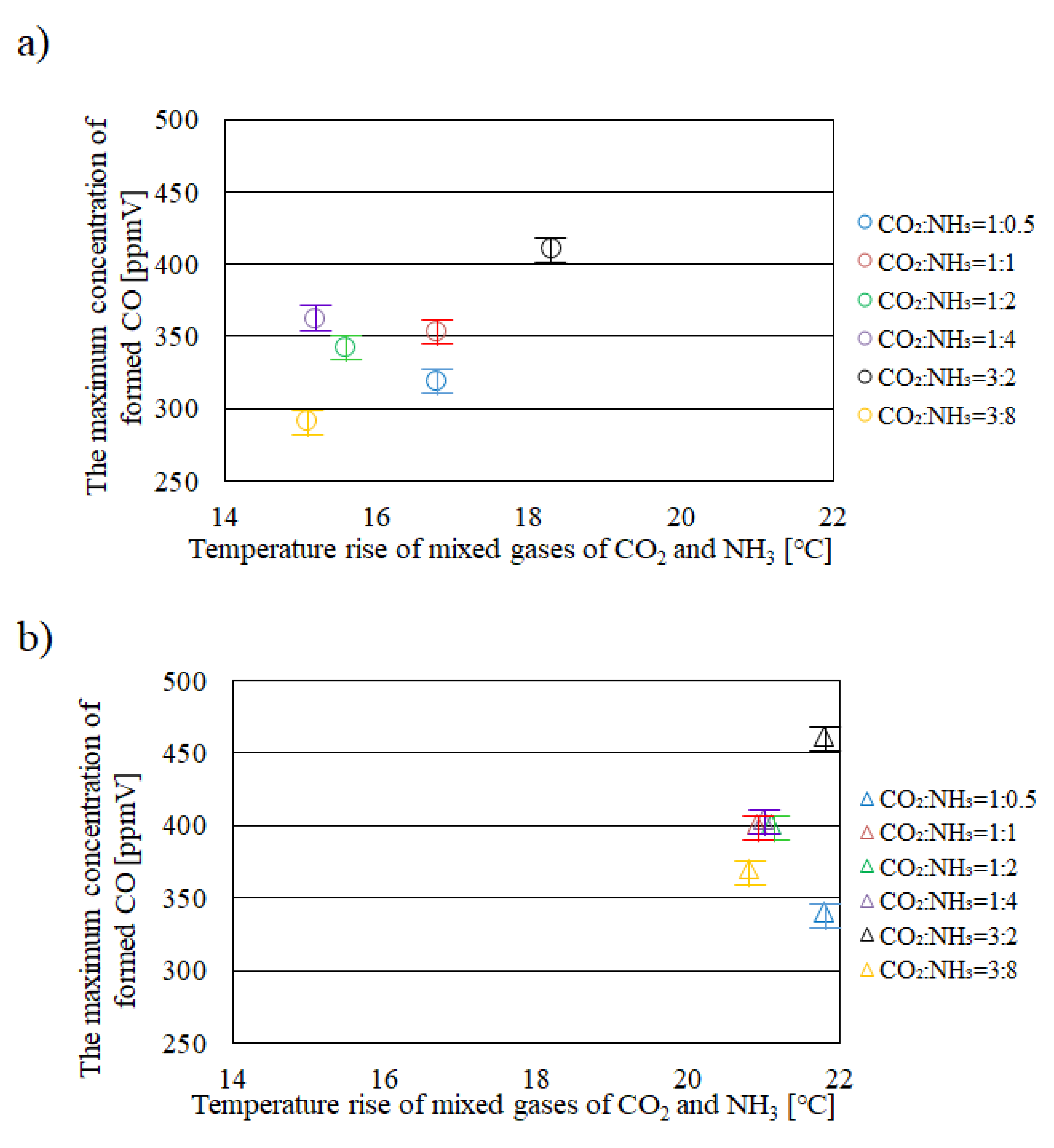
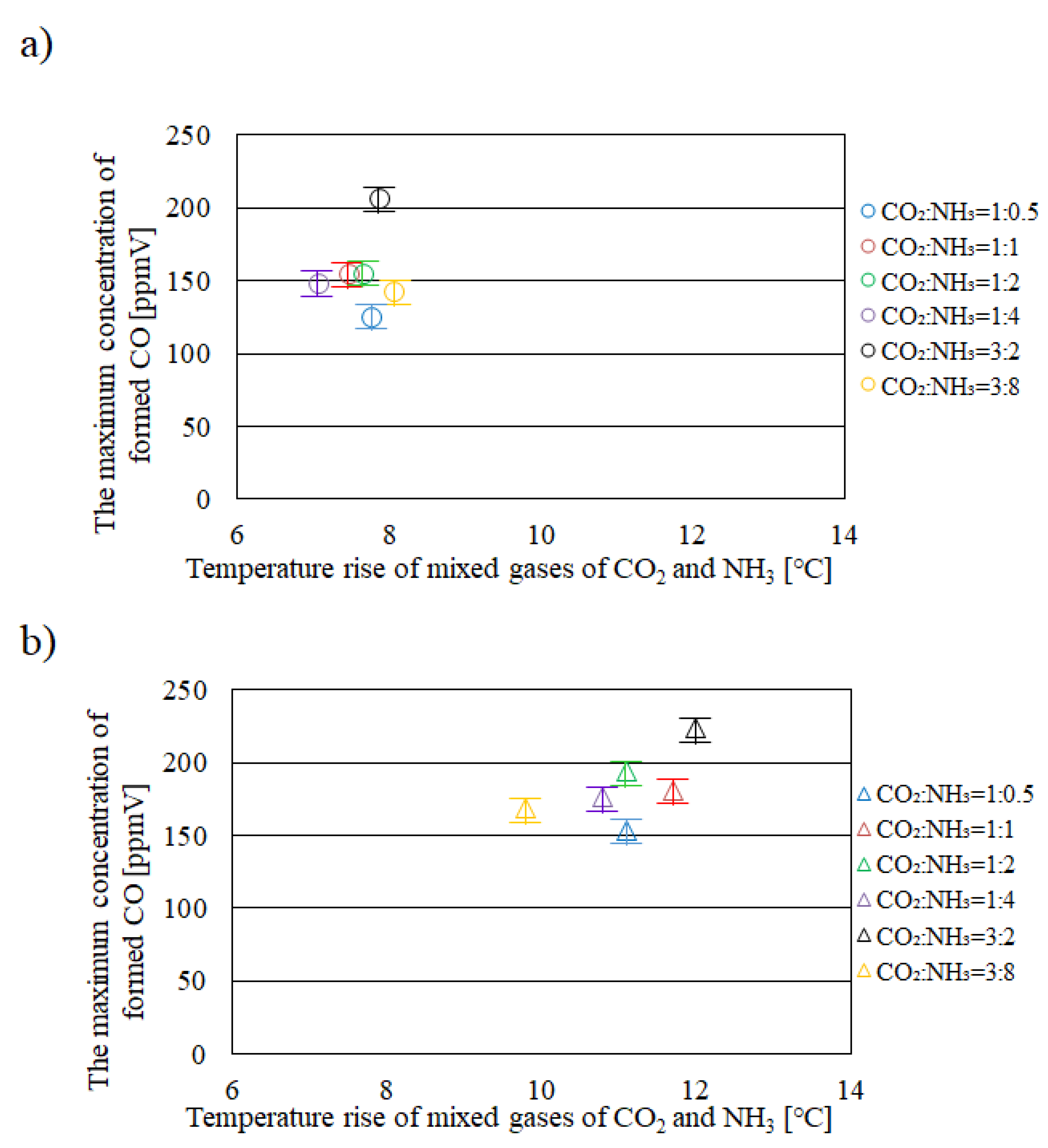
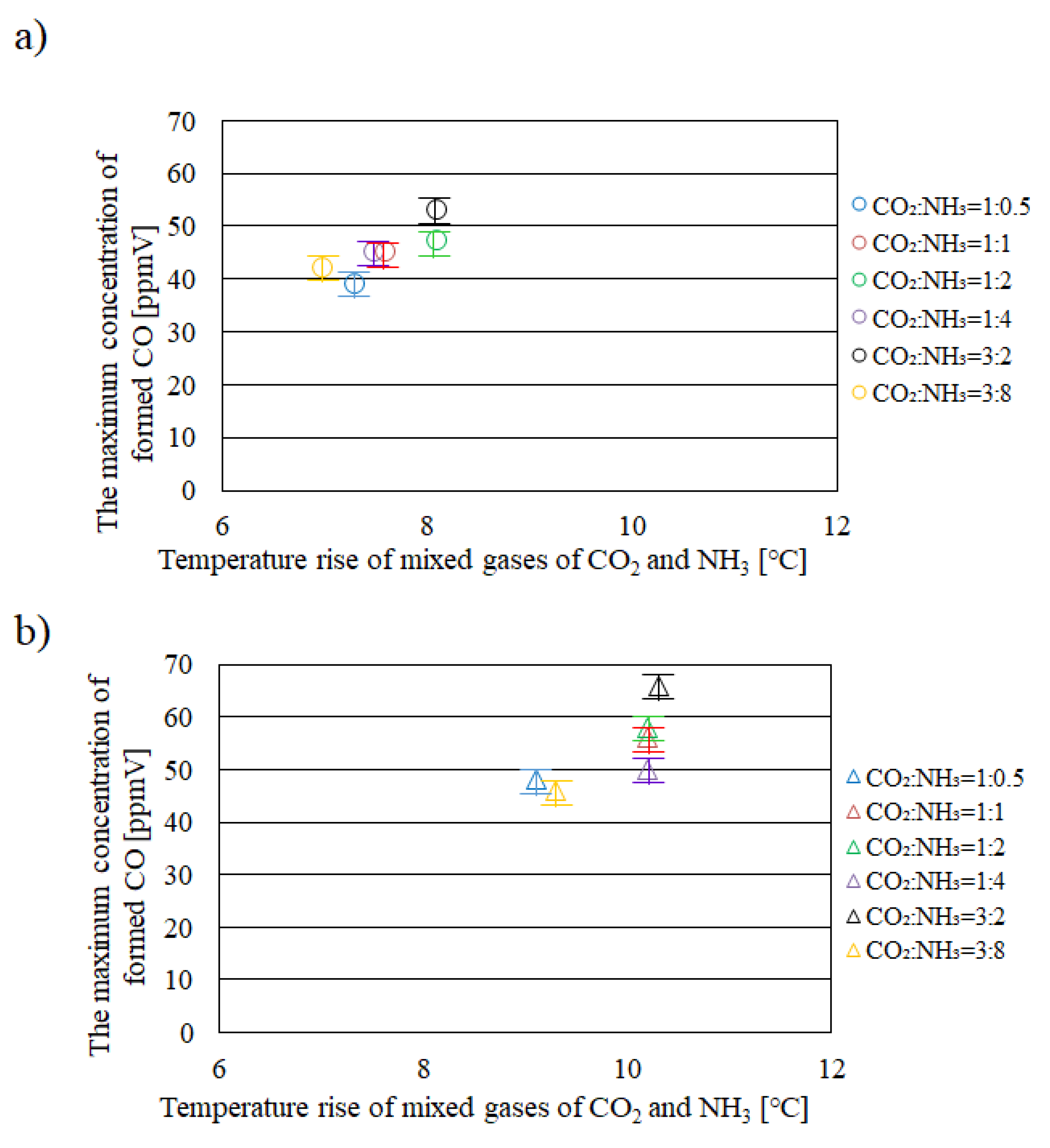
2.1. Relationship between Temperature Rise of Mixed Gases of CO2 and NH3 in Reactor and the Maximum Concentration of Formed CO
2.2. Heat Transfer Mechanism to Heat up Temperature of Mixed Gases of CO2 and NH3 around P4O10/TiO2 Photocatalyst due to Black Body Material
| UV + VIS + IR | |||||
| CO2 [mol] | NH3 [mol] | Tin [°C] | Tg [°C] | Te [°C] | Tg – Te [°C] |
| 1 | 0.5 | 24.7 | 36.7 | 46.5 | 9.8 |
| 1 | 1 | 24.0 | 36.5 | 44.9 | 8.4 |
| 1 | 2 | 24.1 | 36.8 | 45.2 | 8.4 |
| 1 | 4 | 24.2 | 36.9 | 45.2 | 8.3 |
| 3 | 2 | 24.1 | 36.4 | 45.9 | 9.5 |
| 3 | 8 | 24.4 | 37.1 | 45.2 | 8.1 |
| VIS + IR | |||||
| CO2 [mol] | NH3 [mol] | Tin [°C] | Tg [°C] | Te [°C] | Tg – Te [°C] |
| 1 | 0.5 | 25.4 | 36.3 | 36.8 | 0.5 |
| 1 | 1 | 25.0 | 36.3 | 36.7 | 0.4 |
| 1 | 2 | 24.5 | 36.0 | 35.6 | -0.4 |
| 1 | 4 | 24.6 | 36.1 | 35.4 | -0.7 |
| 3 | 2 | 24.3 | 35.4 | 35.5 | 0.1 |
| 3 | 8 | 24.4 | 35.9 | 34.2 | -1.7 |
| IR only | |||||
| CO2 [mol] | NH3 [mol] | Tin [°C] | Tg [°C] | Te [°C] | Tg – Te [°C] |
| 1 | 0.5 | 25.3 | 34.2 | 34.8 | 0.6 |
| 1 | 1 | 24.6 | 33.8 | 34.8 | 1.0 |
| 1 | 2 | 24.6 | 33.9 | 34.9 | 1.0 |
| 1 | 4 | 24.4 | 33.7 | 35.6 | 1.9 |
| 3 | 2 | 24.7 | 33.7 | 35.0 | 1.3 |
| 3 | 8 | 25.0 | 34.3 | 34.3 | 0 |
| 1.6 Q | |||
| CO2 [mol] | NH3 [mol] | Tg [°C] | Tg – Te [°C] |
| 1 | 0.5 | 44.0 | 2.5 |
| 1 | 1 | 44.1 | 0.8 |
| 1 | 2 | 44.4 | 0.8 |
| 1 | 4 | 44.5 | 0.7 |
| 3 | 2 | 43.8 | 2.1 |
| 3 | 8 | 44.7 | 0.5 |
| 1.7 Q | |||
| CO2 [mol] | NH3 [mol] | Tg [°C] | Tg – Te [°C] |
| 1 | 0.5 | 45.2 | 1.3 |
| 1 | 1 | 45.3 | -0.4 |
| 1 | 2 | 45.7 | -0.5 |
| 1 | 4 | 45.8 | -0.6 |
| 3 | 2 | 450 | 0.9 |
| 3 | 8 | 46.0 | -0.8 |
3. Experiments
3.1. Preparation Procedure of P4O10/TiO2 Film and Black Body Material
3.2. The Experimental Procedure of CO2 Reduction and Temperature Measurement
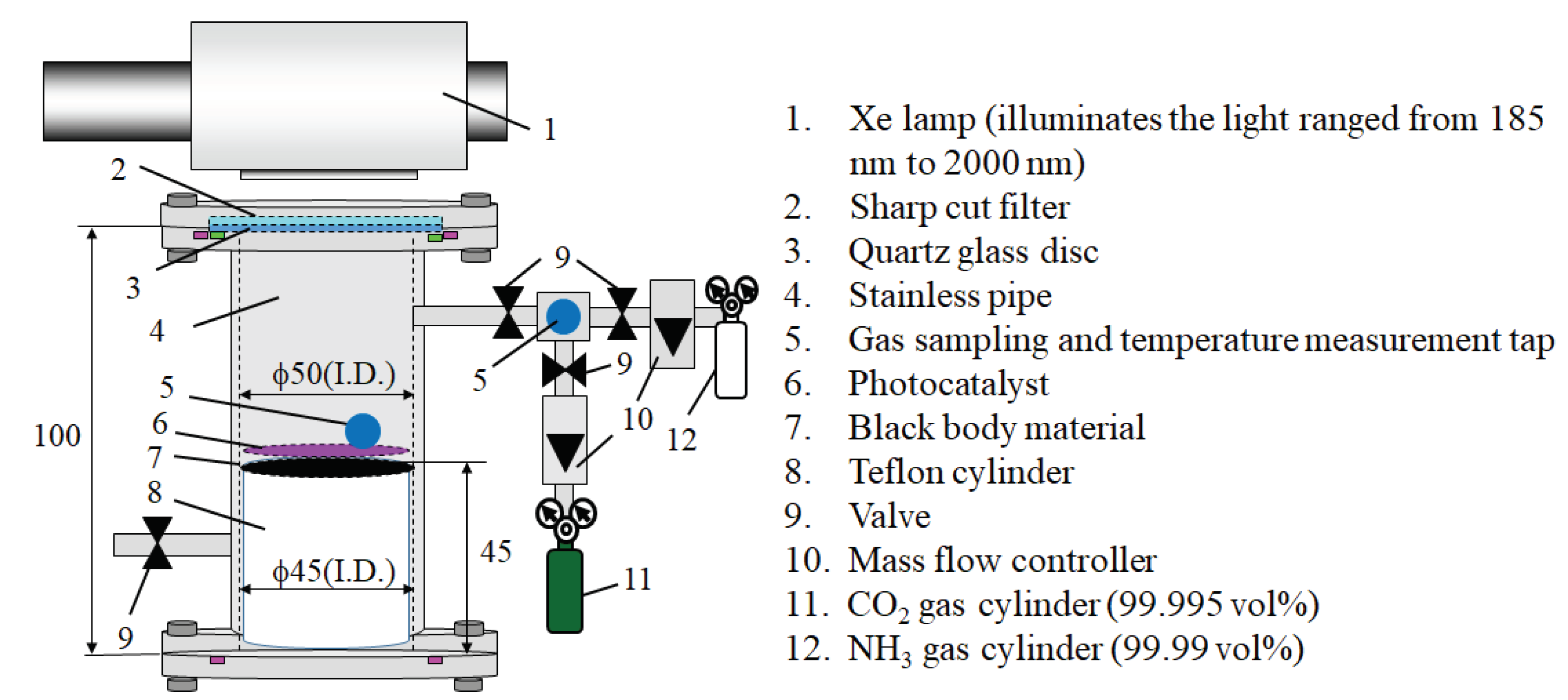
4. Calculation Procedure for Heat Transfer Analysis
4.1. Heat Transfer Formulas
5. Conclusions
- (i)
- It is revealed that the heat transfer model proposed by this study can predict Tg well under the illumination condition with VIS + IR and IR only.
- (ii)
- It is revealed that Tg – Te is larger, e.g. 10 °C under the illumination condition with UV + VIS + IR compared with that under the illumination conditions with VIS + IR and IR only.
- (iii)
- Tg – Te under the illumination condition with UV + VIS + IR becomes smaller by increasing the heat absorbed by black bod materials by 1.6 times or 1.7 times as large as the case of calculation using the light intensity measured by the light intensity meter.
- (iv)
- The mass transfer surrounding P4O10/TiO2 photocatalyst is promoted by the natural thermosiphon movement of the gases around P4O10/TiO2 photocatalyst created by black body material according to the heat transfer analysis conducted in this study.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jesic, D.; Jurkovic, L. D.; Pohar, A.; Suhadolnik, L.; Likozar, B. Engineering Photocatalytic and Photoelectrocatalytic CO2 Reduction Reactions: Mechanisms, Intrinsic Kinetics, Mass Transfer Resistances, Reactors and Multi-scale Modeling Simulations. Chemical Engineering Journal 2021, 407. [Google Scholar] [CrossRef]
- Kaushik, R.; Singh P., K.; Halder, A. Modulation Strategies in Titania Photocatalyst for Energy Recovery and Environmental Remediation. Catalysis Today, 2022; 384-386, 45–69. [Google Scholar]
- Wang, Z. W.; Shi, Y. Z.; Liu, C.; Kang, Y. Y.; Wu, L. Cu+-Ti3+ Interface Interaction Mediated CO2 Coordination model for Controlling the Selectivity of Photocatalytic Reduction CO2. Applied Catalysis B: Environmental, 2022; 301. [Google Scholar] [CrossRef]
- Remiro-Buenamanana, S.; Garcia, H. Photoassisted CO2 Conversion into Fuels. Chem. Cat Chem. Minirev. 2019, 11, 342–356. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, F.; Guo, Y.; Guo, F.; Shi, W.; Yang, S. Near-infrared Light Driven ZnIn2S4-based Photocatalysts for Environmental and Energy Applications: Progress and Perspectives. molecules 2023, 28. [Google Scholar] [CrossRef]
- Tahir, M. Synergistic Effect in MMT-dispersed Au/TiO2 Monolithic Nanocatalyst for Plasma-absorption and Metallic Interband Transitions Dynamic CO2 Photoreduction to CO. Appl. Catal. B Environ 2017, 219, 329–343. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N. A. S. Synergistic Effect in Plasmonic Au/Ag Alloy NPs Co-coated TiO2 NWs toward Visible-light Enhanced CO2 Photoreduction to Fuels. Appl. Catal. B Enviorn 2017, 204, 548–560. [Google Scholar] [CrossRef]
- Hong, L. F.; Guo, R. T.; Yuan, Y.; Ji, X. Y.; Lin, Z. D.; Gu, J. W.; Pan, W. G. Urchinlike W18O49/g-C3N4 Z-Scheme Heterojunction for Highly Efficient Photocatalytic Reduction of CO2 under Full Spectrum Light. Energy Fuels 2021, 35, 11468–11478. [Google Scholar] [CrossRef]
- Dai, W.; Yu, J.; Luo, S.; Hu, X.; Yang, L.; Zhang, S.; Li, B.; Luo, X.; Zou, J. WS2 Quantum Dots Seeding in Bi2S3 Nanotubes: A Novel Vis-NIR Light Sensitive Photocatalyst with Low-Resistance Junction Interface for CO2 Reduction. Chemical Engineering Journal 2020, 389. [Google Scholar] [CrossRef]
- Gan, J.; Wang, H.; Hu, H.; Su, M.; Chen, F.; Xu, H. Efficient Synthesis of Tunable Band-Gap CuInZnS Decorated g-C3N4 Hybrids for Enhanced CO2 Photocatalytic Reduction and Near-Infrared- Triggered Photordegradation Performance. Applied Surface Science 2021, 564. [Google Scholar] [CrossRef]
- Yu, M.; Lv, X.; Idris, A. M.; Li, S.; Lin, J.; Lin, H.; Wang, J.; Li, Z. Upconversion Nanoparticles Coupled with Hierarchical ZnIn2S4 Nanorods as a Near-Infrared Responsive Photocatalyst for Photocatalytic CO2 Reduction. Journal of Colloid and Interface Science 2022, 612, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Mae, H.; Kato, T.; Hu, E. Utilization from ultraviolet to infrared light for CO2 reduction with P4O10/TiO2 photocatalyst. Physics & Astronomy International Journal, 2022; 6, 145–154. [Google Scholar]
- Nishimura, A.; Mae, H.; Hannyu, R.; Hu, E. Impact of loading amount of P4O10 on CO2 reduction performance of P4O10/TiO2 with H2O extending absorption range from ultraviolet to infrared light. Physics & Astronomy International Journal, 2022; 6, 186–194. [Google Scholar]
- Nishimura, A.; Kato, T.; Mae, H.; Hu, E. Impact of black body material enhanced gas movement on CO2 photocatalytic reduction performance. catalysts 2022, 12. [Google Scholar] [CrossRef]
- Nishimura, A.; Hanyu, R.; Mae, H.; Hu, E. Impact of Black Body Material on CO2 Reduction Performance of P4O10/TiO2 with NH3. Journal of Physics and Chemistry Research 2023, 5. [Google Scholar]
- Nishimura, A.; Komatsu, N.; Mitsui, G.; Hirota, M.; Hu, E. CO2 Reforming into Fuel Using TiO2 Photocatalyst and Gas Separation Membrane. Catalysis Today 2009, 148, 341–349. [Google Scholar] [CrossRef]
- Holman, J. P. Heat Transfer, 8th ed.; McGRAW-HILL, INC.: New York, USA, 1997; p. 400. [Google Scholar]
- Japan Society of Mechanical Engineering. Heat Transfer Hand Book, 1st ed.; Maruzen: Tokyo, Japan, 1993; p. 238, 367-369. [Google Scholar]
- Tahir, M.; Amin, N.S. Photocatalytic Reduction of Carbon Dioxide with Water Vapors over Montmorillonite Modified TiO2 Nanocomposites. Appl. Catal. B. Environ. 2013; 142-143, 512–522. [Google Scholar]
- Nishimura, A.; Mitsui, G.; Nakamura, K.; Hirota, M.; Hu, E. CO2 reforming characteristics under visible light response of Cr- or Ag-doped TiO2 prepared by sol-gel and dip-coating process. Int. J. Photoenergy. 2012, Article ID 184169,. [CrossRef]
- Aihara, T. Heat Transfer Engineering, 1st ed.; Syokabo: Tokyo, Japan, 1994; p. 107, 274. [Google Scholar]
- Hasatani, M.; Kimura, J. Basis and Application for Combustion, 2nd ed.; Kyoritsu Shuppan: Tokyo, Japan, 1986; p. 276. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).






