Submitted:
25 October 2023
Posted:
26 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
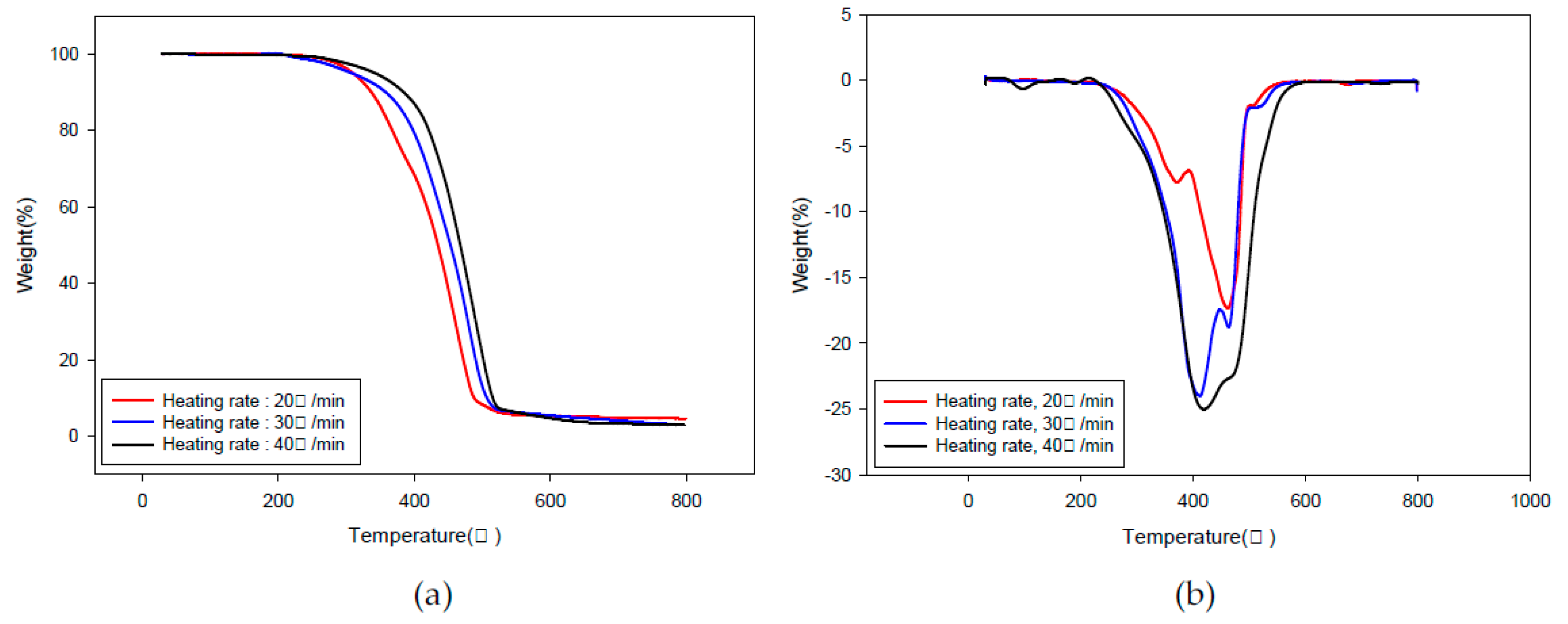
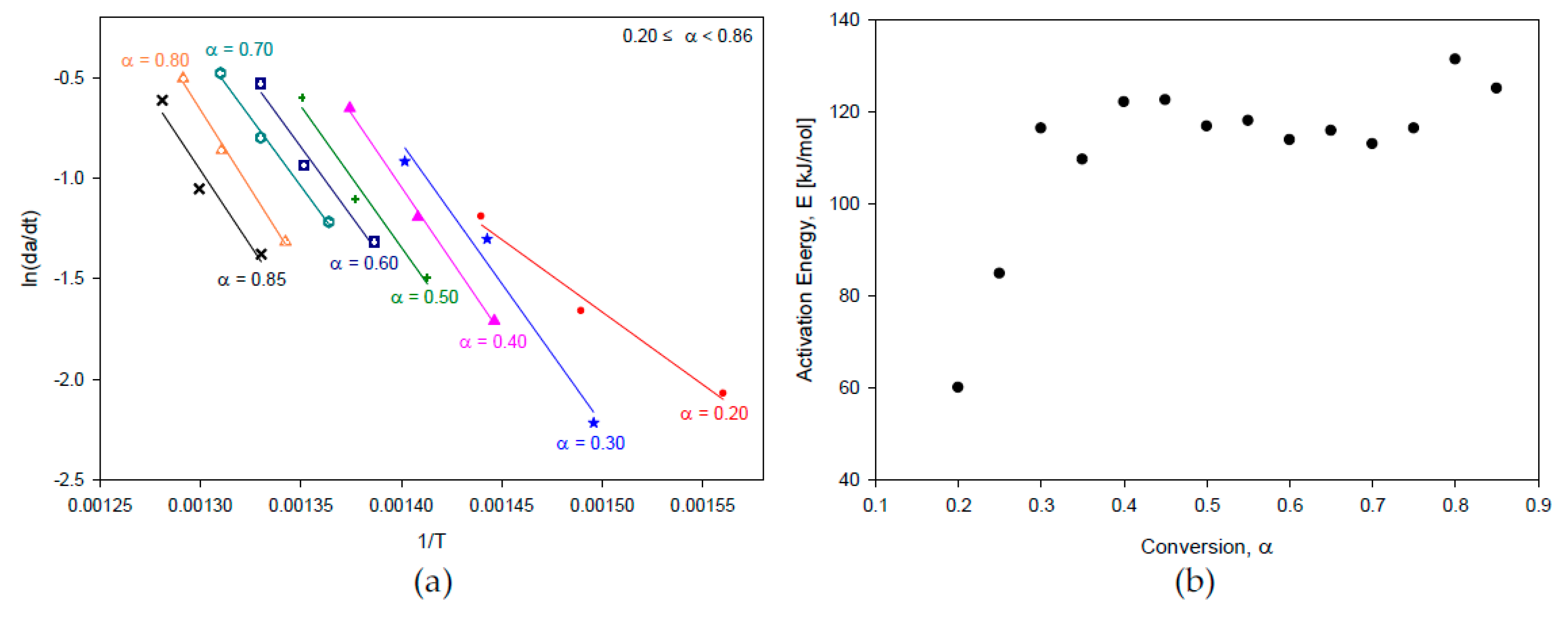
2.1. Kinetic Analysis
2.2. Product Analysis
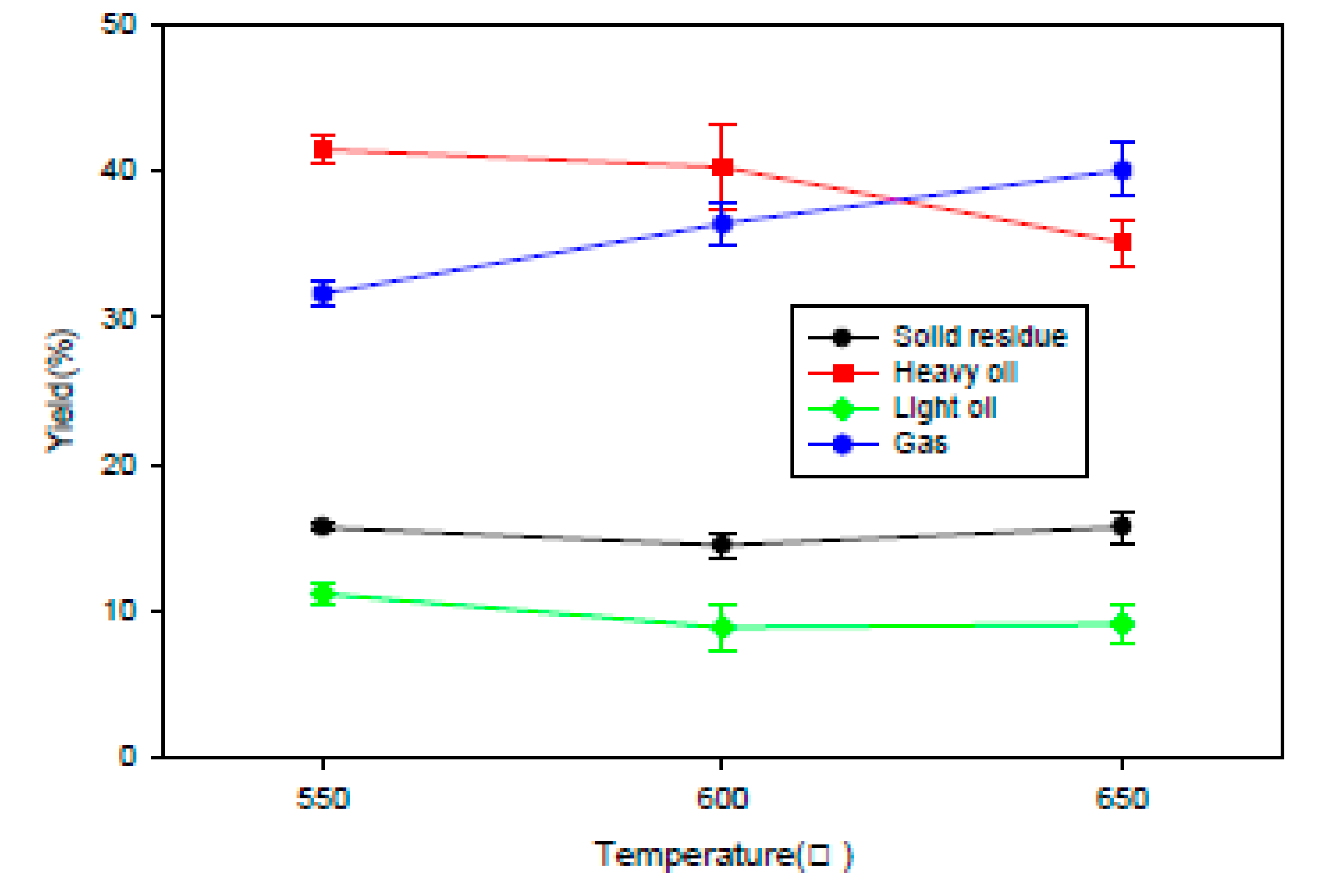
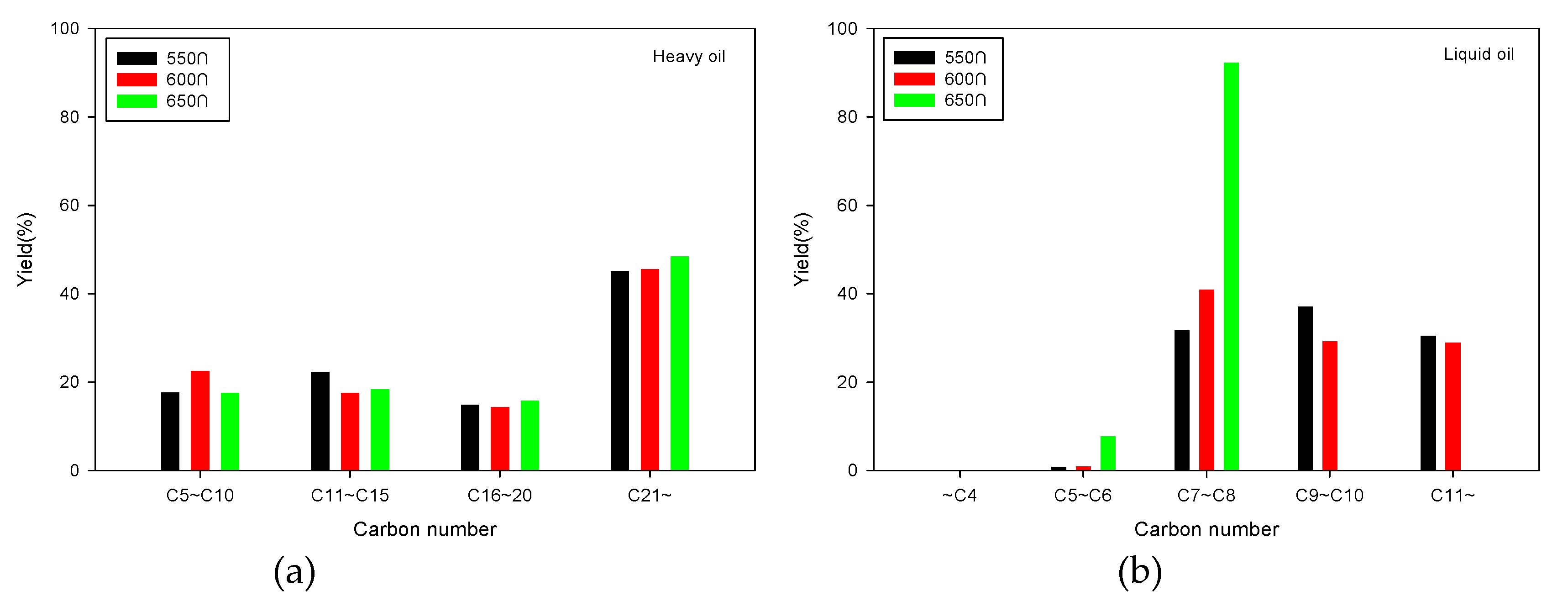
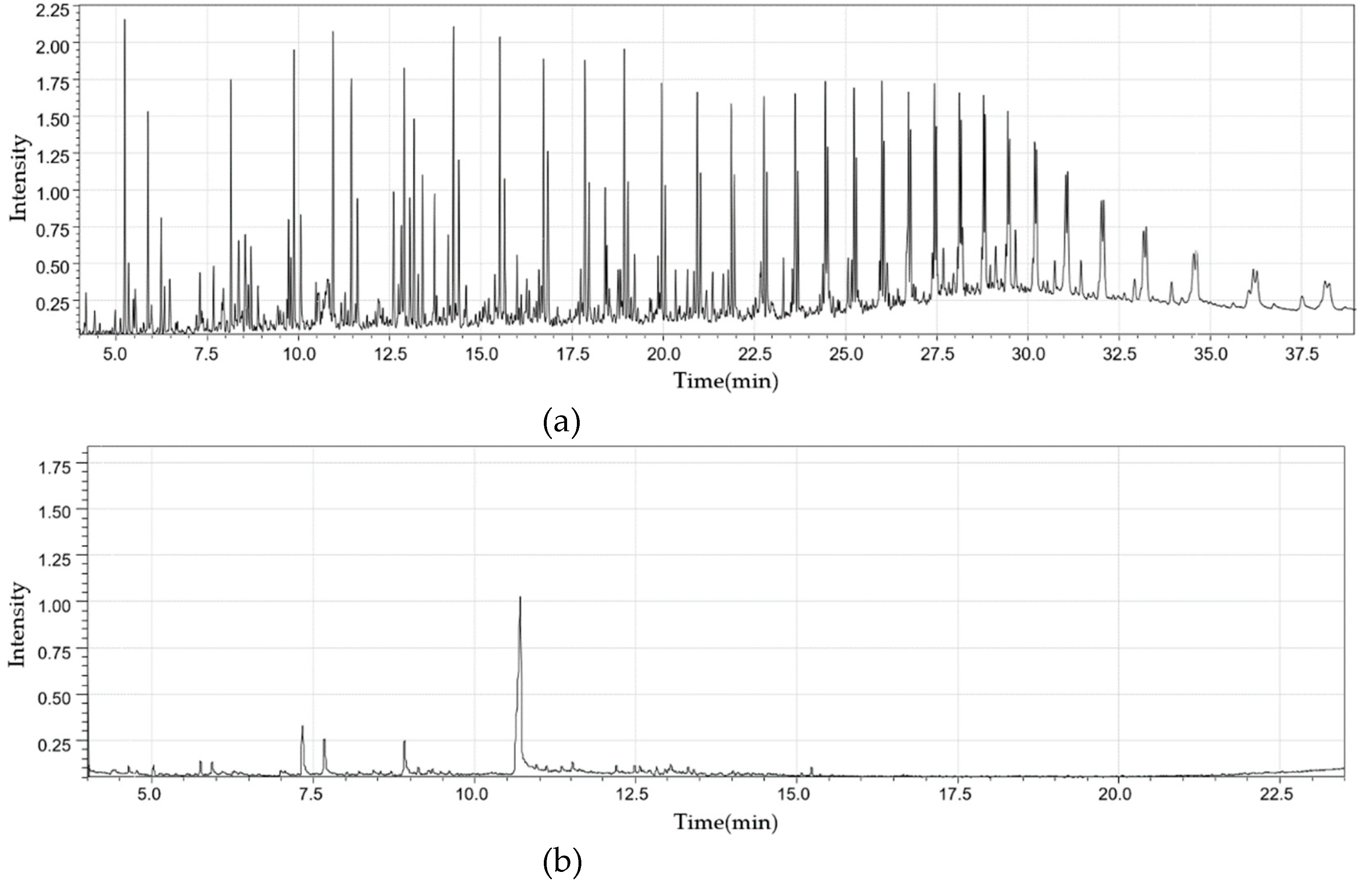
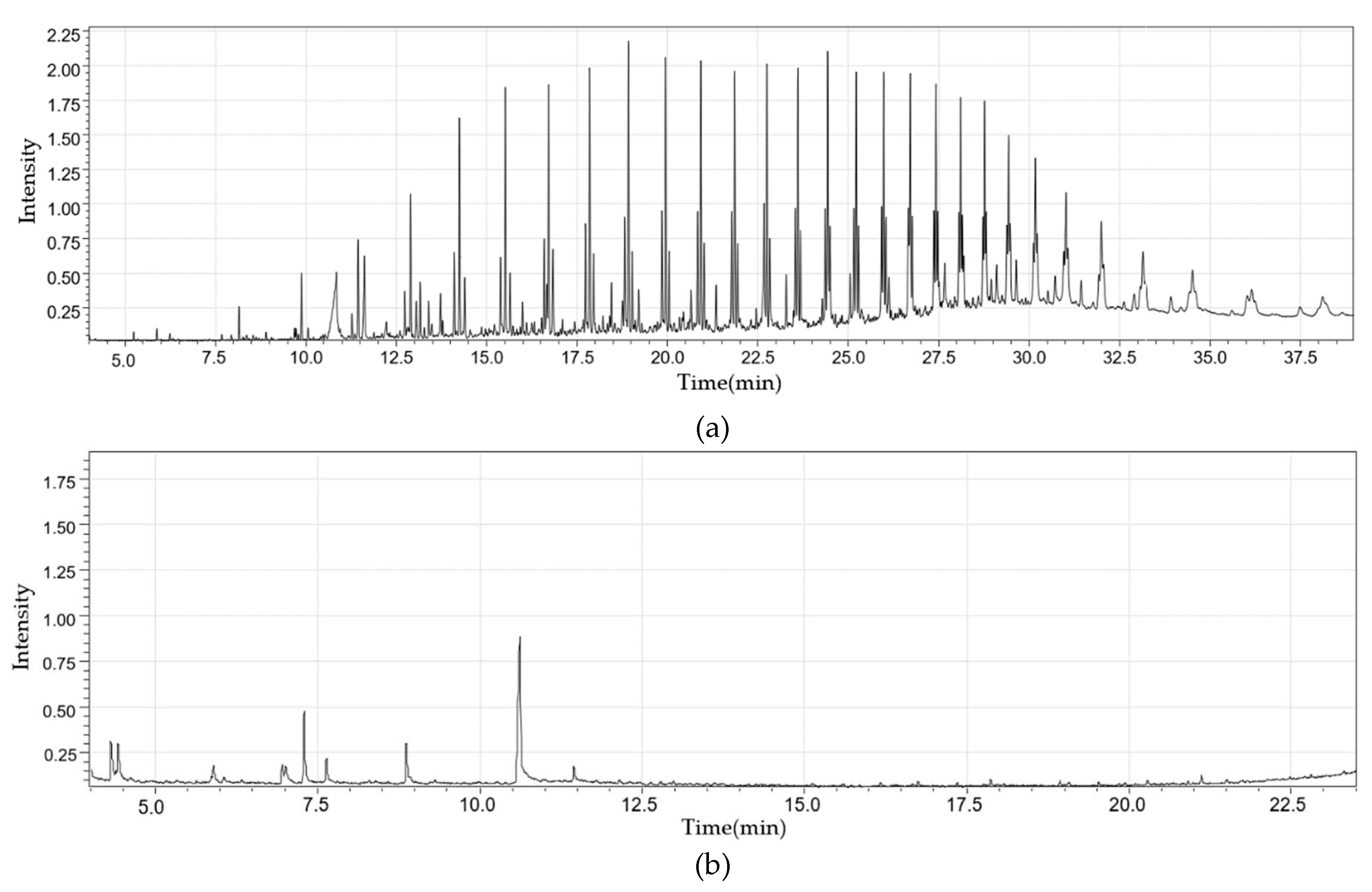
2.2.1. Fixed Bed Reactor
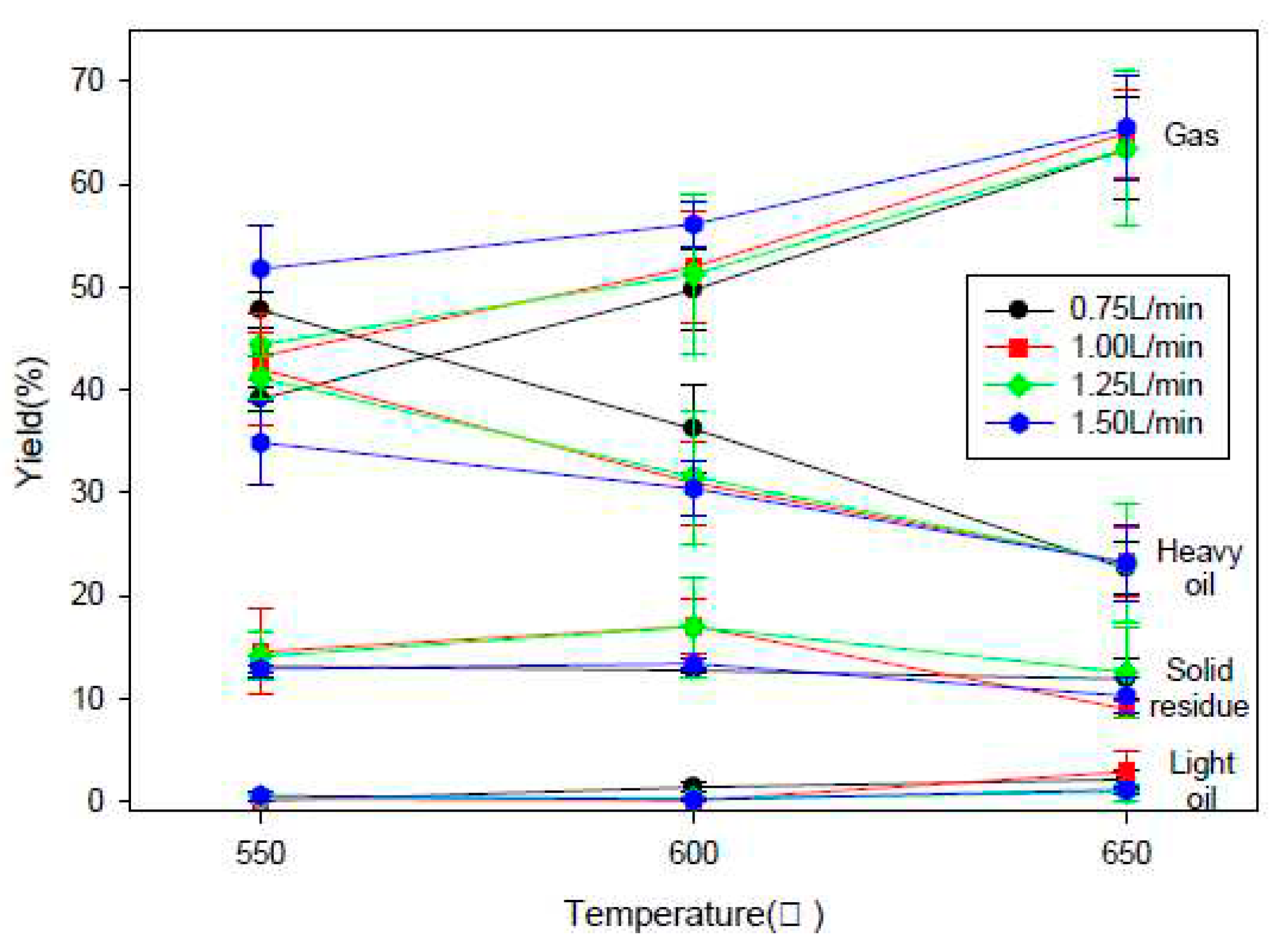
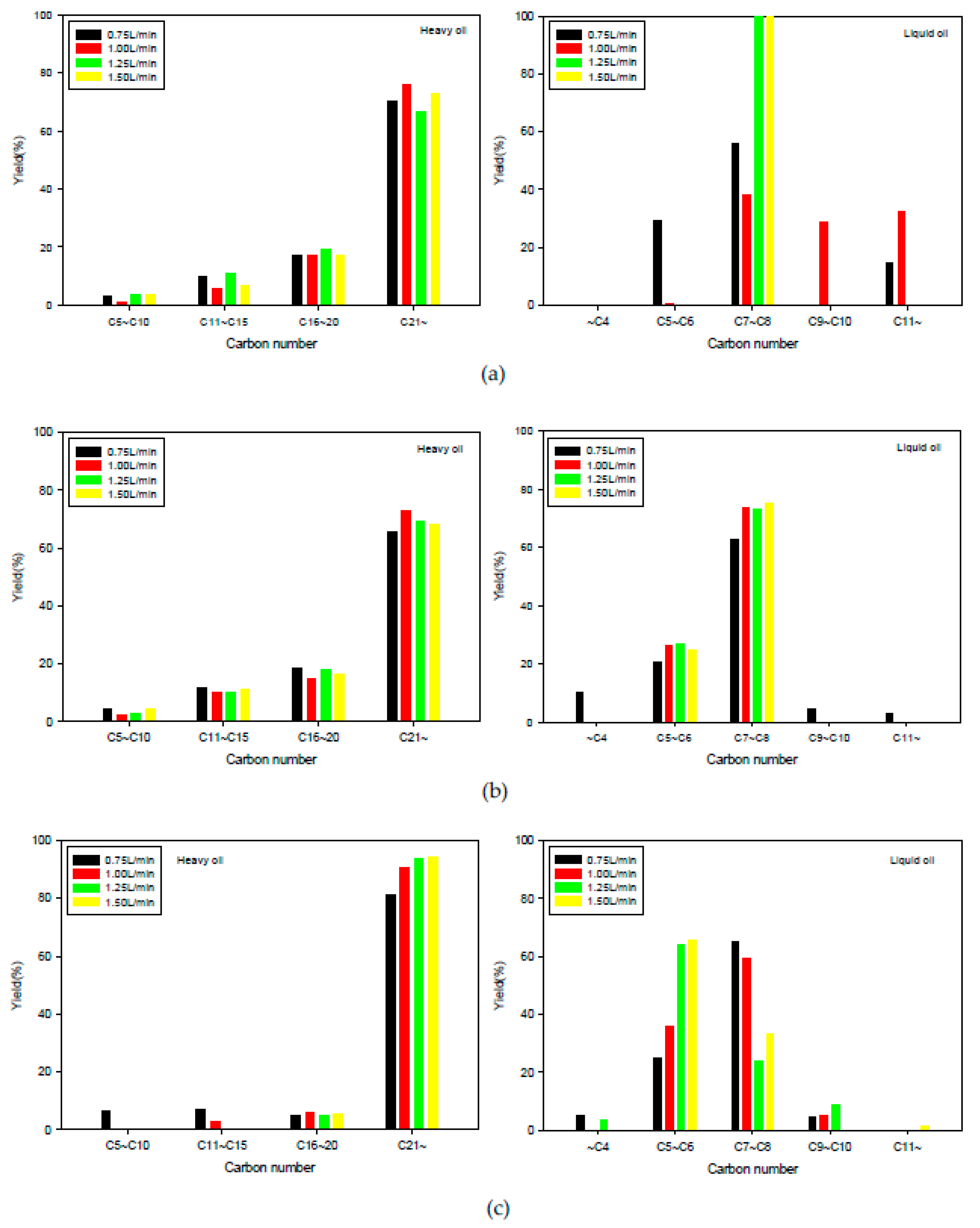
2.2.2. Fluidized Bed Reactor
3. Materials and Methods
3.1. Materials
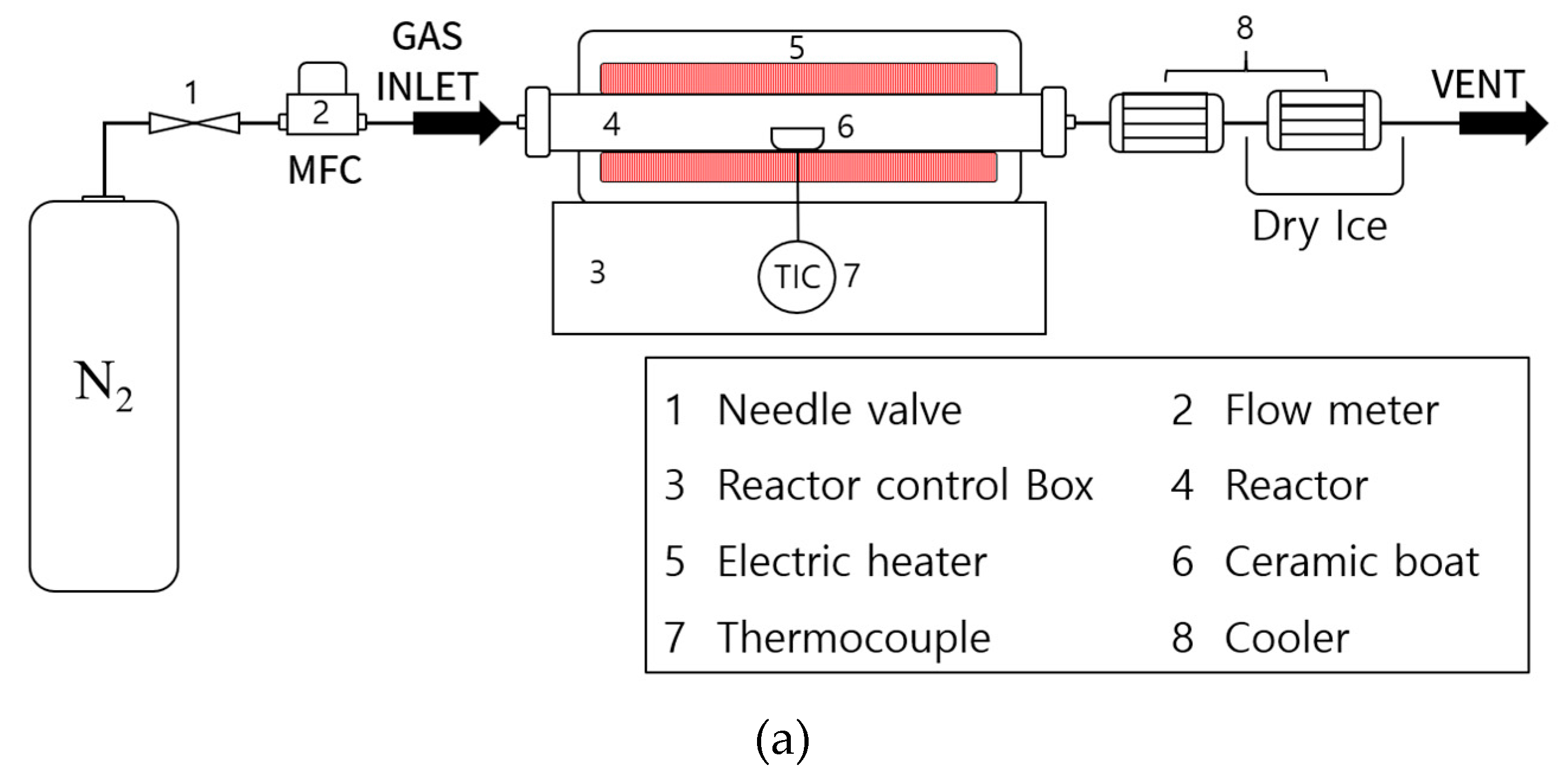
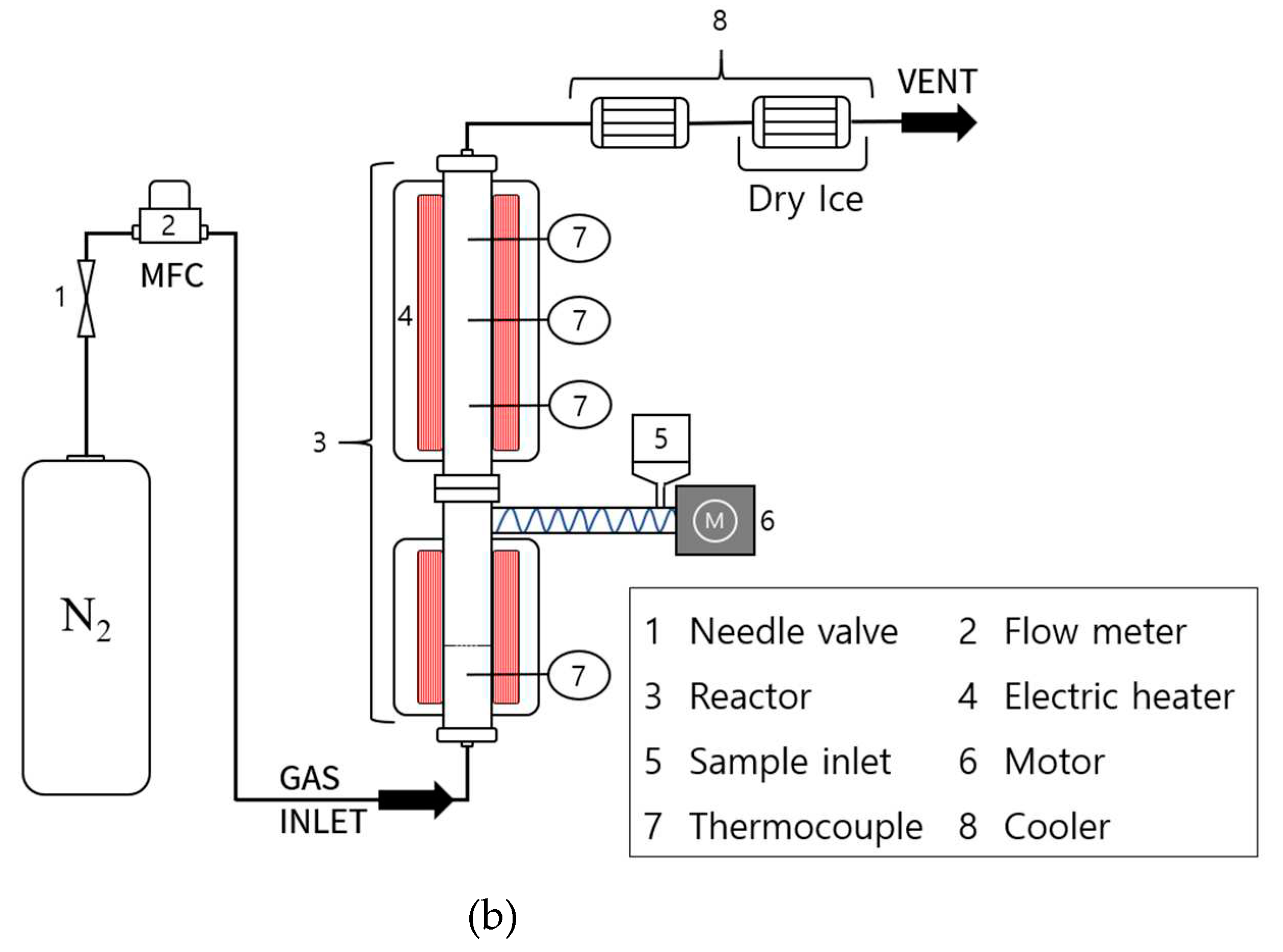
3.2. Experimental Methods
3.3. Kinetic Analysis
3.3.1. Differential Method
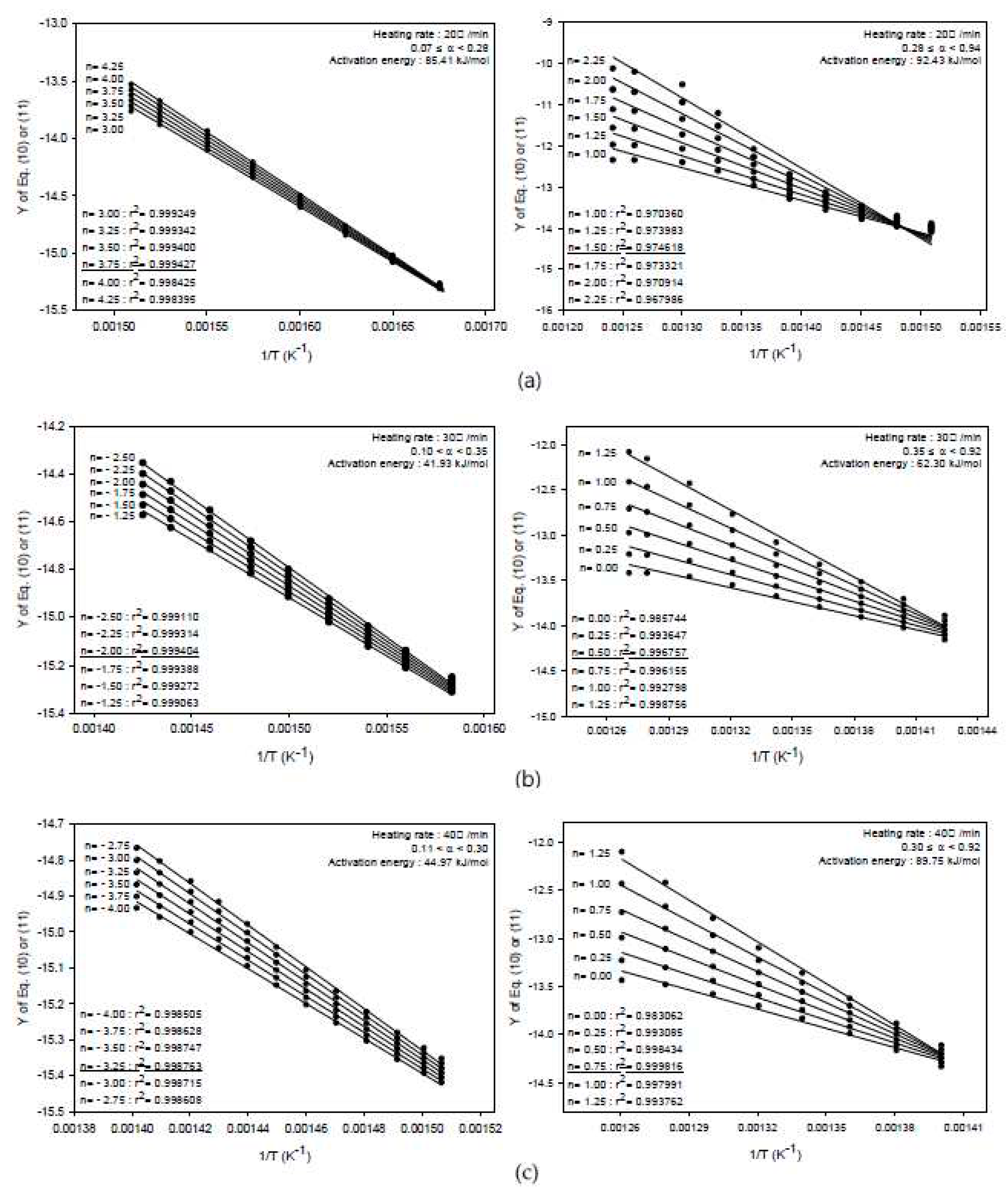
3.3.2. Integral Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Horodyska, O.; Vakdes, F.J.; Fullana, A. Plastic flexible films waste management-A state of art review. Waste Management 2018, 22, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Meys, R.; Frick, F.; Westhues, S.; Sternberg, A.; Klankermayer, J.; Bardow, A. Towards a circular economy for plastic packing waste-the environmental potential of chemical recycling. Resources, Conservation & Recycling 2020, 162, 105010. [Google Scholar]
- Park, K.B.; Jeong, Y.S.; Guzelciftci, B.; Kim, J.S. Two-stage pyrolysis of polystyrene: Pyrolysis oil as a source of fuels or bezene, toluene, ethylbenzene, and xylene. Applied Energy 2020, 259, 114240. [Google Scholar] [CrossRef]
- Mangesh, V.L.; Padmanabhan, S.; Tamizhdural, P.; Ramesh, A. Experimental investigation to identify the type of waste plastic pyrolysis oil suitable for conversion to diesel engine fuel. Journal of Cleaner Production 2020, 246, 119066. [Google Scholar] [CrossRef]
- Sekar, M.; Ponnusamy, V.K.; Pugazhendhi, A.; Nizetic, S.; Praveenkumar, T.R. Production and utilization of pyrolysis oil from solid plastic wastes: A review on pyrolysis ptocess and influence of reactors design. Journal of Environmental Management 2022, 302 Part B, 114046. [Google Scholar] [CrossRef]
- Signh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. Thermal degradation of waste plastics under non-sweeping atmosphere: Part 1 Effect of temperature, product optimization, and degradation mechanism. Journal of Environmental Management 2019, 239, 395–406. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: a review. Renewable and Sustainable Energy Reviews 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Nimmegeers, P.; Billen, P. Quantifying the separation complexity of mixed plastic waste streams with statistical entropy: A plastic waste case study in Belgium. ACS Sustainable Chemistry & Engineering 2021, 9, 9813–9822. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.F.; Roelands, M.C.P.; White, R.J.; van Harmelen, T.; de Wild, P. , van der Laan, G. P.; Meirer, F.; Keurentjes, J.T.F.; Weckhuysen, B.M. Beyond mechanical recycling: Given new life to plastic waste, Angewandte Chemie International Edition, 2020, 59, 15402–15423. [Google Scholar] [CrossRef]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Conservation and Management 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Jahirul, A.I.; Rasul, M.G.; Schaller, D.; Khan, M.M.K.; Hasan, M.M.; Hazrat, M.A. Transport fuel from waste plastics pyrolysis-A review on technologies challenges and opportunities. Energy Conservation and Management 2022, 258, 115451. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of plastic waste: Opportunities and challenges. Journal of Analytical and Applied Pyrolysis. 2020, 152, 104804. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). Journal of Environmental Management 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Vellaiyan, S. Energy extraction from waste plastics and its optimization study for effective combustion and cleaner exhaust engaging with water and cetane improve: A response surface methodology approach. Environmental Research 2023, 231 Part 1, 116113. [Google Scholar] [CrossRef]
- Kaimal, V.K.; Vijayabalan, P. A detailed study of combustion characteristics of a DI diesel engine using waste plastic oil and its blends. Energy Conservation and Management 2015, 105, 951–956. [Google Scholar] [CrossRef]
- Wathakit, K.; Sukjit, E.; Kaewbuddee, C.; Maithomklang, S.; Klinkaew, N.; Liplap, P.; Arjharn, W.; Srisertpol, J. Characterization and impact of waste plastic oil in a bariable compression ration diesel engine. Energies, 2021, 14, 2230. [Google Scholar] [CrossRef]
- Panda, A.K.; Singh, R.K.; Mishra, D.K. Thermolysis of waste plastics to liquid fuel: A suitable method for plastic waste management and manufacture of value added products-A world prospective. Renewable and Sustainable Energy Reviews 2010, 14, 223–248. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste management 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Harussani, M.M.; Sapuan, S.M.; Rashid, U.; Khalina, A.; Ilyas, R.A. Pyrolysis of polypropylene plastic waste into carbonaceous char: Priority of plastic waste management amidst COVID-19 pandemic. Science of the Total Environment 2022, 803, 149911. [Google Scholar] [CrossRef]
- Marcilla, A.; Garcia-Quesada, J.C.; Sanchez, S.; Ruiz, R. Study of the catalytic pyrolysis behaviour of polyethylene–polypropylene mixtures. J. Anal. Appl. Pyrolysis 2005, 74, 387–392. [Google Scholar] [CrossRef]
- Kim, H.T.; Oh, S.C. Kinetic of thermal degradation of waste polypropylene and high-density polyethylene. J. Ind. Eng. Chem. 2005, 11, 648–656. [Google Scholar]
- Jung, S.-H.; Cho, M.-H.; Kang, B.-S.; Kim, J.-S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process. Technol. 2010, 91, 277–284. [Google Scholar] [CrossRef]
- Park, J.W.; Oh, S.C.; Lee, H.P.; Kim, H.T.; Yoo, K.O. Kinetic analysis of thermal decomposition of polymer using a dynamic model. Korean J. Chem. Eng. 2000, 17, 489–496. [Google Scholar] [CrossRef]
- Coats, A.; Redfern, J. Kinetic parameters from thermogravimetric data. Nature, 1964, 201, 68–69. [Google Scholar] [CrossRef]
| Differential Method | Integral Method | |||
|---|---|---|---|---|
| Conversion (α) | Activation Energy (kJ/mol) | Heating Rate | Conversion (α) | Activation Energy (kJ/mol) |
| 0.20≤a<0.40 | 59.94 ~ 116.32 | 20℃/min | 0.07≤a<0.28 | 85.41 |
| 0.28≤a<0.94 | 92.43 | |||
| 30℃/min | 0.10≤a<0.35 | 41.93 | ||
| 0.40≤a<0.85 | 112.47 ~ 131.32 | 0.35≤a<0.92 | 62.30 | |
| 40℃/min | 0.11≤a<0.30 | 44.97 | ||
| 0.30≤a<0.92 | 89.75 | |||
| Elements (wt%) | C | 63.1 |
| H | 9.8 | |
| N | 0.6 | |
| O | 16.4 | |
| S | 0 | |
| Other | 10.1 | |
| Volatile (%) | 90.60 | |
| Fixed Carbon (%) | 6.07 | |
| Ash (%) | 3.33 | |
| Item | Conditions |
|---|---|
| Column Over Temp | 40.0℃ |
| Injection Temp | 250.0℃ |
| Injection Mode | Split |
| Flow Control Mode | Linear Velocity |
| Pressure | 60.1 kPa |
| Total Flow | 234.1 mL/min |
| Column Flow(He) | 1.15 mL/min |
| Linear Velocity | 38.6 cm/sec |
| Purge Flow | 3.0 mL/min |
| Split Ratio | 200.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




