1. Introduction
The baby leaf is harvested earlier than traditionally for consumption, so the leaves are still young and not fully expanded. These vegetables are a rich source of health-promoting phytochemicals compared to their mature counterparts [
1]. The phytochemicals chlorophylls, phenolic compounds, carotenoids, ascorbate (vitamin C), and anthocyanins [
2], which are present in baby leaf kale, can potentially reduce the risk of chronic and degenerative diseases [
3].
Kale (Brassica oleracea var. sabellica L.) has a high antioxidant capacity among Brassica vegetables, and this is due to kale being a rich source of carotenoids and glucosinolates among green leafy vegetables [
4]. Environmental factors such as irradiance levels and temperature can strongly influence the accumulation of glucosinolates and plant pigments [
5,
6].
Light is one of these environmental factors that play an essential role in the development and biosynthesis of health-promoting compounds. The ideal light environment helps to increase photosynthesis, photomorphogenesis, and the growth of horticultural crops [
7,
8]. Plants can perceive light and respond to these stimuli through photoreceptors such as phytochromes and cryptochromes, generating various physiological responses [
9]. Most artificial lights for plants have photosynthetically active radiation ranging from 400 to 700 nm. Chlorophyll biosynthesis is regulated by blue light photoreceptors and phytochrome proteins that interact with red and extreme red light [
10,
11]. In a natural environment, in forests or understories, the leaves of taller plants absorb red wavelengths of light and allow blue to pass through to the lower leaves, which stimulates an increase in the amount of chlorophyll b in these taller plants. lowered or shaded [
12]
The management of light from solar radiation for plant development is a way of improving the conditions to which plants are subjected in the growing environment, so techniques such as the use of growing benches covered with reflective material aim to make better use of solar radiation. Growing benches with reflective materials aim to reflect part of the photosynthetically active radiation that reaches the benches onto the leaves, providing a greater amount of light energy and improving the use of this energy to conduct photosynthesis. Studies were carried out showing the effect of reflective benches on different species such as
Humulus lupulus [
8],
Solanum lycopersicum var.
cerasiforme [
13] and
Dipteryx alata [
14] with promising results.
Given the above, this study aimed to evaluate the growth and bioactive compounds production of baby leaf kale on colored reflective growing benches with different wavelengths to improve the production of biomass and bioactive compounds.
2. Materials and Methods
The experiments with baby leaf kale (Brassica oleracea var. sabellica L.) were conducted at the State University of Mato Grosso do Sul (UEMS), Unit of Cassilândia-MS, in two production cycles. The first cycle was from May 24 to June 29, 2022 (Cycle 1), and the second was from October 18 to November 17, 2022 (Cycle 2). The site is at latitude -19.1225º (19º07'21"S), longitude of -51.7208º (51º43'15" W), and an altitude of 516 m (CASSILANDIA-A742 automatic station).
The experiments were conducted in an agricultural greenhouse 18.0 m long x 8.0 m wide x 4.0 m high under the gutter (144 m2 area), covered with 150-micron low-density polyethylene (LDPE) film, light diffuser, anti-drip, with a zenith opening sealed with 50% white mesh, with side and front monofilament mesh with 50% shading. Underneath the LDPE film was a 50% shading aluminized thermo-reflective mobile screen, which was closed for 30 days in cycle 1 and opened for 15 days in cycle 2.
Inside the protected environment, colored laminated reflective materials (Fórmicas®) with different wavelengths were assessed on the growing benches in a completely randomized design, with five treatments and five replications: a control bench without reflective material, a bench with reflective bright white laminate, a bench with reflective bright yellow laminate, a bench with reflective bright red laminate, and a bench with reflective bright blue laminate. The colors of the growing benches were obtained with reflective bright Formica®.
The growing benches were 1.40 m wide x 3.50 m long x 0.80 m high, and each reflective material covered an area of 1.0 m x 1.2 m (1.20 m2). For the production of baby leaf kale, 1.0 L pots were used, containing substrate (Carolina Soil®). Three seeds were sown per pot. After emergence, the plants were thinned out (using scissors), leaving just one plant per pot. The seedlings were irrigated twice daily, morning and afternoon, when necessary.
When the baby leaves reached 13 to 16 cm in length, the following assessments were conducted: number of leaves (NL), plant height (PH), shoot fresh mass (SFM), shoot dry mass (SDM), root dry mass (RDM), total dry mass (TDM), and contents of chlorophyll a (CHA), chlorophyll b (CHB), total chlorophyll (CHT), and carotenoid (CRT). In the second production cycle, internal CO2 concentration (Ci), transpiration (E), stomatal conductance (gs), CO2 assimilation rate or net photosynthesis (A), water use efficiency (WUE), and instantaneous carboxylation efficiency (EiCi) were also assessed.
Plant heights (PH) were measured using a ruler, measuring the distance from the soil surface to the apex of the meristem stem, and the number of leaves was obtained by counting (NL). Shoot fresh mass (SFM), shoot dry mass (SDM), and root dry mass (RDM) were determined using an analytical balance with a precision of four decimal places. The masses were dried in an air-forced circulation oven at 65ºC for 72 hours.
Chlorophylls (a and b) and carotenoids were extracted following the methodology of Lichtenthaler [
15]. A sample of 0.5 g of fresh plant material was weighed, 5 mL of 80% acetone was added, and the material was stored in 14 mL test tubes for 48 hours in a refrigerator at 25 °C. After this period, the test tubes were centrifuged for 15 minutes at 4,000 rpm, and then the extract supernatant was diluted in a ratio of 0.3 mL of extract to 1.7 mL of 80% acetone. Measurements were made on a spectrophotometer at wavelengths of 470 nm, 647 nm, 653 nm, 663 nm, and 665 nm.
To determine internal CO2 concentration (Ci), transpiration (E), stomatal conductance (gs), and CO2 assimilation rate (A) or net photosynthesis, a portable infrared gas exchange meter (LCi, ADC Bioscientific, Hertfordshire, UK) was used at 9 a.m. (Amazon time - AMT). Subsequently, the water use efficiency (WUE) (ratio between net photosynthesis and transpiration) and the instantaneous carboxylation efficiency (EiCi) (ratio between net photosynthesis and internal intracellular CO2 concentration) were calculated.
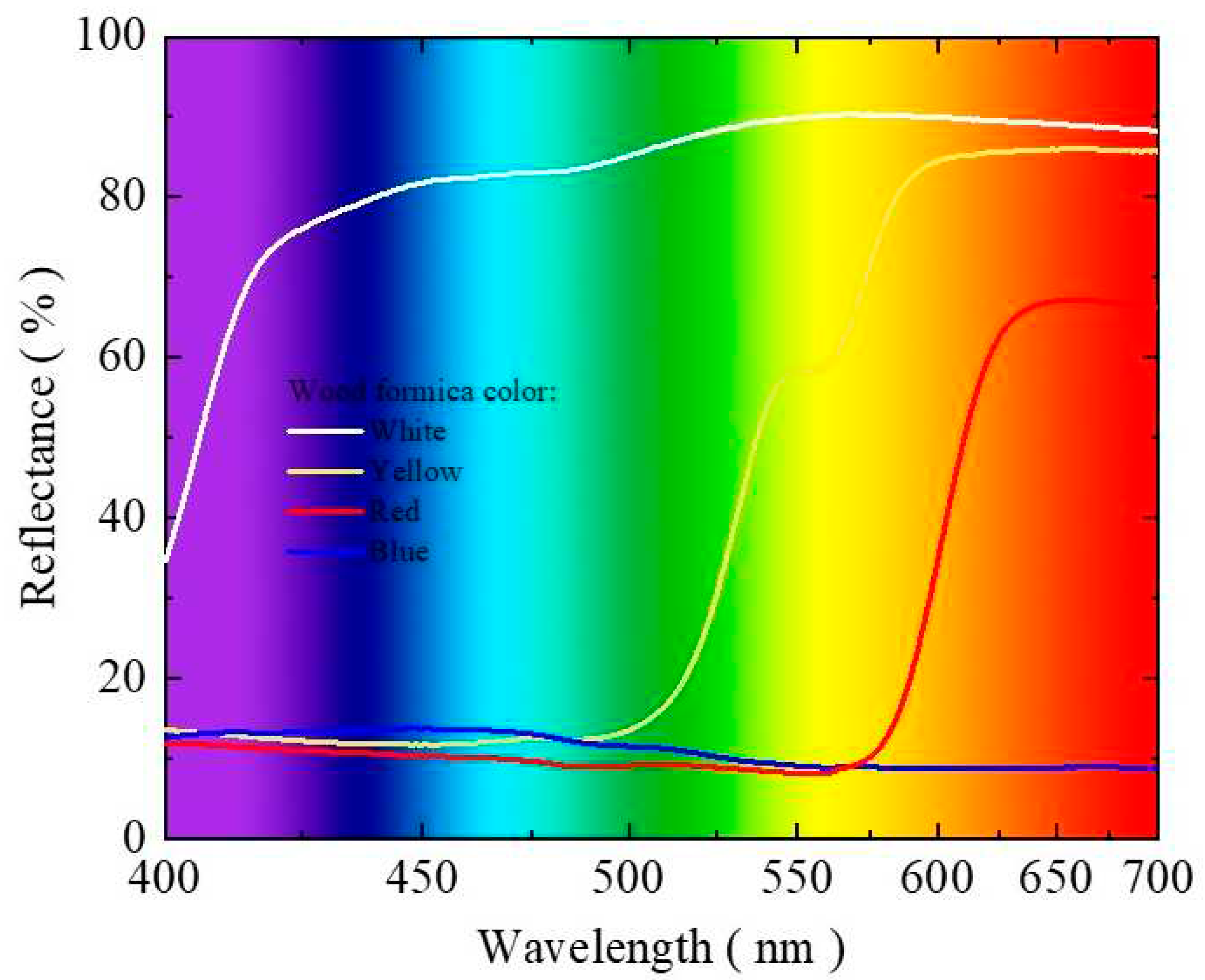
The reflected photosynthetically active radiation (RPA) (μmol m-² s-¹) of the growing benches was monitored with a portable digital pyranometer (Apogee®) every day at 9:30 a.m. (Amazon time - AMT). The RPA data was compared in a randomized block design with six replications of 7 days in cycle 1 and 5 days in cycle 2. The reflectance spectra of the colored laminate reflective materials (Formicas®) were obtained using a UV-Vis-NIR spectrophotometer (Model Lambda 1050, Perkin Elmer) with a step size of 1nm at 100nm/minute. Small disks of the laminates ( = 1cm) were inserted into the sample holder of a 150mm radius integrating sphere (
Figure 1).
The data were submitted to an analysis of variance, and the means were compared with the LSD test at 5% probability.
3. Results
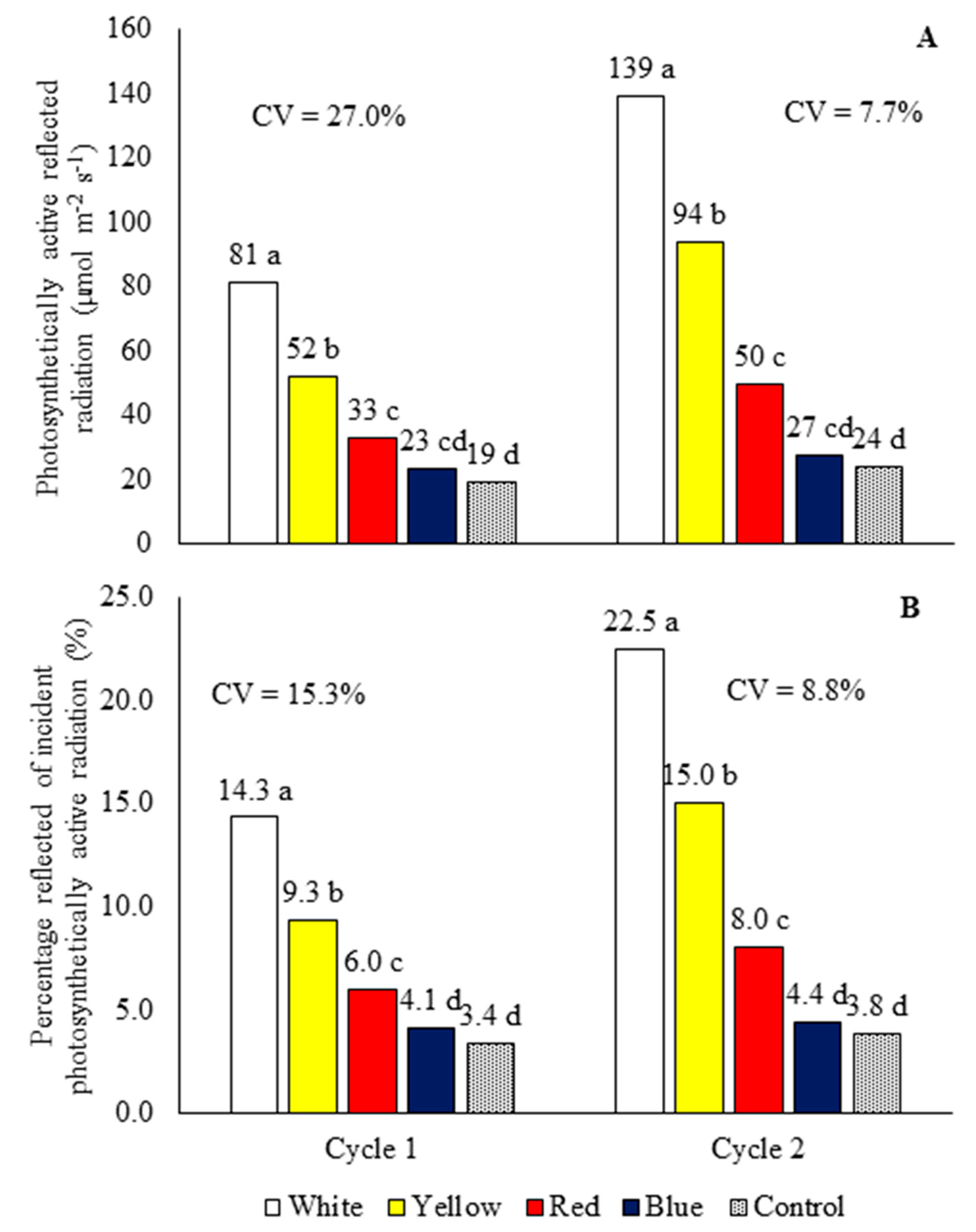
In cycle 1, the RPA under full sun conditions and incident internally in the protected environment were 1,524.41 and 585.9 µmol m
-2 s
-1, respectively. In cycle 2, the RPA under full sun conditions and incident internally were 2,014.83 and 624.79 µmol m-
2 s
-1, respectively. For both cycles, the growing benches covered with white, yellow, and red bright laminates reflected more photosynthetically active radiation than the control (
Figure 2A and 2B). The growing bench covered with bright white laminate had the highest reflectance. The bench covered with bright white laminate increased the internal radiation incident in the protected environment by 14.3% in cycle 1 and 22.5% in cycle 2 (
Figure 2B).
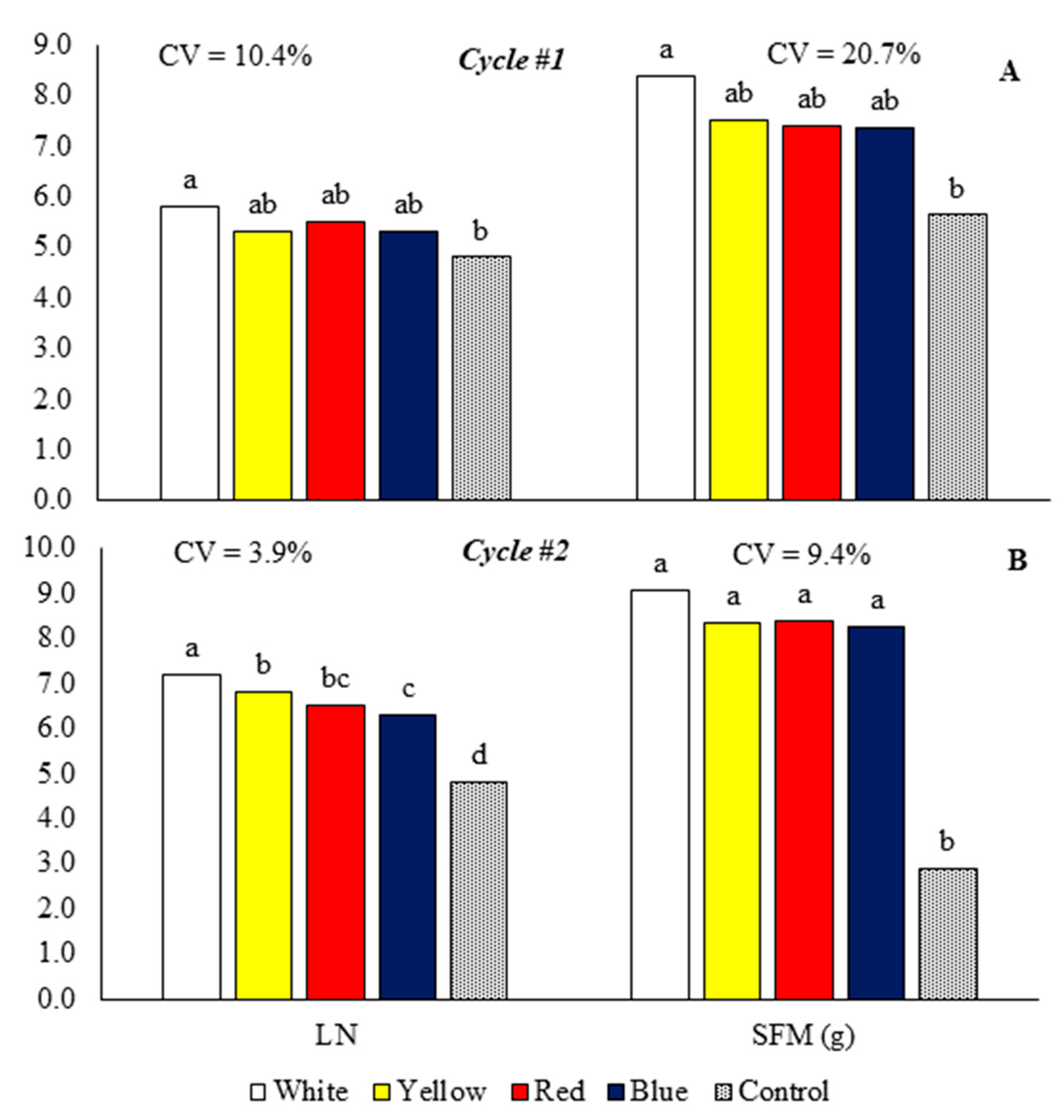
Regarding the number of leaves in cycle 1, the growing bench with reflective bright laminate provided more leaves than the control growing bench (
Figure 3A). In cycle 2, the number of leaves was higher on the bench covered with reflective bright white laminate when compared to the other benches and the control bench (
Figure 3B). For both cycles, the shoot fresh mass was higher in the benches with reflective bright laminate (
Figure 3A and 3B).
In cycle 1, the growing benches with white, yellow, red, and blue laminate increased the number of leaves by 20.8, 10.4, 14.6, and 10.4%, respectively (
Figure 3A). In cycle 2, the growing benches with white, yellow, red, and blue laminate increased the number of leaves by 50.0, 41.7, 35.4, and 31.3%, respectively (
Figure 3B).
In cycle 1, the growing benches covered with white, yellow, red, and blue laminate increased shoot fresh mass by 48.8, 33.3, 31.2, and 30.4%, respectively (
Figure 3A). In cycle 2, the growing benches with white, yellow, red, and blue laminate increased shoot fresh mass by 215.4, 190.3, 191.4, and 187.5%, respectively (
Figure 3B).
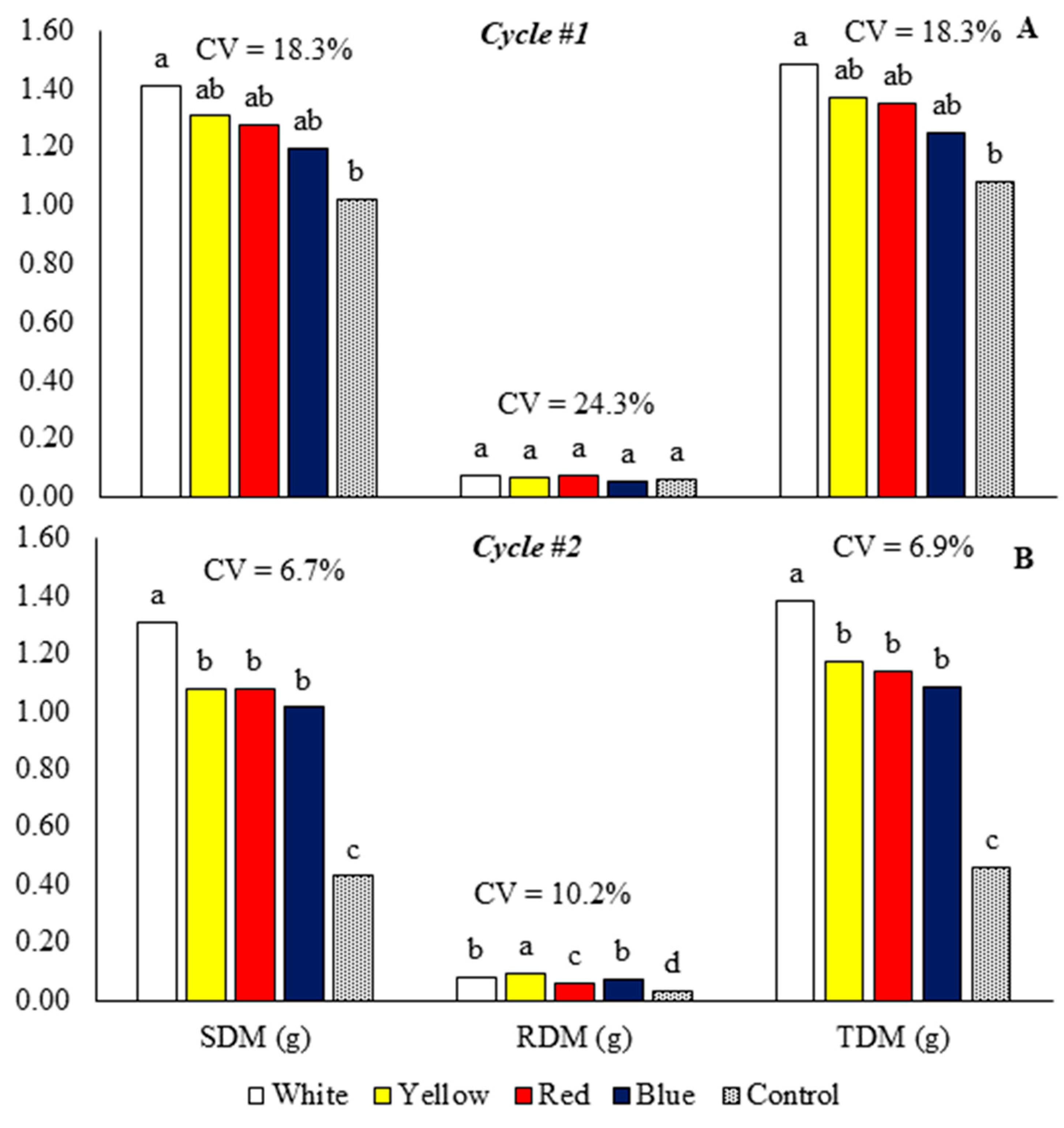
In cycle 1, the growing bench with reflective white laminate increased the shoot and total dry mass, which did not differ significantly from the yellow, blue, and red laminates (
Figure 4A). In cycle 2, the white laminate obtained plants with higher shoot and total dry mass than benches with blue, red, and yellow laminates and the control bench (
Figure 4B).
In cycle 1, the benches with reflective laminates did not differ significantly from the control for the root dry mass variable (
Figure 4A). In cycle 2, the plants on the bench with yellow laminate had higher root dry mass than the benches with blue, white, and red laminates and the control bench (
Figure 4B).
In cycle 1, the benches with white, yellow, red, and blue laminate increased the total dry mass by 37.7, 26.8, 24.8, and 15.4%, respectively (
Figure 4A). In cycle 2, the white, yellow, red, and blue laminate benches increased the total dry mass by 200, 154.3, 146.2, and 135.2%, respectively (
Figure 4B).
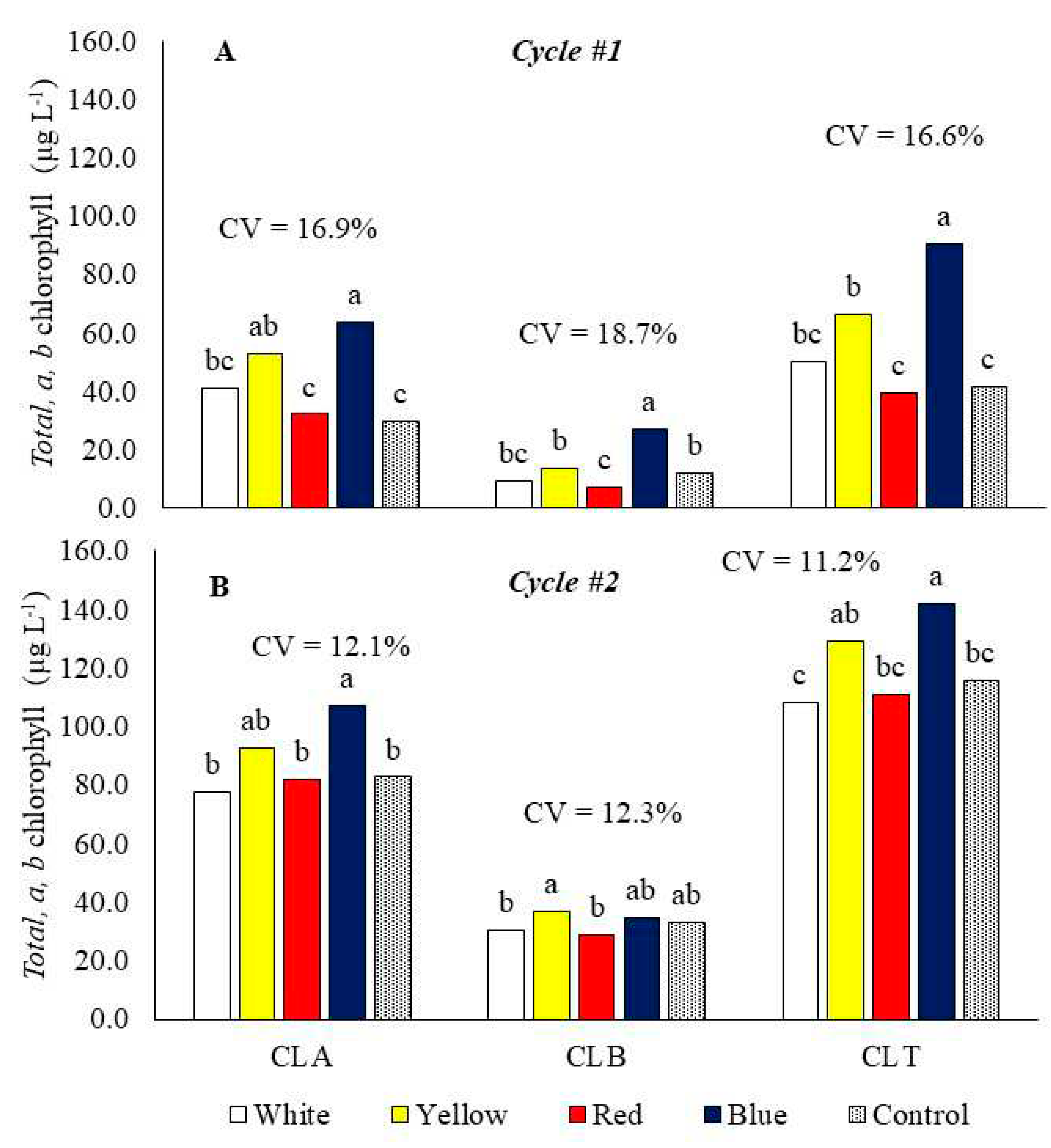
In cycle 1, benches with blue and yellow laminate increased the chlorophyll a content in the plants compared to red laminate and the control (
Figure 5A). In cycle 2, the growing benches with blue and yellow laminate led to increased chlorophyll a content in the plants compared to those with red laminate, white laminate, and the control (
Figure 5B).
In cycle 1, the chlorophyll a content was lower than in cycle 2, however, in cycle 1, the blue reflective bench provided increases of 35.1%, 49.4% and 53.3% compared to the white, red and control benches, respectively (
Figure 5A), and in cycle 2 this increase was 27.8%, 23.6 and 23.0% (
Figure 5B). Chlorophyll b levels were also higher in cycle 2, however, although numerically they were similar when the cabbage was produced on the blue reflective bench, in both cycles with values of 27 µg L
-1 in cycle 1 and 34 µg L
-1 for cycle 2. The increase in chlorophyll b of plants on the blue bench in cycle 1 was 66.2%, 73.8%, 56.3% and 49.2% compared to the white, red, control and yellow benches, respectively (
Figure 5A), and did not vary significantly in cycle 2 between the different benches.
In cycle 1, the growing bench with blue laminate led to an increase in the total chlorophyll content of the plants (
Figure 5A). In cycle 2, the bench with blue and yellow laminate led to a higher total chlorophyll content in the baby leaf kale than in red and white laminates and the control (
Figure 5B).
Therefore, in cycle 1, CHA, CHB, and CHT were increased by 114, 129, and 118% by the bench with blue laminate, respectively (
Figure 4A). In cycle 2, CHA, CHB, and CHT were increased by 30, 4.4, and 22.6% in the bench with blue laminate, respectively (
Figure 5B), when compared to control.
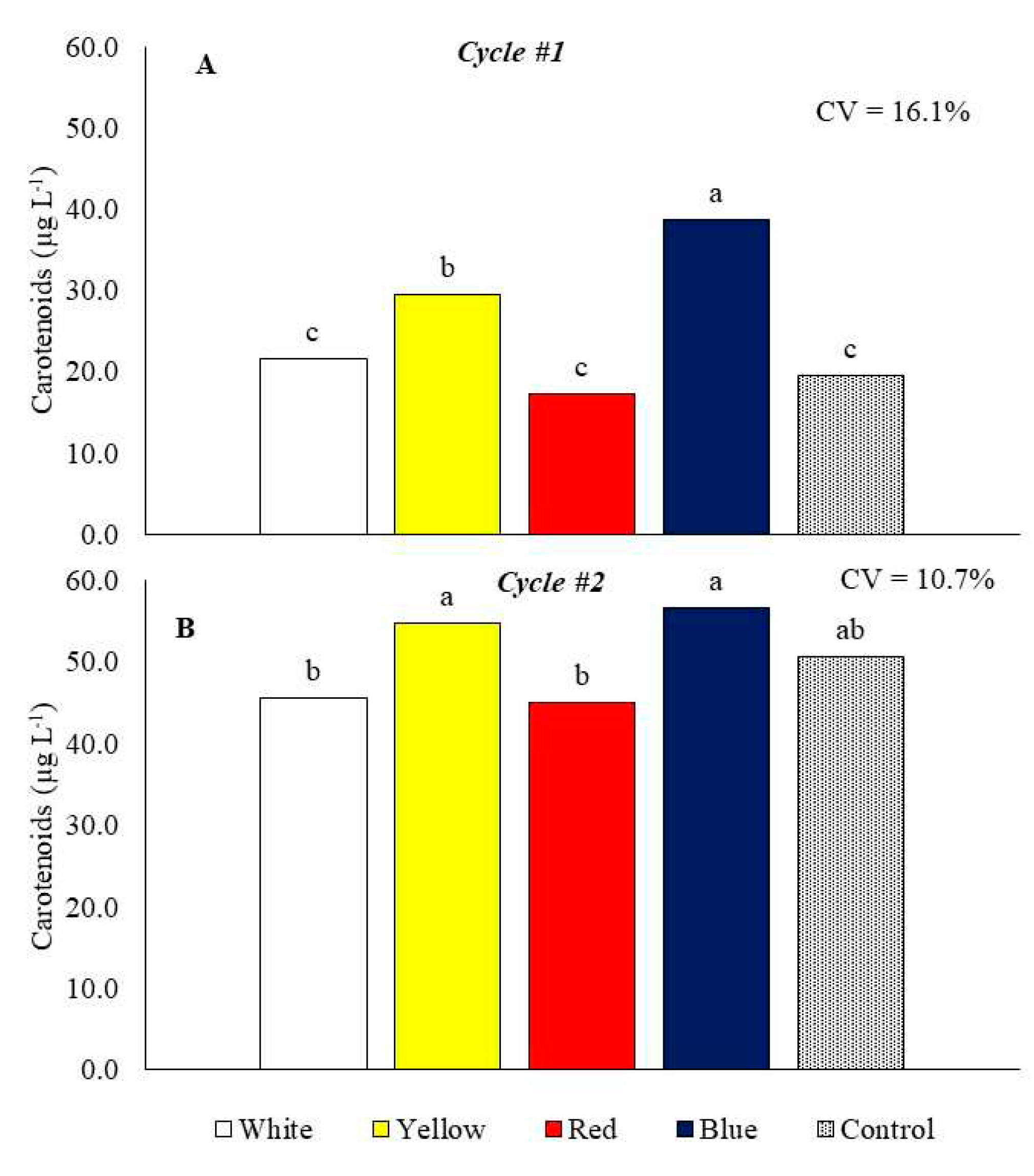
In cycle 1, the highest carotenoid content in the plants was found in the growing bench with blue laminate (
Figure 6A). In cycle 2 there were no significant differences between the benches with blue, yellow and control laminates for the carotenoid content in the plants (
Figure 6B). The growing bench with blue laminate increased carotenoids by 98.2% in cycle 1 and 11.7% in cycle 2 concerning control (
Figures 6A and 6B). As for chlorophylls, plants produced more carotenoids in cycle 2 when compared to cycle 1, and there was an increase on average of 54.9%.
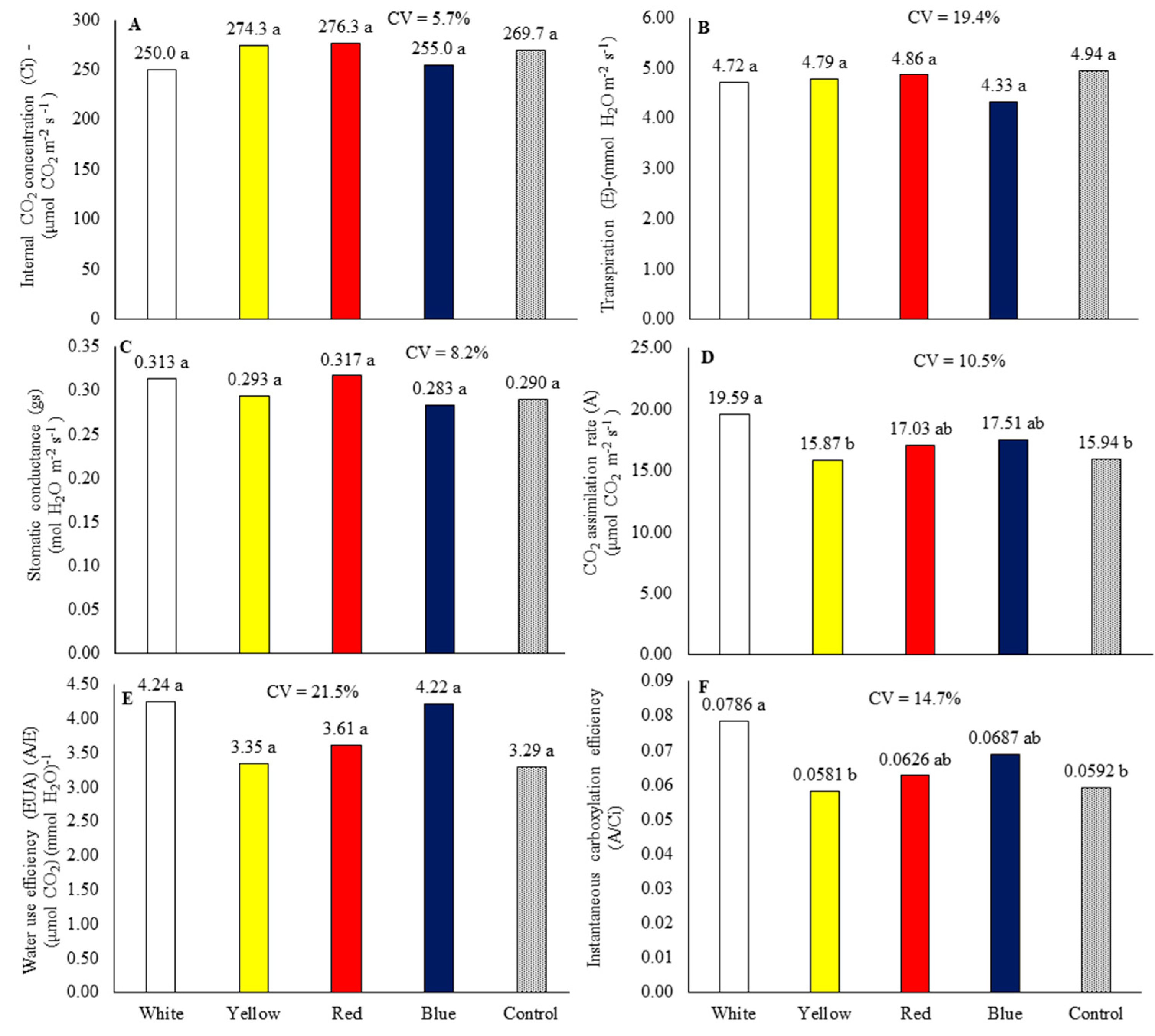
In cycle 2, the intracellular CO2 content (ci) (
Figure 7A), transpiration (E) (
Figure 7B), stomatal conductance (gs) (
Figure 7C), and water use efficiency (WUE) (
Figure 7E) were not influenced by the growing benches. The use of a growing bench with white laminate provided the highest net photosynthesis rate (A) (
Figure 7D) and carboxylation efficiency (EiCi) (
Figure 7F).
4. Discussion
The incidence of photosynthetically active radiation is the micrometeorological factor that most influences plant growth inside protected environments [
16], and the reflectance of the light generated by the growing benches with white reflective material inside the protected environment favored the growth, and photosynthetic activity of baby leaf kale, although the characteristics evaluated showed some different responses depending on the color of the bench.
The results observed in this work confirm the hypothesis of the beneficial effect of reflective benches in increasing the growth and biomass of baby leaf cabbage and also prove the effect of blue reflective benches on the increase in chlorophylls and carotenoids. However, the results observed contradict the hypothesis that colored benches would increase photosynthetic efficiency, since only the white bench provided significantly greater results for the characteristics of photosynthetic activities and intrinsic efficiency of carboxylation, while the results of plants in -more colored benches did not vary in relation to the control bench.
The use of white laminate increased the reflectance of photosynthetically active radiation, providing an increase in shoot fresh mass, shoot dry mass, root dry mass, and total dry mass, related to a greater intrinsic CO
2 carboxylation and assimilation activity, which positively influenced the development of the baby leaf kale. The responses of different species to reflective benches may vary. Thus, it is noteworthy that the literature comments that the use of reflective material on the growing benches increases the photosynthetically active radiation, making it possible to increase photosynthesis in papaya seedlings [
17], however, for baby leaf kale these results were only significant on the white bench favoring the development and quality of baby leaf kale.
However, the results observed in plants grown on a yellow reflective bench, although the efficiency of photosynthetic activity was not maintained at the same level as the red or blue benches, the dry and fresh masses of leaves were similar. Considering that the yellow wavelength has little influence on the excitation/absorption of photosynthetic pigments, these results suggest that the light availability was sufficient to maintain the energy needs of baby leaf kale.
The use of colored growing benches made it possible to increase the number of leaves, even reflecting part of the photosynthetically active radiation that reaches the growing benches to the leaves. The wavelengths provided in the reflection were efficient in activating the photosynthetic pigments components of photosystems I and II (chlorophylls and carotenoids), providing sufficient light energy for photophosphorylation and production of ATP and NADPH2 necessary for the next stage of photosynthesis, with a production of photoassimilates for accumulation of leaf dry mass and bioactive compounds. Furthermore, the reflection of the colored sheets provides greater availability of light energy and an increase in the number of leaves of baby leaf kale, improving their use for photosynthesis [
16].
The biosynthesis of pigments was influenced by the quality and intensity of the light emitted by the growing benches since the photosynthetic process showed the greatest efficiency, especially when a light source with a shorter spectrum of wavelengths was adopted [
18].
The reflection of blue light from the growing bench onto the baby leaf kale corresponded to an increase in the production of chlorophyll a, b, and total because blue light photoreceptors regulate chlorophyll biosynthesis, as well as phytochrome proteins that relate to red light [
10,
11]. The use of blue laminate in the growing bench is interesting due to the wavelength of blue light, which is related to the photosynthetic process since chlorophylls absorb light mainly in the red and blue range of the visible spectrum [
19].
It is interesting to note that baby leaf kale reacted differently to blue light depending on the radiation intensity. Under greater radiation, as observed in Cycle 2 cultivation, chlorophyll a had a significant increase, different from the result observed for chlorophyll b, however, under less radiation, as observed in cycle 1, chlorophyll b showed a proportionally greater increase when compared to the increase in chlorophyll a, considering that when calculating the chlorophyll a/b ratio in the first cycle, an average of 2.4 was observed, while in cycle 2 the average was 3.1. The literature comments that plants under less radiation, evaluated in the understory or seedlings grown under artificial shading, tend to receive a much greater proportion of wavelengths in the blue range and this leads to a pronounced increase in chlorophyll b [
12,
20].
The growing bench with blue laminate stimulated the production of chlorophyll a, b, total, and carotenoids in baby leaf kale because radiation with shorter wavelengths, such as blue and ultraviolet light, stimulates the production of pigments that absorb light and influence leaf color, such as chlorophylls and carotenoids [
21]. In addition, in some leafy crops, such as lettuce, blue light also increases the production of health-promoting compounds such as antioxidants (phenolic acids, carotenoids, flavonoids, anthocyanins) and vitamins [
22].
In summary, we have the growing bench with reflective bright white laminate was the bench that most amplified the photosynthetically active radiation, and although white laminate increased CO2 assimilation (A), intrinsic carboxylation efficiency (A/Ci) and the number of leaves, this increase was not accompanied by an increase in photosynthetic pigments (chlorophylls and carotenoids). Therefore, it is believed that baby leaf cabbage can be quite productive in terms of carotenoids even with lower photosynthesis, as was observed in plants grown on the yellow and blue reflective benches.
The cultivation bench with laminated reflective material increased the growth of baby leaf kale. Thus, on average, in the two cultivation cycles, the benches with white, yellow, red and blue laminates increased the number of leaves by 35.5, 26, 25 and 20.8% and the fresh mass of the aerial part increased by 132, 112, 112 and 109%, respectively to plants grown on benches without laminates (control). Likewise, evaluating the two cultivation cycles, the cultivation bench with bright blue reflective laminate increased photosynthetic and bioactive pigments in kale by 72, 67 and 70%, 55%. Chlorophyll a, b, total and carotenoids respectively.
It is worth noting that cultivation on a reflective bench regardless of color in periods of lower radiation or on a white bench in times of higher radiation increases the number of leaves, this increase increases productivity per unit of seedling. However, if the producer's focus is production with an interest in bioactive compounds such as carotenoids, among others that were not evaluated in this work, the choice of countertop color may vary depending on the radiation intensity. Thus, cultivation in times of lower radiation as characterized in this work by Cycle 1, cultivation is favored on a blue reflective bench, but if cultivation occurs in times of higher radiation, the blue or yellow bench would provide similar results.
The use of reflective materials is an alternative method that provides plants with better conditions for assimilating photosynthetically active radiation, increasing the development, yield, and production of bioactive compounds that promote human health.
Author Contributions
Conceptualization: Dantas T, Araújo TAN, Costa E, Vendruscolo EP, Binotti FFS. Data curation: Dantas T, Araújo TAN, Vendruscolo EP, Binotti FFS, Scalon SPQ. Formal analysis: Dantas T, Araújo TAN, Costa E, Lima SM, Andrade LHC. Funding acquisition: Costa E, Vieira GHC, Binotti FFS, Martins MB, Lima SM, Andrade LHC, Scalon SPQ. Investigation: Dantas T, Araújo TAN, Vendruscolo EP. Methodology: Dantas T, Costa E, Vendruscolo EP, Binotti FFS. Project administration: Dantas T, Costa E, Vieira GHC. Resources: Costa E, Vieira GHC, Binotti FFS, Martins MB, Lima SM, Andrade LHC, Scalon SPQ. Supervision: Costa E, Vendruscolo EP, Binotti FFS. Writing-original draft: Dantas T, Araújo TAN. Writing-review & editing: Dantas T, Costa E, Vendruscolo EP, Binotti FFS, Martins MB, Lima SM, Andrade LHC, Scalon SPQ.
Acknowledgments
To the Foundation for Supporting the Development of Education, Science and Technology of the State of Mato Grosso do Sul – FUNDECT (FUNDECT/CNPq/PRONEM – MS, Process n. 59/300.116/2015 – Nº FUNDECT 080/2015), and to the National Council for Scientific and Technological Development (CNPq), and the Higher Education Personnel Improvement Coordination (CAPES).
Conflicts of Interest
“The authors declare no conflict of interest.”
References
- Xiao, Z.; Lester, G. E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. Journal of Agricultural and Food Chemistry 2012, 60(31): 7644-7651. [CrossRef]
- Xiao, Z.; Rausch, S.R.; Luo, Y.; Sun, J.; Yu, L.; Wang, Q.; Chen, P.; Yu, L.; Stommel, J.R. Microgreens of Brassicaceae: Genetic diversity of phytochemical concentrations and antioxidant capacity. LWT 2019, 101: 731-737. [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20 (12): 21138-21156. [CrossRef]
- Nornberg, M.L.; Pinheiro, P.N.; Nascimento, T.C.; Fernandes, A.S.; Jacob-Lopes, E.; Zepka, L.Q. Carotenoids profile of Desertifilum spp. in mixotrophic conditions. Brazilian Journal of Development 2021 7(3): 33017-33029. [CrossRef]
- Stoewsand, G.S. 1995. Bioactive organosulfur phytochemicals in Brassica oleracea vegetables: A review. Food and Chemical Toxicology 1995 33 (6): 537–543. [CrossRef]
- Lefsrud, M.G.; Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Irradiance levels affect growth parameters and carotenoid pigments in kale and spinach grown in a controlled environment. Physiologia Planatarum 2006 127(4): 624-631. [CrossRef]
- Kang, J.H.; Yoon, H.I.; Kim, J.; Ahn, T.I.; Son, J.E. Ray-tracing analysis on the far-red induced light-capturing ability of kale. Scientia Horticulturae 2023 311: 111806. [CrossRef]
- Silva , J.B.M.; Vendruscolo, E.P.; Bastos, F.E.A.; Binotti, F.F.S.; Rodrigues Sant’ Ana, G.; Costa, E. Does light supplementation improve the initial growth of hops seedlings in a protected environment? Revista de Agricultura Neotropical 2023, 10(3), e7401. [CrossRef]
- Muneer, S.; Kim, E.J.; Park, J S.; Lee, J.H. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). International Journal of Molecular Sciences 2014, 15(3): 4657- 4670. [CrossRef]
- Liu, X.; Li, Y.; Zhong, S. Interplay between light and plant hormones in the control of Arabidopsis seedling chlorophyll biosynthesis. Frontiers in Plant Science 2017 8:1433. [CrossRef]
- Zheng, L.; He, H.; Song, W. Application of light-emitting diodes and the effect of light quality on horticultural crops: A review. HortScience 2019, 54 (10): 1656-1661. [CrossRef]
- Dou, Haijie; Niu, Genhua; Gu, Mengmeng. Photosynthesis, morphology, yield, and phytochemical accumulation in basil plants influenced by substituting green light for partial red and/or blue light. Hort Science 2019, 54(10): 1769-1776. [CrossRef]
- Costa, E.; Lopes, T. C.; Da Silva, A. G.; Zoz, T.; Salles, J. S.; De Lima, A. H. F.; Binotti, F. F. S.; Da Costa Vieira, G. H. Reflective material in the formation of Dipteryx alata seedlings. Research, Society and Development 2020, 9(8): e430985428-e430985428. [CrossRef]
- Campos, R. S. D.; Costa, E.; Cavalcante, D. F.; Freitas, R. A.; Binotti, F. F. D. S. Ornamental cherry tomatoes in different protected environments and reflector materials in cultivation Bench. Revista Caatinga 2023, 36: 09-20. [CrossRef]
- Lichtenthaler, H. K. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In: PACKER, L., DOUCE, R. (Eds.). Methods in Enzymology. Bad Honnef: Academic, 148: 350-382, 1987. [CrossRef]
- Costa, E.; Silva, B. L.; Aguiar, F.K.; Pereira, T.C.; Binotti, F.F.S. Use of benches with reflective material to favor production of rubber tree rootstock seedlings. Engenharia Agrícola 2021, 41(4): 409-417. [CrossRef]
- Cabral, R.C.; Vendruscolo, E.P.; Martins, M.B.; Zoz, T.; Costa, E.; Silva, A.G. Reflective material on cultivation benches and rice strawover the substratein papaya seedlingproduction. Revista Mexicana de Ciencias Agrícolas 2020, 11(8): 1713-1723, 2020. https://cienciasagricolas.inifap.gob.mx/index.php/agricolas/article/view/2481/3628 (pdf English), (. [CrossRef]
- Gupta, S. D.; Karmakar, A. Machine vision based evaluation of impact of light emitting diodes (LEDs) on shoot regeneration and the effect of spectral quality on phenolic content and antioxidant capacity in Swertia chirata. Journal of Photochemistry and Photobiology B: Biology 2017, 174:162-172. [CrossRef]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Plant Physiology and Development. 6a ed. Sun- derland: Sinauer Associates. 2014. 700p.
- Hooks, T.; Masabni, J.; Sun, L.; Niu, G. Effect of pre-harvest supplemental UV-A/Blue and Red/Blue LED lighting on lettuce growth and nutritional quality. Horticulturae 2021, 7(4): 80. [CrossRef]
- Ohtake, N.; Ishikura, M.; Suzuki, H.; Yamori, W.; Goto, E. Continuous irradiation with alternating red and blue light enhances plant growth while keeping nutritional quality in lettuce. HortScience 2018, 53(12): 1804-1809. [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; Giorgio Gianquinto, G.; Marcelis, L.F.M. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red: blue ratio provided by LED lighting. Scientific Reports 2019, 9 (1): 14127. [CrossRef]
Figure 1.
Reflectance of the laminates used on the cultivation benches for growing and production of baby leaf kale. Source: Dr. Sandro Marcio Lima; Dr. Luis Humberto da Cunha Andrade.
Figure 1.
Reflectance of the laminates used on the cultivation benches for growing and production of baby leaf kale. Source: Dr. Sandro Marcio Lima; Dr. Luis Humberto da Cunha Andrade.
Figure 2.
Reflected photosynthetically active radiation (µmol m-2 s-1) (A) and percentage of internal photosynthetically active radiation reflected (B) by baby leaf kale growing benches in two production cycles. Cycle 1: May 23 to July 5, 2022 (A), Cycle 2: October 18 to November 17, 2022 (B). CV = coefficient of variation. Bars with the same letter do not differ by the LSD test.
Figure 2.
Reflected photosynthetically active radiation (µmol m-2 s-1) (A) and percentage of internal photosynthetically active radiation reflected (B) by baby leaf kale growing benches in two production cycles. Cycle 1: May 23 to July 5, 2022 (A), Cycle 2: October 18 to November 17, 2022 (B). CV = coefficient of variation. Bars with the same letter do not differ by the LSD test.
Figure 3.
Leaf number (LN) and shoot fresh mass (SFM) of baby leaf kale on different reflective colored growing benches in two production cycles. Cycle 1: May 23 to July 5, 2022 (A), Cycle 2: October 18 to November 17, 2022 (B). CV = coefficient of variation. Bars with the same letter do not differ by the LSD test.
Figure 3.
Leaf number (LN) and shoot fresh mass (SFM) of baby leaf kale on different reflective colored growing benches in two production cycles. Cycle 1: May 23 to July 5, 2022 (A), Cycle 2: October 18 to November 17, 2022 (B). CV = coefficient of variation. Bars with the same letter do not differ by the LSD test.
Figure 4.
Shoot dry mass (ADM), root dry mass (RDM), and total dry mass (TDM) of baby leaf kale on different reflective colored growing benches in two production cycles. Cycle 1: May 23 to July 5, 2022 (A), Cycle 2: October 18 to November 17, 2022 (B). CV = coefficient of variation. Bars with the same letter do not differ by the LSD test.
Figure 4.
Shoot dry mass (ADM), root dry mass (RDM), and total dry mass (TDM) of baby leaf kale on different reflective colored growing benches in two production cycles. Cycle 1: May 23 to July 5, 2022 (A), Cycle 2: October 18 to November 17, 2022 (B). CV = coefficient of variation. Bars with the same letter do not differ by the LSD test.
Figure 5.
Chlorophyll a (CHA), chlorophyll b (CHB), and total chlorophyll (CHT) of baby leaf kale on different reflective colored growing benches in two production cycles. Cycle 1: May 23 to July 5, 2022 (A), Cycle 2: October 18 to November 17, 2022 (B). CV = coefficient of variation. Bars with the same letter do not differ by the LSD test.
Figure 5.
Chlorophyll a (CHA), chlorophyll b (CHB), and total chlorophyll (CHT) of baby leaf kale on different reflective colored growing benches in two production cycles. Cycle 1: May 23 to July 5, 2022 (A), Cycle 2: October 18 to November 17, 2022 (B). CV = coefficient of variation. Bars with the same letter do not differ by the LSD test.
Figure 6.
Carotenoids of baby leaf kale on different reflective colored growing benches in two production cycles. Cycle 1: May 23 to July 5, 2022 (A), Cycle 2: October 18 to November 17, 2022 (B). CV = coefficient of variation. Bars with the same letter do not differ by the LSD test.
Figure 6.
Carotenoids of baby leaf kale on different reflective colored growing benches in two production cycles. Cycle 1: May 23 to July 5, 2022 (A), Cycle 2: October 18 to November 17, 2022 (B). CV = coefficient of variation. Bars with the same letter do not differ by the LSD test.
Figure 7.
Internal CO2 concentration (A), transpiration (B), stomatal conductance (C), CO2 assimilation rate (D), water use efficiency (E), and instantaneous carboxylation efficiency (F) of baby leaf kale on different reflective colored growing benches in the second production cycle (October 18 to November 17, 2022). CV = coefficient of variation. Bars with the same letter do not differ by the LSD test.
Figure 7.
Internal CO2 concentration (A), transpiration (B), stomatal conductance (C), CO2 assimilation rate (D), water use efficiency (E), and instantaneous carboxylation efficiency (F) of baby leaf kale on different reflective colored growing benches in the second production cycle (October 18 to November 17, 2022). CV = coefficient of variation. Bars with the same letter do not differ by the LSD test.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).