1. Introduction
A high demand for silver-containing products in different areas of industry such as medicine, pharmaceutics, light, food and cosmetic industry requires the need for carrying out thorough toxiс and hygienic characterization of such a production. For instance, silver nanoparticles are frequently used today as wound dressing coatings for purulent surgery, packing materials for food storage, cosmetics, pharmaceutics, [1–4] etc. Silver containing pharmaceutics applied in Russia are Protargol™ for infectious disease treatment, Argosulfan™ ointment for external use to suppress inflammation, silver sulfadiazine cream for burn wound treating, polyvinylpyrrolidone coated silver nanoparticles Argovit™, etc. The high demand for silver compounds is due to their pronounced antibacterial, antiviral and fungicidal action [1,5]. Besides, silver compounds are able to strengthen the antibiotic action when combined.
Studies in the area of toxicity and biokinetics of silver nanoparticles received significant wide-spread for the last 20 years. Apparently, this is due to Nanotechnology initiative support by different world countries. It is believed that nanoparticles possess different biological properties compared to the substance in the bulk, ionic and molecular forms that determines involvement of different mechanisms of interactions with biological cells [6]. Presumably, such differences are due to the number of the atoms increase on the surface compared to the volume with the particle size decrease and subsequent increase of its chemical activity.
Nowadays, in general, despite the relative novelty of Nanotechnology, silver nanoparticles in the sense of their biological effects are characterized in more details compared to silver ionic form. Several works describe biokinetics and study toxic effects of silver nanoparticles and just a few compares them with its salts. For example, [7] demonstrates higher accumulation of AgNo3 in the blood and differently coated silver nanoparticles sized <20 nm and <15 nm in testes and spleen after 28-day oral exposure into laboratory rat organisms. The main target organs for AgNo3 silver nanoparticles are liver, spleen, testes, kidneys, brain and lungs. Herewith, particles of silver were observed in the tissues after exposure to 14 nm sized silver nanoparticles as well as to AgNo3. Similar profiles of silver accumulation after exposure to polyvinylpyrrolidone coated silver nanoparticles and silver acetate to laboratory rats were shown in [8]. Silver was observed in intestines, liver, kidneys, lungs and brain. However, salt form was more intensively accumulated in comparison to the nanoparticles, which were actively eliminated with feces. Granules of silver and its compounds with sulfur and selenium were observed in ileum after the exposure to the nanoparticles as well as to the salt.
Kinetics of accumulation of 34 nm sized polyvinylpyrrolidone coated silver nanoparticles in laboratory mice organs, brain departments and blood of mice during their oral administration for 30, 60, 120 and 180 days is described in [9]. It was shown that within those regimes silver is mostly accumulated in the testes, brain and lungs of the animals. The clearance of different tissues of laboratory rats after 28-day exposure to 10 nm and 25 nm sized silver nanoparticles was studied in [10]. Low levels of clearance of silver from the testes and brain was shown for the particles of both sizes. Acute intravenous exposure to 15 nm silver nanoparticles of laboratory rats resulted in higher accumulation of silver in the spleen, liver, lungs and kidneys. Herewith, the lowest clearance was observed in the spleen [11]. An extremely low clearance from the brain (~5%) in comparison to liver (~75%) and blood (~80%) after 2-month daily oral exposure to 34 nm sized polyvinylpyrrolidone coated silver nanoparticles and 1 month of washing out was observed in laboratory mice in [12]. We studied age differences influencing accumulation levels of silver in organs of laboratory mice at the 60-day oral exposure to 8.7 nm sized polyvinylpyrrolidone coated silver nanoparticles [13]. Accumulation of silver was observed in all the studied organs of the exposed animals. Herewith, silver accumulation was higher in the testes of elder animals compared to the younger ones. Significant differences of silver accumulation in other organs were not detected. The lowest concentrations of silver were observed in the heart, spleen and kidneys of the experimental animals. Accumulation of silver in the internal organs and its transfer across the placenta from mother to offspring at the oral exposure of dams to 55 nm sized silver nanoparticles in different dosages and AgNo3 during gestation for 14 days were studied in [14]. A dose dependent accumulation of silver was found in the spleen, kidneys, uterus, blood plasma and erythrocytes of the dams. Silver administered as AgNo3 accumulated in higher levels compared to the nanoparticles, in general, and especially in the heart and blood plasma of the dams. The highest concentrations of silver among the pups were observed in the kidneys after their maternal exposure to the nanoparticles and in the lungs after the maternal exposure to AgNo3. Increase of silver concentrations was observed in the blood plasma of the offspring after AgNo3 exposure to the dams compared to silver nanoparticle exposure. The tendency to silver concentration increase after the AgNo3 exposure found in the dam tissues was not observed in other studied pup tissues. Thus, the levels of penetrability of the placental barrier were close to each other for the nanoparticle and ionic forms of silver, which does not correspond to the blood-testes [1,7] and blood-brain [10,14] barriers. The studies let us preliminary conclude that main target organs vary on the period of exposure to silver nanoparticles. Thus, the main targeted organs for the acute exposure to silver nanoparticles are spleen, liver and kidneys while testes, lungs and brain are the targeted organs for long-term periods of exposure to them. It may be concluded that the penetrability of blood-organ barriers varies with time.
It is rather intriguing that granules of silver are observed in the tissues after oral exposure to both forms of silver, nanoparticle and salt [7,8]. It should also be noted that after the exposure of pregnant dams to silver nanoparticles and AgNo3 the levels of penetrability of the placental barrier seemed to be close to each other despite the higher level of silver accumulation in the maternal tissues within the AgNo3 exposure [14]. In this case, it is appropriate to remind the technology of green synthesis when using plants, bacteria, yeast and algae silver nanoparticles are produced from its salts applied as precursor [15,16]. Various active substances in the plants, bacteria, yeast and algae act as reducing and capping agents then. Taking into the account the results of [7,8,14] it might be suggested that an animal organism serves as a reactor to synthesize nanoparticles from salts. Anyway, it is highly likely that metal nanoparticles associate and dissociate in an organism depending on the properties of medium such as pH.
A significant number of researches study silver nanoparticle and silver salt toxicity. Oxidative stress markers were significantly increased in dam rats and their pups after the exposure of dams to AgNo3, while exposure of the dams to silver nanoparticles did not significantly change their level [14]. A hippocampal sclerosis was observed in dams exposed to silver nanoparticles and AgNo3 at the same time. Hepatotoxicity was not observed in [7], nor in [14]. A decrease of the number of microglial cells in the brain of the offspring of the dams exposed to silver nanoparticles during gestation in comparison to control ones was observed in [17]. As a rule, salt form of silver demonstrated more pronounced toxicity compared to the nanoparticles [1]. It is frequently supposed that the main mechanism of silver nanoparticle and silver salt toxic action is the oxidative stress induced by an increased generation of reactive oxygen species [1,17–19].
Due to the silver nanoparticle and ionic silver tropism to the brain manifested in the high accumulation of silver in it [7,10] and low clearance of silver from the brain [10,12] as well as the ability of silver nanoparticles [19,21] and silver salts [18,20] to induce oxidative stress the studies on the neurotoxicity of silver compounds receive a special importance. There are works demonstrating the influence of silver nanoparticles on the behavioral and cognitive functions of laboratory mammals. Negative adverse effects [22–25] as well as improvement of behavioral functions [26,27] at silver nanoparticle exposure are shown. For example, a memory impairment, learning and motor function as well as social behavior change were observed after intravenous exposure of mice to silver nanoparticles in [22]. Significant decreases of long-term and short-term memory were found in rats orally exposed to 20 nm bovine serum albumin coated silver nanoparticles during 28 days, while behavior functions remain the same [23]. Along this, a selective accumulation of silver in the brain departments responsible for the special memory was observed. A decrease of spacial memory associated with changes in dopamine and serotonin ratios in rats exposed to 20 nm sized silver nanoparticles and AgNo3 were observed [24]. Herewith, the result was coating dependent. 3-staged changes in the behavioral and cognitive functions in mice at the prolonged oral exposure to polyvinylpyrrolidone coated silver nanoparticles were observed in [25]. Anxiety increase observed at the early stages was followed by improvement of exploration behavior beside the increased anxiety and a significant impairment of long-term contextual memory against the background of non-changed behavior at the final. Silver accumulation in that mice was observed in hippocampus, cerebellum and cortex of the brain [9], which contradicts to [23], where significant silver accumulation was found just in the hippocampus. In contrary to that, an increase of locomotor activity and decrease of anxious-phobic level in a stressful environment as well as impairment of short-term addiction of rats after 2-month exposure to 16.7 nm sized beta-cyclodextrin coated nanosilver were observed. Extra 3 months of washing out resulted in no differences in behavioral functions and the ability for associative learning between experimental and control animals [26]. While silver particles in the brain regions of rats were detected in 2 months of the exposure using electron microscopy, however none of them were found in the extra 3 months of washing out. It allows to suggest a high clearance rate in the nervous tissue in contrary to the earlier studies [7,10,12]. The result can be caused by a relatively low representativeness of electron microscopy. An influence of silver nanoparticles synthesized using typical green synthesis approach with the application of a green tea extraction on behavioral functions of mice with induced inflammation as well as the inflammation itself were studied in [27]. The “green synthesis” term is not mentioned within the manuscript [27], however the nanoparticles produced by its means are designated as green tea silver nanoparticles along it, i.e. as a principally new object. We believe that the use of the new term does not reflect the sense of the study in this case. The authors observed reduction of temperature hypersensitivity and edema, anxiety decrease and locomotor activity increase in the exposed to silver nanoparticles, so called ‘green tea silver nanoparticles’ mice. Dose dependent anti-inflammatory effects of silver nanoparticles were also detected.
Despite the ability of ionic silver to accumulate in the brain [7] as well as its more pronounced toxic action in vivo compared to silver nanoparticles [14], the problem of ionic silver to influence behavioral and cognitive functions of laboratory animals is weakly studied. There is a research investigating the influence of non-lethal doses of AgNo3 exposure on cognitive and behavioral functions of Danio rerio [28]. The exposure changed social preferences, reduced social recognition, learning and memory but did not influence the anxiety and aggressiveness levels as well as the shoaling behavior. A dose dependent silver accumulation in the brains of fishes was observed. It was shown that AgNo3 exposure reduced neural activity in dorsal and medial zones of the dorsal telencephalic area. We have not found any examples of such investigations using laboratory mammals.
However, such studies could shed light onto the discussible questions on the mechanisms of interactions between silver nanoparticles with living beings as well as to identify the possible risks and effects from silver containing preparations usage. Besides, colloidal solutions of silver nanoparticles may contain a certain fraction of the unreacted silver salts as well as dissociated silver ions. As [7] demonstrates <15 nm sized silver nanoparticles relatively fast dissociated into the ions in the exposure solutions. The ionic fraction increased up to 45% after 7 days of the solution preparation. Therefore, it is important to understand and differentiate the possible risks from silver in different forms.
The influence of silver citrate onto behavioral and cognitive functions of laboratory mice for the salt neurotoxicity assessment was studied in the present work. Herewith, the conditions of the analogous experiment on the influence of the prolonged oral administration of polyvinylpyrrolidone coated silver nanoparticles into mice on the same functions were reproduced [25]. It lets us compare the results and identify the role of nanoform in the sense of neurotoxicity.
2. Results
2.1. Physiology
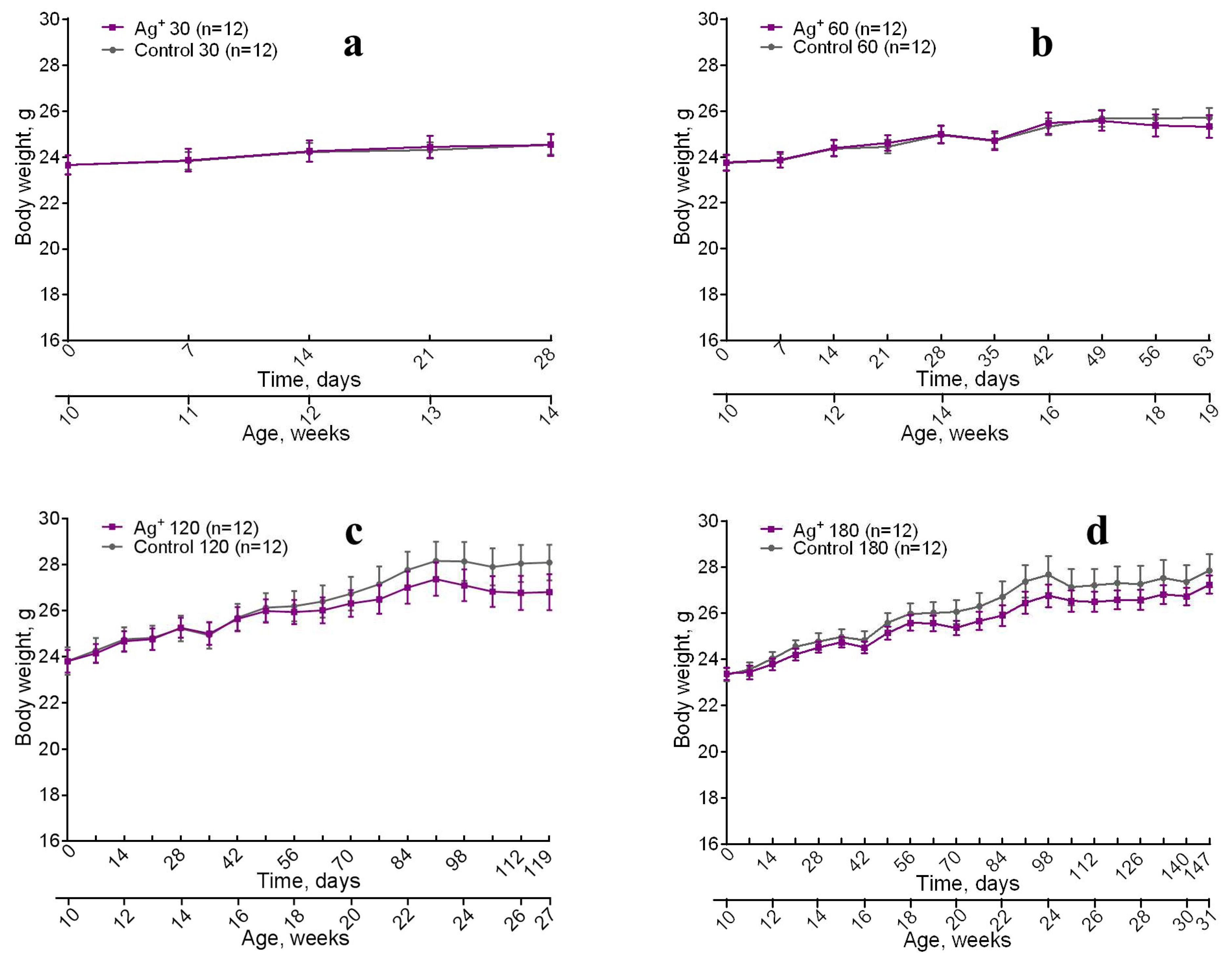
All the animals have been gradually growing and gained body mass during the whole research (
Figure 1). No significant differences between control and experimental mice of the same age by the parameter were detected. However (
Figure 1 c,d) shows insignificant weight decrease for the mice exposed to silver citrate. Lethality was not observed throughout the study.
2.2. Behavioral and cognitive functions
The following parameters were measured and compared in the behavioral tests:
OF: total moved distance; distance moved in the peripheral, intermediate and central areas; number of entries into the center;
EPM: distance moved in the open arms, closed arms, center; time spent in open arms; number of head dips;
LDB: number of looking outs from the dark chamber; number of transitions between chambers; distance moved in the light chamber; latency to exit from the light chamber.
Long-term contextual memory was assessed by the time of freezing (%) in 24 h after the learning in the contextual fear conditioning task as mentioned above.
The results demonstrating the silver citrate influence on the behavioral functions and long-term contextual memory of mice are listed below by the period of exposure increase. Only statistically significantly differing data (*p < 0.05; **p < 0.01) and tendencies (0.05 < p < 0.07) are presented. The other studied parameters did not show significant differences and tendencies between experimental and control animals within each time point.
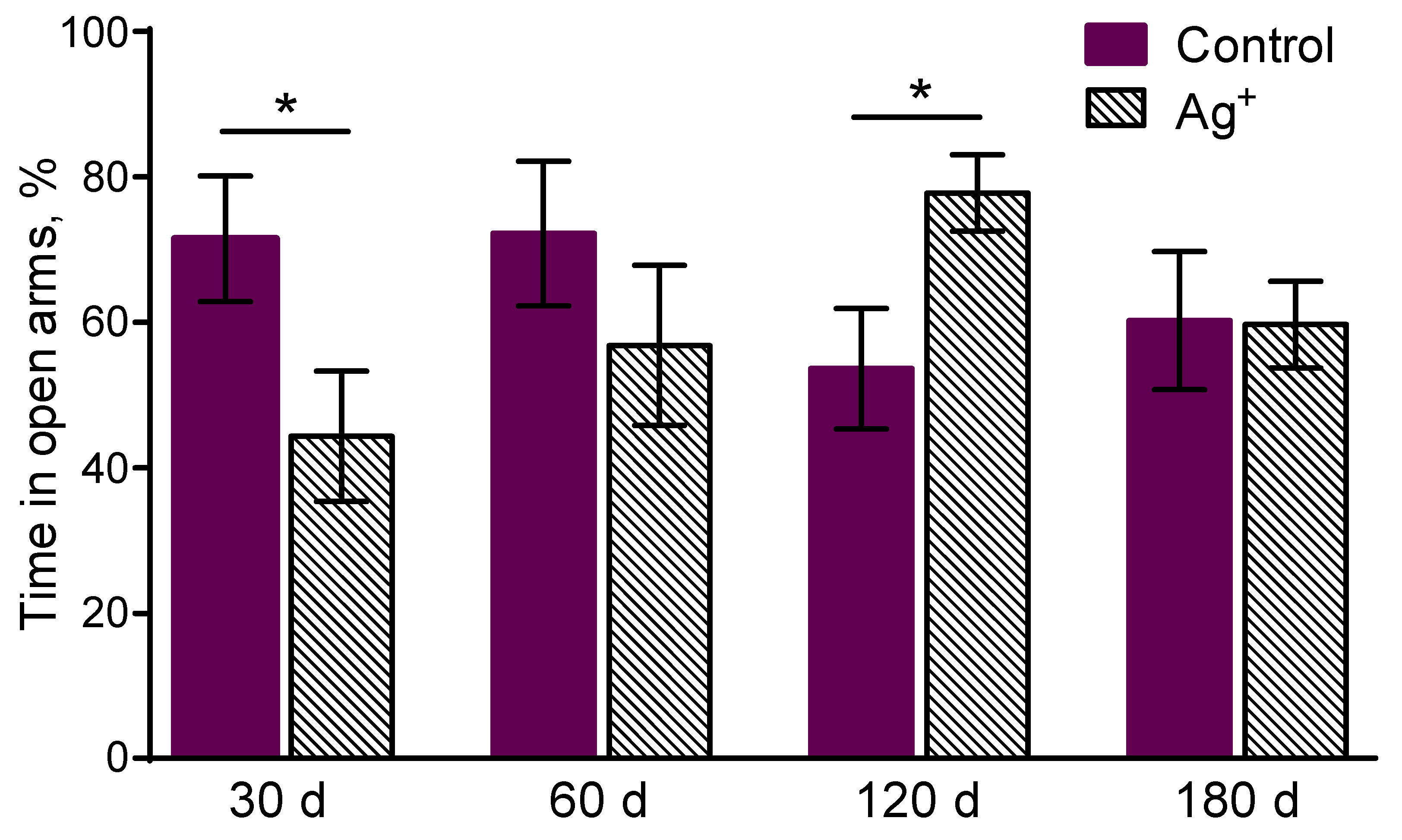
The experimental mice spent less time in the open arms of the EPM compared to the controls in 30 days of the exposure (p = 0,040) (
Figure 2). It demonstrates anxiety increase in «Ag+ 30» group.
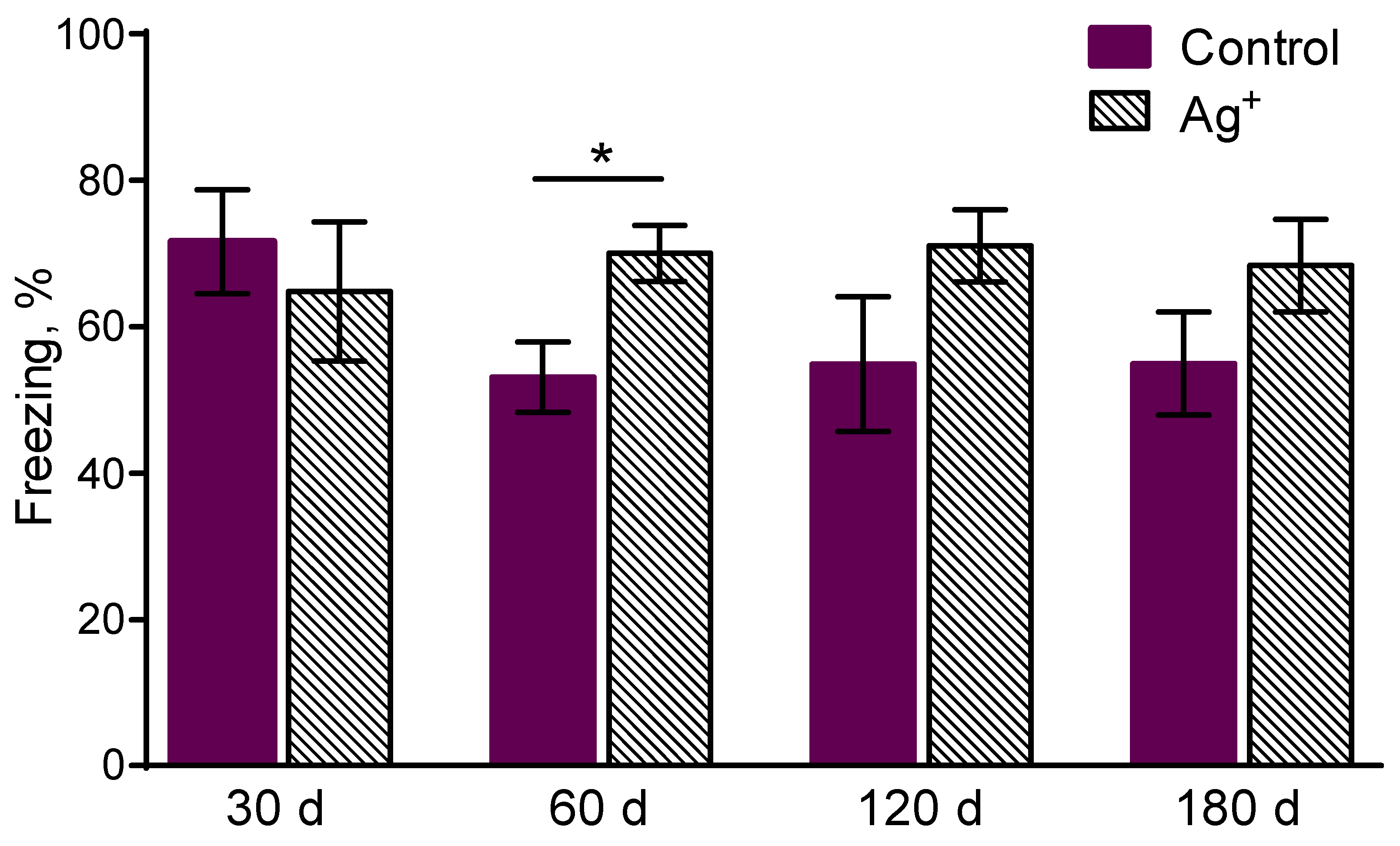
Freezing was enhanced for mice exposed to silver citrate during 60 days compared to the controls in 1 min after learning in the contextual memory task (p = 0,017) (
Figure 3). The character of ratios by the parameter for the following groups is similar, however without significant differences. It may evidence about sensitivity, perceptivity to an irritant in the form of negative experience increase in the silver citrate exposed mice. It might be associated with anxiety increase.
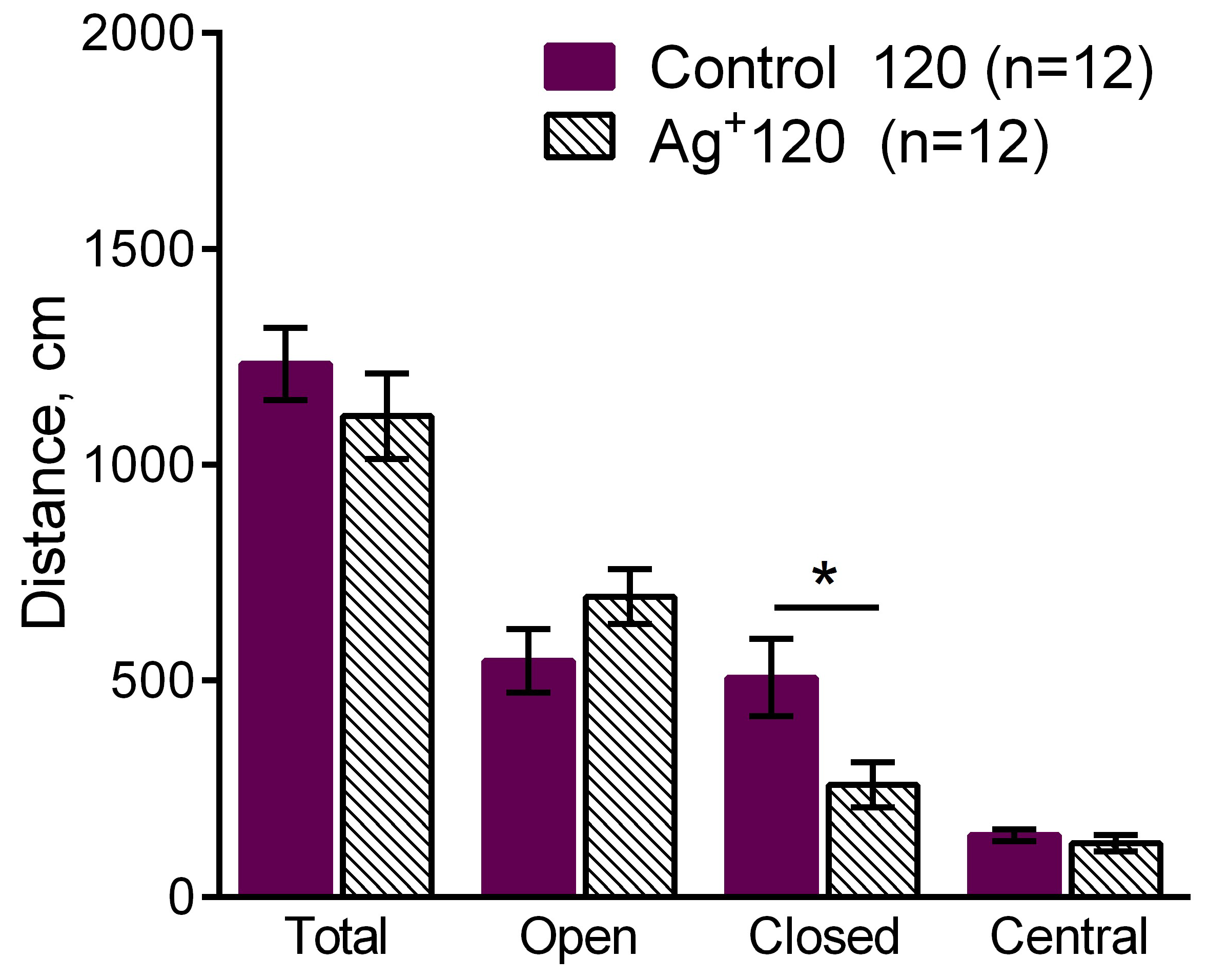
The time spent in the open arms of EPM was enhanced in «Ag+ 120» compared to «Control 120» (p = 0,022) mice (
Figure 2). It demonstrates anxiety decrease in the mice exposed to silver citrate during 120 days. It might also be regarded as exploration behavior level increase in the silver citrate exposed animals. A decrease of the distance moved in the closed arms of EPM in «Ag+ 120» compared to «Control 120» mice was detected (p = 0,025) (
Figure 4). It shows anxiety decrease in the «Ag+ 120» mice. The tendency of freezing enhancement in «Ag+ 120» compared to «Control 120» (p= 0.0617) mice was observed as well. It evidences about the tendency of long-term contextual memory improvement in the mice exposed to silver citrate during 120 days. Any significant differences in the long-term memory were not detected for all the periods of exposure.
Figure 4.
The comparison of the distance moved in the EPM such as total distance moved, distance moved in the open, closed arms and in the center between «Ag+ 120» and «Control 120» mice. Values are presented as mean ± SEM. *p< 0.05. The decrease of the distance moved in the closed arms of the EPM was observed in the mice exposed to silver citrate during 120 days. It demonstrates anxiety decrease in «Ag+ 120» mice.
Figure 4.
The comparison of the distance moved in the EPM such as total distance moved, distance moved in the open, closed arms and in the center between «Ag+ 120» and «Control 120» mice. Values are presented as mean ± SEM. *p< 0.05. The decrease of the distance moved in the closed arms of the EPM was observed in the mice exposed to silver citrate during 120 days. It demonstrates anxiety decrease in «Ag+ 120» mice.
Figure 5.
Freezing in 24 h after the learning in the contextual fear conditioning task. It assesses the long-term contextual memory in mice. Values are presented as mean ± SEM. *p< 0.05. The tendency of freezing enhancement was observed in the «Ag+ 120» compared to «Control 120» mice (p= 0.0617). It could point to the improvement of the long-term contextual memory in the mice exposed to silver citrate during 120 days. Any significant differences in the long-term memory were not detected for all the periods of exposure.
Figure 5.
Freezing in 24 h after the learning in the contextual fear conditioning task. It assesses the long-term contextual memory in mice. Values are presented as mean ± SEM. *p< 0.05. The tendency of freezing enhancement was observed in the «Ag+ 120» compared to «Control 120» mice (p= 0.0617). It could point to the improvement of the long-term contextual memory in the mice exposed to silver citrate during 120 days. Any significant differences in the long-term memory were not detected for all the periods of exposure.
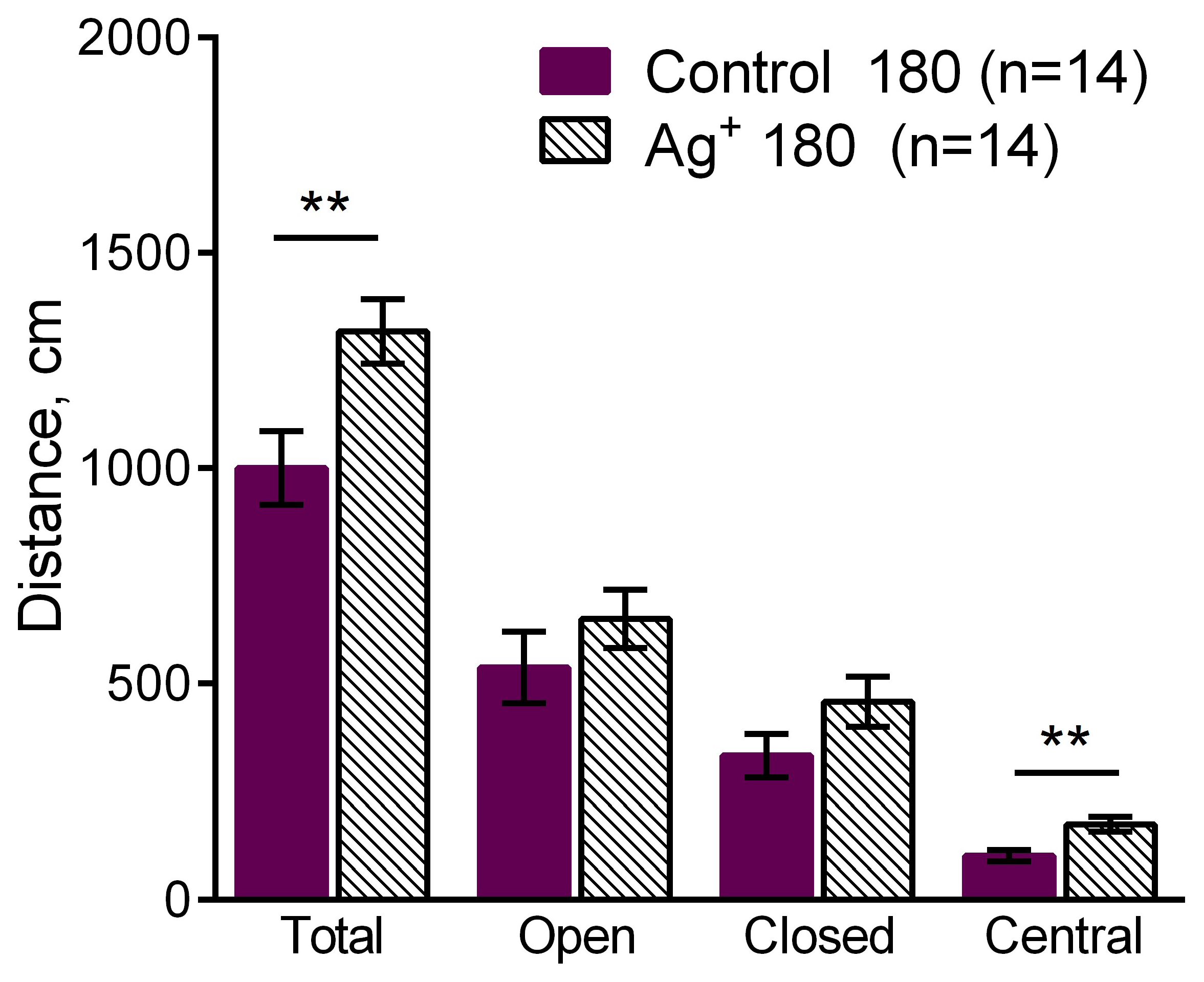
The increase of the total distance moved (p = 0,009) as well as the increase of the distance moved in the central area (p = 0,002) of the EPM were observed in the «Ag+ 180» compared to «Control 180» mice. It demonstrates locomotor activity enhancement in the mice exposed to silver citrate during 180 days.
Figure 6.
The comparison of the distance moved in the EPM such as total distance moved, distance moved in the open, closed arms and in the center between «Ag+ 180» and «Control 180» mice. Values are presented as mean ± SEM. **p< 0.01. The increases of the total distance moved (p = 0,009) and distance moved in the center (p = 0,002) were observed in the mice exposed to silver citrate during 180 days. It demonstrates the enhancement of the locomotor activity of them.
Figure 6.
The comparison of the distance moved in the EPM such as total distance moved, distance moved in the open, closed arms and in the center between «Ag+ 180» and «Control 180» mice. Values are presented as mean ± SEM. **p< 0.01. The increases of the total distance moved (p = 0,009) and distance moved in the center (p = 0,002) were observed in the mice exposed to silver citrate during 180 days. It demonstrates the enhancement of the locomotor activity of them.
3. Discussion
The change dynamics of the influence of daily oral exposure to silver citrate at the period of its administration growth on the behavioral and cognitive functions of laboratory mice have been demonstrated. An analogous study on the silver nanoparticle influence was held earlier and is described in [25]. The present research is important to identify the role of nanosize in the neurotoxic properties of silver as well as for the choice of silver form for antibacterial, antiviral and fungicidal therapy and prevention optimization.
The previous research [25] was due to the recently determined phenomena such as silver accumulation in brain as well as low clearance of it from the brain [7–10,12]. The following change dynamics of the influence of silver nanoparticles exposure on the behavioral and cognitive functions of the C57BL/6 male mice was observed. An anxiety increase was followed by an exploration behavior increase against the background of the increased anxiety and long-term contextual memory impairment besides the absence of behavioral changes at the final stage. Thus, a rather pessimistic picture was observed. Despite the exploration behavior increase, which can be regarded as adaptation and hormesis [29] manifestation, at the background of increased anxiety, the growth of the exposure period until 180 days led to a significant long-term contextual memory impairment in the mice exposed to silver nanoparticles.
We reproduced the experiment conditions from [25], however in the present study we used silver citrate as a potential xenobiotic. Some studies demonstrate silver accumulation in the brain of laboratory rodents after exposure to silver salts [7,8,14], even superior to that for silver nanoparticles. However, the influence of silver salts onto behavioral and cognitive functions of laboratory rodents have not been investigated yet before.
We defined anxiety increase in the mice after 30 days of the exposure to silver citrate. The analogous effect was identified at the silver nanoparticle daily exposure of mice during the same period [25]. We observed sensitivity, perceptivity in 60 days of the exposure, which might be associated with anxiety increase. The assessment by the parameter is our supposition. Common signs of the anxiety increase were not detected at this period of exposure. The criterion likely reflects the further observed adaptation processes. An anxiety decrease as well as a premise to exploration behavior increase and a tendency to long-term contextual memory improvement were observed in 120 days of the silver citrate exposure. The locomotor activity increase was observed in 180 days of the exposure. Thus, a rather optimistic picture of the change dynamics of behavioral and cognitive functions, reflecting the organism adaptation to a chronic exposure to the xenobiotic, was detected in the present study. A hormetic effect and the reverse of the behavior change vector from a pronounced negative to a positive one were observed. Anxiety increase likely connected to an increased sensitivity is replaced by the anxiety decrease, locomotor activity growth and a tendency to cognitive function improvement. Increased sensitivity may also be regarded as a positive marker of a productive adaptation. An increase of locomotor activity is regarded as the compensatory to increased anxiety [26]. The locomotor activity increase as well as the tendency to the long-term memory improvement can be considered as the compensatory to the anxiety increase in the present case. From the other point of view, as the changes of behavioral and cognitive functions of the mice exposed to silver citrate are compared to the controls, the imaginary improvement of their behavior might be considered as tigmotaxis impairment [30], the typical rodent behavior. However, from the other point of view, the improvement can also be considered as manifestation of ageing neutralization [31] by the perturbance in the form of silver citrate exposition.
Thus, from the one point of view, silver citrate can be regarded as less toxic compared to silver nanoparticles xenobiotic, from the other it may be considered as an effective medicine triggering and activating the own resources of an organism, which is the most benevolent property. Apparently, nanosize impacts the toxicity of a compound. The experimental research shows neurotoxicity decrease of a salt form compared to nanoparticles at the example of silver compounds [25]. Nonetheless, the other, not regarded in the present research, toxic effects should be took into the account as well to receive the overall toxicity assessment. Anyway, the obtained result demonstrates higher biocompatibility of silver citrate compared to silver nanoparticles, which is proven by a more pronounced ability of mice to adapt to silver citrate than to the nanoparticles.
A similar effect was observed in [27]. Exposure of rats to the silver nanoparticles produced by the green synthesis technique using green tea extraction resulted in the reduction of induced inflammation similarly to diclofenac effect and repaired behavioral functions. We suppose that the authors of [27] as well as us observed a typical hormetic effect. Hormesis may be viewed as something of a biological corollary of the words of German philosopher Friedrich Wilhelm Nietzsche (1844 -1900): “That which does not kill us makes us stronger.” The key role of hormesis should not be excluded from the mechanism of therapeutic action of various medicals.
We supposed in the Introduction the ability of a living organism to synthesize nanoparticles from ionic form, for example, from silver similarly to green synthesis technology. The present results demonstrate different characters of the behavioral and cognitive functions influence of silver citrate and silver nanoparticles [25]. At the same time, the publications reviewed in the present manuscript evidence the significant dependence of biological effects on size, shape and surface chemistry of nanoparticles. The results of the present work do not contradict to the made assumption that a living organism may play a role of a reactor to synthesize nanoparticles from ions due to the ability of nanoparticles or the granules formed by the salts to associate and dissociate in it. It is likely that silver compounds introduced into a living organism as silver citrate or nanoparticles form granules of different sizes and shapes in it. It can cause the observed differences.
There is no doubts that biological effects are sensitive to various conditions. When considering effects of medicine and xenobiotics, nanosized as well, it is not fully taken into the account that an organism being a synergetic complex object itself is sensitive to changes of exogenous and endogenous factors. For example, meal may cause pH change that may influence the nanoparticle introduced into the organism size [32,33], in its turn and the following may determine their absorption, elimination and biodistribution of them. Due to the complexity of such a synergetic system as a living organism and the presence of a great number of cause-and-effect relationships the result of a biological experiment can be unpredictable. It also may contribute to the significant difference on the toxicity and toxicokinetics of silver nanoparticles received by different researchers as mentioned in [19].
4. Materials and Methods
4.1. Materials
We used silver citrate (SilverSalt, St. Petersburg, Russia) in the study as silver salt form. It is a colorless water solution hermetically sealed in an opaque glass. The chemical formulae of silver citrate is Ag3C6H5O7.
We have chosen silver citrate due to its commercial availability and presumably higher bioavailability compared to some other silver salts, for example, to silver nitrate. The bioavailability may be caused by the strength of the acids the salts formed by. The dissociation constants quantitatively characterize the strength of acids. Thus, the value of dissociation constant for silver nitrate is 4·10-4 and are 7,45·10-4, 1,7·10-5, 4,0·10-7 for a lemon acid [34]. The ability of the anion as well as the cation in a salt to contribute into the overall toxicity should be taken into account [35].
The exposure solutions were obtained dissolving the initial solution of silver citrate by a purified water produced by the purification (OOO “Pharmsystemy”, Besedy, Moscow region, Russia) of tap water to the necessary concentration.
C57BL/6 male mice were used as the mammalian model (“Stolbovaya” branch of the Federal Medical Biological Agency of Russia, Moscow, Russia). The animals were received from the supplier aged 2 months, weighing 20-22 g.
4.2. Methods
Electronic scales Adventurer Pro (Ohaus Corporation, Pine Brook, NJ, USA) were used to determine the body weight of the animals.
Open Field (OF), Elevated Plus Maze (EPM), Light-Dark Box (LDB) were used to study the behavioral functions of mice. We accessed locomotor activity, anxiety and research behavior of the trainees in the tests. A contextual fear conditioning task was used to estimate the long-term contextual memory of the animals.
4.3. Experiment itinerary
On the delivery into the National Research Center Kurchatov Institute the mice were let adapting to the new conditions during 2 weeks. The animals were kept in the individual cages in the room with automatically maintained temperature of 23 ± 2 °C and a 12/12-h day/night cycle during the whole experiment. The animals had an unlimited access to food and water. All the manipulations with them were carried out according to Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes as well as the requirements of the Local Ethical Committee on Biomedical Research of the National Research Center “Kurchatov Institute” approved this research (Protocol No. 2, 07 October 2021).
Silver citrate exposure was implemented in the composition of drinking water in the amount about 50 µg/day/animal during 30, 60, 120 and 180 days. The control mice received purified water, none of chemicals were additionally administered to controls. Thus, we had 4 experimental groups of mice (n=12: «Ag+ 30», «Ag+ 60», «Ag+ 120»; n=14: «Ag+ 180») and 4 control groups of mice (n=12: «Control 30», «Control 60», «Control 120»; n=14: «Control 180») separated by the exposure periods. The mice were weekly weighted during the whole study to estimate the influence of silver citrate onto their physiology.
The assessment of the behavioral and cognitive functions of the animals identifying the silver citrate influence towards them was held since 31st, 61st, 121st and 181st days respectively. A number of objective parameters received from the tests and characterizing the behavioral and cognitive functions of the mice was measured, the parameters were compared between control and experimental groups within each time point, i.e. the period of exposure. The specific parameters are listed in the Results section. The test conditions of the OF, EPM, LDB matches those conditions described in details in [25]. Silver citrate administration was maintained during testing to avoid its washing out.
To assess the influence of silver citrate on the formation and retention of the long-term contextual memory in the contextual fear conditioning task the learning and then testing of the animals were implemented using Video fear conditioning system (MED Associates Inc., USA) and a software Video Freeze (MED Associates Inc., USA).
Videorecording of the mice behavior and determination of the number and duration of the mice freezing implemented automatically were carried out during learning and testing. Freezing was defined as the specific form of mice behavior with a complete absence of movement except breathing. Freezing was automatically identified using Video Freeze (MED Associates Inc., USA) software. To identify freezing the following thresholds were applied – the maximal number of pixels changed in the given frame in relation to the previous one is 15 (image digitization rate 30 frames per second and resolution 320x240; the minimal duration of immobility is 0.5 s.
The animals were put into the experimental chamber (Chamber, MED Associates Inc., USA) sized 300х230х210 mm with two opaque walls (one metallic wall and one polyvinyl chloride wall) and one transparent (plexiglass) wall, plexiglass ceiling and an electrode floor (ENV-005-QD Quick Disconnect Harness, MED Associates Inc., США) consisting of 36 steel rods with the diameter of 2 mm located 5 mm far from each other. The electrode floor was connected with the managing electrostimulator ENV414S Aversive Stimulator (MED Associates Inc., USA). A diffuse white light source giving an average in-chamber illumination level of 87 lux and a near-infrared light source connected to the control light stimulator NIR-100 Light Controller (MED Associates Inc., USA) were placed above the chamber. The chamber was wiped with 70% ethanol solution (ZAO Bryntsalov, Russia) in advance to the placement of each next animal. The home cages were used for the transportation of the animals from the home area to the experimental place and back.
The mice from each group were separated into 2 subgroups such as Active Control (AC) (n=6; n=7 in «Ag+ 180», «Control 180») and Freezing Conditions (FC) (n=6; n=7 in «Ag+ 180», «Control 180»). The AC mice were placed into the experimental chamber for the environment exploration for 6 mins without electrodermal irritation. The FC mice were placed into the experimental chamber for 6 mins totally such as 3 mins for the free environment exploration, 3 electrodermal irritations with the durability of 2 secs and a time interval between them of 1 min (Current is 1 mA) and 1 min of free exploration for the memory consolidation. The assessment of memory formation was carried out 1 min after learning in the conditions of the reflex (fear) reproduction in the same environment without electrodermal irritations. The intervals of interest (0-178 sec and 300-360 sec of the experiment) were set in the Video Freeze software and the freezing was automatically calculated as the ratio of total duration of freezing in an interval to the total interval durability for the animal behavior analysis.
The animals were tested to assess the memory retention in 24 h after learning. Each animal was placed into the experimental chamber for 3 mins to test the long-term contextual memory. The interval of interest (0-180 sec of the experimentа) was set in the Video Freeze software and freezing was automatically calculated as the ratio of total duration of freezing in the interval to the total interval durability for the animal behavior analysis.
We should note that the fraction of freezing in the conditions of the fear to the context reproduction in 1 min after learning more likely shows not the memory but the level of the sensitivity to the irritant caused by a formed memory for electrodermal irritations. Likely it may be associated with anxiety. Thus, its increase might point to the anxiety increase. The parameter was used as an additional measure for behavioral functions assessment in the present research.
The animal testing and learning in all the tests and the task were carried out daily without breaks for rest days.
4.4. Statistical Analysis
Statistical analysis was performed using GraphPad Prizm 6.01 (La Jolla, San Diego, CA, USA). The unpaired, two-sided Student’s t-test was performed. The data are presented as the mean ± SEM. The differences were considered to be significant at values of *p < 0.05 and **p <0.01.
5. Conclusions
The change dynamics of behavioral and cognitive functions of laboratory mice orally exposed to silver citrate is studied in the present research. The reverse of the character of such a dynamics within the regarded time period is found. Increased anxiety of the experimental mice at the early stage was replaced by the anxiety reduction, locomotor activity increase and the tendency to the long-term contextual memory improvement likely associated to a sensitivity enhancement. Apparently, the anxiety increase is compensated by the locomotor activity increase and the memory improvement as the own organism’s resources activation. We observed a typical hormetic effect of silver citrate and adaptation of the mice to it.
Apparently, such a term as ionic form of silver is not fully correct when considering an action of its salt. Salts of silver are able to form granules and nanoparticles in a living organism following their oral exposure as it is shown in [7,8]. Namely, the effects and mechanisms of interactions of such particles with biological organisms should be considered.
We demonstrated that nanoform of silver is more toxic compared to silver citrate in the sense of its influence onto behavioral and cognitive functions of the exposed organisms. The biological action significantly depends on the particle’s size. A living organism might play a role of a green synthesis reactor to produce silver nanoparticles from silver salts. The relatively low neurotoxicity of silver citrate found in the study may be due to the bioavailability of its anion. Namely, chelated forms of silver are reccommended for in vivo use according to the accepted standards.
The present work opens the directions for the further investigations to choose the optimal, less toxic silver compound for a therapy as well as basic problems on the ability of a living organism to synthesize metallic nanoparticles from their salts for a deeper understanding of the mechanisms of living systems functioning.
Author Contributions
Conceptualization, P.K.K. and A.A.A.; methodology, M.Yu.K.; validation, M.A.L.; formal analysis, M.Yu.K.; investigation, M.Yu.K.; resources, A.A.A. and M.Yu.K.; writing—original draft preparation, A.A.A.; writing—review and editing, M.Yu.K.; visualization, M.Yu.K.; supervision, P.K.K.; project administration, A.A.A.; funding acquisition, P.K.K. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
The research was partially financed by RFBR and Moscow city Government according to the project: No. 21-315-70016.
Institutional Review Board Statement
The study was conducted according to Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes and approved by the Local Ethical Committee on Biomedical Research of the National Research Center “Kurchatov Institute” (Protocol No. 2, 07 October 2021).
Informed Consent Statement
Not applicable.
Acknowledgments
The authors are grateful to the leading researcher of the Federal Research Center of Nutrition and Biotechnology Ivan V. Gmoshynskiy for valuable recommendations on the experiment planning, familiarization with the manuscript and valuable comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Antsiferova, A.A.; Kashkarov, P.K.; Kovalchuk, M.V. Effect of Different Forms of Silver on Biological Objects. Nanobiotechnology Reports 2022, 17, 155–164. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Railean, V.; Buszewska-Forajta, M.; Rodzik, A.; Gołębiowski, A.; Pomastowski, P.; Buszewski, B. In Vivo Efficacy of Wound Healing under External (Bio)AgNCs Treatment: Localization Case Study in Liver and Blood Tissue. Int. J. Mol. Sci. 2023, 24, 434. [Google Scholar] [CrossRef]
- Urnukhsaikhan, E.; Bold, BE.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Scientific Reports 2021, 11, 21047. [Google Scholar] [CrossRef]
- Desai, A.S.; Singh, A.; Edis, Z.; Haj Bloukh, S.; Shah, P.; Pandey, B.; Agrawal, N.; Bhagat, N. An In Vitro and In Vivo Study of the Efficacy and Toxicity of Plant-Extract-Derived Silver Nanoparticles. J. Funct. Biomater. 2022, 13, 54. [Google Scholar] [CrossRef]
- Antsiferova, A.; Kashkarov, P. Modern Techniques to Study Biokinetics of Nanoobjects in Living Organisms In Research Highlights in Science and Technology Vol. 1. Edited by Prof. Figen Balo: B P International, Hooghly, West Bengal, India, United Kingdom, 2023. pp. 151-186. [CrossRef]
- van der Zande, M.; Vandebriel, R.J.; Van Doren, E.; Kramer, E.; Herrera Rivera, Z.; Serrano-Rojero, C.S.; Gremmer, E.R.; Mast, J.; Peters, R.J.B.; Hollman, P.C.H.; Hendriksen, P.J.; Marvin, H.J.; Peijnenburg, A.A.; Bouwmeester, H. Distribution, Elimination, and Toxicity of Silver Nanoparticles and Silver Ions in Rats after 28-Day Oral Exposure. ACS Nano 2012, 6, 7427–7442. [Google Scholar] [CrossRef]
- Loeschner, K.; Hadrup, N.; Qvortrup, K.; Larsen, A.; Gao, X.; Vogel, U.; Mortensen, A.; Rye Lam, H.; Larsen, E.H. Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Particle and Fibre Toxicology 2011, 8, 18. [Google Scholar] [CrossRef]
- Antsiferova, A.A.; Kopaeva, M.Y.; Kochkin, V.N.; Kashkarov, P.K. Kinetics of Silver Accumulation in Tissues of Laboratory Mice after Long-Term Oral Administration of Silver Nanoparticles. Nanomaterials 2021, 11, 3204. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.S.; Song, K.S.; Ryu, H.R.; Sung, J.H.; Park, J.D.; Park, H.M.; Song, N.W.; Shin, B.S.; Marshak, D.; Ahn, K.; Lee, J.E.; Yu, I.J. Biopersistence of silver nanoparticles in tissues from Sprague–Dawley rats. Part Fibre Toxicol 2013, 10, 36. [Google Scholar] [CrossRef]
- Xue, Y; Zhang, Sh.; Huang, Y.; Zhang, T.; Liu, X.; Hu, Y.; Zhang, Zh.; Tang, M. Acute toxic effects and gender-related biokinetics of silver nanoparticles following an intravenous injection in mice. J Appl Toxicol. 2012, 32, 890–899. [Google Scholar] [CrossRef]
- Antsiferova, A.; Buzulukov, Yu; Demin, V; Kashkarov, P; Kovalchuk, M; Petritskaya, E. Extremely low level of Ag nanoparticle excretion from mice brain in in vivo experiments. IOP Conf. Series: Materials Science and Engineering 2015, 98. [CrossRef]
- Antsiferova, A.A.; Kopaeva, M.Y.; Kochkin, V.N.; Reshetnikov, A.A.; Kashkarov, P.K. Neurotoxicity of Silver Nanoparticles and Non-Linear Development of Adaptive Homeostasis with Age. Micromachines 2023, 14, 984. [Google Scholar] [CrossRef]
- Charehsaz, M.; Hougaard, K.S.; Sipahi, H.; Ekici, A.I.; Kaspar, Ç.; Culha, M.; Bucurgat, Ü.Ü.; Aydin, A. Effects of developmental exposure to silver in ionic and nanoparticle form: A study in rats. Daru 2016, 24, 1, 24. [Google Scholar] [CrossRef]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids and Surfaces B: Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef]
- Restrepo, C.V.; Villa, C.C. Synthesis of silver nanoparticles, influence of capping agents, and dependence on size and shape: A review. Environmental Nanotechnology, Monitoring & Management 2021, 15, 100428. [Google Scholar] [CrossRef]
- Lyu, Zh.; Ghoshdastidar, Sh.; Rekha, K.R.; Suresh, Dh.; Mao, J.; Bivens, N.; Kannan, R.; Joshi, T.; Rosenfeld, Ch.S.; Upendran, A. Developmental exposure to silver nanoparticles leads to long term gut dysbiosis and neurobehavioral alterations. Scientific Reports 2021, 11, 6558. [Google Scholar] [CrossRef]
- Dąbrowska-Bouta, B.; Sulkowski, G.; Strużyński, W.; Strużyńska, L. Prolonged Exposure to Silver Nanoparticles Results in Oxidative Stress in Cerebral Myelin. Neurotox Res. 2019, 35, 495–504. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Salama, B.; Alzahrani, K.J.; Alghamdi, K.S.; Al-Amer, O.; Hassan, K.E. Elhefny, M.A.; Albarakati, A.J.A.; Alharthi, F.; Althagafi, H.A.; Al Sberi, H.; Amin, H.K.; Lokman, M.S.; Alsharif, K.F.; Albrakati, A.; Abdel Moneim, A.E.; Kassab, R.B.; Fathalla, A.S. Silver Nanoparticles Enhance Oxidative Stress, Inflammation, and Apoptosis in Liver and Kidney Tissues: Potential Protective Role of Thymoquinone. Biol Trace Elem Res 2023, 201, 6, 2942-2954. [CrossRef]
- Dziendzikowska, K.; Wilczak, J.; Grodzicki, W.; Gromadzka-Ostrowska, J.; Węsierska, M.; Kruszewski, M. Coating-Dependent Neurotoxicity of Silver Nanoparticles—An In Vivo Study on Hippocampal Oxidative Stress and Neurosteroids. Int. J. Mol. Sci. 2022, 23, 1365. [Google Scholar] [CrossRef]
- Greish, K.; Alqahtani, A.A.; Alotaibi, A.F.; Abdulla, A.M.; Bukelly, A.T.; Alsobyani, F.M.; Alharbi, G.H.; Alkiyumi, I.S.; Aldawish, M.M.; Alshahrani, T.F.; et al. The Effect of Silver Nanoparticles on Learning, Memory and Social Interaction in BALB/C Mice. Int. J. Environ. Res. Public Health 2019, 16, 148. [Google Scholar] [CrossRef]
- Węsierska, M.; Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Dudek, J.; Polkowska-Motrenko, H.; Audinot, J.N.; Gutleb, A.C.; Lankoff, A.; Kruszewski, M. Silver ions are responsible for memory impairment induced by oral administration of silver nanoparticles. Toxicol Lett. 2018, 290, 133–144. [Google Scholar] [CrossRef]
- Dziendzikowska, K.; Węsierska, M.; Gromadzka-Ostrowska, J.; Wilczak, J.; Oczkowski, M.; Męczyńska-Wielgosz, S.; Kruszewski, M. Silver Nanoparticles Impair Cognitive Functions and Modify the Hippocampal Level of Neurotransmitters in a Coating-Dependent Manner. Int. J. Mol. Sci. 2021, 22, 12706. [Google Scholar] [CrossRef]
- Antsiferova, A.; Kopaeva, M.; Kashkarov, P. Effects of Prolonged Silver Nanoparticle Exposure on the Contextual Cognition and Behavior of Mammals. Materials 2018, 11, 558. [Google Scholar] [CrossRef]
- Egorova, E.M.; Krupina, N.A.; Kaba, S.I.; Khlebnikova, N.N.; Shirenova, S.D.; Sviridkina, N.B.; Paltsyn, A.A. The Effect of Aqueous Solutions of Silver Nanoparticles on Rat Behavior. Nanobiotechnology Reports 2022, 17, 2, 248–260. [Google Scholar] [CrossRef]
- Ninsiima, H.I.; Eze, E.D. Ssekatawa, K.; Nalugo, H.; Asekenye, C.; Onanyang, D.; Munanura, E.I.; Ariong, M.; Matama, K.; Zirintunda, G.; Mbiydzenyuy, N.E.; Ssempijja, F.; Afodun, A.M.; Mujinya, R.; Usman, I.M.; Asiimwe, O.H.; Tibyangye, J.; Kasozi, K.I. Green tea silver nanoparticles improve physiological motor and cognitive function in BALB/c mice during inflammation. Heliyon 2023, 9, 3, e13922. [CrossRef]
- Fu, C.W.; Horng, J.L.; Tong, S.K.; Cherng, B.W.; Liao, B.K.; Lin, L.Y.; Chou, M.Y. Exposure to silver impairs learning and social behaviors in adult zebrafish, Journal of Hazardous Materials 2021, 403, 124031. [CrossRef]
- Pomatto, L.C.D.; Davies, K.J.A. The role of declining adaptive homeostasis in ageing. J. Physiol. 2017, 595, 7275–7309. [Google Scholar] [CrossRef]
- Lotosh, N.Y.; Kulikov, E.A.; Kulikova, I.S.; Selishcheva, A.A.; Ogurtsov, D.P.; Krynsky, S.A.; Malashenkova, I.K.; Kryuchkova, A.V. Effect of Nanoemulsions Containing Astaxanthin or Its Esters on the Spacial Behavior of 5XFAD Mice. Nanobiotechnology Reports 2022, 17, 227–234. [Google Scholar] [CrossRef]
- Antsiferova A.A., Kopaeva M. Yu., Kashkarov P. K. Relationships between Effects of Ag Nanoparticles and Ag Salton Behavioral and Cognitive Functions of Mice and Ageing. Nanobiotechnology Reports 2022, 17, 6, 857–865. [CrossRef]
- Axson, J.L.; Stark, D.I.; Bondy, A.L.; Capracotta, S.S.; Maynard, A.D.; Philbert, M.A.; Bergin, I.L.; Ault, A.P. Rapid Kinetics of Size and pH-Dependent Dissolution and Aggregation of Silver Nanoparticles in Simulated Gastric Fluid. J Phys Chem C Nanomater Interfaces 2015, 119, 20632–20641. [Google Scholar] [CrossRef]
- Tai, J.T.; Lai, C.S.; Ho. H.C.; Yeh. Y.S.; Wang, H.F.; Ho, R.M.; Tsai, D.H. Protein-silver nanoparticle interactions to colloidal stability in acidic environments. Langmuir 2014, 30, 43, 12755-64. [CrossRef]
- https://www.chem-astu.ru/chair/study/anchem/r_pril_4.htm (Accessed on 2023/09/13).
- Petritskaya E.N., Rogatkin D.A., Rusanova E.V. Comparative Characteristics of Antibacterial Effects of Silver and Nanosilver In Vitro. Almanach of Clinical Medicine 2016, 44, 2, 221–226. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).