1. Introduction
Cut flowers of Gerbera jamesonii are known for their widespread use for various decorative purposes due to their attractive flower colors and longer shelf life [1]. These plant species belong to the Asteraceae family and have both decorative and therapeutic significance [2]. In the global flower trade, gerbera occupies the fifth place and therefore its cultivation has increased dramatically in the recent past [3,4]. Gerbera growers often suffer major losses in flower production because the plant is sensitive to various types of biotic and abiotic stress. [5] To reduce the losses and increase productivity, polyhouse or nethouse cultivation has been mainly used to maintain controlled conditions. Although greenhouse cultivation promises high yields, repeated fertilization leads to enormous accumulation of salt on the soil surface, resulting in a toxic ecosystem [6]. Soil salinization is a major threat to crop growth and output, impacting approximately 1,125 million hectares worldwide [7]. Half of the irrigated land worldwide is forecast to be salinized by 2050, with the quantity of arable land suitable for conventional crop farming decreasing by three hectares every minute [8,9]. Salinity in plants, and particularly in Gerbera, weakens the ability of roots to absorb water, leading to reduction in its overall growth [11]. Both, reduced water uptake and elevated Na+/Cl- ions intracellular levels through roots lead to creation of an ionic gradient in the tissues causing an osmotic shock and nutrient imbalance [12]. Gerbera possess different mechanisms to overcome salinity stress, such as scavenging the reactive oxygen species with superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX) and glutathione peroxidase (GPX), thereby minimizing the intracellular damage [11,13]. One of the ROS intermediates, peroxides, accumulate in excess amounts and cause lipid peroxidation of cell membranes, which in turn causes cell leakage and cell death [14]. Based on the level of plant salt tolerance, antioxidative defense and ROS scavenging activity reach different levels [15].
Previous research in our laboratory has provided an indication that there is cultivar-specific salt tolerance in gerbera, with yellow-flowering gerbera cultivars being more salt tolerant than white-flowering gerbera cultivars [16]. Plant growth regulators (PGRs) modulate the response of plants to biotic and abiotic stresses and regulate their growth and developmental progression. Thiourea (TU) or thiocarbamide, a non-physiological thiol-based ROS scavenger is a synthetic compound containing nitrogen (as -NH2) and sulfur (as -SH), which positively enhance plant growth under abiotic stress conditions [17,18]. Several methods such as seed priming, foliar spray or in vitro supplementation were employed as TU treatments to relieve stress in plants [19]. Thus, thiourea is a bio-fertilizer with plant growth enhancer properties and abiotic stress alleviator in several crop and ornamental plants [14]. In the current work we studied the role of TU application in improving salt tolerance of white flowered Gerbera cultivar.
The aim of this study was to analyze the enzymatic and non-enzymatic defense activities in the two gerbera cultivars (yellow and white flowering) and to investigate the priming effect of TU application (1.0 mM, 5.0 mM &10 mM) on the acquisition of salt tolerance in gerbera treated with 200 mM NaCl. Studies on TU -induced salt tolerance in gerbera have not been conducted before, which prompted us to investigate the physiology of gerbera pretreated with TU and exposed to salinity.
2. Results and Discussion
Upon completion of the treatment phase (thiourea application followed by NaCl 200mM), a prominent bleaching was observed in the leaves of T2 group of both yellow and white varieties and it was minimal in T1 (control), T3, T4 and T5 plants (Fig. 1). While T3, T4 & T5 were found to demonstrate a similar increase in both leaf size and shape, there was a remarkable decrement in T2. The phenotypic traits observed in T3, T4 and T5, demonstrates the beneficial role of TU in recovering from the salt stress injury. Previous research conducted in our lab has demonstrated that salinity has an adverse impact on Gerbera growth, resulting in a decrease in leaf width and leaf length [4]. In the current study, application of TU evaded the unfavorable effects experienced by Gerbera during salt stress with stunted plant growth. The development of fresh leaves was one of the visible signs in T3, T4 & T5 in contrast to T2 indicating the growth promoting role of TU in Gerbera (Fig 1) as observed in several studies [17,18,19].
Figure 1.
Effect of salinity & TU application on the morphological characteristics of white and yellow Gerbera cultivars. Salinity denotes the treatment of plants with 200 mM NaCl (T2 treatment group: white and yellow), whereas TU treatment denotes the application of an exogenous foliar spray containing 1, 5 and 10 mM TU (T3, T4 & T5 treatment groups: C, D & E). The T1 treatment group-A's untreated leaves served as the experimental control.
Figure 1.
Effect of salinity & TU application on the morphological characteristics of white and yellow Gerbera cultivars. Salinity denotes the treatment of plants with 200 mM NaCl (T2 treatment group: white and yellow), whereas TU treatment denotes the application of an exogenous foliar spray containing 1, 5 and 10 mM TU (T3, T4 & T5 treatment groups: C, D & E). The T1 treatment group-A's untreated leaves served as the experimental control.
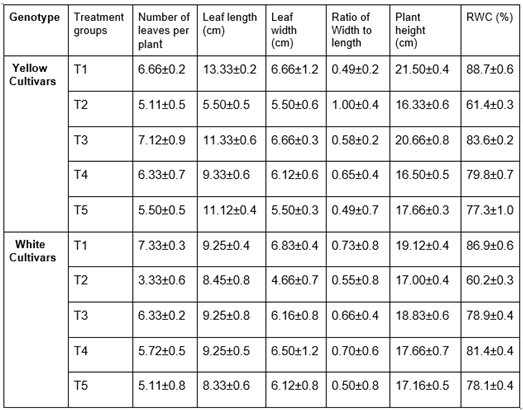
Height of plantlets, leaf length, and leaf width were evaluated before and after treatments in order to further examine the priming effect of TU on plant morphology of Gerbera (
Table 1). In short, plant height in T2 was significantly lower than all the other treatment groups whereas T3, T4 and T5 displayed increased parameters but were lower than T1. Similar kind of observations was noticed with respect to other measurable parameters like leaf length and width. Our results are in corroboration with TU involved salinity studies in Brassica juncea, Wheat & Maize wherein application of TU resulted in improved plant height and growth [30,31,32,33].
While quantifying the RWC of leaves the T2 showed signs of decrease, the other treatment groups (T3-5) were found to be similar to the T1. In brief, RWC in T2 decreased by 27.3% in white and 26.7% in yellow, respectively, when compared to T1. However, an increase in RWC was seen in T3, T4, and T5 when compared with T2 (
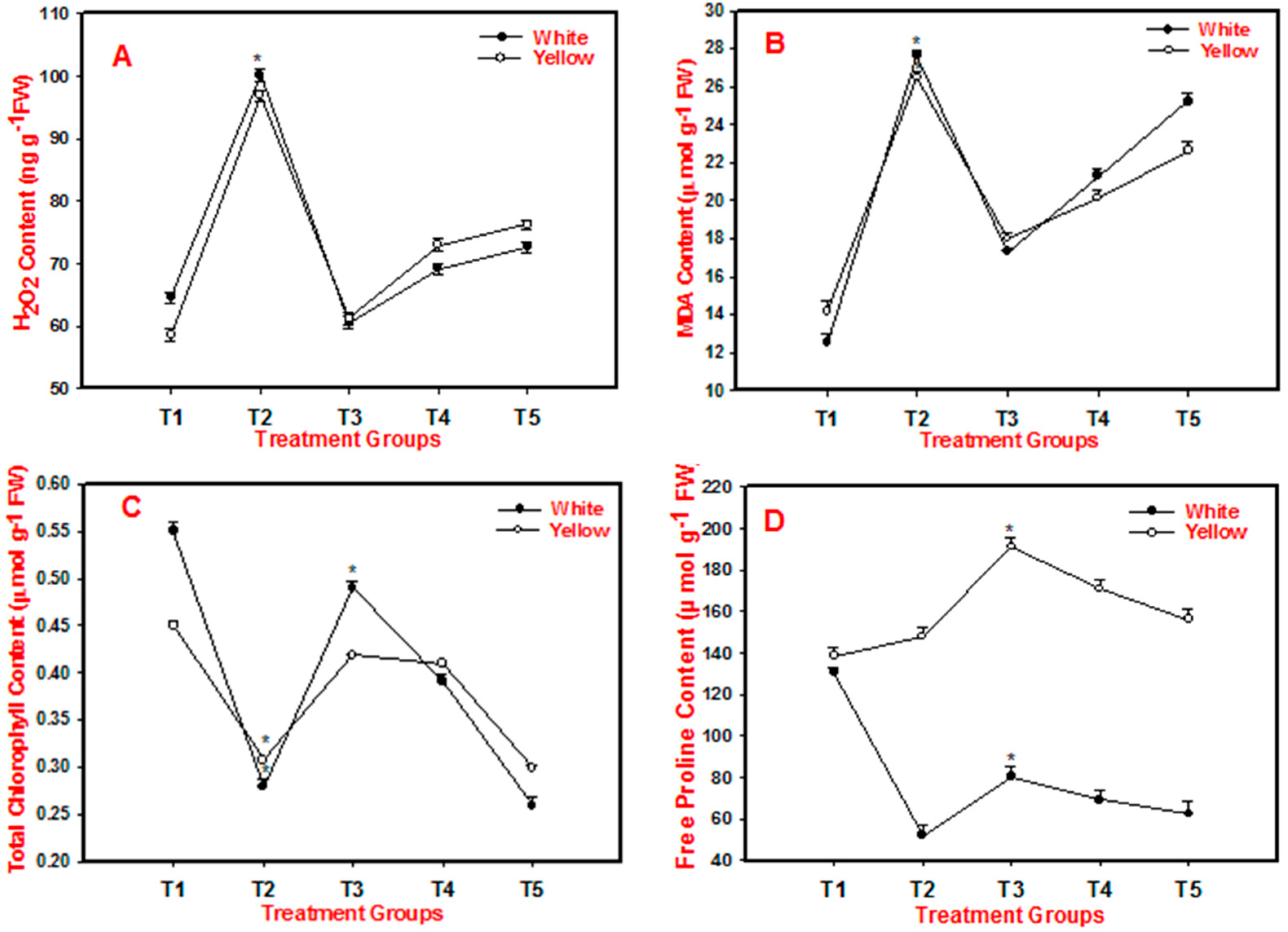
Table 1). These results suggest that TU might play a crucial role in increment of the biomass content by retaining water content in Gerbera. Similar to our observations, in leaves of maize seedlings Plant FW, DW, and RWC improved as a result of exogenous application of TU (3.5 and 7.0 mM) [18,34]. To further investigate the effect of TU on salinity alleviation in Gerbera, we examined H2O2 and MDA (malondialdehyde) levels which were found to be high in T2 of both cultivars. The H2O2 formation in plants indicates intracellular physiological damage and triggers the plant defense system to activate stress response genes such as CAT, POD, and several others [35]. The MDA accumulation is caused by lipid peroxidation in cell membranes and signifies oxidative damage [35]. In the current study, H2O2 and MDA levels in T3, T4, and T5 were found to be lower than in T2 and significant when compared to T2, indicating that cell damage was minimized in plants pre-treated with Thiourea (Fig 2a & 2b). Similar to our reports, salinity stress in Sunflower and Maize resulted in increased MDA which was reduced to negligible amounts when TU (10 and 20 mM) and (3.5 and 7.0 mM) was applied [34,37].
Figure 2.
Figure 2. TU-induced salinity stress alleviation. A–D shows the impact of salinity and TU on the contents of H2O2, MDA (lipid peroxidation), total chlorophyll, and free proline in the white and yellow cultivars of Gerbera. Salinity refers to plants treated with 200 mM NaCl (T2 treatment group), while TU treatment refers to exogenous foliar sprays of 1, 5 mM and 10 mM TU (T3, T4, and T5). The T1 treatment group's experimental control consisted of the untreated leaves. Each bar displays the mean average and standard error of three replicates of each treatment performed at random times. When there are asterisks next to the bars, one-way ANOVA (Holm-Sidak technique) has established that the values are statistically significant.
Figure 2.
Figure 2. TU-induced salinity stress alleviation. A–D shows the impact of salinity and TU on the contents of H2O2, MDA (lipid peroxidation), total chlorophyll, and free proline in the white and yellow cultivars of Gerbera. Salinity refers to plants treated with 200 mM NaCl (T2 treatment group), while TU treatment refers to exogenous foliar sprays of 1, 5 mM and 10 mM TU (T3, T4, and T5). The T1 treatment group's experimental control consisted of the untreated leaves. Each bar displays the mean average and standard error of three replicates of each treatment performed at random times. When there are asterisks next to the bars, one-way ANOVA (Holm-Sidak technique) has established that the values are statistically significant.
We also measured the total chlorophyll and proline content in both cultivars and found that T2 had a significant drop in chlorophyll content, which was particularly prominent in White cultivars. Any oxidative stress would reduce chlorophyll production and cause complexes to break down [38]. An increase in chlorophyll content was seen in T3, T4 and T5 of both cultivars, which was substantial when compared to T2 and was more prominent in T4 (Fig 2c). Similarly, proline, which acts as a compatible solute in stressed plants, accumulated less in white than in yellow in T2, indicating that white is more salt sensitive than yellow. Furthermore, we found an increase in accumulated free proline levels in both cultivars in T3, T4, and T5, indicating that TU may play a role in preserving growth in salinity-exposed plants (Fig 2d). Proline operates as a significant organic osmolyte that accumulates in stressed plant tissues, safeguarding cell membranes from salt-induced oxidative stress through improving the activity of multiple antioxidants [39]. Studies on Thiourea-induced salt tolerance in Maize support our findings, which show that exposing NaCl-stressed seedlings to (3.5 and 7.0 mM) TU increased chlorophyll and proline levels [18,34].
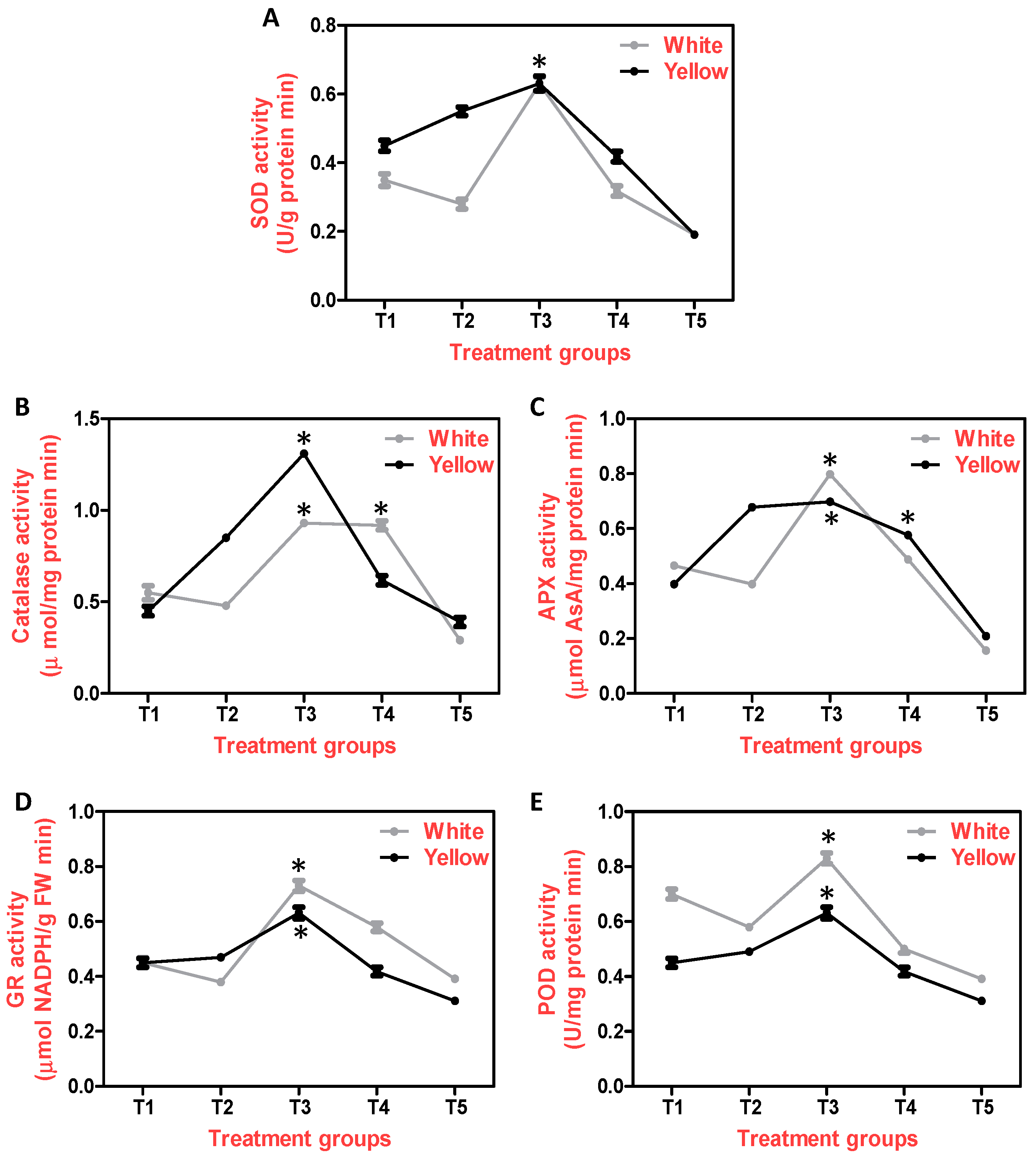
To understand the function of Thiourea in the overall negative consequences of salinity, we explored the activity of several antioxidant enzymatic defense systems like SOD, CAT, APX and GR. During this study, we observed a significant increase in SOD activity in T2 when compared to T1 in yellow cultivars, but a significant decrease in SOD levels was observed in white cultivars, demonstrating the white cultivar's sensitivity to salinity stress as reported by Uzma et al. 2023. When compared to T1 and T2, the SOD activity was shown to be greater in T3 plants of both cultivars (Fig 3a). The SOD is believed to be the first line of defense in the cellular environment of plants exposed to salinity, and the foliar spray of Thiourea has dramatically influenced SOD levels thus helping Gerbera to combat salinity.
Figure 3.
TU induced anti-oxidant capacity in gerbera grown under salinity stress. A-E: Effect of salinity & TU on antioxidant enzyme activities of A) Superoxide Dismutase, B) Catalase C) Ascorbate Peroxidase D) Glutathione Reductase and E) Peroxidase in white and yellow cultivars of Gerbera. Salinity indicates plants treated with 200 mM NaCl (T2 treatment group) and TU treatment indicates exogenous foliar spray of 1, 5 mM & 10 mM concentration of TU (T3, T4 & T5 treatment groups). The untreated leaves served as experimental control (T1 treatment group). Each bar is represented as mean average ± standard error of three replicates per treatment performed randomly at different time periods. Asterisks on bars indicate that the values are statistically significant as determined by one-way ANOVA (Holm-Sidak method).
Figure 3.
TU induced anti-oxidant capacity in gerbera grown under salinity stress. A-E: Effect of salinity & TU on antioxidant enzyme activities of A) Superoxide Dismutase, B) Catalase C) Ascorbate Peroxidase D) Glutathione Reductase and E) Peroxidase in white and yellow cultivars of Gerbera. Salinity indicates plants treated with 200 mM NaCl (T2 treatment group) and TU treatment indicates exogenous foliar spray of 1, 5 mM & 10 mM concentration of TU (T3, T4 & T5 treatment groups). The untreated leaves served as experimental control (T1 treatment group). Each bar is represented as mean average ± standard error of three replicates per treatment performed randomly at different time periods. Asterisks on bars indicate that the values are statistically significant as determined by one-way ANOVA (Holm-Sidak method).
We observed CAT activity and noticed a significant increase in T2. In yellow cultivars, a significant increase in its activity was recorded when compared to T1, whereas in white cultivars, there was an insignificant decrease in T2 and a significant increase in T3&T4, indicating the promising role of TU in enhancing the activity of a potent defense enzyme, CAT (Fig 3b). The CAT and SOD activities were increased in response to NaCl exposure, which was further increased when Thiourea was exogenously applied in Indian mustard, Wheat and Sesame (Sesamum indicum L.) [18,40,41].
Further, we questioned the role of APX, a cellular redox homeostasis enzyme in decreasing the toxic effects of NaCl and found that its activity increased in T2 which was significantly more pronounced in yellow cultivars. This type of increase was also observed in T3 & T4 which was higher and significant when compared to T1 (Fig 3c). We also observed a significant decrease in GR activity in T2 when compared to T1 in White and in T2 of yellow on significant decrease was seen across the T2 of Yellow Cultivars. In T3 & T4, a significant increase in GR activity was observed in both the cultivars (Fig 3d). Finally, we also observed a rise in the activity of POD in T2 which further increased in T3 when compared to T1 & T2 in yellow cultivars whereas in white decrease in T2 and significant increase in T3 POD activity was observed (Fig 3e). Our results are in corroboration with hybrid maize and where TU (400µM) led to reduction in the salt induced damage which was evident from the fact that, activities of APX, GR & POD got enhanced in them [42].
4. Materials and Methods
4.1. Experimental Design
For carrying out the present study, we chose five sets of treatment groups (T1, T2, T3, T4 and T5) each containing three plants in three replicates. The whole set-up was a completely randomized block design in which T1 & T2 received watering on a regular basis while T3, T4 and T5 were subjected to treatments with TU in 1.0 mM, 5.0 mM & 10.0 mM concentrations for a period of five days. From 6th day onwards, the T2, T3, T4 and T5 saplings, NaCl treatment (200mM) was initiated to impose salinity stress which lasted for five more days. T1 (control) received regular water as usual. Therefore, the whole experimental model comprised of a control (T1-0mM NaCl), salt treatment without TU (T2- 200mM NaCl) and three treatment groups (T3 - 1.0 mM TU+200 mM NaCl, T4 - 5.0 mM TU+200 mM NaCl and T5 - 10.0 mM TU+200 mM NaCl). According to Es-sbihi et al. 2021, TU was administered as a foliar spray and the NaCl through soil drenching. After completion of treatment period i.e., on 10th day, secondary leaves were collected, which were then frozen in liquid nitrogen and kept at -20°C to perform further study.
4.2. Measurement of Relative Water Content (RWC):
Making use of the method described by Sarker & Oba [20], the leaves' relative water content (RWC) was measured. On the tenth day after treatment, leaves were collected and their fresh weight (FW) was calculated. Following that, the turgor weight (TW) of the same leaves was measured after they had been placed in covered petri dishes and submerged in distilled water for 4 hours. Further, to determine the dry weight (DW), leaves were baked in a preheated oven for 24 hours at 70°C. The formula shown below was used to calculate the RWC.
4.3. Estimation of MDA Content
With a few minor modifications, the amount of malondialdehyde (MDA) was determined based on the method of Heath and Packer [21]. In a nutshell, frozen leaf tissue (1gm) was homogenized in 1ml of 0.5% TCA buffer (trichloroacetic acid), and the supernatant was collecteds after centrifugation (19000g) for 20 minutes. This was then heated at 95°C for 15 minutes after adding equal amounts of TBA (thiobarbituric acid), a reagent made by combining 0.25M HCl with 15% TCA (w/v) and 0.375% TBA (w/v). Post heat treatment, by placing the samples on ice, the reaction was stopped. After centrifuging the mixture at a rate of 15,000 g for 15 minutes, the absorbance at 532 nm was measured. By deducting the absorbance measured at 600 nm from the real absorbance, the resultant turbidity was eliminated. The quantity of MDA was determined using the extinction coefficient of 155mm-1cm-1, and values were values were presented in mole per g FW.
4.4. Assessment of H2O2 Content
A frozen leaf sample (100 mg) was homogenized in 100 mM K-Phosphate buffer (pH 7.8) and centrifuged at a speed of 19,000 g for 20 minutes in order to measure the H2O2 content. 0.5ml of 0.1% trichloroacetic acid (TCA), 100mM Na-phosphate buffer (pH 7.8), and 2ml of 1.0M KI reagent were added to the supernatant and incubated for an hour in the dark. At 390 nm, the absorbance was measured, and H2O2 production was quantified.
4.5. Determination of Proline Content
In accordance with Bates et al. [22], the proline content was determined using the Ninhydrin technique. After being homogenized in 3% sulfo-salicylic acid (5 µl/mg FW) for 100 mg of frozen leaf sample, the mixture was centrifuged at 5000 g for 15 min. 3% sulfo-salicylic acid, 200 µl of glacial acetic acid, 200 µl of acidic ninhydrin, and 100 µl of supernatant were added to 100 µl of supernatant. After mixing, samples were incubated for 60 minutes at 96°C. By keeping the tubes on cold, the process was stopped. Toluene (1 ml) was then added to extract the proline. After addition, samples underwent 20 seconds of vortexing before being left to stand to separate into their organic and aqueous phases. The proline-containing chromophore was gathered, its absorbance was assessed at 520 nm, and results were reported in µmole per g FW
4.6. Measurement of Chlorophyll Content
Chlorophyll content was measured in accordance with Lichtenthaler and Wellburn [23], extracted frozen leaf samples with acetone and measured their absorbance at 646 nm before subtracting their measured turbidity at 663 nm. The formula below was used to compute the total chlorophyll content, and values were represented as µmole per gram of fresh weight (FW).
4.7. Evaluation of Antioxidant Enzyme Activities
Frozen leaf samples (100 mg) were homogenized in ice-cold extraction buffer (1 ml) to figure out antioxidant enzyme activities. 100mM potassium phosphate buffer (pH 7.0) and 1mM EDTA made up the extraction buffer. In addition, 1% PVP (polyvinyl pyridine) and 0.5% (v/v) Triton X were added to the mixture to increase SOD (superoxide dismutase) activity as well as APX (ascorbate peroxidase) activity. The homogenate was centrifuged for 15 minutes at 5000g, and the supernatant now referred to as enzyme extract was employed in subsequent steps.
Bovine serum album (BSA) (Sigma Aldrich co, USA) was used as a reference to evaluate the total soluble protein content of the enzyme extract using Lowry's technique (1951), and absorbance was measured in mg/g FW.
i) Measurement of Catalase activity: Catalase activity (CAT) was measured in accordance with Aebi et al. [24] with a few minor modifications. Phosphate buffer (50 mM) and H2O2 (0.1 mM) were added to 100 g of enzyme extract, and absorbance was measured at 240 nm. For three minutes, the drop in substrate (H2O2) concentration was measured and its molar extension coefficient (36 M-1 cm-1) was computed. The CAT activity was expressed as µmoles of H2O2 decreased min-1mg-1protein.
ii) Measurement of APX activity: With a few minor adjustments, APX activity was determined according to Nakano and Asada [25]. Ascorbate (ASA-0.1mM), H2O2 (0.3mM), and potassium phosphate buffer (50mM - pH 7.0) were all added to 100µg of enzyme extract. Ascorbate's molar extension coefficient of 2.8 mM-1 cm-1 was used to quantify the absorbance of this reaction mixture. The activity of the APX enzyme was measured as µmoles of ASA reduced min-1 mg-1 protein.
iii) Measurement of Glutathione Reductase Activity: Activity of Glutathione Reductase (GR) was measured mostly in accordance with Foyer and Halliwell's [26] guidelines. In an experiment mixture containing 100mM potassium phosphate buffer pH 7.0, 1.0mM oxidized glutathione (GSSG), 0.08mM DTNB, 0.1mM NADPH, and 100µg of enzyme extract, DTNB(5,5'-dithio-bis-2-nitrobenzoicacid) was reduced. The calibration factor for the absorbance measured at 340 nm was the molar extinction coefficient of NADPH (6.2 mM-1 cm-1). The definition of one unit of GR was 1 µmole ml-1 GSSG decreased min-1.
iv) Measurement of SOD Activity: SOD activity was measured using Beyer and Fridovich's [27] method, in which the photochemical reduction of nitro blue tetrazolium (NBT) was observed at a wavelength of 560 nm. The reaction mixture contained 50µg of enzyme extract, 1.5 mM Na2CO3, 200 mM of methionine, 3.0 mM of EDTA, 2.25 mM of NBT, and 100 mM of potassium phosphate buffer (pH 7.0). The reaction was started by adding 60µM riboflavin to the abovementioned mixture and exposing it to two 15 W fluorescent lamps in an illuminated chamber for 15 min. Removal of the light source stopped the reaction, and the quantity of SOD used during the reaction—enough to block 50% of NBT—was given as one unit of SOD activity.
v) Measurement of Peroxidase (POD) activity: Activity of POD was assayed according to Shannon et al and M. A. Fieldes [28,29], with slight adjustments where, 0.05M guaicol and 0.8M H2O2 were added to 100μg of enzyme extract and rate of change in absorbance was recorded at 470 nm for 180 sec.
4.8. Statistical Analysis
The results from five treatment groups that were completed in triplicate at various times are averaged out in the data that are shown in figures. To determine the significance using the Holm-Sidak technique, the data were exposed to several statistical parameters (mean, standard error, and one-way ANOVA) using Sigma plot software version 12.0.5.
5. Conclusions
Horticulture worldwide suffers greatly from salinity, and to date, studies on salt stress in gerbera species are still in the early stages. Although there are many reports studying the consequences of salt-induced plant growth inhibition in ornamental and crop plants, there are few works in gerbera. In this regard, we attempted for the first time to reduce the negative effects of salinity in Gerbera by foliar application of thiourea (TU). According to our hypothesis, we observed an improvement in the growth conditions of gerbera pretreated with TU and exposed to salinity (200 mM NaCl). The results of the current studies suggest that thiourea can be used as an economical and environmentally friendly means to improve the cultivation of gerbera in polyhouses, as it can counteract the detrimental effects of salinity stress.
This research report on TU induced salt tolerance in Gerbera may be further extended to other ornamental plants which are grown in poly/net houses. Furthermore, elucidating genes and metabolic pathways associated with salt tolerance in Gerbera during Thiourea treatment may shed light on molecular mechanism to benefitting Gerbera production under suboptimal environments.
Author Contributions
MP and UJ conceived the presented idea wherein MF and UJ developed the protocols and performed the wet lab experiments. MP, MF and UJ verified the biochemical/analytical methods and UJ supervised the findings of this work. Writing and draft preparation: GAP, MP, MF and UJ. All authors discussed the results and contributed to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank, Head, Department of Biotechnology for allowing us to utilize the central instrumentation facility of the Biotechnology Department, Telangana University, Nizamabad.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cioc, M.; Dziurka, M.; Pawlowska, B. Changes in Endogenous Phytohormones of Gerbera jamesonii Axillary Shoots Multiplied under Different Light Emitting Diodes Light Quality. Mol. 2022, 27, 1804. [Google Scholar] [CrossRef]
- El-Dein, S.N.; Hussein, A.; Abu-Bakr, M.S.; El-Dein, A.N.; Awad, H. M.; Ragab, E. A. Phytochemical analysis and determination of antioxidant, anti-cholesterol, anti-inflammatory and anti-proliferative activities of Gerbera jamesonii flowers. Adv. trad. med. 2023, 23, 863–875. [Google Scholar] [CrossRef]
- Singh, P.; Bhardwaj, A.; Kumar, R.; Singh, D. Evaluation of Gerbera Varieties for Yield and Quality under Protected Environment Conditions in Bihar. Int. J Curr. Microbial. App. Sci. 2017, 6(9):112-116. [CrossRef]
- Reinten, E.; Coetzee, J.; Van Wyk, B. E. The potential of South African indigenous plants for the international cut flower trade. S. Afr. J. Bot. 2011, 77, 934–946. [Google Scholar] [CrossRef]
- Uzma, J.; Talla, S.K.; Mamidala, P. Insights into the impact of spermidine in reducing salinity stress in Gerbera jamesonii. J. Appl. Boil. 2023, 11(4), 141–147. [Google Scholar] [CrossRef]
- Hafez, M.; Alexander, I.; Rashad, M. Evaluation of the Effects of New Environmental Additives Compared to Mineral Fertilizers on the Leaching Characteristics of Some Anions and Cations under Greenhouse Plant Growth of Saline-Sodic soils. Open Agric. 2020, 14, 246–256. [Google Scholar] [CrossRef]
- Hossain, M. S. Present scenario of global salt affected soils, its management and importance of salinity research. Int. Res. J. Biol. Sci. 2019, 1, 1–3. [Google Scholar] [CrossRef]
- Shabala, S.; Wu, H.; Bose, J. Salt stress sensing and early signaling events in plant roots: current knowledge and hypothesis. Plant Sci. 2015, 241, 109–119. [Google Scholar] [CrossRef]
- Singh, G. Salinity-related desertification and management strategies: Indian experience. Land Degrad. Dev. 2009, 20(4), 367–385. [Google Scholar] [CrossRef]
- Sharma, D. K.; Chaudhari, S.K.; Singh, A. In salt-affected soils agroforestry is a promising option. Indian Farming, 2014, 63(11): 19-22. https://krishi.icar.gov.in/jspui/handle/123456789/3365.
- Uzma, J.; Talla, S.K.; Madam, E.; Mamidala, P. Salinity-driven oxidative stress in Gerbera jamesonii cv. Bolus. J. Appl. Hortic. 2022b, 24(2):240-244. [CrossRef]
- Yang, Y.; and Guo, Y. Unraveling salt stress signaling in plants. J. Integrat. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Waqas, M.A.; Kaya, C.; Riaz, A.; Farooq, M.; Nawaz, I. Potential Mechanisms of Abiotic Stress Tolerance in Crop Plants Induced by Thiourea. Front. Plant Sci. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Uzma, J.; Talla, S.K.; Madam, E.; Mamidala, P. Assessment of Salinity Tolerance Deploying Antioxidant Defense Systems in Gerbera Jamesonii. Biosci Biotech Res Asia. 2020a, 19(1), 243–254. [Google Scholar] [CrossRef]
- Garg, B. K.; Burman, U.; Kathju, S. Influence of thiourea on photosynthesis, nitrogen metabolism and yield of clusterbean (Cyamopsis tetragonoloba (L.) Taub. under rainfed conditions of Indian arid zone. Plant Growth Regul. 2006, 48, 237–245. [CrossRef]
- Dhillon, S. A.; Mujahid, N.; Shahbaz, M.; Debez, A. Response of sesame (Sesamum indicum L. ) to foliar-applied thiourea under saline conditions. Intl. J. Appl. Exp. 2023, 2, 167–177. [Google Scholar] [CrossRef]
- Perveen, T.; Nawaz, K. Modulation of morpho-physiological and biochemical attributes of Solanum Melongena L. (Brinjal) by Exogenous Application of Thiourea under salinity stress.Pak. J. Bot., 2021, 53(6), 1969-1978. [CrossRef]
- Sarker, U.; Oba, S. Drought Stress Enhances Nutritional and Bioactive Compounds, Phenolic Acids and Antioxidant Capacity of Amaranthus Leafy Vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.; Packer, L. Photoperoxidation in isolated chloroplasts. Kinetics and stochiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bates, L. S.; Waldren, R. P.; Teare, I. D. Rapid determination of free proline for water stress studies Plant Soil. 1973, 39, 205–7. [CrossRef]
- Lichtenthaler, K.; Welburn, A. R. Determination of Total Carotenoids and Chlorophylls A and B of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Aebi, H. Catalases. In: Methods of Enzymatic Analysis Vol 2. Bergmeyer Hu. (Ed), 1974, 673–684. New York: Academic Press. [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in Spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef]
- Beyer, W. F. Jr.; Fridovich, I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161(2), 559–66. [Google Scholar] [CrossRef]
- Shannon, L.M.; Kay, E.; Lew, J.L. Peroxidase Isozymes from Horse radish Roots : isolation and physical properties. chemistry and metabolism of macromolecules. The J. Biol. Chem. 1966, 241(9), 2166–2172. [Google Scholar] [CrossRef]
- Fieldes, M. A.; Bashour, N.; Deal, C. L.; Tyson, H. Isolation of peroxidase isozymes from two flax genotypes by column chromatography. Canad. J. Bot. 1976, 54(11), 1180–1188. [Google Scholar] [CrossRef]
- Pandey, M.; Srivastava, A. K.; D’Souza, S. F.; Penna, S. Thiourea, a ROS scavenger, regulates source-to-sink relationship to enhance crop yield and oil content in Brassica juncea (L.). PLoS One. 2013, 8, e73921. [CrossRef]
- Srivastava, A. K.; Srivastava, S.; Penna, S.; D’Souza, S. F. Thiourea orchestrates regulation of redox state and antioxidant responses to reduce the NaCl-induced oxidative damage in Indian mustard (Brassica juncea (L.) Czern.). Plant. Physiol. Biochem. 2019, 49, 676–686. [CrossRef]
- Suryavanshi, P.; Buttar, G. S. Effects of exogenous osmoprotectants on physiological characteristics of wheat. Int.J.Curr.Microbiol.App.Sci. 2018, 7, 1077–1089. [Google Scholar]
- Granaz; Shaukat, K.; Baksh, G.; Zahra, N.; Hafeez, M.B.; Raza, A.; Samad, A.; Nizar, M.; Wahid, A. Foliar application of thiourea, salicylic acid, and kinetin alleviate salinity stress in maize grown under etiolated and de-etiolated conditions. Discov. Food. 2022, 2, 27. [CrossRef]
- Kaya, C.; Sonmez, O.; Aydemir, S.; Ashraf, M.; Dikilitas, M. Exogenous application of mannitol and thiourea regulates plant growth and oxidative stress responses in salt-stressed maize (Zea mays L.). J Plant Interact. 2013, 8(3), 234–41. [CrossRef]
- Angon, P.B.; Tahjib-Ul-Arif, M.; Samin, S.I.; Habiba, U.; Hossain, M.A.; Brestic, M. How do plants respond to combined drought and salinity stress?—A systematic review. Plants. 2022, 11(21):2884. [CrossRef]
- Radhwan, A.; Hussein, K.B. ; Al-Kaaby; Adul-Qadir L.H. Antioxidant responses in wheat plants (Tritticum Aestivum L.) Treated with Thiourea. Plant Arch. 2020, 20(2),717-722. e-ISSN:2581-6063 (online), ISSN:0972-5210.
- Akladious, S. A. Influence of thiourea application on some physiological and molecular criteria of sunflower (Helianthus annuus L.) plants under conditions of heat stress. Protoplasma, 2014, 251, 625–638. [CrossRef]
- Mbarki, S.; Sytar, O.; Cerda, A.; Zivcak, M.; Rastogi, A.; He, X. Strategies to mitigate the salt stress effects on photosynthetic apparatus and productivity of crop plants, In: Salinity Responses and Tolerance in Plants. Volume 1: Targeting Sensory, Transport and Signaling Mechanisms, 2018, 85-136. [CrossRef]
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V. Regulation of L-proline biosynthesis, signal transduction, transport,accumulation and its vital role in plants during variable environmental conditions. Heliyon 2019, 5(12), e02952. [CrossRef]
- Srivastava, K.; Srivastava, S.; Penna, S.; D’Souza, S.F. Thiourea orchestrates regulation of redox state and antioxidant responses to reduce the NaCl-induced oxidative damage in Indian mustard (Brassica juncea (L.). Plant Physiol. Biochem. 2011, 49(6), 676-686. [CrossRef]
- Farzana, S.; Rasel, M.; Tahjib-Ul-Arif, M.; Al-Galib, M. A.; Sarker, K. K.; Hossain, M. A. Exogenous salicylic acid and thiourea ameliorate salt stress in wheat by enhancing photosynthetic attributes and antioxidant defense. J. Bangladesh Agric. Univ. 2020, 18(2), 272–282. [Google Scholar] [CrossRef]
- Sanaullah, T.; Wahid, A.; Sadia, B.; Hanif, A.; Maqbool, N.; Arshad, T.; Kabir, Z. Exogenous Application of Thiourea Ameliorates Salt Stress Effects by Alleviation of Oxidative Damage in Hybrid Maize. J. agric. Sci. Technol. 2016, 6, 220–231. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).