1. Introduction
Gerbera jamesonii commonly called Gerbera is well known across the globe for its cut flowers which are extensively used in many decorative applications due to their diversity of floral colors (white, yellow, pink, orange, red, etc.) with extended shelf life [
1]. These plant species are members of the Asteraceae family which are well known for ornamental traits in floriculture. Gerberas are used as cut flowers and occupy fifth place in the world’s floral trade exhibiting high sales around the year [
2]. Although Gerbera is very popular in the floral industry, farmers to date have experienced huge losses due to its vulnerability to biotic and abiotic stress [
3]. In view of the above, Gerbera growers prefer polyhouses which offer controlled conditions for sustainable production. Despite the high yields that poly house cultivation offers, frequent fertigation practices may cause a reduction in growth and productivity after three years of plantation because of salt build-up on the soil's surface, making it saline [
4].
Salinity not only impairs Gerbera’s ability to absorb water but also shows drastic effects on the growth of Gerbera [
5]. The decreased water absorption, combined with increased penetration of Na
+ and Cl
- into the plant cell, leads to altered plant metabolism and generates excessive Reactive oxygen species (ROS) [
6]. Peroxides are one of the ROS intermediates that build up in large quantities and cause the lipid peroxidation of cell membranes, resulting in cell leakage and cell death [
7]. During stress, plants elevate their antioxidative defense mechanism, which are engaged in scavenging the excess ROS produced [
8]. Recent studies in our laboratory suggested that different Gerbera cultivars have different salinity tolerance levels, with yellow flowered Gerbera cultivars being more tolerant than white flowered Gerbera cultivars [
3]. Employing certain antioxidants and plant growth inducers like Ascorbic acid (AsA) to reduce the lethal effects of salinity is well documented in several crop and ornamental plants [
9,
10].
The AsA, commonly referred to as vitamin C, is a cyclic molecule with a six-carbon ring comprising a conjugated enediol group, which is responsible for the antioxidant properties of AsA as it can donate electrons to neutralize free radicals [
11]. Free radicals are unstable molecules involved in intracellular damage, including DNA [
12]. Further, AsA plays a role in photosynthesis, growth, and, development, and is also involved in cell division and elongation, as well as the synthesis of cell wall components of plants to tolerate abiotic stresses such as salinity, drought, and, heat [
13,
14]. The AsA helps plants tolerate salinity stress in several ways. First, it acts as an antioxidant to scavenge free radicals generated by salt stress. Second, it helps to regulate ion homeostasis by maintaining the balance of sodium and potassium ions in plant cells. Third, it enhances photosynthesis by protecting the photosynthetic apparatus from salt-induced damage [
15]. Finally, it improves water relations by increasing the water uptake and retention capacity of plant cells and also reduces oxidative stress, regulating ion homeostasis and, enhancing stress response [
16,
17]. Therefore, as the AsA can help plants overcome the deleterious effects of salinity when grown in saline-rich environments as per the literature reports mentioned, in the current study we have employed AsA as an exogenous foliar spray and an attempt was made to identify the putative role of AsA in developing salt tolerance in Gerbera cultivars.
The current study aimed to investigate the priming effects of AsA (1.0 mM, 2.0 mM, & 4.0 mM) on acquiring salt tolerance in Gerbera treated with 200 mM NaCl, by analyzing the antioxidative enzymatic activities of Superoxide Dismutase (SOD), Catalase (CAT), Ascorbate Peroxidase (APX), Glutathione Reductase (GR) and Peroxidase (POD) and non-enzymatic defensive activities like proline and chlorophyll contents in the two cultivars of Gerbera. In addition, we also monitored H2O2 and MDA accumulation levels as they are considered to be the major indicators of oxidative damage in plants exposed to biotic or abiotic stress.
2. Results and discussion
2.1. Morphological improvements observed in Gerbera plants upon exposure to NaCl post-AsA pretreatment
2.1.1. Changes seen in the visual appearance and other parameters of leaves
The plants in both the cultivars white and yellow (W/Y) - E2 group displayed clear signs of leaf bleaching and stunting accompanied by leaf thickening, while those in the W/Y E1, W/Y E3, W/Y E4, and W/Y E5 showed minimal effects in the above-mentioned criteria (
Figure 1). Besides this, plants in the W/Y E3, W/Y E4, and W/Y E5 groups also exhibited an increment in leaf size and shape, while those in the W/Y E2 group showed a marked decrement. This observation might explain that the application of AsA as an exogenous foliar spray in Gerbera prior to its exposure to salt stress is making the plants resist and defend against the adverse effects and consequences caused due to salinity. Moreover, the W/Y E4 group performed best with maximum improvements in leaf characteristics, suggesting that 2.0 mM of AsA can be used as an optimal concentration to improve growth and productivity in Gerbera.
Previous research has shown that salinity negatively impacts Gerbera growth, reducing leaf width and length and it can be improved by the application of exogenous growth promoters such as polyamines [
5]. In the current study, AsA application prevented the unfavorable effects of salt stress on Gerbera, such as stunted growth and reduced new leaf development, which was observed in the W/Y E2 group but not in the W/Y E3, W/Y E4, and W/Y E5 groups (
Figure 1). In both the cultivars, YE1 and WE1 displayed 6.6 leaves per plant whereas YE2 and WE2 showed a decrease in 6.1 and 4.2 leaves per plant. With the supplementation of AsA, we observed incremental patterns in yellow flowering cultivars (0.5%, 1.1%, and 1%) and in white flower cultivars (1.1%, 3.1% and 2.4%) in E3, E4, and E5 respectively. These findings are in line with earlier research on safflower (
Carthamus tinctorious L.) and maize (
Zea mays L.) by Ali et al. 2015 and Farooq et al. 2020, [
18,
19] which demonstrated that application of AsA increased plant height and growth.
To further examine the priming effect of AsA on Gerbera plant morphology; plant height, leaf length, and leaf width were evaluated before and after treatments. AsA priming significantly improved all three parameters, but the increases were not as pronounced as in the control group in both cultivars (W/Y E1). In brief, there was a noticeable incremental display in terms of plant height in YE3, YE4, and YE5 when compared with YE2 which exhibited a 2.2%, 2.2%, and 0.6% increase and similarly, in WE3, WE4, and WE5 an enhancement of 2.0%, 2.8%, and 0.9% were when compared to WE2 in terms of plant height.
Due to huge variations across the control and AsA treatment groups on the number of leaves and plant height, we have also obtained the ratio of Width to length (RLW) of each plant leaf. Intriguingly, the RLW has shown increased patterns in all the AsA treatment groups in both cultivars when compared to the salt treatment group. In YE3, YE4, and YE5 (0.09%, 0.1%, and 0.03%) increment and in WE3 WE4 and WE5 (0.15%, 0.19%, and 0.06%) increment when compared to their respective E2 groups in both the cultivars. However, the existing data is not sufficient to understand the effects of higher concentrations of AsA (4.0 mM) on RWL values in both cultivars.
2.1.2. Improvement in the RWC
Similarly, AsA priming also increased relative water content (RWC) in leaves of salt stressed white cultivar where, we observed 84.7% of RWC in control which reduced to 65.8% when treated with NaCl. While, in plants pre-treated with AsA and then subjected to NaCl stress the values recorded were 75.8% which gives us evidence that AsA might help in improving the water content in leaves. A similar kind of observation was noticed in the yellow cultivar, we observed 86.2% of RWC in control which reduced to 69.1% when treated with NaCl. While in plants pre-treated with AsA and then subjected to NaCl stress the values recorded were 84.1% (
Table 1). In brief, The RWC in E2 decreased by 18.9% in white and 17.1% in yellow, respectively, when compared to E1. These results suggest that AsA may increase biomass content by retaining water. This is consistent with previous studies in Barley (
Hordeum vulgare L.) and Canola (
Brassica napus L.), which showed that AsA application improved the RWC [
20].
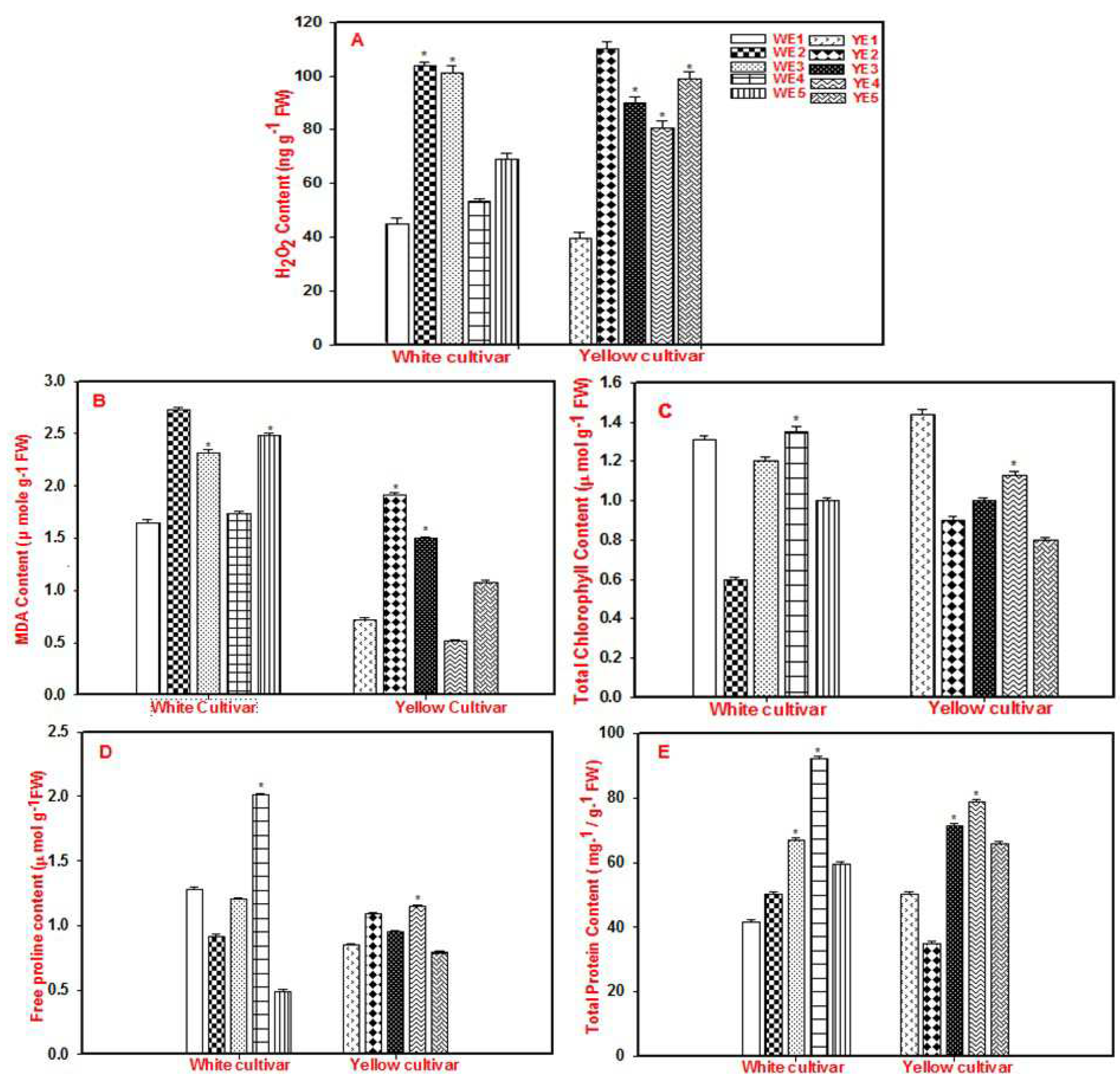
2.2. Measurement of the differential accumulation of oxidative stress indicators (MDA and H2O2) across treatments
To further investigate the effect of AsA on salt stress alleviation in Gerbera, we measured hydrogen peroxide (H2O2) and malondialdehyde (MDA) levels, which are biomarkers of oxidative stress.
H
2O
2 formation in plants is a hallmark of intracellular physiological damage and triggers the plant defense system to activate stress response genes such as CAT, APX, POD, etc., [
21]. Enzymes such as CAT, and APX will detoxify H
2O
2, a ROS intermediate into H
2O and O
2 whereas GR and POD will help in replenishing the reducing agents like AsA, NADPH, etc. required for the catalytic activity of APX. MDA accumulation is caused by lipid peroxidation in cell membranes and is a sign of oxidative damage [
22].
In the current study, H
2O
2 content in WE2 and YE2 was more than two-fold higher when compared to their respective controls (WE1 and YE1) indicating the devastating nature of NaCl in increasing oxidative damage in Gerbera. Upon application of AsA at a concentration of 1.0 mM did not improve the growth as well and the H
2O
2 levels were similar to that of WE2. Whereas, application of AsA at a concentration of 2.0 mM (WE4) reduced the H
2O
2 levels with its concentration similar to that of WE1 indicating that cell damage was minimized in plants pre-treated with AsA at a concentration of 2.0 mM. This suggests that AsA can help white-flowered Gerbera to tolerate salt stress by reducing oxidative stress and cell damage. In the Yellow flower cultivar, no reduction in H
2O
2 was seen even in E4; though it was lesser than YE2. Application of H
2O
2 at a concentration of 4.0 mM in both cultivars (WE5 and YE5) however, did not result in much improvement. This result gives us a conclusion that though yellow flowered Gerbera cultivar can tolerate salt stress up to some extent (150 mM NaCl) as per Uzma et al. 2023, it cannot tolerate higher levels of it (200 mM NaCl) (
Figure 2a,b).
MDA content in WE2 and YE2 was nearly two-fold and three-fold higher when compared to their respective controls (WE1 and YE1) which display the detrimental effects of NaCl on Gerbera. When AsA is applied at a concentration of 1.0 (W/Y E3) and 4.0 mM (W/Y E5) in both cultivars; there was a reduction in MDA levels which was significant in the yellow cultivar. The best results were obtained when AsA, at a concentration of 2.0 mM was applied both in yellow and white cultivars wherein, the levels of MDA in these treatments (WE4 and YE4) were more or less similar to WE1 and YE1. Our findings are consistent with previous studies on other plant species. For example, Mukhtar et al. 2016 reported that AsA application (75 and 150 mg L⁻¹) significantly reduced H
2O
2 and MDA levels in cauliflower (
Brassica oleracea L. var. Botrytis) plants under salt stress. [
23] Azzedine et al. 2011 [
24] also showed that AsA was effective in alleviating the adverse effects of salt stress in wheat by decreasing H
2O
2 levels in plant tissues.
2.3. Influence of AsA in improving the levels of chlorophyll and proline across treatments
Total chlorophyll content was drastically reduced to half in WE2 and to a quarter in YE2 when compared to WE1 and YE1 and this reduction was significant in white cultivar. Pre-treatment with AsA (1.0 and 2.0 mM concentrations) (W/Y - E3 and E4) prior to salt treatment resulted in improved chlorophyll content in both cultivars which was higher than E2 but lower than E1. Whereas, application of AsA at a concentration of 4.0 mM resulted in no improvement in both the cultivars when compared to the control (E1) but in white it was higher than E2. Chlorophyll content was significantly reduced in E2, particularly in the white cultivar. This suggests that oxidative stress reduced chlorophyll production and caused a complex breakdown (
Figure 2c) [
25].
Proline is a significant organic osmolyte that accumulates in plant tissues and protects cell membranes from stress-induced redox reactions by enhancing the activity of multiple antioxidants [
26]. The levels of proline in WE2 were lesser than WE1 whereas YE2 was higher than YE1 indicating that yellow might be accumulating more osmo-protectants like proline in higher amounts during salt stress than white and is helping the plants to overcome the stress. Considering pre-treatment of Gerbera cultivars with AsA at a concentration of 1.0 mM and then subjecting to salt stress resulted in improved proline accumulations in both the cultivars (WE3 and YE3) when compared to W/Y E1 and E2. A significant reduction is observed in plants pre-treated with 4.0 mM AsA in both cultivars which indicates that higher concentrations of AsA may not help plants to accumulate more osmo-protectants. To finalize, proline accumulation was lower in the white cultivar than in the yellow cultivar in E2, indicating that the white cultivar is more salt-sensitive. In contrast, proline levels increased in both cultivars in E3, especially in the white cultivar. This suggests that AsA can help preserve growth in salt-stressed plants by increasing proline accumulation (
Figure 2d). Our studies are in similar line with previous studies where the application of 1.0 mM of AsA in salt-stressed Barley improved the content of proline and was recorded as 1.74 µmol per gram [
27] by Agami et al. 2014 and the application of 1.0 mM of AsA in drought-stressed
Satureja hortensis resulted in 1.04 µmol per gram of proline [
28]. While, in our study, we noticed that the application of 2.0 mM of AsA (E4) led to 2.0 µmol per gram of proline accumulation in the white cultivar and 1.2 µmol per gram of proline accumulation in the yellow cultivar.
2.4. Improvements in the soluble protein content and antioxidative enzyme activities across treatments
The total soluble protein concentrations in leaf samples of white and yellow cultivars pre-treated with AsA (1.0, 2.0, and 4.0 mM) i.e., W/Y E3-E5 were more pronounced when compared to their respective controls (W/Y E1). Although protein content was higher at all concentrations, in WE4 and YE3 this increment was significant when compared to their respective controls (
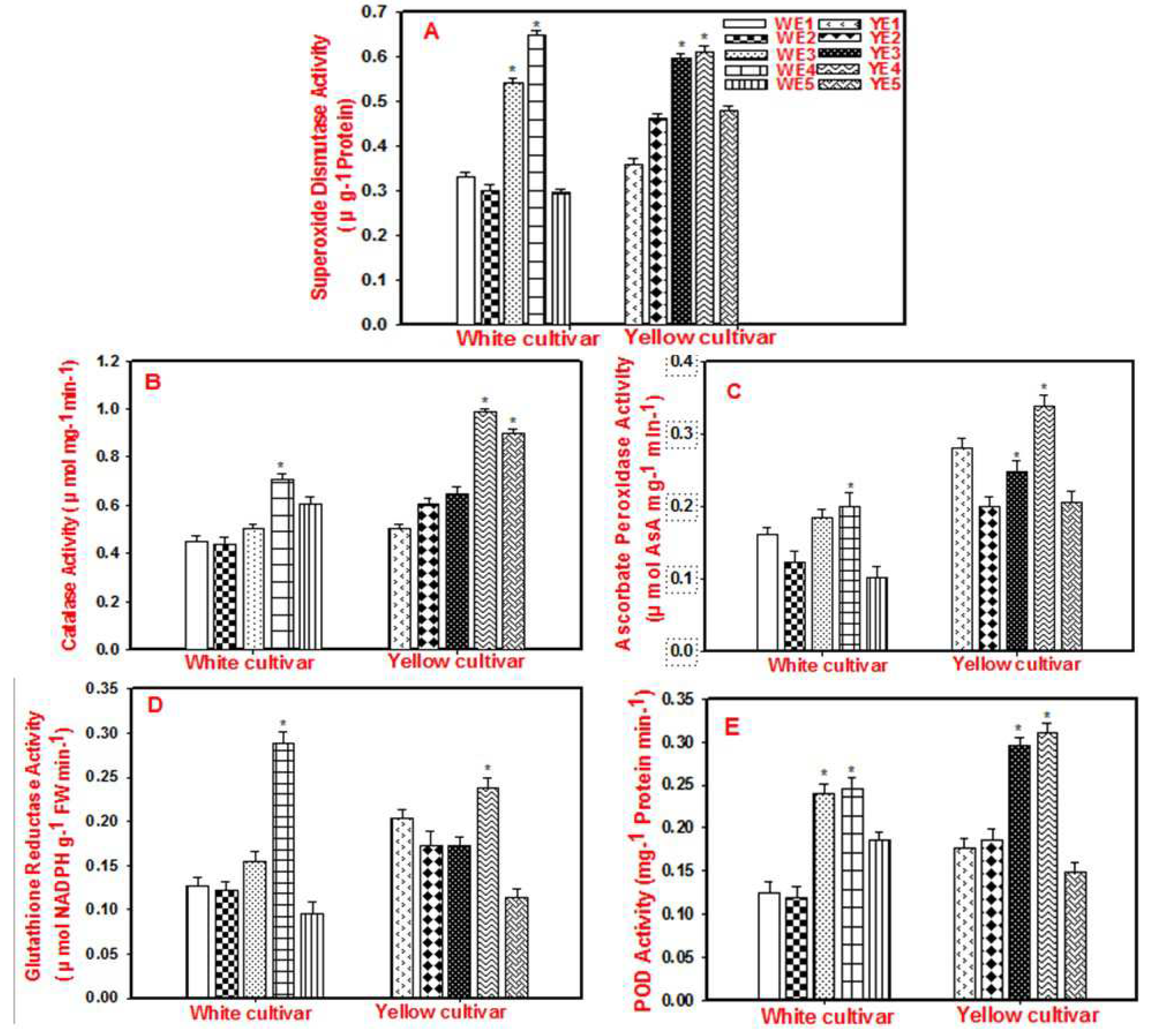
Figure 2e). To further investigate the role of AsA in mitigating the negative effects of salinity stress, we measured the activity of several antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), and peroxidase (POD).
2.4.1. Alterations in the activity of SOD
The activity of SOD depicted a two-fold significant enhancement in WE3 and WE4 whereas in WE5 there was a decrement noticed when compared to control (WE1) and NaCl-treated samples (WE2). In the yellow cultivar, similar results were recorded but the values at YE4 were higher than YE1 and lower than YE2 (
Figure 3a). As SOD is believed to be the first line of defense in the cellular environment of plants exposed to salinity, and because AsA plays a beneficial role in enhancing its activity, it can be believed that AsA might help plants to combat salinity.
Our findings are consistent with previous studies in Wheat, where application of 100 mg/L AsA in salt stressed cultivar MH97 lead to enhancement of SOD activity from 23 units per mg protein (as observed in control) to 30 units per mg protein (in AsA treated) [
29], whereas in our study the values of SOD activity were elevated from nearly 0.3 units per gram protein to 0.65 units per gram protein in white and 0.38 units per gram protein to 0.6 units per gram protein in the yellow cultivar. Similarly, in Sugarcane, AsA application (0.5 mM and 1 mM, respectively) increased SOD activity from 65 units per gram protein to 95 and 75 units per gram protein respectively [
30], which indicates that the application of AsA will improve the antioxidative defense in different plant species subjected to salt stress.
2.4.2. Improvements in the activity of CAT
The activity of CAT in all the treatments exhibited an increased trend while in WE4 we noticed a two-fold significant increment when compared to WE1 and WE2. The same type of response was also noticed in yellow but the activity of CAT in YE2 was slightly higher than in YE1 but was non-significant (
Figure 3b). CAT activity was significantly higher by approximately 20% and 30% in the yellow cultivar than in the white cultivar in the E2 and E4 groups. This suggests that AsA can enhance the activity of CAT, a powerful antioxidant enzyme. Our studies are in line with recent research carried out in a salt-sensitive cultivar of wheat - MH97 where application of AsA (100 mg/L) to salt-stressed seedlings (120 mM NaCl) resulted in fivefold increase in the activity of CAT when compared to control [
29].
2.4.3. Modifications in the activity of APX
Further, we questioned the role of APX, a cellular redox homeostasis enzyme in decreasing the toxic effects of NaCl. The APX activity in WE2 was highest which did not lead to any increment upon exposure to various concentrations of AsA. While, in the yellow cultivar, the activity of APX was two-fold higher in E3 and was significant when compared to E1 but it does not exhibit any good response in E2, E4, and E5 when compared to E1(
Figure 3c). Results obtained in our studies are in line with previous research carried out in Peanuts where, supplementation of AsA (50 mM) played an effective role in culminating salt stress (50 mM NaCl) indicated by the enhanced activity of APX, an antioxidative defense-related enzyme which plays a prominent role in ascorbate glutathione cycle operative in plants with efficient defense potentials [
31].
2.4.4. Increased activity of GR
The activity of GR depicted a three-fold increment in WE4 when compared to control and NaCl-treated plants (WE1 and WE2). Its activity in WE3 was negligibly high whereas in WE5 it was lowest. In the yellow cultivar, a similar type of response was observed (
Figure 3d). In corroboration with our observations, similar results were reported in a study related to figuring out the role of AsA (50 mM) in culminating salt stress (50 mM NaCl) in peanuts where AsA enhanced the activity of GR in salt-stressed plants [
31].
2.4.5. Enhancements in the activity of POD
Finally, we also observed an increase in peroxidase (POD) activity in the W/Y E2 groups which explains that the plants in these groups are elevating their defense potentials, which further increased in the W/Y E3 groups compared to the W/Y E1 groups. The increment in POD activity in W/Y E3 groups signifies that at a concentration of up to 2.0 mM, AsA is modulating the cellular antioxidative enzymatic defense systems to protect the plants against salt stress. However, POD activity did not change much in the E4 and E5 groups compared to the E2 group in the white cultivar, and a slight decrease was observed in the yellow cultivar (
Figure 3e). This decrement may be because higher concentrations of AsA may not be tolerated by the cellular environment in plants. In brief, The POD activity in white and yellow showed a similar response wherein WE3 and YE3 displayed an incremental response when compared to all other treatments and were significant when compared to their respective controls. Our results are consistent with previous studies on low-temperature stressed Maize cultivars where the activity of POD enhanced by 0.03 units per gram protein when compared to control upon application of 40 mg/L AsA [
32]. Similar reports were noticed in Barley, where the POD activity values enhanced from 0.65 units per gram protein in control to 1.16 units per gram protein in 200 mM NaCl-treated samples pre-treated with 1.0 mM AsA [
27]. These reports suggest that AsA when applied at optimal concentrations mitigates salt-induced damage, as evidenced by the enhanced activities of APX, GR, and POD.
4. Materials and Methods
4.1. Chemicals and reagents
Sodium chloride (NaCl), Hydrochloric acid (HCl), potassium iodide (KI), Hydrogen peroxide (H2O2), Sulfosalcylic acid, Acetic acid, Ninhydrin, Toluene, Acetone, Na2Co3 (Sodium carbonate), NaOH (Sodium hydroxide) were obtained from Himedia Laboratories pvt. Ltd. India.
TCA (trichloroacetic acid), TBA (thiobarbituric acid), Na-K Tartarate, CuSo4 (copper sulfate), Folin's reagent, Oxidised glutathione (GSSG), 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB), Nicotinamide adenine dinucleotide phosphate hydrogen (NADPH), Nitroblue tetrazolium (NBT), Riboflavin, Guaiacol, Ascorbate (AsA), Triton X, polyvinyl pyridine (PVP), Ethylene diamine tetra acetic acid (EDTA), Bovine serum albumin (BSA), Methionine were procured from Sigma Aldrich, India.
4.2. Bio-analysis and calculations
Five sets of experimental groups (E1, E2, E3, E4, and E5) for each cultivar were chosen for the current investigation, each of which had three plants in three replicates. Therefore, this study consisted of ten groups (WE1-WE5 and YE1-YE5) where W and Y denote white and yellow flowered Gerbera cultivars. The complete setup was organized as a random block, where W/YE1 and W/YE2 were watered daily, whereas W/YE3, W/YE4, and W/YE5 were subjected to 10 days of AsA treatment at concentrations of 1.0 mM, 2.0 mM, and 4.0 mM. Post-treatment with AsA, for four groups (W/YE2 - W/YE5), 200 mM NaCl dissolved in distilled water was given from the 11th day onwards till the 20th day. Meanwhile, W/YE1 continued to receive water and served as control. In brief, the whole experimental setup for each cultivar comprised, a control (W/YE1-0 mM NaCl), a salt treatment without AsA (W/YE2- 200 mM NaCl), and three treatment groups (W/YE3 - 1.0 mM AsA+200 mM NaCl, W/YE4 - 2.0 mM AsA+200 mM NaCl and W/YE5 - 4.0 mM AsA+200 mM NaCl).
4.2.1. Sampling and data collection:
The fully expanded secondary leaves from all plants that were subjected to treatments were harvested on the 20th day of treatment, according to the experimental design. These leaf samples were washed in distilled water, blotted dry with tissue paper, immediately crushed in liquid nitrogen, and stored at -20°C for further use. This whole procedure was carried out in the early morning to avoid other kinds of stress factors getting imposed on the samples.
4.2.2. Determination of fresh, turgor, dry weights, and relative water content:
Further, on the same day, using the other secondary leaves, the fresh weight (FW), turgor weight (TW), and dry weight (DW) was determined. To elaborate, the FW of leaf samples was measured using a digital weighing balance, and then the leaf samples were suspended for 4 h in distilled water using petri dishes to calculate the TW. Furthermore, to determine the DW, the leaf samples were kept in an oven for 72 h at 65 °C and were measured. In addition, the heights of plants ranging from roots to shoot apex were measured using a measuring tape, following the method described by Es-Sbihi et al. 2021 [
33]. After determining the above parameters, the relative water content (RWC) of the leaf samples was calculated using the formula (Eq: 1) given by Sarker & Oba 2018 [
34].
4.3. The assay for estimating Malondialdehyde (MDA) content:
To assess the level of intracellular damage occurred in plants due to stress; monitoring the MDA accumulation will serve as a useful parameter. In the current study, the method of Heath and Packer 1968 [
35] was followed to determine MDA levels. During the assay, 1 gm of frozen leaf tissue was homogenized in 1 ml of 0.5% TCA buffer (trichloroacetic acid), and the supernatant was collected following centrifugation (19000 rpm) for 20 min. After adding equal volumes of TBA (thiobarbituric acid), a reagent comprising 0.25M HCl with 15% TCA (w/v) and 0.375% TBA (w/v), it was heated at 95°C for 15 minutes. After completion of the incubation time, it was centrifuged for 15 min at a rate of 15,000 g and the absorbance at 532 nm was calculated. By taking down the absorption at 600 from the real absorbance, the resultant turbidity was eliminated. The quantity of MDA was determined using the extinction coefficient of 155mm
-1cm
-1, and values where presented in mole per gram fresh weight (FW).
4.4. The assay for estimation of H2O2 Content:
To assess the amount of primary ROS accumulated in plants during stress, the determination of H
2O
2 will be an appropriate parameter, as H
2O
2 is one of the primary ROS generated in plants during stress. For this, 100 mg frozen leaf sample was taken and crushed in Potassium-Phosphate buffer (100 mM, pH 7.8) following the method of Alexieva et al. 2001 [
36]. Later, it was centrifuged at 19, 000 rpm for 20 minutes. The supernatant was allowed to react with 2ml of 1.0M Potassium Iodide (KI) reagent and 0.5ml of 0.1% trichloroacetic acid (TCA) and incubated in dark conditions for an hour. Following this, the absorbance values were recorded at 390 nm to calculate the amount of H
2O
2 produced.
4.5. The assay for the estimation of Proline content:
Proline is one of the osmoprotectants and a compatible solute accumulated inside a cell during unfavorable conditions experienced by plants. To estimate the content of proline, the nutrition assay-based protocol was given by Bates et al. 1973 [
37]. For 100 mg of a frozen leaf sample, 3 % sulfo-salicylic acid (5 µl/mg FW) was added and crushed to a fine paste, which was then subjected to centrifugation at 5000 rpm for 15 min. Following centrifugation, to the supernatant, a mixture of 3% sulfo-salicylic acid, 200µl of glacial acetic acid, 200µl of acidic ninhydrin was added and incubated for 1 h at 96°C. The reaction was stopped by keeping the samples on ice. The proline was subsequently extracted using 1 ml of toluene, wherein toluene was added to samples and vortexed for 20 to allow organic and aqueous phases to separate. Absorbance was recorded for the proline-containing chromophore at 520 nm, and results were reported in µmole per gram Fresh weight (FW).
4.6. The assay for the estimation of chlorophyll content
the amount of chlorophyll present in the sample was calculated in accordance with Lichtenthaler and Wellburn 1983 [
38] using acetone. In brief, 100 mg of frozen leaf samples were crushed in 1 ml of 100 mm potassium phosphate buffer, and the supernatant obtained after centrifugation at 5000 g for 20 min was vortexed after mixing with equal amounts of acetone. Their absorbance at 646.6 nm was measured, and their measured turbidity at 663.6 nm was subtracted. The total chlorophyll content was calculated using the formula (Eq: 2), and values were expressed as moles per gram fresh weight (FW).
4.7. Total soluble protein content measurement:
Frozen leaf samples (100mg weight) were pulverized in a mortar and pestle which acted as leaf extract. To this sample, protein content was assayed as per Lowry et al. (1951). As per the protocol, solution I which comprised 2% Na
2CO
3 in 0.1 N NaOH, 2% Na-K tartarate, and 1% CuSo4 in a 23:1:1 ratio was prepared. To 20µl of leaf extract, 980µl of distilled water and 1ml of solution I was mixed well and incubated for 15 minutes. After completion of the incubation period, 200µl of Folin’s reagent was added to it and mixed which was followed by 30 minutes of dark incubation. After incubation, absorbance for the samples at 750 nm was taken using a UV-Vis spectrophotometer (Shimadzu UV-1800) and the optical density values were used to plot a standard graph using Bovine serum albumin as a standard. The amount of protein was given by mg per gram FW (fresh weight) as per Uzma et al. 2022a [
3].
4.8. Evaluation of antioxidant enzyme activities:
To ascertain antioxidant enzyme activities, leaf extract from the previous step was homogenized in ice-cold extraction buffer (1 ml). The extraction buffer contained 100 mM potassium phosphate buffer (pH 7.0) and 1 mM EDTA. For the assay of SOD (superoxide dismutase) and APX (ascorbate peroxidase) activity, 1% PVP (polyvinyl pyridine) and 0.5% (v/v) Triton X were also added to the mixture. The homogenate was centrifuged at 5000 g for 15 minutes, and the supernatant (now known as enzyme extract) was used in the next procedures as per Uzma et al. 2023 [
5].
4.8.1. The assay for determining Catalase (CAT) activity
the standard protocol of Aebi et al. (1974) with a few alterations was deployed to estimate the activity of CAT. To elaborate, 100mg of enzyme extract was mixed with (50 mM) phosphate buffer and (0.1 mM) H
2O
2, and absorbance at 240 nm was recorded. The molar extension coefficient (36 M
-1 cm
-1) of the decrease in substrate (H
2O
2) concentration during the course of three minutes was calculated. Molecules of µmoles of H
2O
2 reduced (min
-1 mg
-1 protein) were used to express the CAT activity by Uzma et al. 2022b [
4].
4.8.2. The assay for determining Ascorbate Peroxidase (APX) activity
APX activity was assayed employing the protocol of Nakano and Asada (1981), with a few changes. 100 µg of enzyme extract was mixed with potassium phosphate buffer (50 mM), 0.3 mM H
2O
2, and 0.1 mM ascorbate. The absorbance of this reaction mixture was measured using the molar extension coefficient of AsA i.e. 2.8 mM
-1 cm
-1. The amount of µmoles of ASA decreased per minute per milligram of protein was used to measure the APX enzyme's activity [
5].
4.8.3. The assay for determining Glutathione Reductase (GR) Activity:
By following the procedure of Foyer and Halliwell's (1976) the activity of GR was monitored. A reaction mixture containing potassium phosphate buffer (100mM- pH 7.0), oxidized glutathione (GSSG-1.0mM), DTNB (0.08mM), NADPH (0.1mM), and of enzyme extract (100µg) was prepared. To this, absorbance at 340 nm was measured and to calculate the activity of GR the calibration factor used was the molar extinction coefficient of NADPH (6.2 mM
-1 cm
-1). The definition of one unit of GR consumed in the reaction was given by 1 µmole ml
-1 GSSG decreased min
-1 [
3].
4.8.4. The assay for determining SOD activity
applying the principles and protocols of the method developed by Beyer and Fridovich (1987) we calculated the activity of SOD. In short, to 50 µg of enzyme extract, 1.5 mM of Na
2CO
3, 200 mM of methionine, 3.0 mM of EDTA, 2.25 mM of NBT and 100 mM of potassium phosphate buffer (pH 7.0) were added. To this, 60µM riboflavin was added to begin the reaction, and it was then exposed to two 15 W fluorescent lamps in an illuminated chamber for 15 min. The reaction was stopped by turning off the light source and absorbance was recorded at 560 nm. The amount of SOD consumed to photo-chemically reduce 50% of NBT in the reaction was given as one unit of SOD for micrograms of protein [
3].
4.8.5. The assay for determining Peroxidase (POD) activity
as per the standard method of Shannon et al. (1966) to 100µg of enzyme extract, 0.05M guaiacol and 0.8M H
2O
2 were added, and enzyme kinetics of POD were calculated by recording the absorbance at 470nm for 180 seconds. The data were expressed as POD Activity for mg-1 protein per min-
1 [
39].
4.9. Statistical Analysis
The observations and data depicted in the figures and graphs are the mean average of the results revealed from five experiment groups that were performed in triplicate at different time intervals. Using Sigma plot software version 12.0, the data were exposed to a number of statistical parameters (mean, standard error, and one-way ANOVA) in order to establish the significance using the Holm-Sidak method as per Uzma et al. [
3,
4,
5].
5. Conclusions
Our study demonstrated that ascorbic acid (AsA) pretreatment can enhance salt tolerance in Gerbera jamesonii plants by improving plant growth and alleviating oxidative stress. AsA pretreatment significantly promoted plant growth as evaluated by an increase in leaf fresh and dry weight, relative water content, total protein content, free proline, and chlorophyll contents. This was accompanied by a decline in the levels of H2O2 and MDA, which indicates less cellular damage in both the cultivars used in the study. In addition, a noticeable enhancement in the activities of antioxidant enzymes (CAT, APX, SOD, POD, and GR) suggests that AsA is efficient in improving salt tolerance in G. jamesonii, particularly at a concentration of 1.0 mM. Thus, we conclude that a foliar spray comprising 1.0 mM AsA might be helpful to protect G. jamesonii plants from the negative effects of salt stress and increase their production capacity, even in fertigation-affected soils. This kind of approach to employing exogenous plant growth enhancers like AsA in optimal concentrations can improve production and offer sustainable development in the floriculture industry.
Author Contributions
MP and UJ conceived the presented idea wherein MF and UJ developed the protocols and performed the wet lab experiments. MP, MF and UJ verified the biochemical/analytical methods and UJ supervised the findings of this work. Writing and draft preparation: GAP, MP, MF and UJ. All authors discussed the results and contributed to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Authors thank the Head, Department of Biotechnology, Telangana University for providing all the facilities to carry out the investigations of the current study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cioc, M.; Dziurka, M.; Pawłowska, B. Changes in Endogenous Phytohormones of Gerbera jamesonii Axillary Shoots Multiplied under Different Light Emitting Diodes Light Quality. Molecules 2022, 27, 1804. [Google Scholar] [CrossRef]
- Singh, P.; Bhardwaj, A.; Kumar, R.; Singh, D. Evaluation of Gerbera Varieties for Yield and Quality under Protected Environment Conditions in Bihar. Int. J Curr. Microbial. App. Sci. 2017, 6, 112–116. [Google Scholar] [CrossRef]
- Uzma, J.; Talla, S.K.; Madam, E.; Mamidala, P. Assessment of Salinity Tolerance Deploying Antioxidant Defense Systems in Gerbera Jamesonii. Biosci Biotech Res Asia 2022, 19, 243–254. [Google Scholar] [CrossRef]
- Uzma, J.; Talla, S.K.; Madam, E.; Mamidala, P. Salinity-driven oxidative stress in Gerbera jamesonii cv. Bolus. J. Appl. Hortic. 2022, 24, 240–244. [Google Scholar] [CrossRef]
- Uzma, J.; Talla, S.K.; Mamidala, P. Insights into the impact of spermidine in reducing salinity stress in Gerbera jamesonii. J. Appl. Biol. 2023, 11, 141–147. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Waqas, M.A.; Kaya C.; Riaz A.; Farooq M.; Nawaz I. et al. Potential Mechanisms of Abiotic Stress Tolerance in Crop Plants Induced by Thiourea. Front. Plant Sci 2019 10:1336.
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signaling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Amjad, S.F.; Saleem, M.H.; Yasmin, H.; Imran, M.; Riaz, M.; Ali, Q.; Joyia, F.A.; Mobeen; Ahmed, S; Ali, S.; Alsahli, A.A.; Alyemeni, M.N. Foliar application of ascorbic acid enhances salinity stress tolerance in barley (Hordeum vulgare L.) through modulation of morpho-physio-biochemical attributes, ions uptake, osmo-protectants and stress response genes expression. Saudi J. Biol. Sci. 2021, 28(8), 4276-4290. https://doi.org/10.1016/j.sjbs.2021.03.045. [CrossRef]
- Khan, I.; Muhammad, A.; Chattha, M.U.; Skalicky, M.; Chattha, M.B.; Ayub, M.A.; Anwar, M.R.; Soufan, W.; Hassan, M.U.; Rahman, M.A.; Brestic, M.; Zivcak, M.; Sabagh, A.E. Mitigation of Salinity-Induced Oxidative Damage, Growth, and Yield Reduction in Fine Rice by Sugarcane Press Mud Application. Front Plant Sci. 2022, 13, 840900. [Google Scholar] [CrossRef]
- Wang, M.; He, J.; Li, S.; Cai, Q.; Zhang, K.; She, J. Structural basis of vitamin C recognition and transport by mammalian SVCT1 transporter. Nat Commun. 2023, 14, 1361. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Ascorbate and glutathione: versatile molecules in plants under abiotic stress. Redox Biol. 2013, 1, 1–10. [Google Scholar]
- Nazar, R.; Iqbal, N.; Masood, A.; Khan, N. A.; Sayeed, S.; Khan, M.I.R. Ascorbic acid and salinity tolerance in plants: a review. Front. Plant Sci. 2016, 7, 486. [Google Scholar]
- Sharma, P.; Dubey, R.S. Ascorbic acid-induced salinity tolerance in plants: a review. J. Plant Physiol. 2012, 169, 577–590. [Google Scholar]
- Ali, D.; Torlak, E. Foliar Ascorbic Acid Alleviates Salt-induced Oxidative Stress in Maize. J. Stress Physiol. Biochem. 2021, 17(1), 24-31. ISSN 1997-0838.
- Jamil, M.; Iqbal, N.; Ashraf, M.; Rehman, S. Ascorbic acid-mediated modulation of growth, physiology and biochemical characteristics in maize under salinity stress. Acta Physiol. Plant. 2015, 37(6), 104. [Google Scholar]
- Akram, N. A.; Aisha, R.; Shafiq, S.; Ashraf, M. Foliar-applied ascorbic acid enhances antioxidative potential and drought tolerance in cauliflower (Brassica oleracea L. var. Botrytis). Agrochimica. 2016, 60, 100–113.
- Noman, A.; Ali, S.; Naheed, F.; Ali, Q.; Farid, M.; Rizwan, M. Foliar application of ascorbate enhances the physiological and biochemical attributes of maize (Zea mays L.) cultivars under drought stress. Arch. Agron. Soil Sci. 2015, 61(12), 1659-1672. [CrossRef]
- Farooq, A.; Bukhari, S.A.; Akram, N.A.; Ashraf, M.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Exogenously Applied Ascorbic Acid-Mediated Changes in Osmoprotection and Oxidative Defense System Enhanced Water Stress Tolerance in Different Cultivars of Safflower (Carthamus tinctorious L.). Plants 2020, 9, 104. [CrossRef]
- Shafiq, S.; Akram, N. A.; Ashraf, M.; Arshad, A. Synergistic effects of drought and ascorbic acid on growth, mineral nutrients and oxidative defense system in canola (Brassica napus L.) plants. Acta Physiol. Plant. 2014, 36, 1539–1553. [CrossRef]
- Angon, P.B.; Arif, M.T.; Samin, S.I.; Habiba, U.; Hossain, M.A.; Brestic, M. How Do Plants Respond to Combined Drought and Salinity Stress?—A Systematic Review. Plants 2022, 11, 2884. [Google Scholar] [CrossRef]
- Jawad Hassan, M.; Ali Raza, M.; Ur Rehman, S.; Ansar, M.; Gitari, H.; Khan, I.; ... & Li,; Z. . Effect of cadmium toxicity on growth, oxidative damage, antioxidant defense system and cadmium accumulation in two sorghum cultivars. Plants, 2020 9(11), 1575.
- Mukhtar, A.; Akram, N. A.; Ashraf, M.; Shafiq, S.; & Aisha, R. Foliar-applied ascorbic acid enhances antioxidative potential and drought tolerance in cauliflower (Brassica oleracea L. var. Botrytis). Foliar-applied ascorbic acid enhances antioxidative potential and drought tolerance in cauliflower (Brassica oleracea L. var. Botrytis), International Journal of Plant Chemistry, Soil Science and Plant Nutrition 2016 100-113.
- Azzedine, F.; Gherroucha, H.; Baka, M. Improvement of salt tolerance in durum wheat by ascorbic acid application. J. Stress Physiol. Biochem. 2011, 7, 27–37. [Google Scholar]
- Mbarki, S.; Sytar, O.; Cerda, A.; Zivcak, M.; Rastogi, A.; and He, X.; et al. Strategies to mitigate the salt stress effects on photosynthetic apparatus and productivity of crop plants, in Salinity Responses and Tolerance in Plants. Volume 1: Targeting Sensory, Transport and Signaling Mechanisms. 2018, 85-136.
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon. 2019, 5(12): e02952. [CrossRef]
- Agami, R. A. Applications of ascorbic acid or proline increase resistance to salt stress in barley seedlings. Biol. Plant. 2014, 58, 341–347. [Google Scholar] [CrossRef]
- Yazdanpanah, S.; Baghizadeh, A.; Abbassi, F. The interaction between drought stress and salicylic and ascorbic acids on some biochemical characteristics of Satureja hortensis. Afric. J. Agric. Res. 2011, 6, 798–807. [Google Scholar]
- Athar, H.; Khan. A; Ashraf, M. Including salt tolerance in wheat by exogenously applied ascorbic acid through different modes. J. Plant Nutr. 2009, 32, 1799–1817. [CrossRef]
- Ejaz, B.; Sajid, Z. A.; Aftab, F. Effect of exogenous application of ascorbic acid on antioxidant enzyme activities, proline contents, and growth parameters of spp. Hybrid cv. Hsf-240 under salt stress. Turk. J. Biol. 2012, 36, 1201–1237. [CrossRef]
- Alves, de Cássia, R.; Oliveira, K. R.; Lúcio, J. C. B.; Santos Silva, dos. J.; Carrega, W. C.; Queiroz, S. F;, & Gratão, P. L. Exogenous foliar ascorbic acid applications enhance salt-stress tolerance in peanut plants throughout an increase in the activity of major antioxidant enzymes. South African Journal of Botany, 2022 150, 759-767.
- Ahmad, I.; Basra, S. M. A.; Wahid, A. Exogeneous application of ascorbic acid, salicylic acid, hydrogen peroxide improves the productivity of hybrid maize at low temperature stress. Int. J. Agric. Biol. 2014, 16, 825–830. [Google Scholar]
- Es-sbihi, F. Z.; Hazzoumi, Z.; Aasfar, A. Improving salinity tolerance in Salvia officinalis L. by foliar application of salicylic acid. Chem. Biol. Technol. Agric. 2021, 8, 25. [Google Scholar] [CrossRef]
- Sarker, U.; and Oba, S. Drought Stress Enhances Nutritional and Bioactive Compounds, Phenolic Acids and Antioxidant Capacity of Amaranthus Leafy Vegetable. BMC Plant Biology, 2018 18, 258. [CrossRef]
- Heath, R.; Packer, L. Photoperoxidation in isolated chloroplasts. Kinetics and stochiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Moskova, I.; Todorova, D.; Alexieva, V.; & Segiev, I. Protective effect of hydrogen peroxide against paraquat toxicity in young pea plants: Possible role of endogenous polyamines. American Journal of Plant Sciences, 2014 5(22), 3408.
- Bates, L. S.; Waldren, R. P.; Teare, I. D.; Rapid determination of free proline for water stress studies Plant Soil. 1973, 39: 205–7. [CrossRef]
- Lichtenthaler, K.; Welburn, A. R. Determination of Total Carotenoids and Chlorophylls A and B of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Chi, Z. L.; Yu, G. H.; Kappler, A.; Liu, C. Q.; & Gadd, G. M. Fungal–mineral interactions modulating intrinsic peroxidase-like activity of iron nanoparticles: Implications for the biogeochemical cycles of nutrient elements and attenuation of contaminants. Environmental Science & Technology, 2021 56(1), 672-680.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).