Submitted:
26 October 2023
Posted:
27 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental site, and treatments of the OMET system
2.2. Establishment of seedling on the OMET system
2.3. Growth and yield data collection
2.4. Determination of total irrigation water
2.5. Determination of bioactive compounds and HPLC-MS-QTOF untargeted metabolites.
2.6. Determination of protein
2.7. Determination of minerals or trace elements
2.10. Determination of amino acids
2.11. Data analysis
3. Results
3.1. Effect between growth conditions and species on growth, flowering, irrigation water and yield attributes of three Amaranth species
3.2. Interactive effect between growth condition and species on bioactive compounds and HPLC-MS-QTOF metabolites
3.2.1. Bioactive compounds
3.2.2. HPLC-MS-TOF metabolites
3.3. Interactive effect between growth condition and species on nutritional composition
3.3.1. Protein and nutritive minerals
3.3.2. Amino acid composition
4. Discussion
5. Conclusion and recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bvenura, C., and Afolayan, A. J., 2015. The role of wild vegetables in household food security in South Africa: A review. Food Research International, 76(P4), 1001–1011. [CrossRef]
- South-East Asia Region and WHO, 2021. Regional Nutrition Strategy : Addressing malnutrition and micronutrient deficiencies.
- Taia23TP0F, W.K., 2021. Vegetative morphological variations within some Egyptian Amaranthus L. species. Jordan Journal of Biological Sciences, 14(1). [CrossRef]
- Sarker, U. and Oba, S., 2019. Salinity stress enhances colour parameters, bioactiv leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. Journal of the Science of Food and Agriculture, 99(5), 2275-2284. [CrossRef]
- Mateos-Maces, L., Chávez-Servia, J.L., Vera-Guzmán, A.M., Aquino-Bolaños, E.N., Alba-Jiménez, J.E. and Villagómez-González, B.B., 2020. Edible leafy plants from Mexico as sources of antioxidant compounds, and their nutritional, nutraceutical and antimicrobial potential: A review. Antioxidants, 9(6), 541. [CrossRef]
- Jimoh, M. O., Afolayan, A. J., and Lewu, F. B., 2018. Suitability of Amaranthus species for alleviating human dietary deficiencies. South African Journal of Botany, 115, 65–73. [CrossRef]
- Ngoroyemoto, N., Gupta, S., Kulkarni, M. G., Finnie, J. F., and Van Staden, J., 2019. Effect of organic biostimulants on the growth and biochemical composition of Amaranthus hybridus L. South African Journal of Botany, 124, 87–93. [CrossRef]
- Sarmadi, B., Rouzbehan, Y., and Rezaei, J., 2016. Influences of growth stage and nitrogen fertilizer on chemical composition, phenolics, in situ degradability and in vitro ruminal variables in Amaranth forage. Animal Feed Science and Technology, 215,73–84. [CrossRef]
- Ferreira, J., 2013. OMET farming better than hydroponics? Farmer’s weekly.
- Mokgalabone, T.T., Mpai, S. and Ndhlala, A.R., 2023. Organic Medium Enclosed Trough Growing Technique Improves Abelmoschus esculentus (Okra) Growth, Yield and Some Nutritional Components. Applied Sciences, 13(9), p.5645. [CrossRef]
- Mpai, S., Mokganya, L.M., Raphoko, L., Masoko, P. and Ndhlala, A.R., 2022. Untargeted metabolites and chemometric approach to elucidate the response of growth and yield attributes on different concentrations of an amino acid based biostimulant in two lettuce cultivars. Scientia Horticulturae, 306, p.111478. [CrossRef]
- Mpai, S., Du Preez, R., Sultanbawa, Y. and Sivakumar, D., 2018. Phytochemicals and nutritional composition in accessions of Kei-apple (Dovyalis caffra): Southern African indigenous fruit. Food chemistry, 253, 37-45. [CrossRef]
- Mpai, S. and Sivakumar, D., 2020. Influence of growing seasons on metabolic composition, and fruit quality of avocado cultivars at ‘ready-to-eat stage’. Scientia Horticulturae, 265, p.109159. [CrossRef]
- Makkar, H.P., Siddhuraju, P. and Becker, K., 2007. Plant secondary metabolites (Vol. 393, pp. 1-122). Totowa, NJ, USA: Humana Press.
- Tambe, V.D. and Bhambar, R.S., 2014. Estimation of total phenol, tannin, alkaloid, and flavonoid in Hibiscus tiliaceus Linn. Wood extracts. Journal of Pharmacognosy and Phytochemistry 2(4): 2321-6182.
- Managa, M.G., Sultanbawa, Y. and Sivakumar, D., 2020. Effects of different drying methods on untargeted phenolic metabolites, and antioxidant activity in Chinese cabbage (Brassica rapa L. subsp. chinensis) and nightshade (Solanum retroflexum Dun.). Molecules, 25(6), 1326. [CrossRef]
- Helrich, K., 1990. Official methods of analysis of the Association of Official Analytical Chemists. Association of official analytical chemists.
- Mathipa, M.M., Mphosi, M.S. and Masoko, P., 2022. Phytochemical Profile, Antioxidant Potential, Proximate and Trace Elements Composition of Leaves, Stems and Ashes from 12 Combretum spp. Used as Food Additives. International Journal of Plant Biology, 13(4), pp.561-578. [CrossRef]
- Yahaya, Y., Birnin-Yauri, U.A., Bagudo, B.U. and Noma, S.S., 2012. Quantification of macro and micro elements in selected green vegetables and their soils from Aliero agricultural fields in Aliero, Kebbi State, Nigeria. Journal of Soil science and Environmental Management, 3(8): 207-215. [CrossRef]
- Manyelo, T.G., Sebola, N.A. and Mabelebele, M., 2020. Nutritional and phenolic profile of early and late harvested Amaranth leaves (Amaranthus cruentus) grown under cultivated conditions, 432(10): 05. [CrossRef]
- Saeed, R. and Ahmad, R., 2009. Vegetative growth and yield of tomato as affected by the application of organic mulch and gypsum under saline rhizosphere. Pakistan Journal of Botany, 41(6), 3093-3105.
- Ferrini, F., Fini, A., Frangi, P. and Amoroso, G., 2008. Mulching of ornamental trees: Effects on growth and physiology. Arboric Urban Forum, 34: 157. [CrossRef]
- Ngala, J.M., Ndiso, J.B. and Mundi, E.M., 2019. Effects of selected organic mulches on growth and yield of Amaranth in Kilifi country. International Journal of Agriculture, Environment and Bioresearch. Volume 4, No. 06. [CrossRef]
- Department of Agriculture, Forestry and Fisheries (DAFF), 2010. Amaranthus production guideline. Pretoria, RSA.
- Scharenbroch, B.C. and Lloyd, J.E., 2006. Particulate organic matter and soil N availability in urban landscapes. Arboric Urban Forum. 32: 180. [CrossRef]
- Kazimierczak, R., Hallmann, E. and Rembiałkowska, E., 2015. Effects of organic and conventional production systems on the content of bioactive substances in four species of medicinal plants. Biological Agriculture & Horticulture, 31(2), pp.118-127. [CrossRef]
- Coley, P.D., Bryant, J.P. and Chapin III, F.S., 1985. Resource availability and plant anti-herbivore defense. Science, 230(4728), pp.895-899.
- Da Silva Ferreira, V. and Sant’Anna, C., 2017. Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World Journal of Microbiology and Biotechnology, 33(1): 1-8. [CrossRef]
- Skotnica, J., Matoušková, M., Nauš, J., Lazár, D. and Dvořák, L., 2000. Thermoluminescence and fluorescence study of changes in Photosystem II phytochemistry in desiccating barley leaves. Photosynthesis Research, 65(1): 29-40. [CrossRef]
- Kwenin, W.K.J., Wolli, M. and Dzomeku, B.M., 2011. Assessing the nutritional value of some African indigenous green leafy vegetables in Ghana.
- Hanif, R., Iqbal, Z., Iqbal, M., Hanif, S. and Rasheed, M., 2006. Use of vegetables as nutritional food: role in human health. Journal of Agricultural and Biological Science, 1(1): 18-22.
- Wijewardana, C., Reddy, K.R. and Bellaloui, N., 2019. Soybean seed physiology, quality, and chemical composition under soil moisture stress. Food Chemistry, 278: 92-100. [CrossRef]
- Bellaloui, N., Mengistu, A., Fisher, D.K. and Abel, C.A., 2012. Soybean seed composition constituents as affected by drought and Phomopsis in phomopsis susceptible and resistant genotypes. Journal of Crop Improvement, 26(3), 428-453. [CrossRef]
- Farooq, M., Wahid, A., Kobayashi, N.S.M.A., Fujita, D.B.S.M.A. and Basra, S.M.A., 2009. Plant drought stress: effects, mechanisms and management. In Sustainable agriculture (153-188). Springer, Dordrecht. [CrossRef]
- Rouphael, Y., Cardarelli, M., Schwarz, D., Franken, P. and Colla, G., 2012. Effects of drought on nutrient uptake and assimilation in vegetable crops. In Plant responses to drought stress (171-195). Springer, Berlin, Heidelberg. [CrossRef]
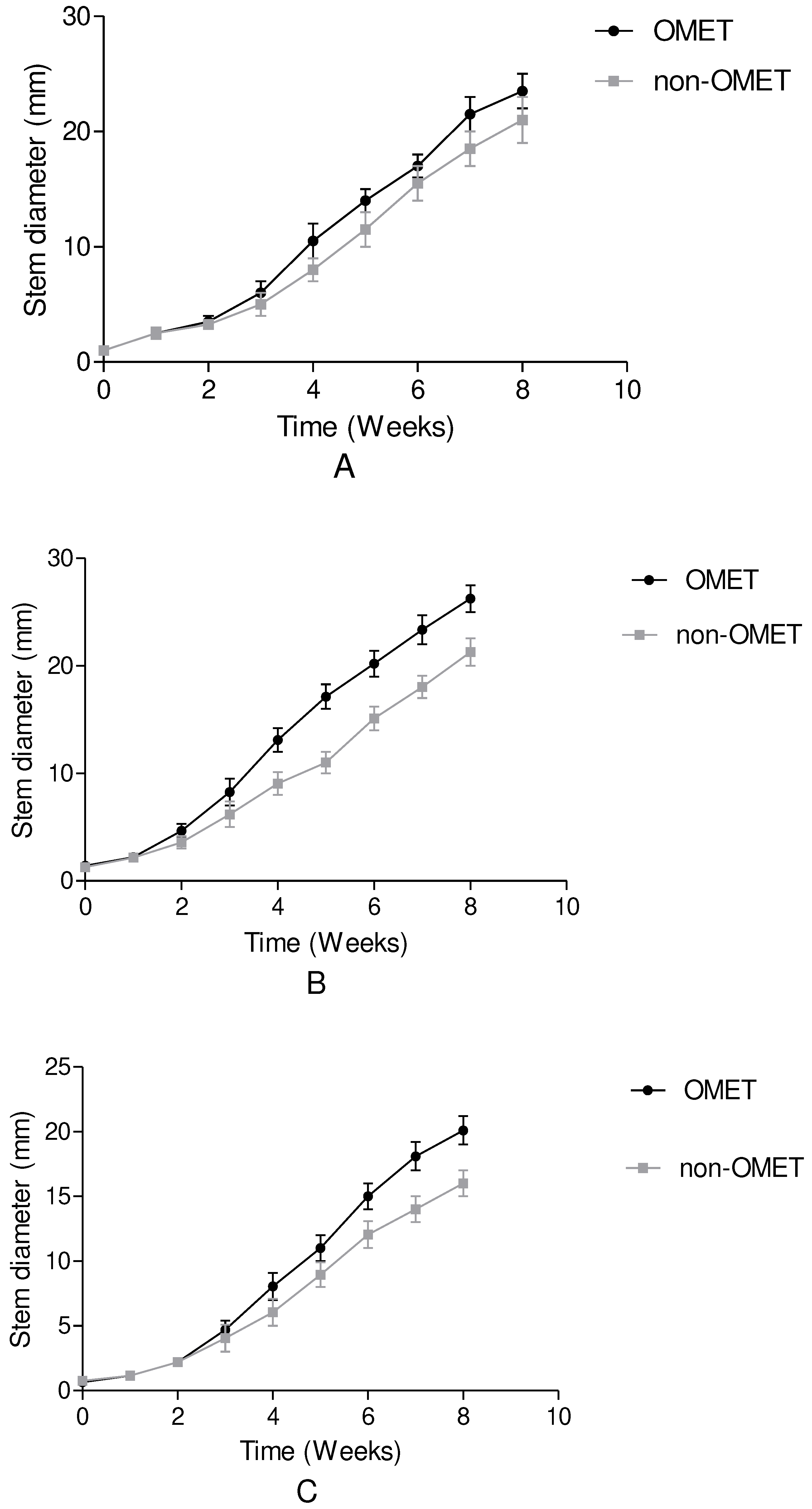
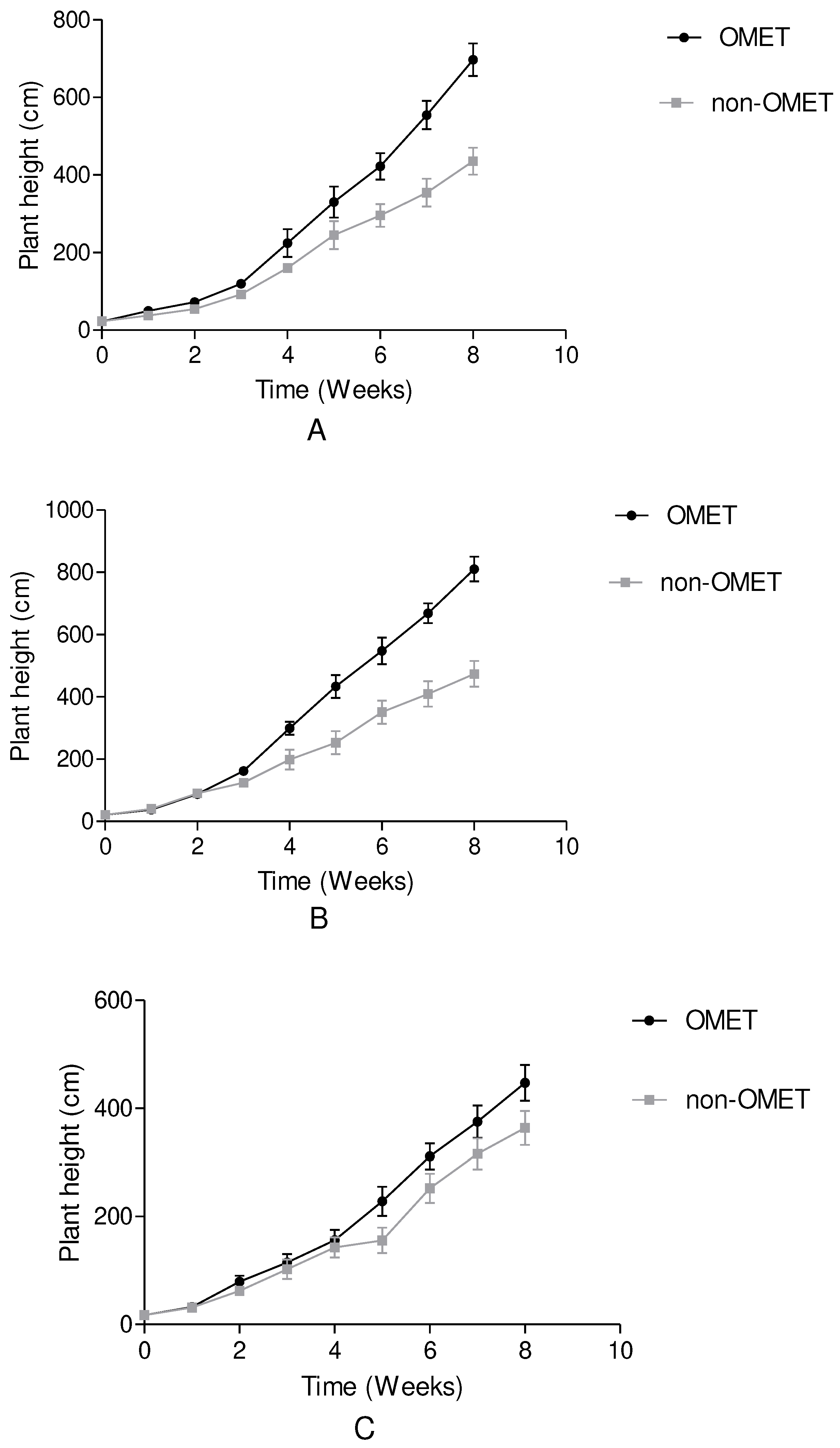
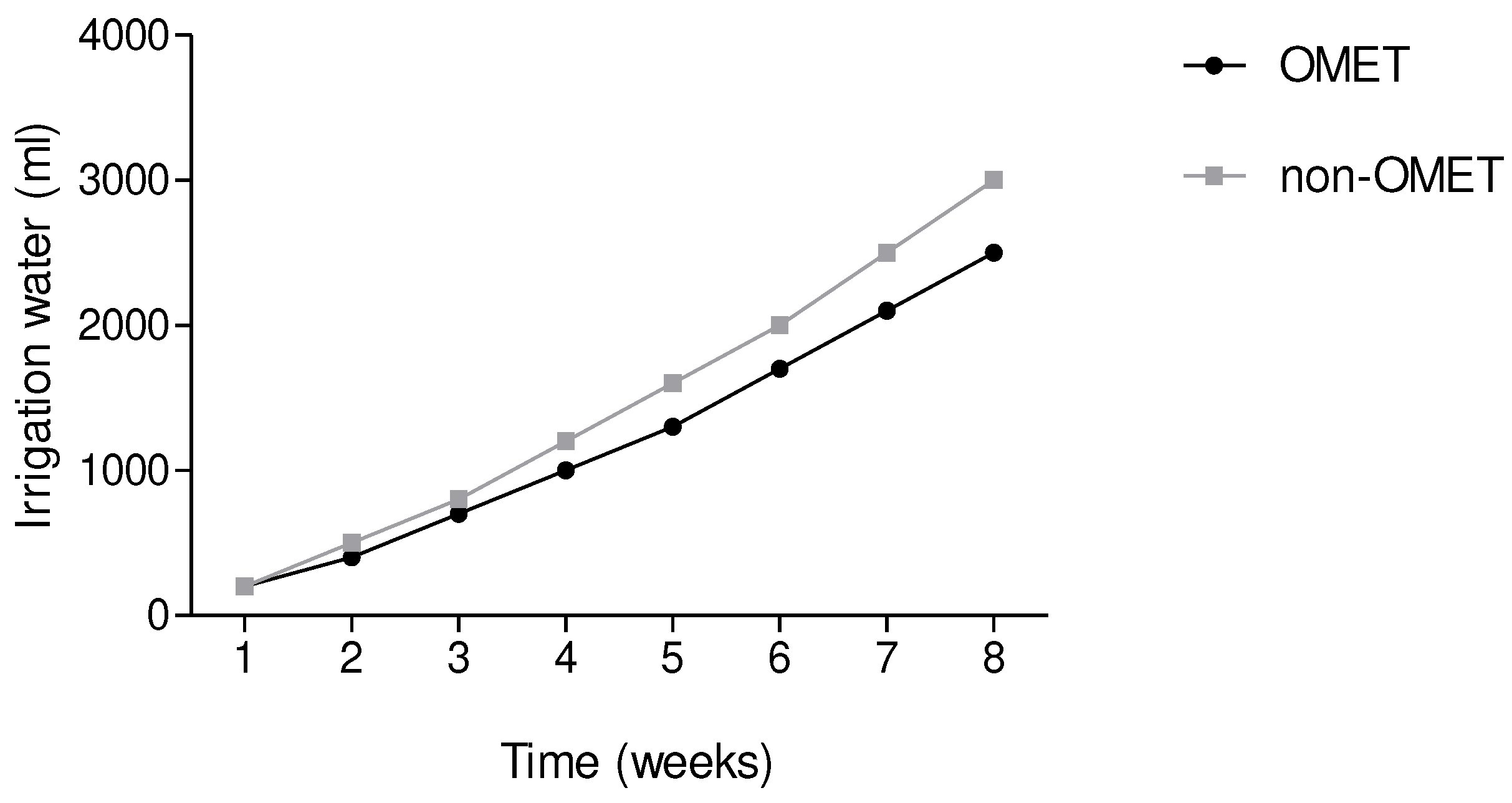
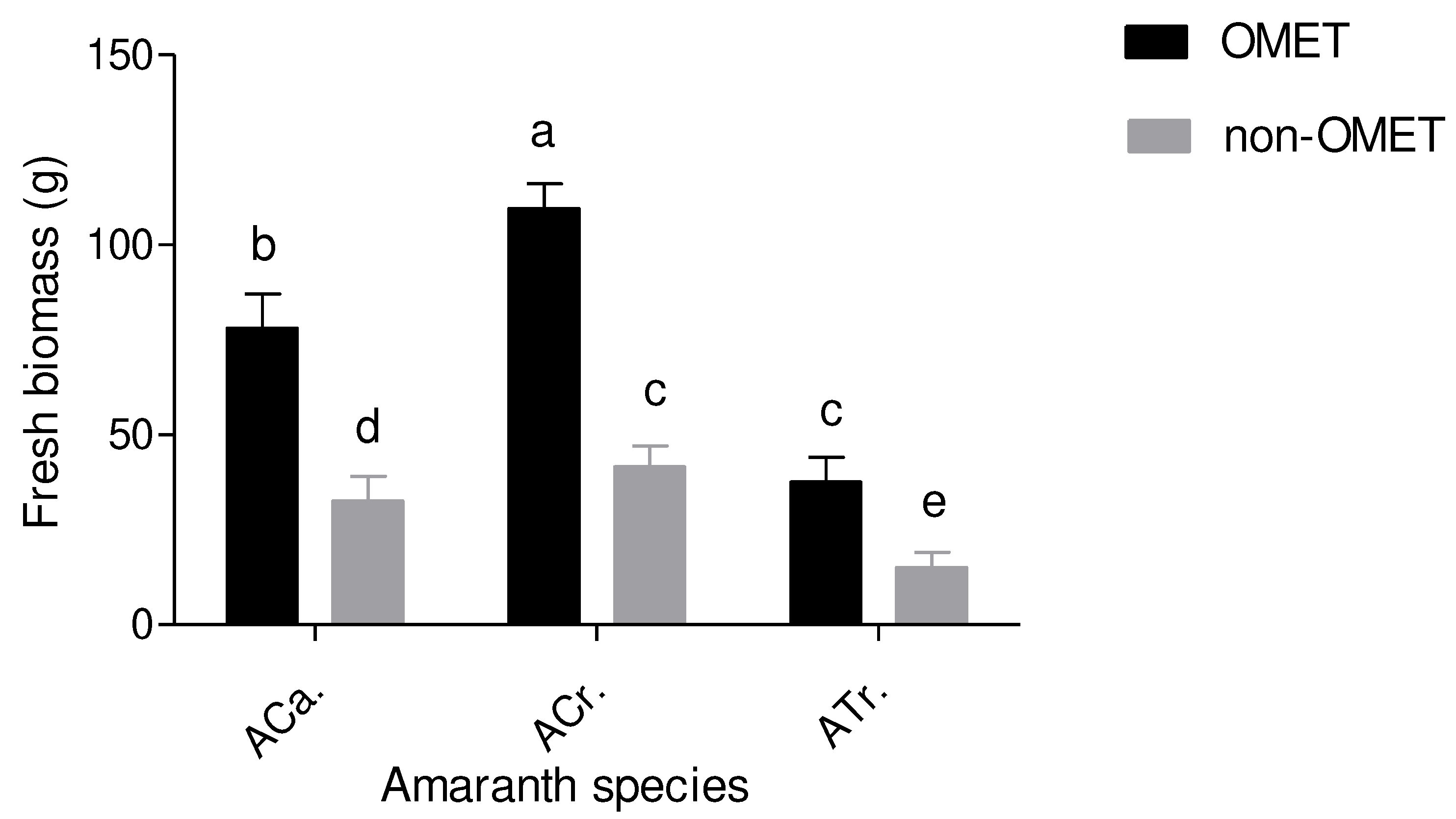
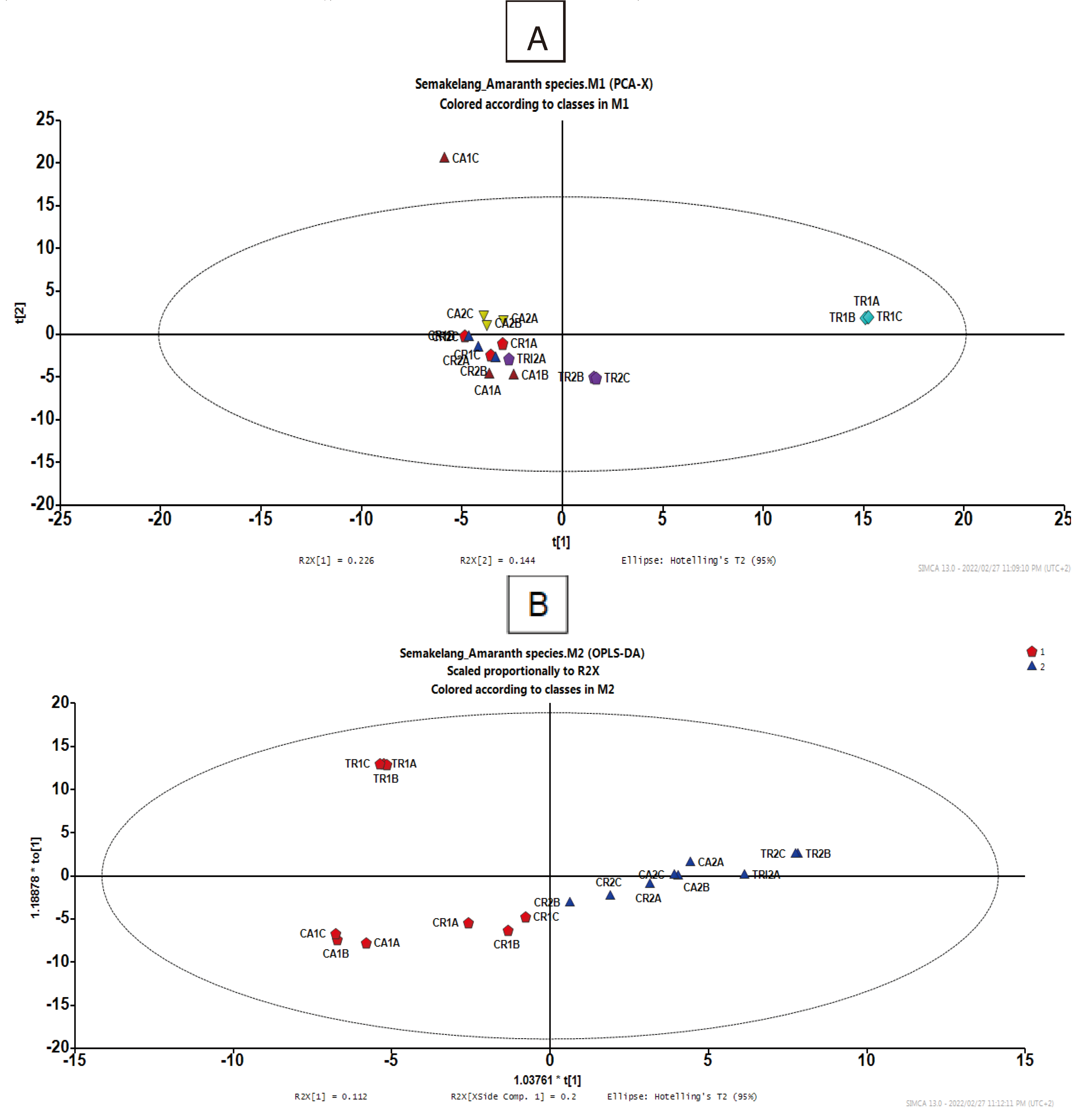
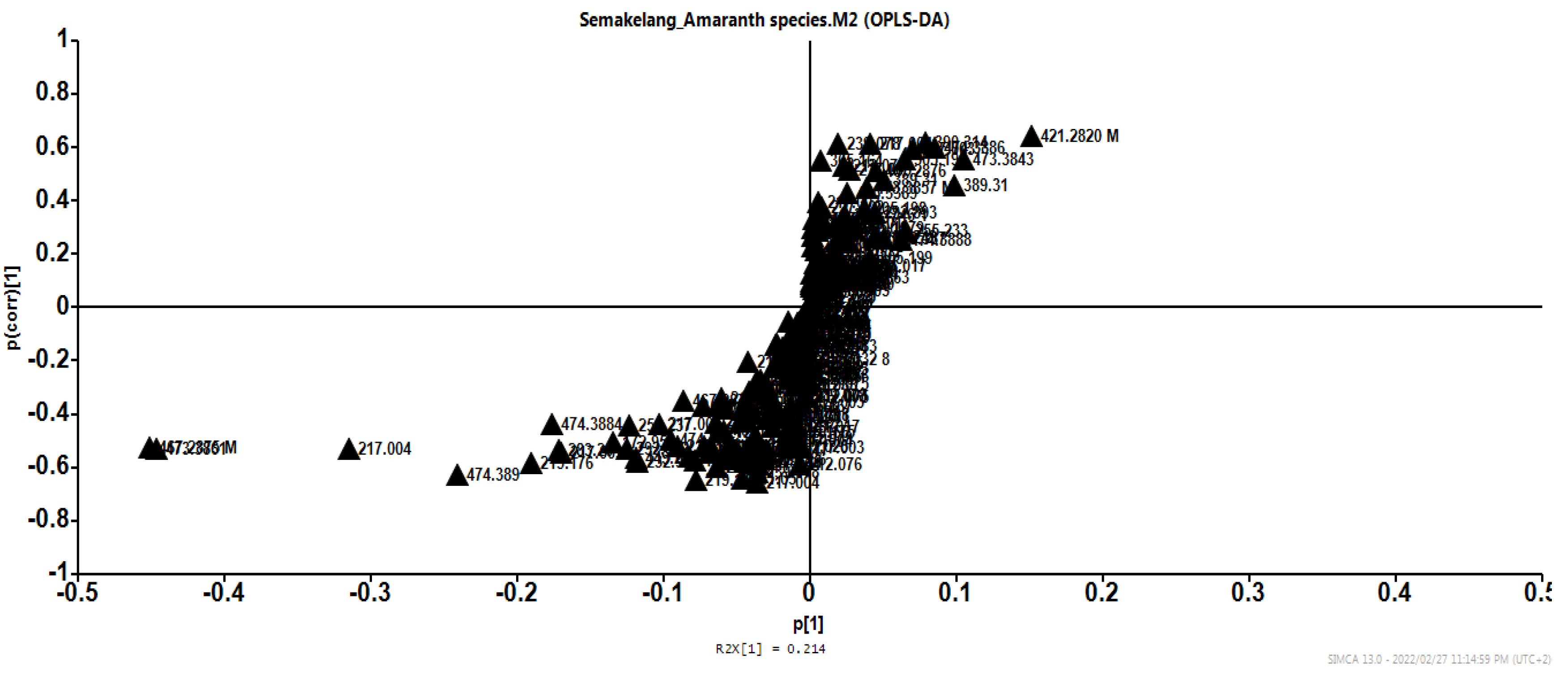
| Treatment | Amaranth species | Time of flowering (Weeks) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| OMET system | Amaranthus caudatus | X | |||||||
| Amaranthus cruentus | X | ||||||||
| Amaranthus tricolor | X | ||||||||
| non-OMET system | Amaranthus caudatus | X | |||||||
| Amaranthus cruentus | X | ||||||||
| Amaranthus tricolor | X | ||||||||
| Amaranth species & treatment | Total phenolic (mg GAE/100g DW) | Total flavonoids (mg CA/100g DW) |
Total tannins (mg GAE/100g DW) | |
| A. caudatus | OMET | 210.24±0.033d | 391.26±0.046d | 4.09±0.042d |
| non-OMET | 259.68±0.046b | 453.34±0.036b | 9.60±0.022a | |
| A. cruentus | OMET | 209.65±0.035d | 382.60±0.047e | 3.98±0.043d |
| non-OMET | 262.45±0.028a | 473.28±0.021a | 8.78±0.031b | |
| A. tricolor | OMET | 207.70±0.0.036e | 364.24±0.038f | 3.61±0.038d |
| non-OMET | 257.24±0.040c | 447.59±0.042c | 6.89±0.040c | |
| Chl a (mg/kg) | Chl b (mg/kg) | Chl a+b (mg/kg) | ||
| A. caudatus | OMET | 0.306±0.005a | 47.779±0.004a | 48.085±0.005a |
| non-OMET | 0.301±0.0047b | 47.142±0.003a | 47.443±0.007a | |
| A. cruentus | OMET | 0.301±0.0048b | 47.142±0.008a | 47.443±0.008a |
| non-OMET | 0.296±0.0034c | 46.306±0.003b | 46.603±0.004a | |
| A. tricolor | OMET | 0.289±0.044d | 45.253±0.048c | 45.543±0.045a |
| non-OMET | 0.197±0.036e | 30.749±0.041d | 30.946±0.039b | |
| Retention time (Min) |
Exact Mass (g/mol) |
Mass generated ESI (-) TOF MS (g/mol) | Fragmentation | Chemical Formula | Tentative structural assignment |
|---|---|---|---|---|---|
| 15.658 | 474.4557 | 474.08423 | 474.08758:246 475 | C21H30O12 | 6-Feruloylglucose 2,3,4-trihydroxy-3-methylbutylglycoside |
| 8,093 | 219.23 | 218.10211 | 218.10655:4843 219 | C9H17NO5 | Pantothenic acid |
| 21.15 | 468.46 | 467,15897 | 467.13629:1292 468 | C22H28O11 | 6-O-(4-Hydroxybenzoyl)-ajugol |
| 17.63 | 420.41 | 420.3132 | 268.03810:269.04341 | C21H24O9 | Apigenin 7-O-glucoside |
| Amaranth species & treatment | Protein (%) | |
|
A. caudatus |
OMET | 24.1±0.039b |
| non-OMET | 21.4±0.044d | |
|
A. cruentus |
OMET | 28.6±0.46a |
| non-OMET | 24.1±0.040b | |
|
A. tricolor |
OMET | 22.7±0.036c |
| non-OMET | 20.3±0.042d | |
| P-value | 0.04 | |
| 3A | |||||||||||
| Amaranth species & treatment | Macro elements (mg/kg DW) | ||||||||||
| Ca | Mg | K | P | ||||||||
| A. caudatus | OMET | 104±0.043b | 77.3±0.032b | 251±0.041c | 29.7±0.045b | ||||||
| non-OMET | 104±0.043b | 62.6±0.030d | 244±0.038d | 21.8±0.037c | |||||||
| A. cruentus | OMET | 130±0.038a | 63.3±0.029d | 254±0.046b | 34.7±0.043a | ||||||
| non-OMET | 88.3±0.033d | 56.6±0.025e | 217±0.033f | 22.2±0.039c | |||||||
| A. tricolor | OMET | 104±0.039b | 82.5±0.038a | 276±0.05a | 30.3±0.041b | ||||||
| non-OMET | 90.5±0.041c | 66.2±0.032c | 226±0.035e | 21.5±0.036c | |||||||
| 3B | |||||||||||
| Microelements (mg/kg DW) | |||||||||||
| Cu | Mn | Fe | Se | Zn | |||||||
| A. caudatus | OMET | 0.89±0.023c | 1.73±0.046b | 2.62±0.05b | 8.03±0.049a | 1.11±0.024c | |||||
| non-OMET | 0.84±0.021d | 1.46±0.043d | 2.5±0.050c | 7.01±0.048d | 1.09±0.036c | ||||||
| A. cruentus | OMET | 1.04±0.034a | 1.78±0.048b | 2.52±0.04c | 8.13±0.050a | 1.66±0.048a | |||||
| non-OMET | 0.89±0.023c | 1.17±0.041e | 2.51±0.04c | 7.69±0.05b | 1.28±0.045b | ||||||
| A. tricolor | OMET | 0.93±0.025b | 2.16±0.05a | 3.41±0.39a | 7.28±0.05c | 1.27±0.047b | |||||
| non-OMET | 0.78±0.022e | 1.64±0.04c | 2.47±0.05c | 6.36±0.048e | 0.95±0.039d | ||||||
| Essential amino acids | |||||||
| Threonine | Valine | Isoleucine | Lysine | Leucine | Phenylalanine | ||
| A. caudatus | OMET | 0.92±0.024b | 1.09±0.043c | 0.97±0.031b | 2.25±0.05a | 1.66±0.044b | 2.36±0.05a |
| non-OMET | 0.66±0.025d | 0.84±0.027d | 0.76±0.026c | 1.3±0.034b | 1.25±0.038c | 1.14±0.035c | |
| A. cruentus | OMET | 1.15±0.034a | 1.45±0.041a | 1.29±0.027a | 2.37±0.05a | 2.21±0.042a | 2.54±0.051a |
| non-OMET | 0.93±0.036b | 1.22±0.042b | 1.12±0.039a | 2.19±0.050a | 1.92±0.051b | 1.95±0.043b | |
| A. tricolor | OMET | 0.88±0.021c | 1.08±0.034c | 0.98±0.028b | 1.62±0.036b | 1.66±0.041b | 1.47±0.038c |
| non-OMET | 0.43±0.021e | 0.52±0.026d | 0.46±0.022d | 0.8±0.012c | 0.74±0.024d | 0.71±0.023d | |
| Non-essential amino acids | |||||||
| Arginine | Serine | Glycine | Aspartate | Glutamate | |||
| A. caudatus | OMET | 1.12±0.047b | 0.97±0.034b | 1.37±0.05b | 1.2±0.041c | 1.52±0.05c | |
| non-OMET | 0.86±0.031c | 0.7±0.030c | 0.95±0.041c | 0.96±0.042c | 1.29±0.049c | ||
| A. cruentus | OMET | 1.37±0.048a | 0.98±0.036b | 1.33±0.044b | 1.33±0.044b | 1.75±0.05b | |
| non-OMET | 1.54±0.05a | 1.16±0.045a | 1.46±0.048a | 2.02±0.05a | 2.55±0.05a | ||
| A. tricolor | OMET | 1.12±0.049b | 0.91±0.042b | 1.21±0.047b | 1.53±0.049b | 1.95±0.05b | |
| non-OMET | 0.53±0.028d | 0.46±0.022c | 0.59±0.029d | 0.6±0.030d | 0.73±0.032d | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




