Submitted:
26 October 2023
Posted:
27 October 2023
You are already at the latest version
Abstract
Keywords:
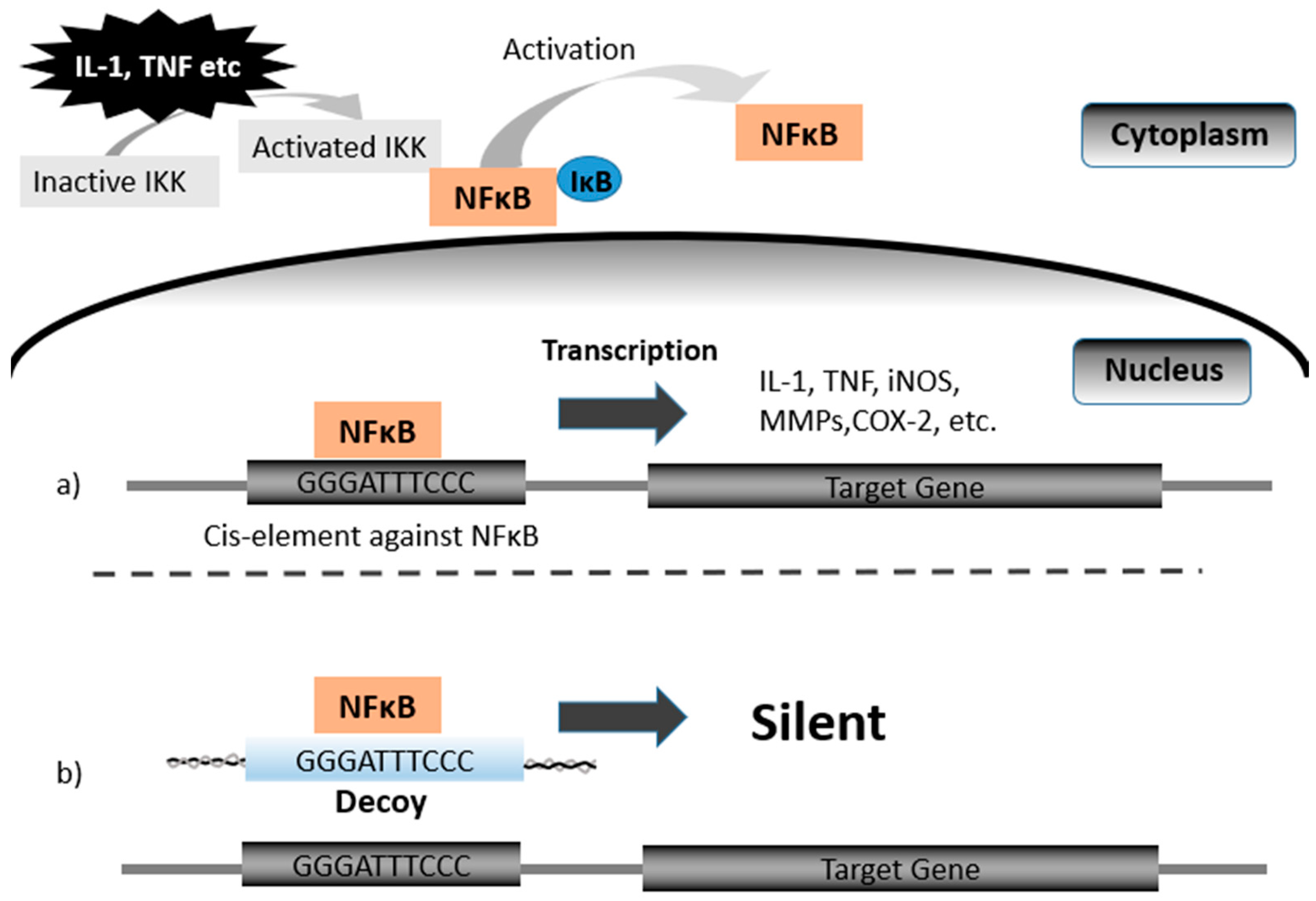
1. Background
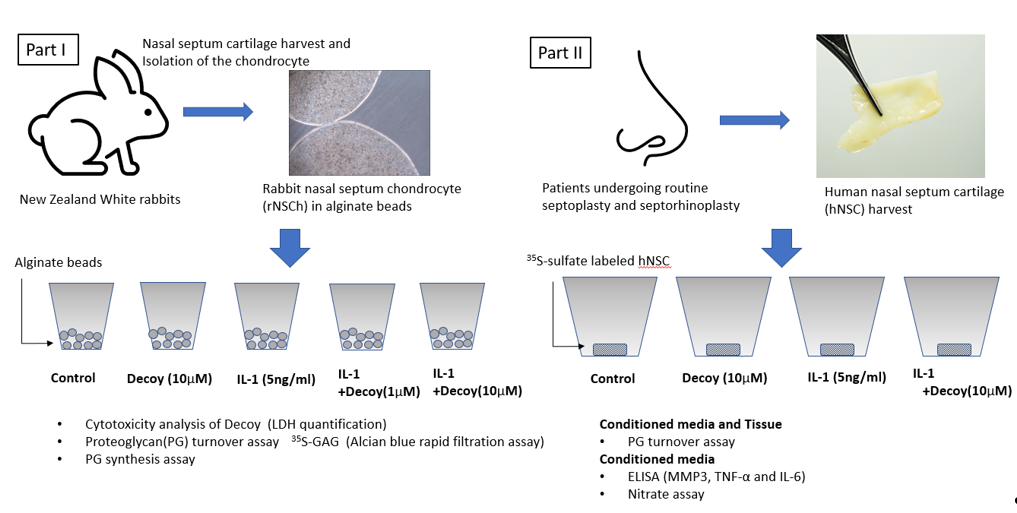
2. Materials and Methods
2.1. Study Design
2.1.1. Safety and determination of an efficacious concentration of Decoy
2.1.1.1. rNSCh Cell Isolation and Culture in Alginate Beads
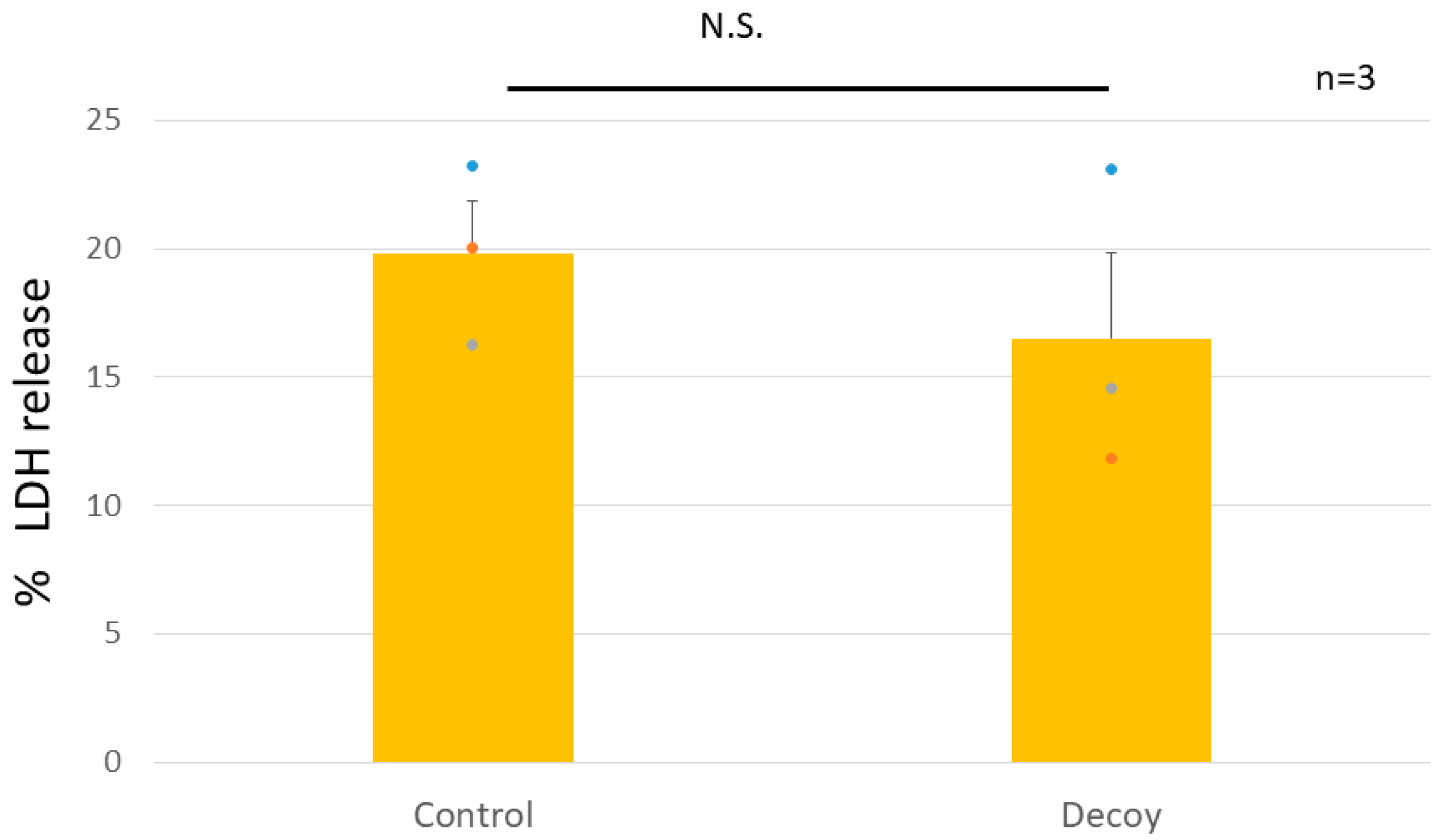
2.1.1.2. Cytotoxicity Analysis of Decoy
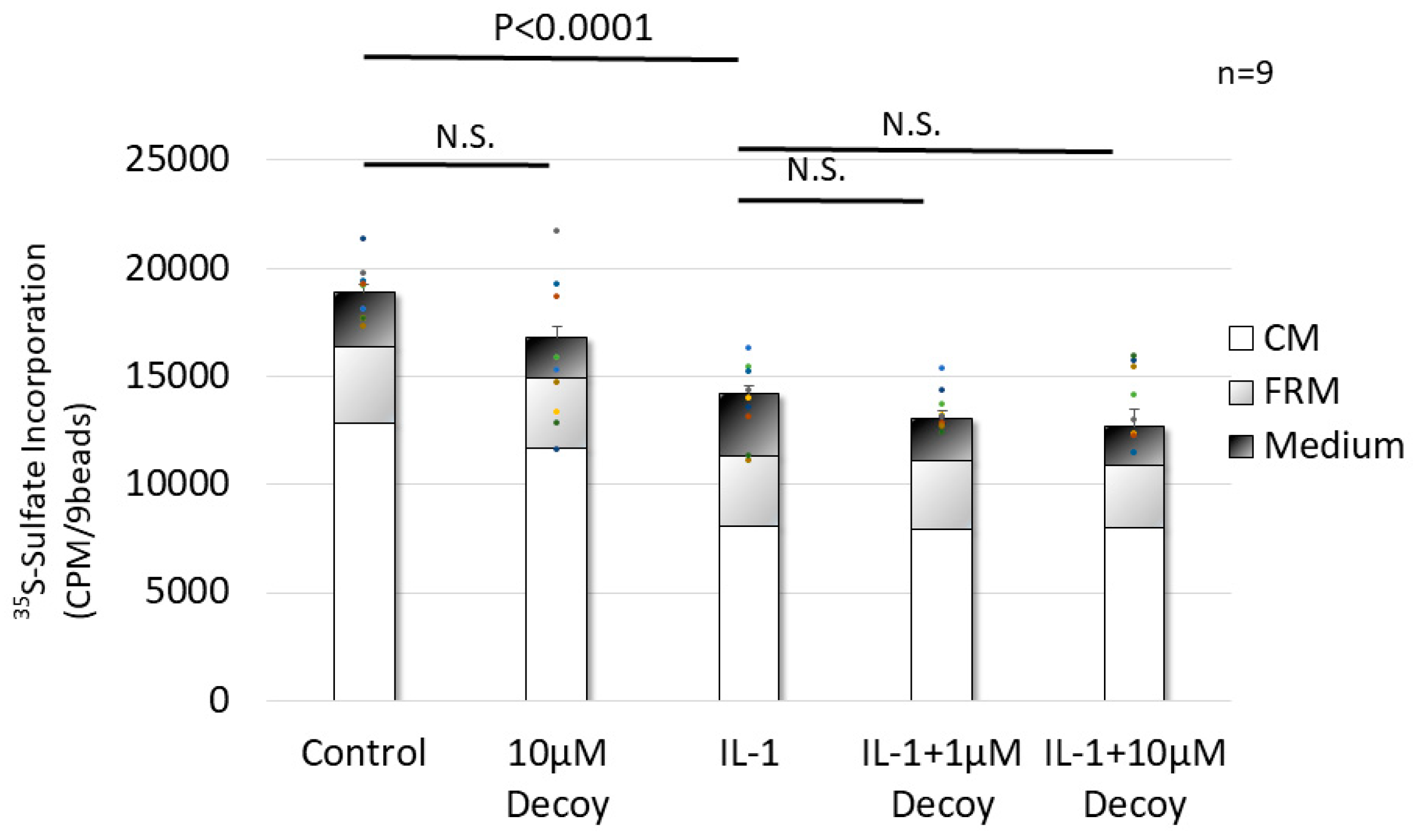
2.1.1.3. PG Synthesis Assay
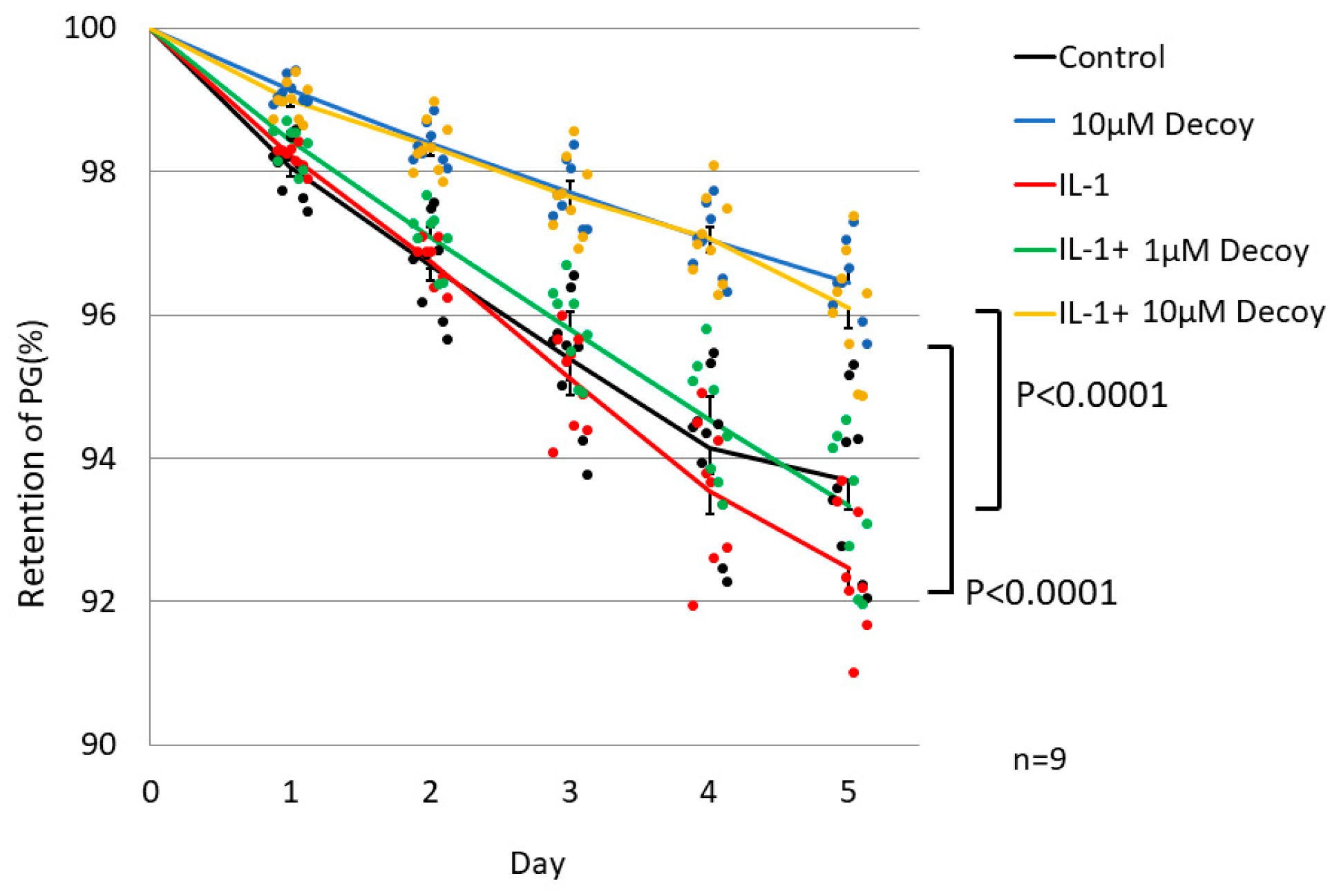
2.1.1.4. PG Turnover Assay
2.1.2. Effect of Decoy on hNSC tissue
2.1.2.1. hNSC Harvest and Tissue Culture
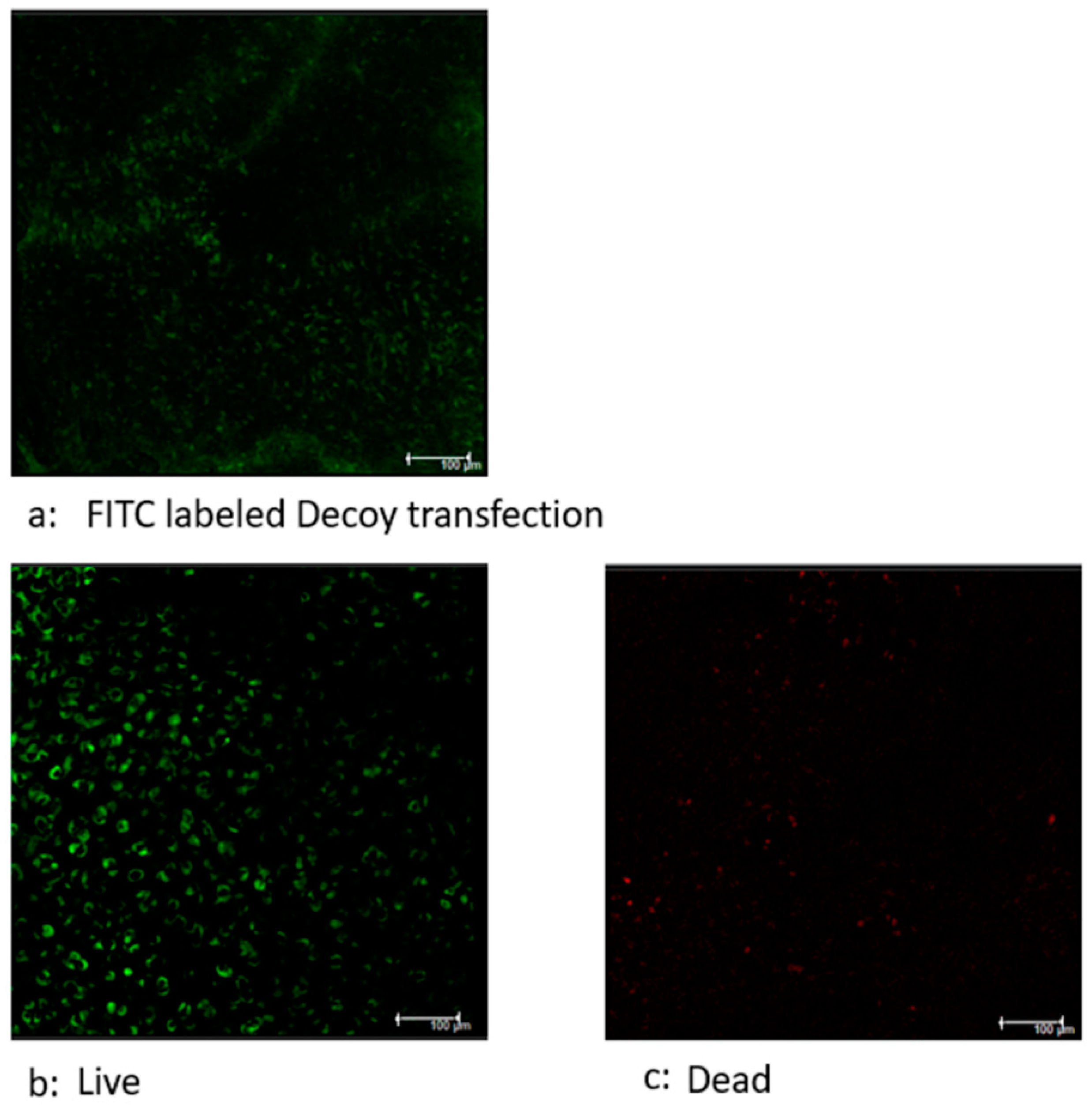
2.1.2.2. Efficiency of Transfection of Decoy
2.1.2.3. PG Turnover Assay
2.1.2.4. Enzyme-Linked Immunosorbent Assay (ELISA) Matrix Metalloprotease 3 (MMP-3), TNF and IL-6
2.1.2.5. Measurement of Nitric Oxide (NO)
2.1.2.6. Statistical Analysis
3. Results
3.1.1. Decoy inhibiting the release of LDH.
3.1.2. Decoy not affecting PG synthesis.
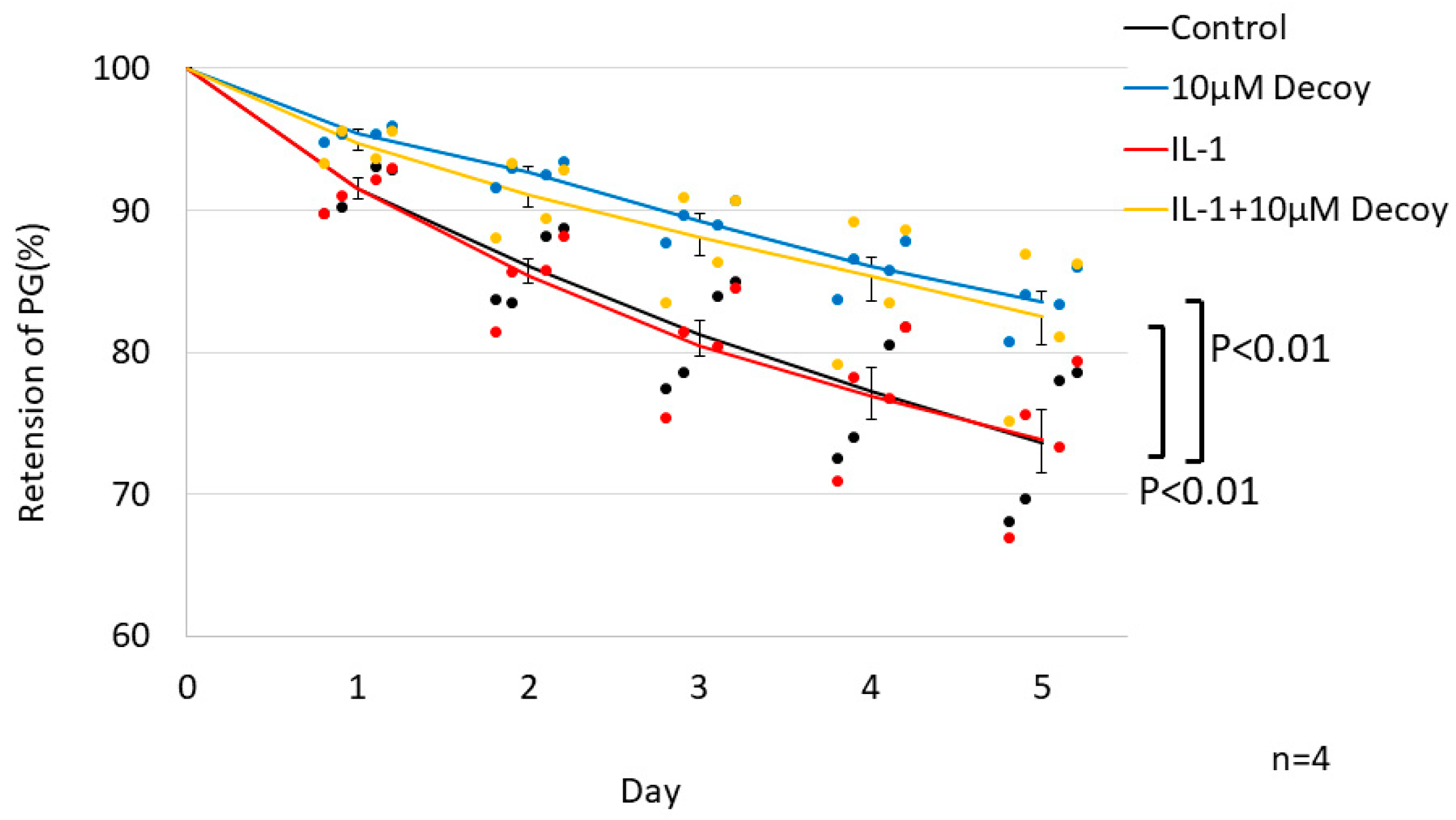
3.1.3. 10 uM Decoy inhibiting PG degradation in rNSCh.
3.2.1. Decoy transfected into viable cells.
3.2.2. 10uM Decoy inhibiting PG degradation in hNSC tissue culture.
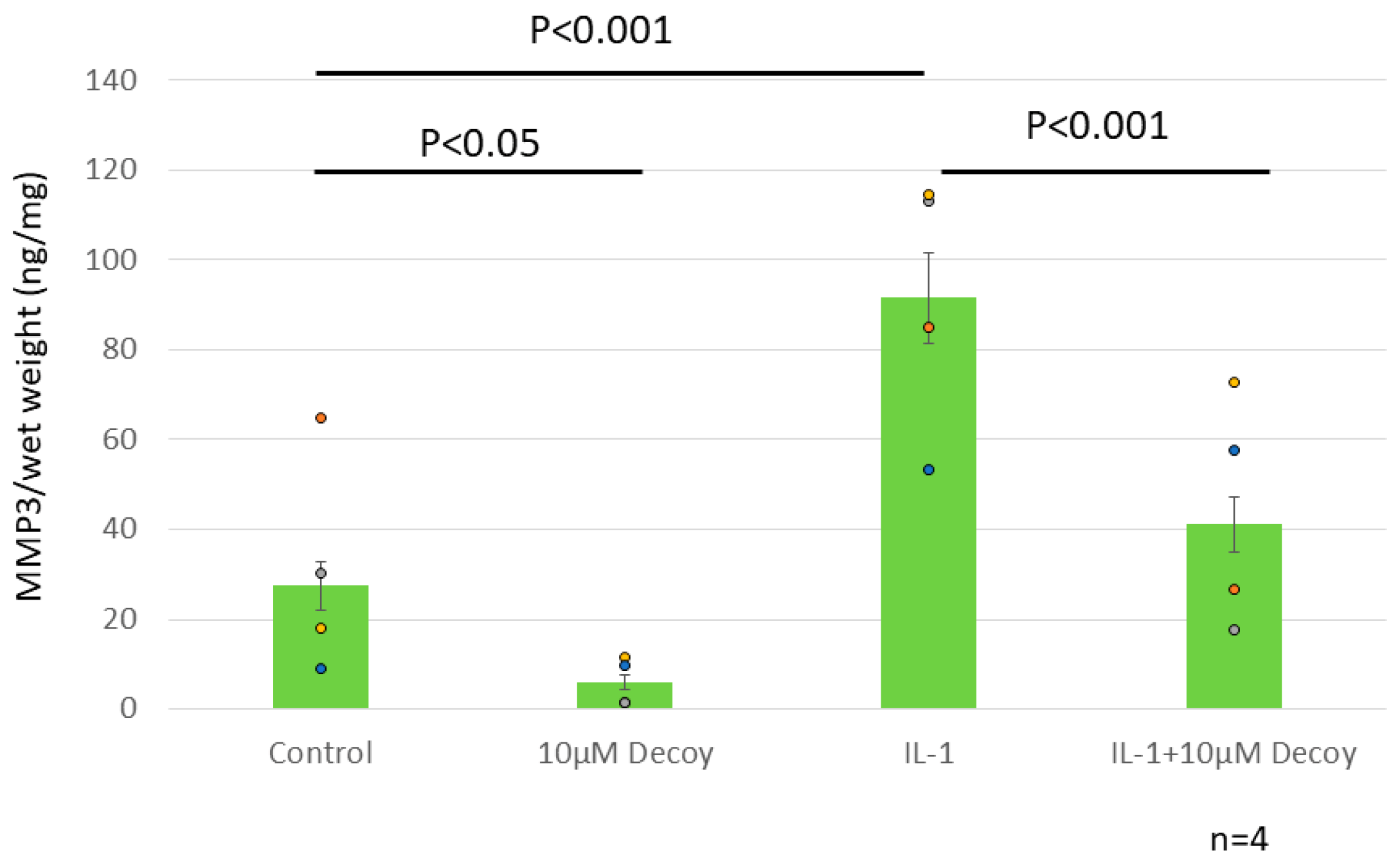
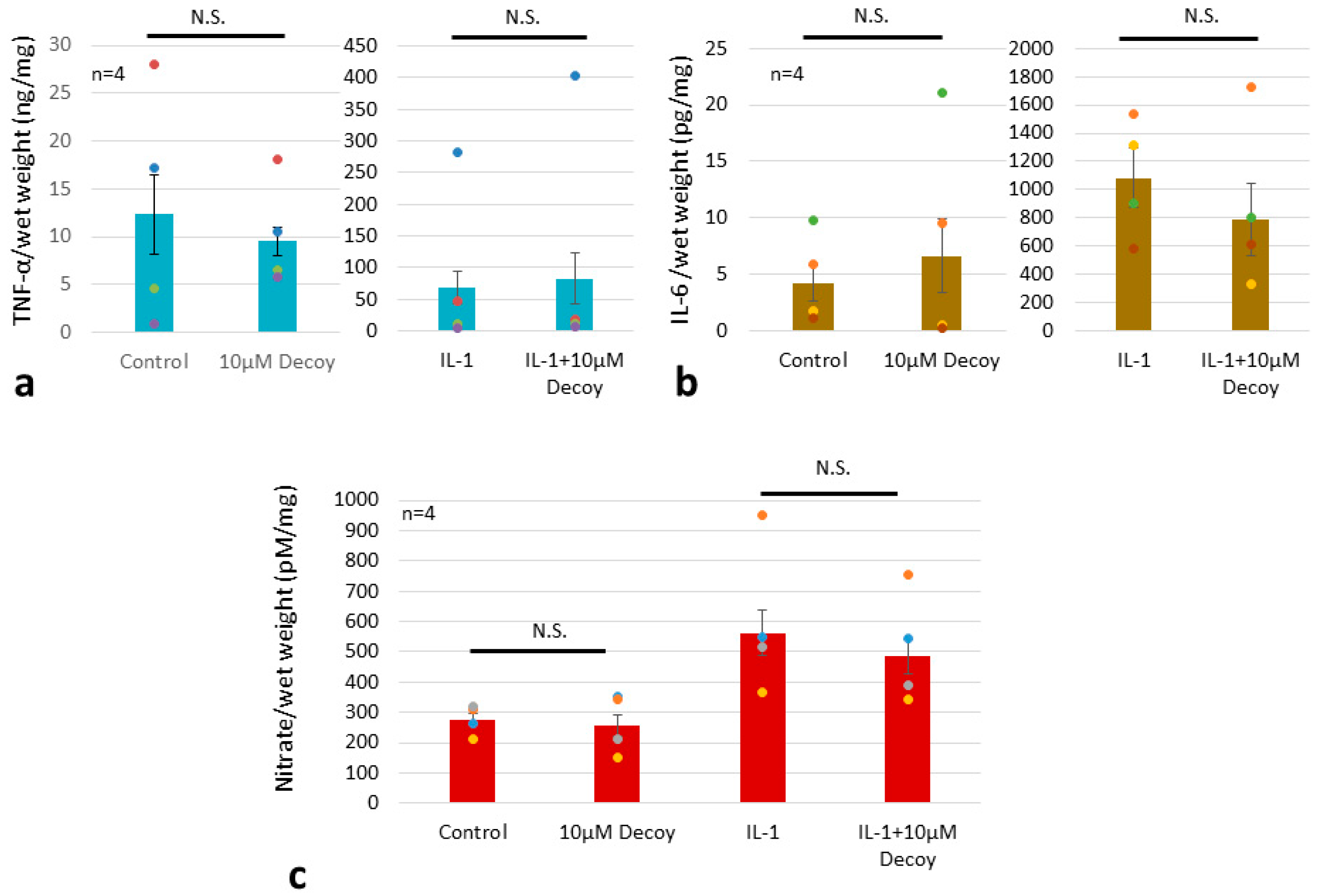
3.2.3. Decoy inhibiting MMP3 production (ELISA and Nitrate Assay)
4. Discussion
5. Conclusion
Author Contributions
Acknowledgments
References
- Fu, Y.; He, A.; Xie, Y.; Zhu, Y.; Li, C.; Zhang, T. The Effect of Fixation Materials on the Long-Term Stability of Cartilage Framework for Microtia Reconstruction. Facial Plast. Surg. Aesthetic Med. 2023, 25, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, X.; Deng, Y. Complications Associated with Autologous Costal Cartilage Used in Rhinoplasty: An Updated Meta-Analysis. Aesthetic Plast. Surg. 2022, 47, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Cervelli, V. Nasal Tip Remodeling Using Autologous Cartilage Grafts: Systematic Review. J. Craniofacial Surg. 2022, 33, 2035–2040. [Google Scholar] [CrossRef] [PubMed]
- Brent, B. The Versatile Cartilage Autograft: Current Trends in Clinical Transplantation. Clin. Plast. Surg. 1979, 6, 163–180. [Google Scholar] [CrossRef] [PubMed]
- De Gabory, L.; Fricain, J.C.; Stoll, D. [Resorption of cartilage grafts in rhinoplasty: fundamental basis]. Revue de laryngologie - otologie - rhinologie 2010, 131, 83–88. [Google Scholar] [PubMed]
- Bujía, J. Determination of the viability of crushed cartilage grafts: clinical implications for wound healing in nasal surgery. Ann. Plast. Surg. 1994, 32, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Hom, D. The wound healing response to grafted tissues. Otolaryngol. Clin. N. Am. 1994, 27, 13–24. [Google Scholar] [CrossRef]
- Janis, J.E.; Harrison, B. Wound Healing: Part I. Basic Science. Plast. Reconstr. Surg. 2014, 133, 199e–207e. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hsu, S.; Chang, M.; Huang, Y. Reducing scar formation by regulation of IL-1 and MMP-9 expression by using sustained release of prednisolone-loaded PDLL microspheres in a murine wound model. J. Biomed. Mater. Res. Part A 2013, 101, 1165–1172. [Google Scholar] [CrossRef]
- Haisch, A.; Gröger, A.; Radke, C.; Ebmeyer, J.; Sudhoff, H.; Grasnick, G.; Jahnke, V.; Burmester, G.; Sittinger, M. Macroencapsulation of human cartilage implants: pilot study with polyelectrolyte complex membrane encapsulation. Biomaterials 2000, 21, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A. S., Gedin, P., Hugo, A., et al. Activation of NF-κB in Synovium versus Cartilage from Patients with Advanced Knee Osteoarthritis: A Potential Contributor to Inflammatory Aspects of Disease Progression. Journal of immunology (Baltimore, Md : 1950) 2018;201:1918-1927. [CrossRef]
- Yamasaki, K., Asai, T., Shimizu, M., et al. Inhibition of NFκB activation using cis-element ‘decoy’of NFκB binding site reduces neointimal formation in porcine balloon-injured coronary artery model. Gene therapy 2003;10:356-364. [CrossRef]
- Morishita, R.; Tomita, N.; Kaneda, Y.; Ogihara, T. Molecular therapy to inhibit NFκB activation by transcription factor decoy oligonucleotides. Curr. Opin. Pharmacol. 2004, 4, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Baltimore, D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 1986, 46, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T. D. The Rel/NF-kappaB signal transduction pathway: introduction. Oncogene 1999;18:6842. [CrossRef]
- Kato, K., Akeda, K., Miyazaki, S., et al. NF-kB decoy oligodeoxynucleotide preserves disc height in a rabbit anular-puncture model and reduces pain induction in a rat xenograft-radiculopathy model. European cells & materials 2021;42:90-109. [CrossRef]
- Nishimura, A., Akeda, K., Matsubara, T., et al. Transfection of NF-κB decoy oligodeoxynucleotide suppresses pulmonary metastasis by murine osteosarcoma. Cancer gene therapy 2011;18:250-259. [CrossRef]
- Cao, C. C., Ding, X. Q., Ou, Z. L., et al. In vivo transfection of NF-κB decoy oligodeoxynucleotides attenuate renal ischemia/reperfusion injury in rats. Kidney international 2004;65:834-845. [CrossRef]
- Xu, M.-Q., Shuai, X.-R., Yan, M.-L., Zhang, M.-M., Yan, L.-N. Nuclear factor-kappaB decoy oligodeoxynucleotides attenuates ischemia/reperfusion injury in rat liver graft. World Journal of Gastroenterology 2005;11:6960. [CrossRef]
- Tomita, T.; Takano, H.; Tomita, N.; Morishita, R.; Kaneko, M.; Shi, K.; Takahi, K.; Nakase, T.; Kaneda, Y.; Yoshikawa, H.; et al. Transcription factor decoy for NFκB inhibits cytokine and adhesion molecule expressions in synovial cells derived from rheumatoid arthritis. Rheumatology 2000, 39, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Abeyama, K., Eng, W., Jester, J. V., et al. A role for NF-κB–dependent gene transactivation in sunburn. Journal of Clinical Investigation 2000;105:1751-1759.
- Uemura, T.; Tsujii, M.; Akeda, K.; Iino, T.; Satonaka, H.; Hasegawa, M.; Sudo, A. Transfection of nuclear factor-kappaB decoy oligodeoxynucleotide protects against ischemia/reperfusion injury in a rat epigastric flap model. J. Gene Med. 2012, 14, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K., Samura, S., Tsuji, K., et al. Oral nuclear factor-κB decoy oligonucleotides delivery system with chitosan modified poly (D, L-lactide-co-glycolide) nanospheres for inflammatory bowel disease. Biomaterials 2011;32:870-878. [CrossRef]
- Morales, T.I.; Hascall, V.C. Factors involved in the regulation of proteoglycan metabolism in articular cartilage. Arthritis Rheum. 1989, 32, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Hascall, V.C.; Handley, C.J.; McQuillan, D.J.; Hascall, G.K.; Robinson, H.; Lowther, D.A. The effect of serum on biosynthesis of proteoglycans by bovine articular cartilage in culture. Arch. Biochem. Biophys. 1983, 224, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.A.; Handley, C.J.; Hascall, V.C.; Campbell, R.A.; Lowther, D.A. Turnover of proteoglycans in cultures of bovine articular cartilage. Arch. Biochem. Biophys. 1984, 234, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Luyten, F.P.; Hascall, V.C.; Nissley, S.; Morales, T.I.; Reddi, A. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch. Biochem. Biophys. 1988, 267, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Fell, H.B.; Jubb, R.W. The Effect of Synovial Tissue on the Breakdown of Articular Cartilage in Organ Culture. Arthritis Rheum. 1977, 20, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Saklatvala, J.; Pilsworth, L.M.C.; Sarsfield, S.J.; Gavrilovic, J.; Heath, J.K. Pig catabolin is a form of interleukin 1. Cartilage and bone resorb, fibroblasts make prostaglandin and collagenase, and thymocyte proliferation is augmented in response to one protein. Biochem. J. 1984, 224, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Morales, T.I.; Hascall, V.C. Effects of Interleukin-1 and Lipopolysaccharides on Protein and Carbohydrate Metabolism in Bovine Articular Cartilage Organ Cultures. Connect. Tissue Res. 1989, 19, 255–275. [Google Scholar] [CrossRef] [PubMed]
- Jenei-Lanzl, Z., Meurer, A., Zaucke, F. Interleukin-1β signaling in osteoarthritis - chondrocytes in focus. Cellular signalling 2019;53:212-223. [CrossRef]
- Bonassar, L.J.; Frank, E.H.; Murray, J.C.; Paguio, C.G.; Moore, V.L.; Lark, M.W.; Sandy, J.D.; Wu, J.; Eyre, D.R.; Grodzinsky, A.J. Changes in cartilage composition and physical properties due to stromelysin degradation. Arthritis Rheum. 1995, 38, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Temple, M.M.; Xue, Y.; Chen, M.Q.; Sah, R.L. Interleukin-1alpha induction of tensile weakening associated with collagen degradation in bovine articular cartilage. Arthritis Rheum. 2006, 54, 3267–3276. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.D.; Mangiapani, D.S.; Zeitler, E.; Bailey, K.N.; Horne, P.H.; Huebner, J.L.; Kraus, V.B.; Guilak, F.; A Olson, S. Targeting pro-inflammatory cytokines following joint injury: acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis Res. Ther. 2014, 16, R134. [Google Scholar] [CrossRef] [PubMed]
- Morishita, R., Sugimoto, T., Aoki, M., et al. In vivo transfection of cis element “decoy” against nuclear factor-κB binding site prevents myocardial infarction. Nature medicine 1997;3:894-899. [CrossRef]
- Sawa, Y.; Morishita, R.; Suzuki, K.; Kagisaki, K.; Kaneda, Y.; Maeda, K.; Kadoba, K.; Matsuda, H. A novel strategy for myocardial protection using in vivo transfection of cis element ’decoy’ against NFkappaB binding site: evidence for a role of NFkappaB in ischemia-reperfusion injury. Circulation 1997;96:II-280-284; discussion II-285.
- Yoshimura, S., Morishita, R., Hayashi, K., et al. Inhibition of intimal hyperplasia after balloon injury in rat carotid artery model using cis-element ‘decoy’of nuclear factor-kB binding site as a novel molecular strategy. Gene therapy 2001;8:1635-1642. [CrossRef]
- Chiba, K.; Andersson, G.B.J.; Masuda, K.; Thonar, E.J.-M.A. Metabolism of the Extracellular Matrix Formed by Intervertebral Disc Cells Cultured in Alginate. Spine 1997, 22, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, D.; Yin, L.; Zhang, C.; Qu, H.; Xu, J. Neuroglobin protects against cerebral ischemia/reperfusion injury in rats by suppressing mitochondrial dysfunction and endoplasmic reticulum stress-mediated neuronal apoptosis through synaptotagmin-1. Environ. Toxicol. 2023, 38, 1891–1904. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Shirota, H.; Thonar, E. Quantification of 35S-Labeled Proteoglycans Complexed to Alcian Blue by Rapid Filtration in Multiwell Plates. Anal. Biochem. 1994, 217, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Mok, S.S.; Masuda, K.; Häuselmann, H.J.; Aydelotte, M.B.; Thonar, E.J. Aggrecan synthesized by mature bovine chondrocytes suspended in alginate. Identification of two distinct metabolic matrix pools. J. Biol. Chem. 1994, 269, 33021–33027. [Google Scholar] [CrossRef] [PubMed]
- Liu, T., Zhang, L., Joo, D., Sun, S. C. NF-κB signaling in inflammation. Signal transduction and targeted therapy 2017;2:17023. [CrossRef]
- Sullenger, B.A.; Gallardo, H.F.; Ungers, G.E.; Gilboa, E. Overexpression of TAR sequences renders cells resistant to human immunodeficiency virus replication. Cell 1990, 63, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Bielinska, A.; Shivdasani, R.A.; Zhang, L.; Nabel, G.J. Regulation of Gene Expression with Double-Stranded Phosphorothioate Oligonucleotides. Science 1990, 250, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H., Ishida, Y., Hosomichi, J., et al. Ultrasound microbubble-mediated transfection of NF-κB decoy oligodeoxynucleotide into gingival tissues inhibits periodontitis in rats in vivo. PloS one 2017;12:e0186264. [CrossRef]
- NIH. AMG0103 in Subjects With Chronic Discogenic Lumbar Back Pain - Full Text View - ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT03263611. Accessed 28, Jan. 2023.
- Miyake, T., Miyake, T., Sakaguchi, M., Nankai, H., Nakazawa, T., Morishita, R. Prevention of Asthma Exacerbation in a Mouse Model by Simultaneous Inhibition of NF-κB and STAT6 Activation Using a Chimeric Decoy Strategy. Molecular therapy Nucleic acids 2018;10:159-169. [CrossRef]
- Allaith, S.; Tew, S.; Hughes, C.; Clegg, P.; Canty-Laird, E.; Comerford, E. Characterisation of key proteoglycans in the cranial cruciate ligaments (CCLs) from two dog breeds with different predispositions to CCL disease and rupture. Veter- J. 2021, 272, 105657. [Google Scholar] [CrossRef] [PubMed]
- Hascall, V., Luyten, F., Plaas, A., Sandy, J. Steady-state metabo-lism of proteoglycans in bovine articular cartilage explants. In A. Maroudas, K. E. Kuettner eds., Methods in Cartilage Research London: Academic Press; 1990:108-112.
- Peng, Z.; Sun, H.; Bunpetch, V.; Koh, Y.; Wen, Y.; Wu, D.; Ouyang, H. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials 2021, 268, 120555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, C.; He, B.; Zhang, X.; Hao, H.; Hou, Y.; Li, A.; Wang, Y.; Wang, Y. Macrophage Migration Inhibitory Factor Promotes Expression of Matrix Metalloproteinases 1 and 3 in Spinal Cord Astrocytes following Gecko Tail Amputation. J. Integr. Neurosci. 2023, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Tyler, J., Bolis, S., Dingle, J., et al. Mediators of matrix metabolism. In K. Kuettner, R. Schleyerbach, J. Peyron eds., Articular Cartilage and Osteoarthritis. New York: Raven Press; 1992:251-264.
- Cawston, T., Curry, V., Summers, C., et al. The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis & Rheumatism 1998;41:1760-1771. [CrossRef]
- Okada, Y. Regulation of Matrix Metalloprotease (in Japanese). Jikken igaku 1992;10:256-262.
- Pichika, R., Akeda, K., Gemba, T., Miyamoto, K., An, H. S., Masuda, K. Transcription Factor Decoy For Nfkb Inhibits The Appearance Of Active Mmps And Adamts4 In The Medium Of Human Intervertebral Disc Cells Cultured In Alginate. Paper presented at: 2005 Annual Meeting of the Orthopaedic Research Society May 20-23; Chicago, Ill.
- Nelson, H. S., Leung, D. Y., Bloom, J. W. Update on glucocorticoid action and resistance. Journal of allergy and Clinical Immunology 2003;111:3-22. [CrossRef]
- Song, Y.W.; Zhang, T.; Wang, W.B. Gluococorticoid could influence extracellular matrix synthesis through Sox9 via p38 MAPK pathway. Rheumatol. Int. 2012, 32, 3669–3673. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




