Submitted:
27 October 2023
Posted:
27 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Characterization of the ZnO/Au and ZnO/Ag nanocomposites
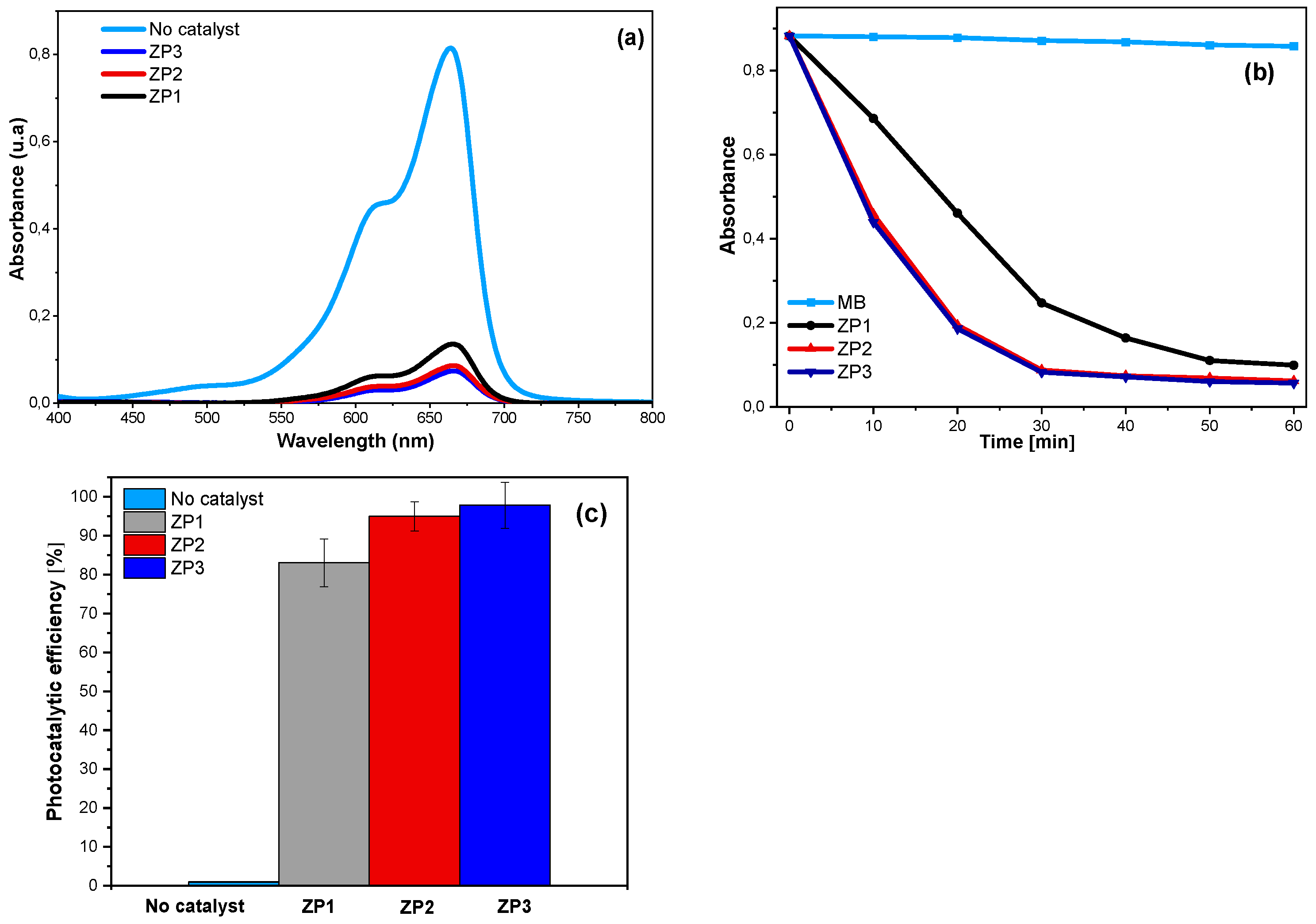
2.2. Optical properties
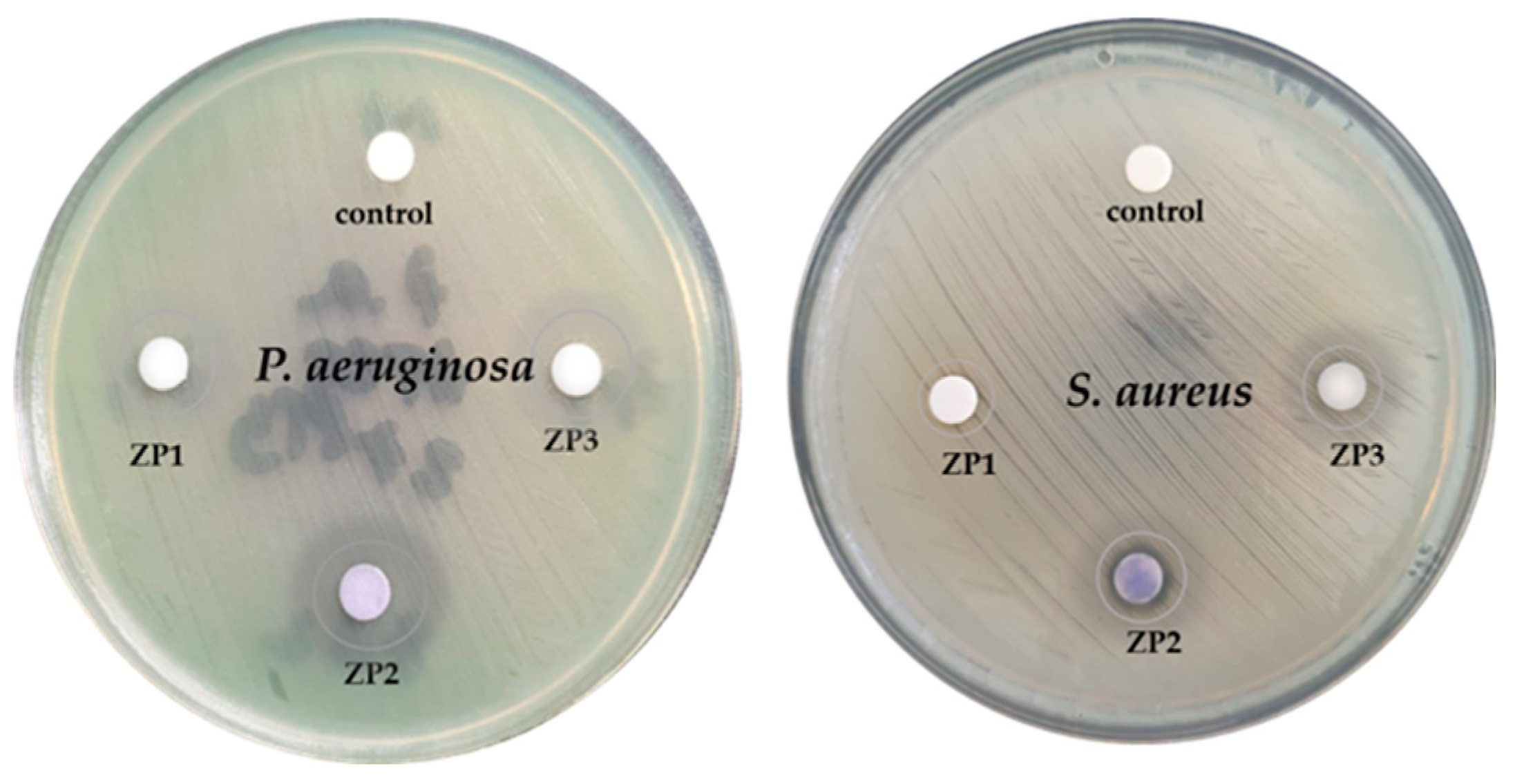
2.3. Antimicrobial activity
2.4. Photocatalytic activity
3. Materials and Methods
3.1. Reagents
3.2. Preparation of ZnO/Au and ZnO/Ag nanocomposites
3.3. Characterisation Techniques
3.4. Antibacterial testing
3.4.1. Antibacterial susceptibility
3.4.2. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) assays
3.5. Photocatalytic testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [CrossRef]
- Hasnat, M.A.; Hossain, M.I.; Ahsan, M.; Islam, M.F. Recent Developments in the Utilization of Nanomaterials for Sensing Platforms. Recent Developments in Green Electrochemical Sensors: Design, Performance, and Applications 2023, 61-99.
- Kumar, R.; Kumar, M.; Luthra, G. Fundamental approaches and applications of nanotechnology: A mini review. Mater. Today: Proc. 2023. [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angewandte Chemie International Edition 2009, 48, 60-103.
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; A. Ahmed, E.; Battah, B.; Abd Ellah, N.H.; Zanetti, S.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413.
- Zhao, Y.; Chen, L.; Wang, Y.; Song, X.; Li, K.; Yan, X.; Yu, L.; He, Z. Nanomaterial-based strategies in antimicrobial applications: Progress and perspectives. Nano Res. 2021, 14, 4417–4441. [CrossRef]
- Dediu, V.; Ghitman, J.; Pircalabioru, G.G.; Chan, K.H.; Iliescu, F.S.; Iliescu, C. Trends in Photothermal Nanostructures for Antimicrobial Applications. Int. J. Mol. Sci. 2023, 24, 9375. [CrossRef]
- Hosny, A.E.-D.M.; A Rasmy, S.; Aboul-Magd, D.S.; Kashef, M.T.; E El-Bazza, Z. The increasing threat of silver-resistance in clinical isolates from wounds and burns. Infect. Drug Resist. 2019, ume 12, 1985–2001. [CrossRef]
- Wu, Z.; Chan, B.; Low, J.; Chu, J.J.H.; Hey, H.W.D.; Tay, A. Microbial resistance to nanotechnologies: An important but understudied consideration using antimicrobial nanotechnologies in orthopaedic implants. Bioact. Mater. 2022, 16, 249–270. [CrossRef]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019, 20, 2468. [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [CrossRef]
- Karagoz, S.; Kiremitler, N.B.; Sarp, G.; Pekdemir, S.; Salem, S.; Goksu, A.G.; Onses, M.S.; Sozdutmaz, I.; Sahmetlioglu, E.; Ozkara, E.S.; et al. Antibacterial, Antiviral, and Self-Cleaning Mats with Sensing Capabilities Based on Electrospun Nanofibers Decorated with ZnO Nanorods and Ag Nanoparticles for Protective Clothing Applications. ACS Appl. Mater. Interfaces 2021, 13, 5678–5690. [CrossRef]
- Gupta, J.; Irfan, M.; Ramgir, N.; Muthe, K.P.; Debnath, A.K.; Ansari, S.; Gandhi, J.; Ranjith-Kumar, C.T.; Surjit, M. Antiviral Activity of Zinc Oxide Nanoparticles and Tetrapods Against the Hepatitis E and Hepatitis C Viruses. Front. Microbiol. 2022, 13, 881595. [CrossRef]
- Melk, M.M.; El-Hawary, S.S.; Melek, F.R.; Saleh, D.O.; Ali, O.M.; A El Raey, M.; Selim, N.M. Antiviral Activity of Zinc Oxide Nanoparticles Mediated by Plumbago indica L. Extract Against Herpes Simplex Virus Type 1 (HSV-1). Int. J. Nanomed. 2021, ume 16, 8221–8233. [CrossRef]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of ZnO nanoparticles—the role of ROS mediated cell injury. Nanotechnology 2011, 22, 105101. [CrossRef]
- Kumar, R.S.; Dananjaya, S.H.S.; De Zoysa, M.; Yang, M. Enhanced antifungal activity of Ni-doped ZnO nanostructures under dark conditions. RSC Adv. 2016, 6, 108468–108476. [CrossRef]
- Marín-Caba, L.; Bodelón, G.; Negrín-Montecelo, Y.; Correa-Duarte, M.A. Sunlight-Sensitive Plasmonic Nanostructured Composites as Photocatalytic Coating with Antibacterial Properties. Adv. Funct. Mater. 2021, 31, 2105807. [CrossRef]
- Lu, J.; Wang, H.; Peng, D.; Chen, T.; Dong, S.; Chang, Y. Synthesis and properties of Au/ZnO nanorods as a plasmonic photocatalyst. Phys. E: Low-dimensional Syst. Nanostructures 2016, 78, 41–48. [CrossRef]
- Kavitha, R.; Kumar, S.G. A review on plasmonic Au-ZnO heterojunction photocatalysts: Preparation, modifications and related charge carrier dynamics. Materials Science in Semiconductor Processing 2019, 93, 59-91.
- Balestri, A.; Cardellini, J.; Berti, D. Gold and silver nanoparticles as tools to combat multidrug-resistant pathogens. Curr. Opin. Colloid Interface Sci. 2023, 66. [CrossRef]
- Dediu, V.; Busila, M.; Tucureanu, V.; Bucur, F.I.; Iliescu, F.S.; Brincoveanu, O.; Iliescu, C. Synthesis of ZnO/Au Nanocomposite for Antibacterial Applications. Nanomaterials 2022, 12, 3832. [CrossRef]
- Hernández-Sierra, J.F.; Ruiz, F.; Pena, D.C.C.; Martínez-Gutiérrez, F.; Martínez, A.E.; Guillén, A.d.J.P.; Tapia-Pérez, H.; Castañón, G.M. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine: Nanotechnology, Biol. Med. 2008, 4, 237–240. [CrossRef]
- Burlibaşa, L.; Chifiriuc, M.C.; Lungu, M.V.; Lungulescu, E.M.; Mitrea, S.; Sbarcea, G.; Popa, M.; Măruţescu, L.; Constantin, N.; Bleotu, C. Synthesis, physico-chemical characterization, antimicrobial activity and toxicological features of AgZnO nanoparticles. Arabian Journal of Chemistry 2020, 13, 4180-4197.
- Mousavi-Kouhi, S.M.; Beyk-Khormizi, A.; Amiri, M.S.; Mashreghi, M.; Yazdi, M.E.T. Silver-zinc oxide nanocomposite: From synthesis to antimicrobial and anticancer properties. Ceram. Int. 2021, 47, 21490–21497. [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [CrossRef]
- Zhang, X.; Chen, Y.L.; Liu, R.-S.; Tsai, D.P. Plasmonic photocatalysis. Reports on Progress in Physics 2013, 76, 046401.
- Deng, Q.; Duan, X.; Ng, D.H.L.; Tang, H.; Yang, Y.; Kong, M.; Wu, Z.; Cai, W.; Wang, G. Ag Nanoparticle Decorated Nanoporous ZnO Microrods and Their Enhanced Photocatalytic Activities. ACS Appl. Mater. Interfaces 2012, 4, 6030–6037. [CrossRef]
- Güy, N.; Özacar, M. The influence of noble metals on photocatalytic activity of ZnO for Congo red degradation. Int. J. Hydrogen Energy 2016, 41, 20100–20112. [CrossRef]
- Putri, A.E.; Roza, L.; Budi, S.; Umar, A.A.; Fauzia, V. Tuning the photocatalytic activity of nanocomposite ZnO nanorods by shape-controlling the bimetallic AuAg nanoparticles. Appl. Surf. Sci. 2020, 536, 147847. [CrossRef]
- Sun, F.; Tan, F.; Wang, W.; Qiao, X.; Qiu, X. Facile synthesis of Ag/ZnO heterostructure nanocrystals with enhanced photocatalytic performance. Mater. Res. Bull. 2012, 47, 3357–3361. [CrossRef]
- Ibănescu, M.; Muşat, V.; Textor, T.; Badilita, V.; Mahltig, B. Photocatalytic and antimicrobial Ag/ZnO nanocomposites for functionalization of textile fabrics. J. Alloy. Compd. 2014, 610, 244–249. [CrossRef]
- Akman, B.; Aras, O. Usability, durability and regeneration of Ag/ZnO coated microreactor for photocatalytic degradation of methylene blue. J. Mol. Struct. 2021, 1251, 132003. [CrossRef]
- Lin, W.-H.; Chiu, Y.-H.; Shao, P.-W.; Hsu, Y.-J. Metal-Particle-Decorated ZnO Nanocrystals: Photocatalysis and Charge Dynamics. ACS Appl. Mater. Interfaces 2016, 8, 32754–32763. [CrossRef]
- Pawinrat, P.; Mekasuwandumrong, O.; Panpranot, J. Synthesis of Au–ZnO and Pt–ZnO nanocomposites by one-step flame spray pyrolysis and its application for photocatalytic degradation of dyes. Catal. Commun. 2009, 10, 1380–1385. [CrossRef]
- Patel, J.B. Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement. 2015.
- Kassim, A.; Omuse, G.; Premji, Z.; Revathi, G. Comparison of Clinical Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing guidelines for the interpretation of antibiotic susceptibility at a University teaching hospital in Nairobi, Kenya: a cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 21–21. [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71, doi:10.1107/s0021889869006558.
- Pecharsky, V.K.; Zavalij, P.Y. Fundamentals of Crystalline State and Crystal Lattice. Fundamentals of Powder Diffraction and Structural Characterization of Materials 2009, 1-15.
- Williamson, G.K.; Smallman, R.E., III. Dislocation densities in some annealed and cold-worked metals from measurements on the X-ray debye-scherrer spectrum. Philos. Mag. 1956, 1, 34–46. [CrossRef]
- An, S.S.A.; Kim, K.; Choi, M.; Lee, J.-K.; Jeong, J.; Kim, Y.-R.; Kim, M.-K.; Paek, S.; Shin, J.-H. Physicochemical properties of surface charge-modified ZnO nanoparticles with different particle sizes. Int. J. Nanomed. 2014, 9, 41–56. [CrossRef]
- Nicholas, N.J.; Franks, G.V.; Ducker, W.A. Selective Adsorption to Particular Crystal Faces of ZnO. Langmuir 2012, 28, 7189–7196. [CrossRef]
- Restrepo, C.V.; Villa, C.C. Synthesis of silver nanoparticles, influence of capping agents, and dependence on size and shape: A review. Environ. Nanotechnology, Monit. Manag. 2021, 15, 100428. [CrossRef]
- Hessien, M.; Da’na, E.; Al-Amer, K.; Khalaf, M.M. Nano ZnO (hexagonal wurtzite) of different shapes under various conditions: fabrication and characterization. Mater. Res. Express 2019, 6, 085057. [CrossRef]
- ucureanu, V.; Munteanu, D. Enhanced optical properties of YAG: Ce yellow phosphor by modification with gold nanoparticles. Ceramics International 2019, 45, 7641-7648.
- Fageria, P.; Gangopadhyay, S.; Pande, S. Synthesis of ZnO/Au and ZnO/Ag nanoparticles and their photocatalytic application using UV and visible light. RSC Adv. 2014, 4, 24962–24972. [CrossRef]
- Abdullahi, S.S.; Güner, S.; Musa, Y.; Adamu, B.I.; Abdulhamid, M.I. Sımple method for the determınatıon of band gap of a nanopowdered sample usıng Kubelka Munk theory. NAMP J 2016, 35, 241-246.
- Niño-Martínez, N.; Salas Orozco, M.F.; Martínez-Castañón, G.-A.; Torres Méndez, F.; Ruiz, F. Molecular Mechanisms of Bacterial Resistance to Metal and Metal Oxide Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2808. [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [CrossRef]
- Hachicho, N.; Hoffmann, P.; Ahlert, K.; Heipieper, H.J. Effect of silver nanoparticles and silver ions on growth and adaptive response mechanisms of Pseudomonas putida mt-2. FEMS microbiology letters 2014, 355, 71-77.
- Regmi, C.; Joshi, B.; Ray, S.K.; Gyawali, G.; Pandey, R.P. Understanding Mechanism of Photocatalytic Microbial Decontamination of Environmental Wastewater. Front. Chem. 2018, 6, 33. [CrossRef]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: a critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295-2350.
- Tian, E.-K.; Wang, Y.; Ren, R.; Zheng, W.; Liao, W. Gold Nanoparticle: Recent Progress on Its Antibacterial Applications and Mechanisms. J. Nanomater. 2021, 2021, 1–18. [CrossRef]
- Dasari, T.S.; Zhang, Y.; Yu, H. Antibacterial activity and cytotoxicity of gold (I) and (III) ions and gold nanoparticles. Biochemistry & pharmacology: open access 2015, 4.
- Lee, H.; Lee, D.G. Gold nanoparticles induce a reactive oxygen species-independent apoptotic pathway in Escherichia coli. Colloids Surfaces B: Biointerfaces 2018, 167, 1–7. [CrossRef]
- Lipovsky, A.; Tzitrinovich, Z.; Friedmann, H.; Applerot, G.; Gedanken, A.; Lubart, R. EPR Study of Visible Light-Induced ROS Generation by Nanoparticles of ZnO. J. Phys. Chem. C 2009, 113, 15997–16001. [CrossRef]
- Pham, T.N.; Hue, N.T.; Lee, Y.-C.; Huy, T.Q.; Thuy, N.T.T.; Van Tuan, H.; Khi, N.T.; Phan, V.N.; Thanh, T.D.; Lam, V.D.; et al. A hybrid design of Ag-decorated ZnO on layered nanomaterials (MgAC) with photocatalytic and antibacterial dual-functional abilities. RSC Adv. 2021, 11, 38578–38588. [CrossRef]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New Insights into the Mechanism of Visible Light Photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [CrossRef]
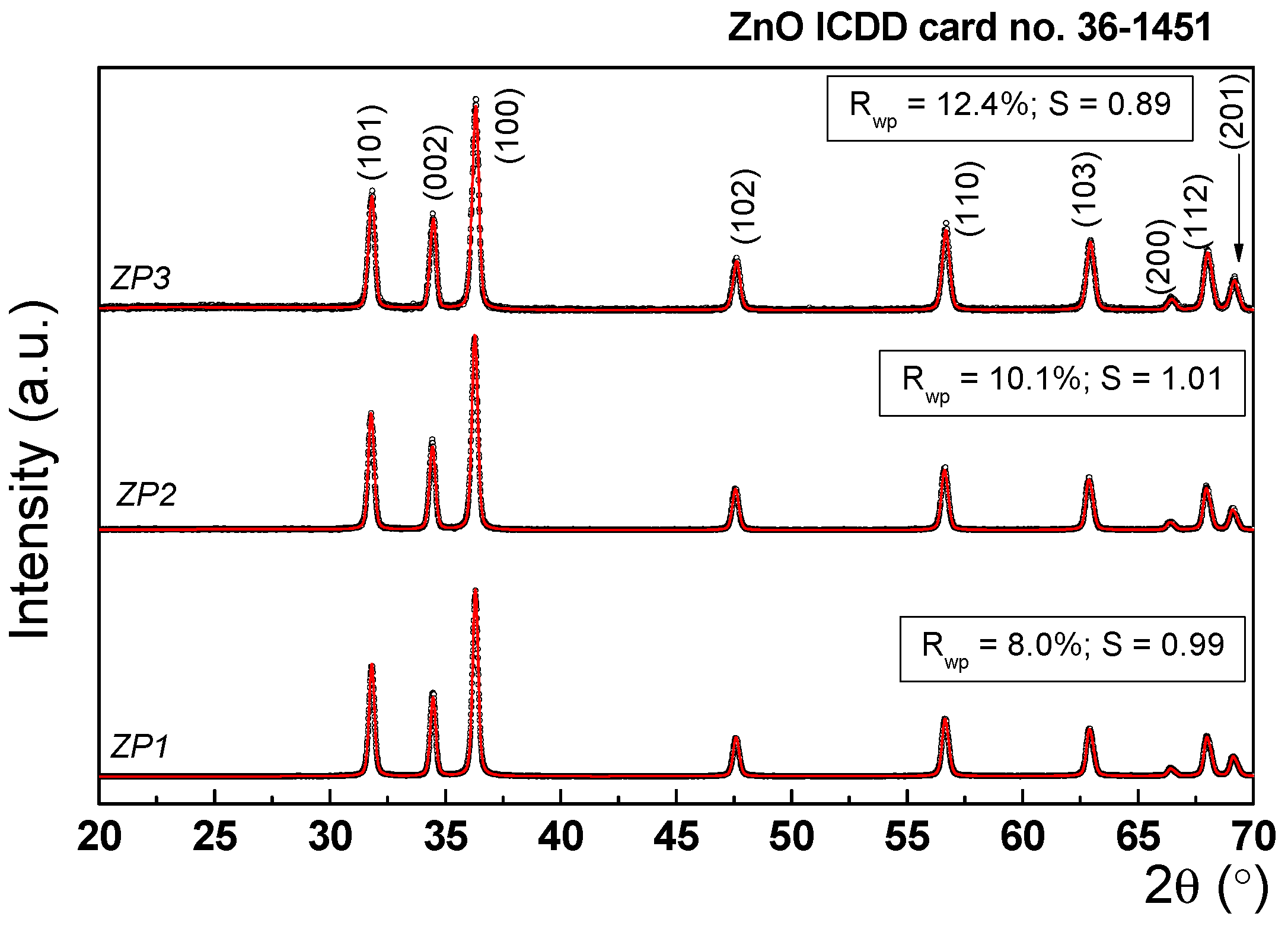
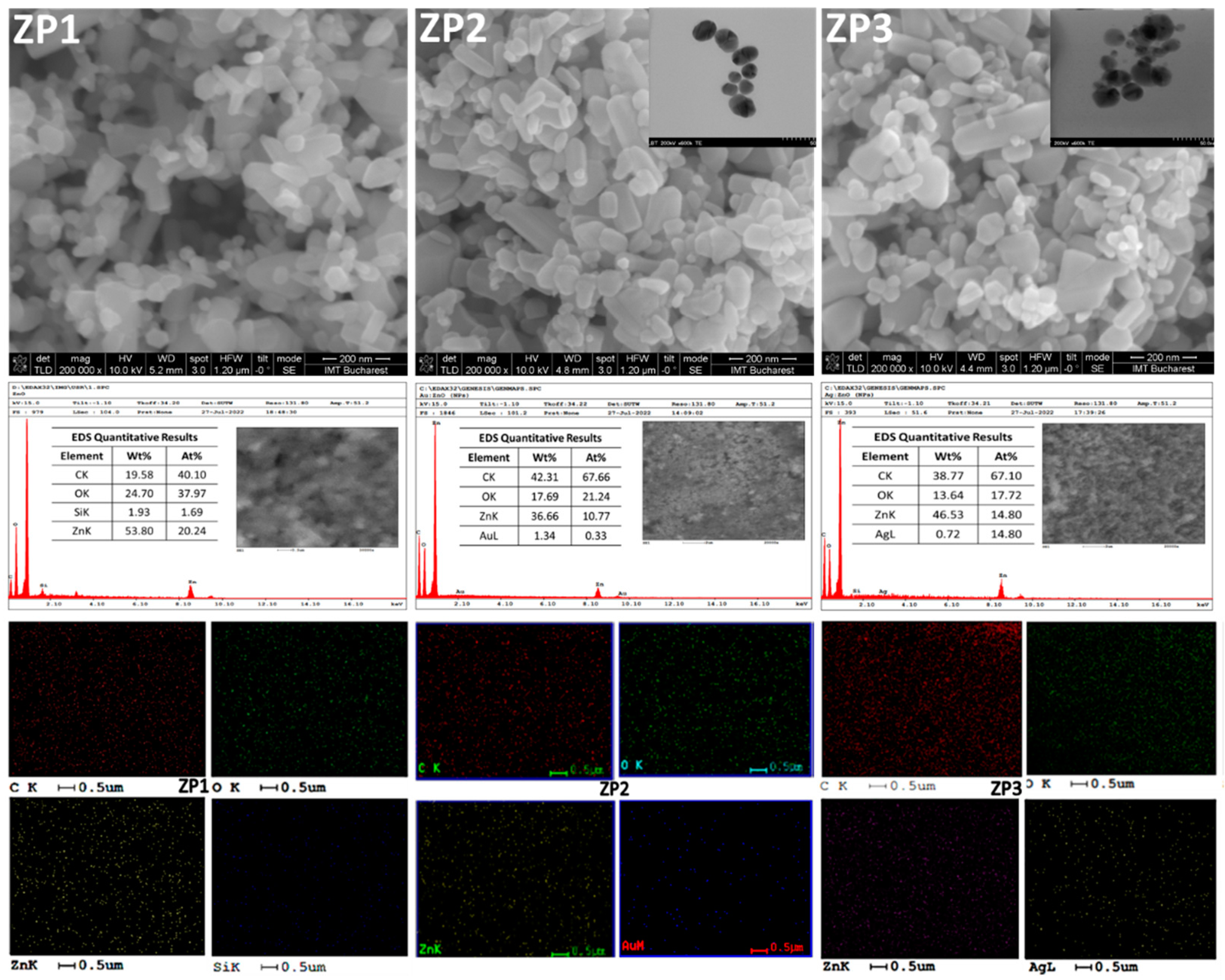
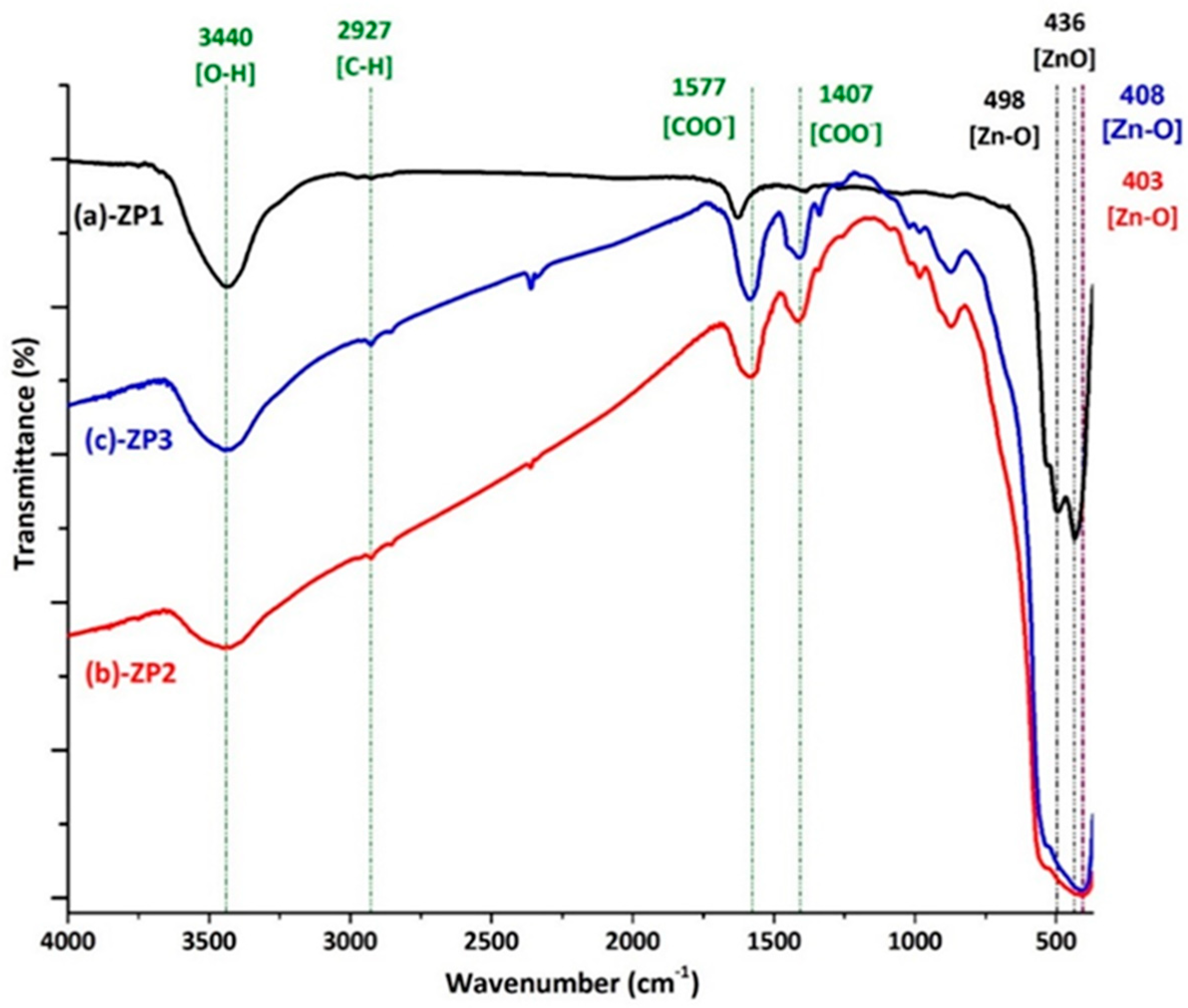
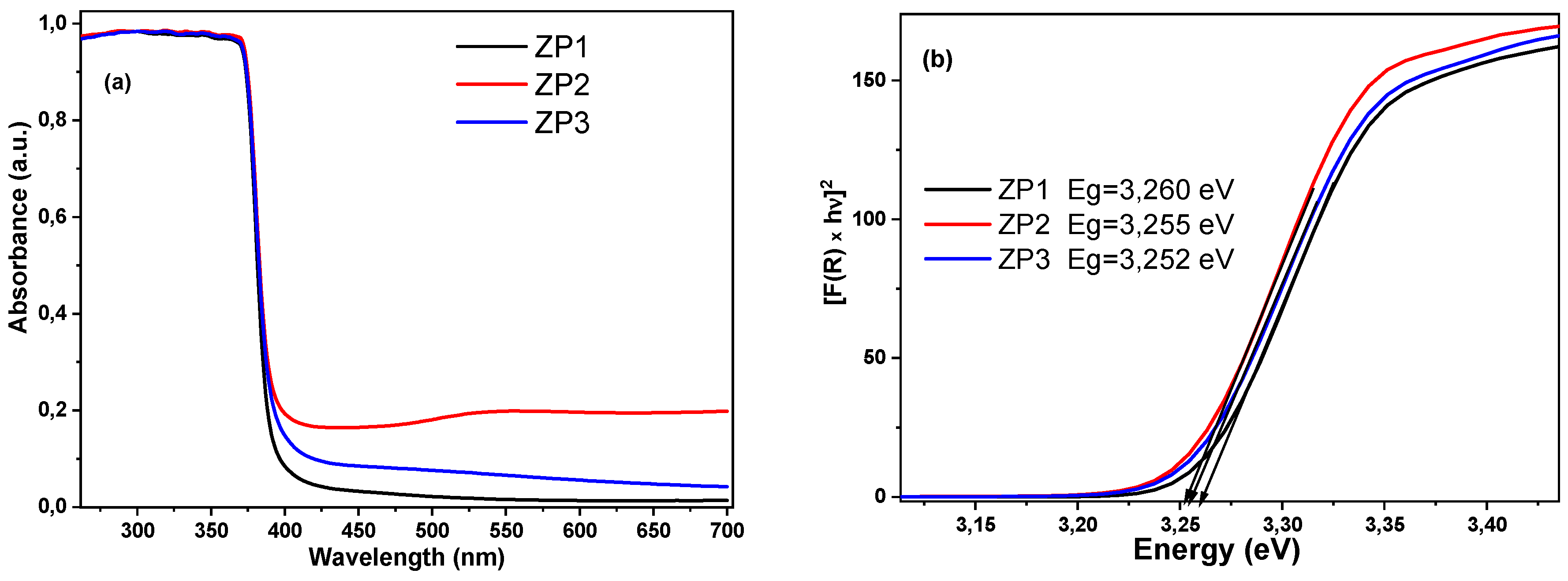
| Sample | Unit cell parameters (nm) | Crystallite size(nm) | Rwp% | S | Lattice strain(%) | |
|---|---|---|---|---|---|---|
| a | c | |||||
| ZP1 | 0.325 | 0.52 | 31.1 | 8.00 | 0.9933 | 0.73 |
| ZP2 | 0.325 | 0.52 | 27.5 | 10.12 | 0.9110 | 0.64 |
| ZP3 | 0.325 | 0.52 | 25.3 | 12.40 | 0.8935 | 0.42 |
| Sample |
Pseudomonas aeruginosa (P. aeruginosa) |
Staphylococcus aureus (S. aureus) |
|---|---|---|
| ZP1 | 10 - diffuse | 9 - clear |
| ZP2 | 18 - diffuse | 9 – clear/14- diffuse |
| ZP3 | 13 - diffuse | 10 – clear/12 - diffuse |
| Sample | P. aeruginosa | MIC | MBC | S. aureus | MIC | MBC | ||
|---|---|---|---|---|---|---|---|---|
| (µg/mL) | Obs. | (µg/mL) | Obs. | |||||
| ZP1 | 50 | - | 12.5 | 25 | 50 | - | 6.25 | 6.25 |
| 25 | - | 25 | - | |||||
| 12.5 | - | 12.5 | - | |||||
| 6.25 | + | 6.25 | - | |||||
| 3.75 | + | 3.75 | + | |||||
| 1.5 | + | 1.5 | + | |||||
| ZP2 | 50 | - | 3.75 | 6.25 | 50 | - | 1.5 | 3.75 |
| 25 | - | 25 | - | |||||
| 12.5 | - | 12.5 | - | |||||
| 6.25 | - | 6.25 | - | |||||
| 3.75 | - | 3.75 | - | |||||
| 1.5 | + | 1.5 | - | |||||
| ZP3 | 50 | - | 6.25 | 12.5 | 50 | - | 3.75 | 6.25 |
| 25 | - | 25 | - | |||||
| 12.5 | - | 12.5 | - | |||||
| 6.25 | - | 6.25 | - | |||||
| 3.75 | + | 3.75 | - | |||||
| 1.5 | + | 1.5 | + | |||||
| Positive (+): Turbidity indicating growth; Negative (–): No turbidity indicating absence of growth. | ||||||||
| Sample code | Nanomaterials | |
|---|---|---|
| ZP1 | Citrate functionalized-ZnO NPs |  |
| ZP2 | ZnO NPs/ Au NPs | |
| ZP3 | ZnO NPs /Ag NPs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





