1. Introduction
Cardiovascular diseases, encompassing ischemic heart disease and stroke, remain the leading cause of death worldwide, contributing to disability and rising healthcare costs [
1]. Coronary heart disease, the leading cause of deaths attributable to cardiovascular disease in the United States, primarily results from vessel occlusion [
2]. Revascularization procedures like coronary angioplasty and stent insertion are vital to relieve occlusion of arteries; however, interventional procedures may injure arterial walls, leading to blood vessel re-occlusion and occurrence of in-stent restenosis [
3]. The incidence of in-stent restenosis and the necessity for revascularization again occur at a rate of 1-2% per year [
4]. Vascular smooth muscle cells (VSMCs) are the predominant component of the arterial medial layer. Apart from the structural role, a main function of VSMCs in the blood vessels is to contract and regulate vascular tone and blood pressure [
5]. In the normal vessel wall, VSMCs exhibit a differentiated, contractile phenotype and proliferate at an extremely low rate. In response to injury, VSMCs within the vessel wall rapidly dedifferentiate and change from a quiescent and contractile phenotype to a synthetic phenotype with enhanced proliferative and migratory properties and increased matrix production, contributing to lesion formation and occlusion of blood vessels [
6,
7,
8]. Therefore, it is crucial to modulate VSMC proliferation and migration under pathological conditions to mitigate intimal hyperplasia.
The mitogen-activated protein kinase (MAPK) cascades transduce extracellular signals from different stimuli to elicit cellular responses [
9]. Of the MAPK cascades, ERK1/2 pathway regulates many cellular processes, affecting physiological functions [
10]. ERK1/2 activation drives actin polymerization, adhesion turnover, and cell contraction for cell movement [
11]. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure in vivo [
12]. In VSMCs, loss of SM22α impairs angiotensin II-induced ERK1/2 phosphorylation, concomitant with reduced vascular contraction [
13]. Furthermore, MAPK pathway activation, particularly ERK1/2 is pivotal in mitogen-induced VSMC proliferation and migration [
14]. In the presence of inflammatory cytokines like IL-1β, the activation of tyrosine kinase receptor leads to the phosphorylation of ERK1/2 [
15], which is associated with activation of genes responsible for cell cycle progression and subsequent proliferation [
16]. Collectively, ample evidence reveal important functions of ERK1/2 pathway in cardiovascular disease [
14,
16]. As such, proper modulation of ERK1/2 signaling to maintain a balance between its activation and inactivation is critical.
Protein phosphatases negatively regulate MAPK signaling [
17]. Dual-specificity phosphatases (DUSPs) are a subfamily of protein tyrosine phosphatases, able to dephosphorylate MAPKs [
18]. Because of their role in modulating MAPK activity, DUSPs have been implicated as major modulators of critical signaling pathways that are dysregulated in various diseases, including cardiac remodeling [
19]. Dual-specificity phosphatase 6 (DUSP6, also named MKP3), a member of the MAPK phosphatase family, specifically dephosphorylate activated ERK1/2 [
20]. DUSP6 interacts with ERK1/2 through a conserved binding motif, enabling it to dephosphorylate dual phosphates at threonine and tyrosine positions, thereby regulating the intensity of the ERK1/2 cascade [
20]. Studies have revealed diverse functions of DUSP6 in different cellular contexts and diseases. DUSP6 can either promote or inhibit cellular proliferation. While DUSP6 induces proliferation in breast cancer, lung cancer, and thyroid carcinoma, DUSP6 inhibits proliferation and suppresses tumor progression in pancreatic, ovarian, and non-small lung cancer [
21]. Interestingly, DUSP6 has been reported to participate in diet-induced obesity and dextran sulfate sodium-induced colitis [
22,
23,
24]. Despite its involvement in various cellular processes and diseases, it is not clear whether DUSP6 plays a role in regulating VSMC function and vascular disease.
In this study, through utilization of an arterial denudation model in wild-type and Dusp6 knockout (Dusp6–/–) mice, we uncovered a new function of DUSP6 within the realm of vascular injury. We showed that compared to wild-type mice, Dusp6–/– mice displayed reduced neointimal formation. This reduction was attributed to the inhibition of VSMC proliferation and migration, mediated via modulation of cell cycle inhibitor p27 and cell-cell adhesion molecule N-cadherin levels by DUSP6.
3. Discussion
In this study, we identified that in VSMCs, the baseline levels of the cell cycle inhibitor p27 and the cell-cell adhesion molecule N-cadherin were higher in the absence of DUSP6. DUSP6 deficiency prevented IL-1β-elicited p27 and N-cadherin downregulation, thus maintaining their levels under inflammatory conditions, resulting in reduced proliferation and migration of VSMCs and reduced neointima formation after vascular injury. Our study unveiled a novel role of DUSP6 in regulating vascular disease.
We found that DUSP6 did not modulate ERK1/2 activation in VSMCs, as lack of DUSP6 did not increase or sustain IL-1β-induced ERK1/2 phosphorylation levels (
Figure 4b). This finding is in contrast to the study that peripheral phosphorylated ERK1/2 levels in MKP3 (
Dusp6) knockout mice remained elevated after injury [
32] and the general belief that DUSP6 is a negative regulator of ERK1/2 activation [
33]. Interestingly, the result that IL-1β-induced ERK1/2 phosphorylation in VSMCs was not affected by DUSP6 is similar to the report that ERK1/2 activation is not increased or prolonged in
Dusp6–/– mouse embryo fibroblasts after stimulation with phenylephrine, although it affects baseline phosphorylated ERK1/2 levels [
34]. Another interesting finding is that IL-1β-induced DUSP6 expression was mediated by ERK1/2 activation (
Figure 4c), indicating ERK1/2 functions upstream of DUSP6 to induce its levels rather than as a substrate of DUSP6 in VSMCs under inflammatory condition. This notion is supported by a previous study that DUSP6 is inducible by fibroblast growth factor (FGF) signaling via ERK1/2 activity in NIH 3T3 cells [
35]. Although Ekerot et al. proposed that DUSP6 acts as a negative regulator of ERK activity to exert a negative-feedback regulation of FGF signaling [
35], our results from
Dusp6–/– VSMCs clearly demonstrated that lack of DUSP6 did not affect IL-1β-ERK1/2 signaling axis and did not serve a negative-feedback function, which differed from the proposition by Ekerot et al. Together, it is conceivable that in VSMCs, additional DUSPs, other than DUSP6, that have the capacity to dephosphorylate ERK1/2 are responsible for the inactivation of ERK1/2 [
36,
37], particularly under pathological conditions. Alternatively, DUSP6 may have phosphatase activity independent functions in VSMCs. Supporting this notion, DUSP6 was found to promote endothelial inflammation independent of ERK1/2 signaling [
38].
DUSP6 deficiency in VSMCs led to an increase in total and phosho-p27 at baseline levels and prevented their downregulation even after IL-1β stimulation (
Figure 5a). p27 is one of the cyclin-dependent kinase inhibitors (CDKs), which control cell cycle progression [
39]. Serine 10 phosphorylation of p27 increases its protein stability [
27]. The increased p27 level was reflected functionally in decreased VSMC proliferation in the knockout or knockdown of
Dusp6 in VSMCs (
Figure 2c, 5a, 5b). Inhibition of VSMC proliferation has been attributed to inhibition of CDKs by p27 via preventing progression of G1 to S phase of the cell cycle, ultimately attenuating intimal hyperplasia following arterial injury [
40,
41]. Transcription factor FKHRL1 or mediators such as baicalin that increase p27 levels in VSMCs prevent injury-induced neointimal hyperplasia [
42,
43]. Our finding adds DUSP6 to the list of molecules that regulate p27 levels in VSMCs. This is likely the mechanisms by which DUSP6 deficiency resulted in reduced proliferative capacity in response to IL-1β stimulation, contributing at least in part, to mitigating neointimal formation in mice after injury.
The live cell imaging of the wound healing experiments revealed a distinctive behavior in neighboring
Dusp6–/– VSMCs compared with wild-type cells. Notably, these
Dusp6–/– VSMCs exhibited a tendency to adhere to each other, and their migration toward the wound site was notably slower than that observed in wild-type cells (Supplementary Video S1). This behavior suggests enhanced cell-cell adhesion in
Dusp6–/– VSMC compared with wild-type cells. Indeed,
Dusp6–/– VSMCs exhibited higher levels of N-cadherin at baseline, and DUSP6 deficiency mitigated IL-1β-reduced N-cadherin levels (
Figure 8). This result suggests that DUSP6 plays a regulatory role in VSMC migration through the N-cadherin axis. Concordant with our results, downregulation of N-cadherin in VSMCs has been demonstrated to induce neointima formation in a porcine restenosis model, potentially through RhoA deactivation towards enhancing VSMC migration capacity [
44]. Mechanistically, during migration, N-cadherin translocates to the posterior-lateral cell edge of VSMCs, and the cell-cell adhesions via N-cadherin determine polarity and consequently decrease migratory capacity [
45]. Nevertheless, contradictory results have been reported in some studies. N-cadherin inhibition with antagonist, neutralizing antibodies, and adenoviral expression of dominant negative N-cadherin reduced VSMC migration and intimal thickening [
46]. The role of N-cadherin in VSMCs remains controversial, further investigations are needed to clarify this issue.
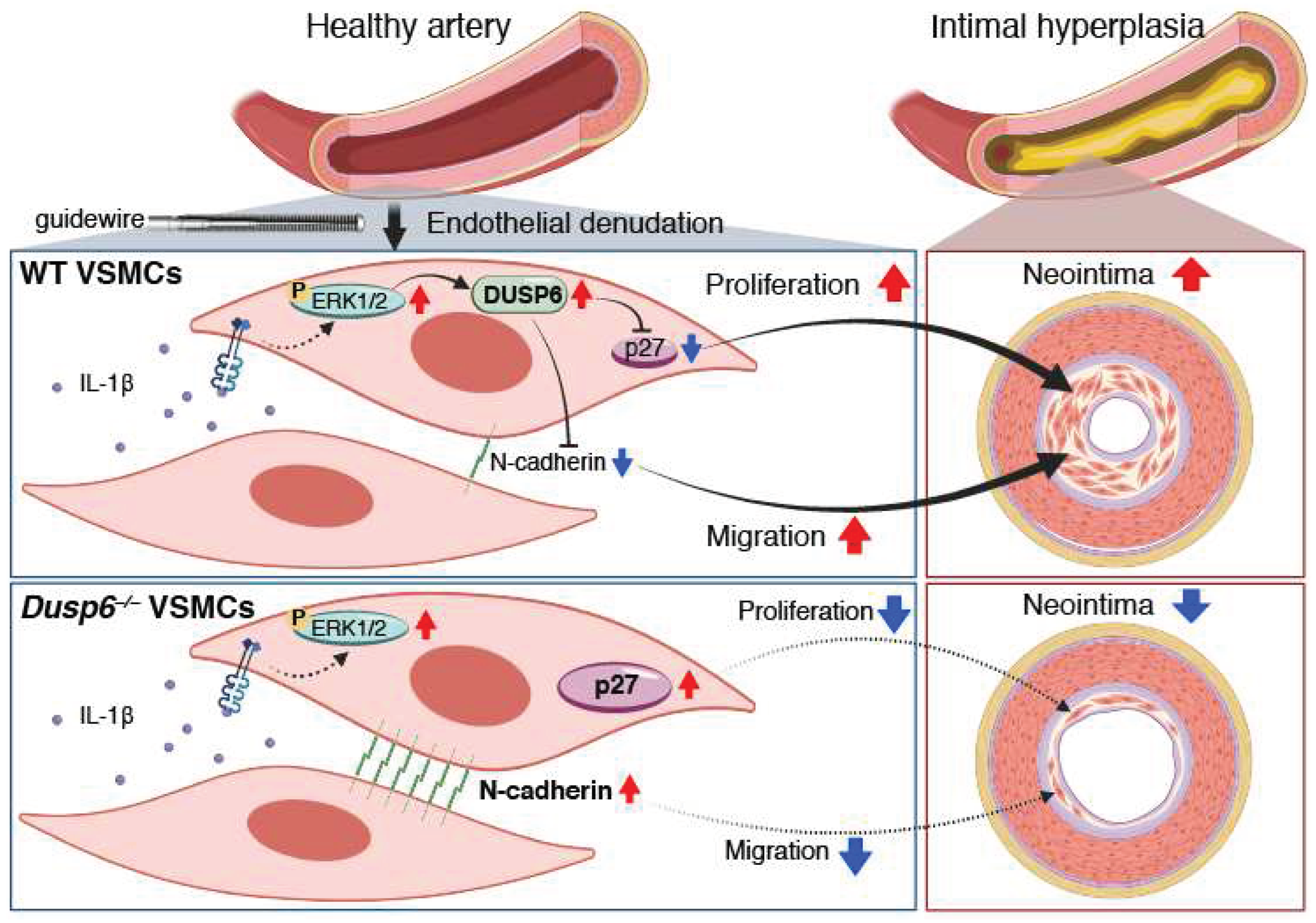
In summary, our study identified a novel role of DUSP6 in VSMCs and vascular injury (
Figure 9). Inflammatory cytokine IL-1β activates ERK1/2 pathway to induce DUSP6 expression, resulting in downregulation of CDK inhibitor p27 and cell-cell adhesion molecule N-cadherin, consequently promoting VSMC proliferation and migration, and subsequent neointima formation and restenosis after arterial injury. On the other hand, absence of DUSP6 increases baseline levels of p27 and N-cadherin. Furthermore, lack of DUSP6 prevents IL-β-elicited downregulation of p27 and N-cadherin, maintaining high levels of these two molecules, leading to decreased VSMC proliferation and migration, and ultimately mitigating arterial injury-induced intimal hyperplasia. Our findings propose DUSP6 as a potential target for development of therapeutic strategies for occlusive vascular disease.
4. Materials and Methods
4.1. Animal Care and Genotyping
All mice were maintained at National Health Research Institutes (NHRI) animal facility in Taiwan (AAALAC accredited #001596, 12 h light-dark cycle). Mice were housed in the ventilated transparent plastic cage (3-4 mice/cage; Bio-Zone, 410 cm
2 enclosure size, Lignocel FS-14 bedding). Water and food were provided ad libitum. Wild-type mice in C57BL/6 background were obtained from the National Laboratory Animal Center (Taiwan) and acclimated for 2 weeks before any experiments.
Dusp6-deficient (
Dusp6–/–) mice in C57BL/6 background [
24] were genotyped as described in JAX Protocol.
4.2. Experimental Mouse Model of Femoral Artery Denudation Injury
All experimental procedures were performed in accordance with NIH guidelines (Guide for the care and use of laboratory animals) and approved by the Institutional Animal Care and Use Committee of National Health Research Institutes, Taiwan (#NHRI-IACUC-107063-A). Animal studies are reported in compliance with the ARRIVE guidelines. Approximately 10-12-week-old male mice were subjected to the neointimal hyperplasia model of femoral artery endoluminal injury as described [
47]. Mice were anesthetized with tribromoethanol solution (Sigma-Aldrich, #T84802; 250 mg/kg) via intraperitoneal injection to achieve proper sedation. Endoluminal injury to the left common femoral artery was then performed with 3-5 passages of a hydrophilic coating guide wire (Abbott, Hi-Torque Cross-it 100XT, 0.014’’ diameter) to denude endothelium. Mice were placed on a heating pad (low-medium heat) until recovered and then housed in individual cages.
4.3. Histological Analysis and Immunohistochemistry
At indicated time points after injury, mice were sacrificed by an overdose of tribromoethanol solution (500-750 mg/kg) via intraperitoneal injection until they no longer displayed a withdrawal reflex in the hind limbs, perfused with saline, followed by 10% formalin (Sigma-Aldrich, #HT501128). In some experiments, to determine cellular proliferation in the injured vessel wall, mice were injected twice with bromodeoxyuridine (BrdU, Sigma-Aldrich) at 16-18 h and 1-2 h before harvest. The contralateral uninjured right femoral artery and injured left femoral artery were carefully dissected, further fixed in 10% formalin at 4°C overnight, processed, and embedded in paraffin for histological analysis. Serial 4-μm cross sections of the femoral arteries were collected. H&E staining on vessel sections was initially performed for assessing morphology and Verhoeff's staining (Sigma) was used to delineate elastin layers. Three sets of cross sections at 150-μm intervals were used for morphometry using NIH ImageJ software. The intimal and medial areas were measured, and the intima-to-media ratio was calculated essentially as described [
48].
For immunohistochemistry, sections were rehydrated, antigen retrieved, blocked with 5% bovine serum albumin, and incubated with primary antibody. To determine DUSP6 expression, sections were incubated with DUSP6 antibody (Abcam ab76310, 1:50) overnight at 4°C, and counterstained with hematoxylin before mounting. DUSP6 expression levels were quantified by colorimetric analysis with ImageJ software and expressed as a percentage of positive area in the neointima or media. To assess in vivo cell proliferation after injury, sections were stained with a monoclonal BrdU antibody (Dako #M0744, 1:100), and counterstained with hematoxylin. The BrdU positive cells were quantified and expressed as percentage of total cells in neointima or media, respectively.
4.4. Primary VSMC Culture, IL-1β Stimulation, and Proliferation Assays
Primary VSMCs were isolated by enzyme digestion from wild-type and
Dusp6–/– mouse aortas and cells of passage 5-8 were used for experiments [
49]. VSMCs were serum starved in DMEM containing 0.2% FBS for 24 h before stimulation with IL-1β (PeproTech, 10 ng/mL) for different periods of time. Total proteins were then prepared for Western blot analysis to detect DUSP6 expression levels. To measure VSMC proliferation, cells were plated in 24-well plate (8 x 10
3 cells/well), serum starved in 0.2% FBS quiescent medium for 24 h, stimulated with or without IL-1β for 24 h, and cellular proliferation assessed using BrdU cell proliferation kit (Millipore, QIA58) or cell counting kit-8 (CCK-8) (Dojindo, CK04-05) according to manufacturer’s instructions.
4.5. Wound Healing Assays
Wound healing assays were performed to measure VSMC migratory capacity. Wild-type and Dusp6–/– VSMCs (2.5 x 105 cells/well) were plated onto 6-well dish, serum starved, and treated with 10 ng/mL mitomycin C (Roche, #10107409001) for 2 h to arrest cell growth prior to wounding with a p200 tip. IL-1β (0 or 10 ng/mL) was then used to induce cell migration. Wound images were captured with microscope (Olympus CKX53, 40X magnification) at time 0 and at 6 h after IL-1β stimulation. Area of wound closure was quantified by Image J software from 3 fields of each treatment and divided by the wound area at time 0, and was presented as percent wound closure. To confirm the effects of DUSP6 on VSMC migration, Dusp6 expression was knocked down with siRNA in wild-type VSMCs and wound healing assays were then performed.
4.6. Western Blot Analysis
Total proteins were prepared from VSMCs [
50] without stimulation or stimulated with IL-1β. Western blotting was then performed to detect protein of interest. The primary antibodies used were: DUSP6 (Abcam, ab76310), phospho-p27 S10 (Abcam, ab62364), p27 (Santa Cruz, sc-1641), phospho-Erk-1/2 Thr202/Tyr204 (Cell Signaling, #9101), Erk-1/2 (Cell Signaling, #4696), N-cadherin (Cell Signaling, #13116), and β-catenin (BD Biosciences, #610154). To verify equivalent loading, the blots were subsequently probed with α-tubulin (GeneTex, GTX112141) or β-actin antibodies (Sigma-Aldrich, MAB1501).
4.7. DUSP6 Overexpression and Knockdown in VSMCs
To overexpress DUSP6 in VSMCs, wild-type VSMCs were electroporated with vector control (pCMV6-AN-3DDK) or expression plasmid pCMV6-AN-3DDK-hDUSP6 (8 µg DNA/1 x 106 cells) with Gene Pulser Xcell™ Electroporation System (Bio-Rad) using 400V, 10 ms, square wave parameters. Transfected VSMCs were then recovered overnight in 10% FBS medium and starved for 24-48 h prior to IL-1β stimulation. Twenty-four h later, total proteins were prepared for cell proliferation assessment or Western blot analysis.
To knock down DUSP6 expression in wild-type VSMCs, ON-TARGET plus SMART pool mouse DUSP6 small interfering RNA (siRNA) (Dharmacon, #L-040050-00) and negative control siRNA (Dharmacon, #D-001810-0X) were used. VSMCs were transfected with 20 nmol/L siRNA in Opti-MEM® I Reduced Serum Medium (Thermo, #31985062) using Lipofectamine RNAiMAX (Thermo, #13778150) as described by the manufacturer. Following overnight recovery, cells were starved, stimulated with or without IL-1β for 24 h. Proliferation (BrdU incorporation) or migration assays, and Western blot analysis were then performed.
4.8. ERK1/2 Signaling and Cell Cycle Inhibitor p27
To evaluate ERK1/2 activation, wild-type and Dusp6–/– VSMCs were serum starved, treated with IL-1β, and total proteins harvested at different time points for Western blotting to detect phospho- and total ERK1/2 and DUSP6. To inhibit ERK1/2 activation, serum-starved cells were pretreated with U0126 (10 µmole/L, Calbiochem) 30 min before stimulation with IL-1β, and total proteins harvested at different time points for Western blotting. To assess the effect of ERK1/2 on VSMC proliferation, cells were pretreated with U0126 (0, 5, or 10 µmole/L) before stimulation with or without IL-1β for 24 h, and BrdU incorporation assays performed to measure proliferation. To detect cell cycle inhibitor p27, starved VSMCs were treated with IL-1β for 12 h and proteins prepared for Western blotting to detect total p27 (Santa Cruz, sc-1641) and phospho-p27(S10) (Abcam, ab62364).
4.9. Cell adhesion, Spreading, and Lamellipodia Formation
To assess VSMC adhesion and spreading, wild-type and Dusp6–/– VSMCs were seeded into μ-Slide VI 0.4 (ibidi, #80601) and allowed to attach for different periods of time. Adhered cells were fixed with 4% paraformaldehyde at the indicated time after plating (15’, 30’, 1 h, 2 h, and 3 h) for 10 min, permeabilized in 0.1% Triton-X for 5 min, blocked with 1% BSA for 1 h, and incubated with phalloidin (Thermo Alexa Fluor™ 594, 1:5000) for 30 min. Cells were then counterstained with DAPI (Invitrogen, #R37606, 1:5000) for 5 min and mounted with 0.5% mounting buffer-ddH2O (DAKO, S3023). Images were captured with fluorescence microscope (Olympus IX71, 40x magnification). The percentage of attached, spread cells was calculated as number of spread cells divided by total cell number (as determined by number of blue nuclei) and multiplied by 100. The cell size of attached cells was measured with ImageJ software. To assess lamellipodia formation, wild-type and Dusp6–/– VSMCs were seeded into ibidi μ-Slide VI 0.4 and serum-starved for 24 h before stimulation with or without IL-1β for 1 h. Cells were then processed and stained with phalloidin as above. The number of lamellipodia was counted in 100 wild-type or Dusp6–/– VSMCs per experiment.
4.10. Statistical Analysis
Data were expressed as mean ± standard error of the mean (SEM) and derived from a minimum of 3 independent experiments. Parametric data were analyzed using two-tailed unpaired Student’s t test or One-way ANOVA, followed by Sidak or Tukey tests for multiple comparisons. These analyses encompassed intima/media (I/M) ratio, BrdU-positive cells, DUSP6 expression, wound closure areas, lamellipodia number, cell spreading, and cell size. Non-parametric data, including protein blot levels, were analyzed using the Mann-Whitney U test or One-way ANOVA followed by the Kruskal-Wallis test. Significance was set at p < 0.05. All analyses were conducted using GraphPad Prism 8 software.
Author Contributions
Conceptualization, C.D.H., M.L.W., and S.F.Y.; methodology, C.D.H., M.L.W., P.Y.G., and H.H.H.; validation, Y.C.H., and C.M.C.; formal analysis, C.D.H. and M.L.W.; investigation, C.D.H., M.L.W., and Y.C.H.; resources, H.C.C. and T.H.T; data curation, C.D.H., M.L.W., and S.F.Y.; writing—original draft preparation, C.D.H.; writing—review and editing, S.F.Y.; visualization, C.D.H., M.L.W., C.M.C., and S.F.Y.; supervision, W.C.J. and S.F.Y.; project administration, S.F.Y.; funding acquisition, S.F.Y. All authors have read and agreed to the published version of the manuscript.
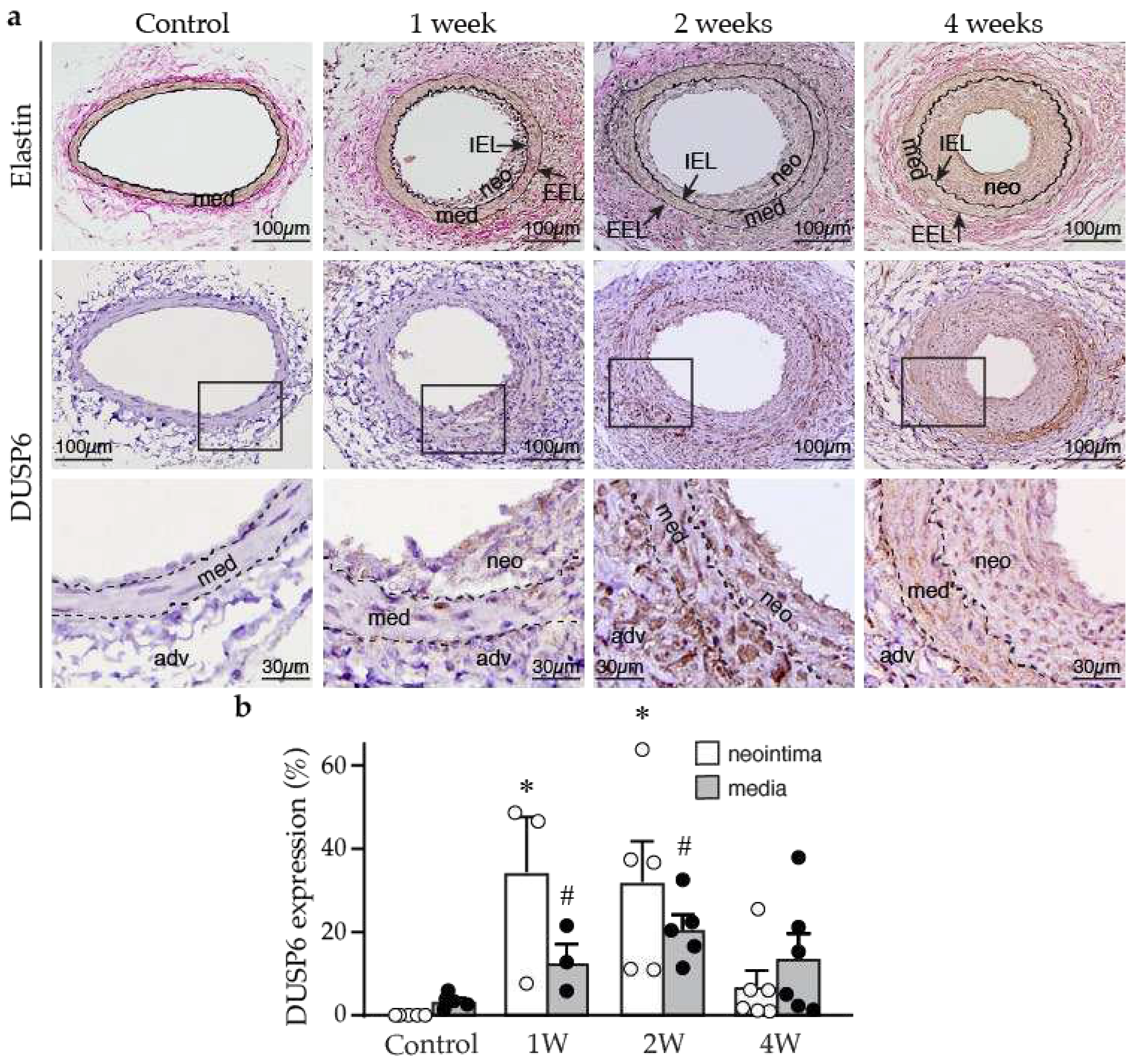
Figure 1.
Arterial injury induces neointima formation and DUSP6 expression in mice. Wild-type mice were subjected to femoral artery injury, and vessels were harvested after 1, 2, and 4 weeks for histological analysis. (a) Verhoeff’s stain and immunostaining with DUSP6 antibody were performed on vessel sections to delineate elastin layers (top row) and DUSP6 expression (brown, middle row), respectively. Bottom row, higher magnification of the box in the middle row, respectively. adv, adventitia; IEL, internal elastic lamina; EEL, external elastic lamina; med, media; neo, neointima. Arrows indicate IEL and EEL, respectively. Dashed lines demarcate medial layer. (b) Quantitation of DUSP6 expression in the media and neointima of arteries after denudation injury. n = 3-6, *p < 0.05 vs. control neointima, #p < 0.05 vs. control media, One-way ANOVA, followed by Tukey’s test.
Figure 1.
Arterial injury induces neointima formation and DUSP6 expression in mice. Wild-type mice were subjected to femoral artery injury, and vessels were harvested after 1, 2, and 4 weeks for histological analysis. (a) Verhoeff’s stain and immunostaining with DUSP6 antibody were performed on vessel sections to delineate elastin layers (top row) and DUSP6 expression (brown, middle row), respectively. Bottom row, higher magnification of the box in the middle row, respectively. adv, adventitia; IEL, internal elastic lamina; EEL, external elastic lamina; med, media; neo, neointima. Arrows indicate IEL and EEL, respectively. Dashed lines demarcate medial layer. (b) Quantitation of DUSP6 expression in the media and neointima of arteries after denudation injury. n = 3-6, *p < 0.05 vs. control neointima, #p < 0.05 vs. control media, One-way ANOVA, followed by Tukey’s test.
Figure 2.
DUSP6 promotes VSMC proliferation. (a) Serum-starved VSMCs were treated with IL-1β (10 ng/mL), and DUSP6 expression was examined at different time points by Western blot analysis. α-Tubulin was detected as an internal control. A representative blot is shown (n=4). Quantitation of DUSP6 levels in VSMCs. *p < 0.05 vs. control, Mann-Whitney U test. (b) VSMCs were transfected with vector pCMV6-AN-3DDK or pCMV6-AN-3DDK-hDUSP6 expression plasmid. Proteins were prepared after 24 h to examine DUSP6 expression by Western blotting (n=4). Transfected cells were serum starved, treated with or without IL-1β for 24 h and proliferation assessed and normalized to vector without IL-1β (n=4). *p < 0.05 vs. vector without IL-1β, #p < 0.05 vs. vector with IL-1β, Mann-Whitney U test. (c) VSMCs were transfected with control siRNA or Dusp6 siRNA and then treated with or without IL-1β for 24 h, and proteins were harvested for Western blotting for DUSP6 to assess knockdown efficiency. α-Tubulin was used as a loading control. Knockdown cells were serum starved, treated with or without IL-1β for 24 h, and proliferation was measured and normalized to vector without IL-1β (n=4). *p < 0.05 vs. siControl without IL-1β, #p < 0.05 vs. siControl with IL-1β, Mann-Whitney U test.
Figure 2.
DUSP6 promotes VSMC proliferation. (a) Serum-starved VSMCs were treated with IL-1β (10 ng/mL), and DUSP6 expression was examined at different time points by Western blot analysis. α-Tubulin was detected as an internal control. A representative blot is shown (n=4). Quantitation of DUSP6 levels in VSMCs. *p < 0.05 vs. control, Mann-Whitney U test. (b) VSMCs were transfected with vector pCMV6-AN-3DDK or pCMV6-AN-3DDK-hDUSP6 expression plasmid. Proteins were prepared after 24 h to examine DUSP6 expression by Western blotting (n=4). Transfected cells were serum starved, treated with or without IL-1β for 24 h and proliferation assessed and normalized to vector without IL-1β (n=4). *p < 0.05 vs. vector without IL-1β, #p < 0.05 vs. vector with IL-1β, Mann-Whitney U test. (c) VSMCs were transfected with control siRNA or Dusp6 siRNA and then treated with or without IL-1β for 24 h, and proteins were harvested for Western blotting for DUSP6 to assess knockdown efficiency. α-Tubulin was used as a loading control. Knockdown cells were serum starved, treated with or without IL-1β for 24 h, and proliferation was measured and normalized to vector without IL-1β (n=4). *p < 0.05 vs. siControl without IL-1β, #p < 0.05 vs. siControl with IL-1β, Mann-Whitney U test.
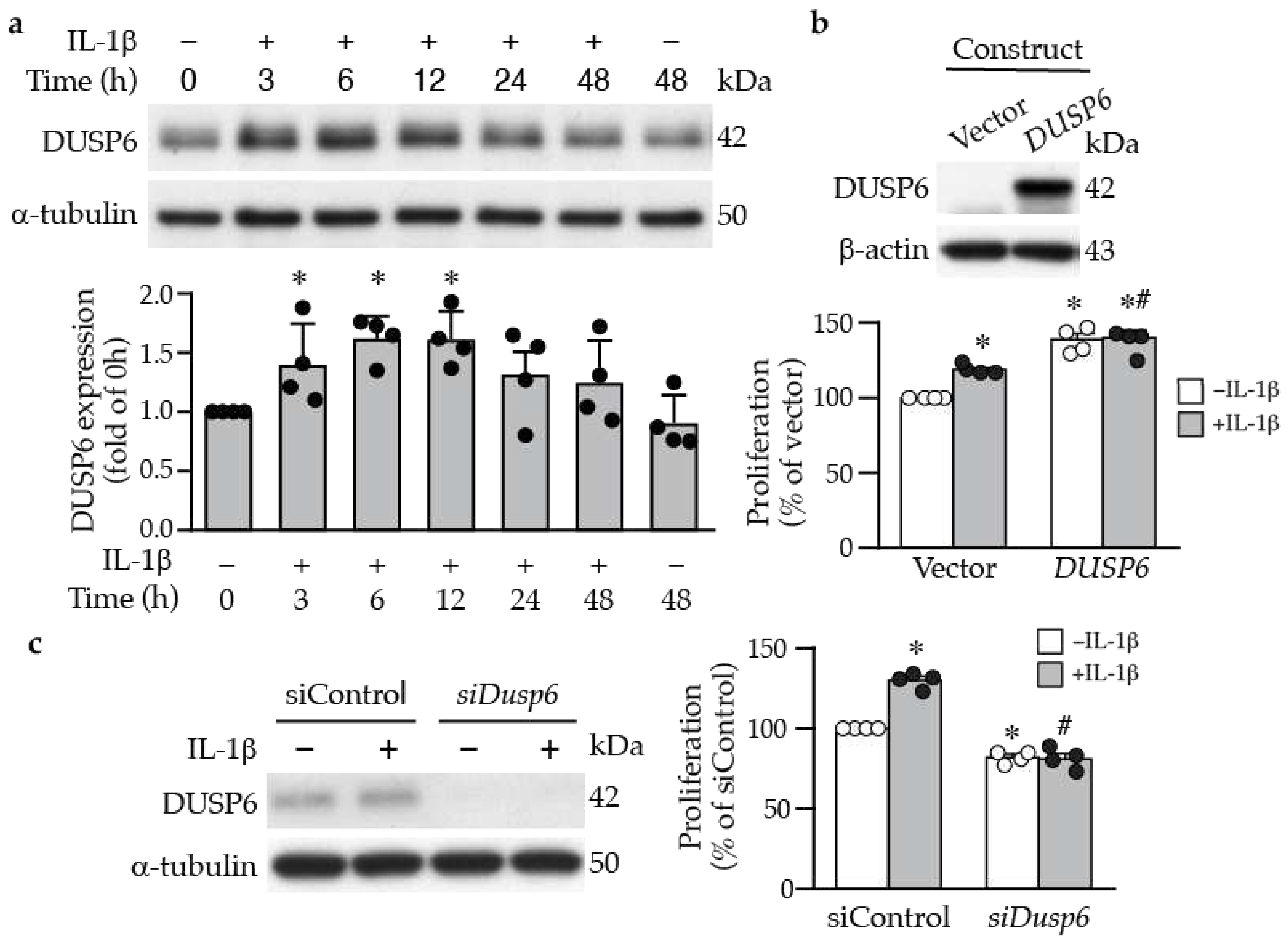
Figure 3.
DUSP6 deficiency reduces neointima formation in response to arterial injury in mice. (a) wild-type (WT) and Dusp6–/– mice were subjected to femoral artery injury. Vessels were harvested 4 weeks later for Verhoeff’s staining for elastin layers. IEL, internal elastic lamina; EEL, external elastic lamina; neo, neointima. Arrows indicate IEL and EEL, respectively. Quantitative morphometric analysis of intimal and medial area and expressed as intima/media ratio (WT, n=7; Dusp6–/–, n=6). *p < 0.05 vs. WT, Two-tailed unpaired Student’s t test. (b) Serum-starved wild-type and Dusp6–/– VSMCs were treated with or without IL-1β. DUSP6 expression and proliferation were assessed by Western blotting and BrdU incorporation assays, respectively (n=3). *p < 0.05 vs. WT without IL-1β, #p < 0.05 vs. WT+IL-1β, Mann-Whitney U test. (c) WT (n=6) and Dusp6–/– mice (n=7) were subjected to femoral artery injury and vessels harvested 2 weeks later. Mice were injected with BrdU at 16-18 h and 1-2 h before harvest. Vessel sections were stained with Verhoeff’s staining for elastin layers (left column) and with BrdU antibody (brown) for proliferating cells (middle column). Right column, higher magnification of the box in the middle column, respectively. Dashed lines demarcate medial layer. med, media; neo, neointima; adv, adventitia. (d) Quantitation of intima/media ratio of WT (n=6) and Dusp6–/– (n=7) mice 2 weeks after injury. *p < 0.05 vs. WT, two-tailed unpaired Student’s t test. (e) BrdU-positive cells were quantified and expressed as % of total cells in the neointima or media (WT, n=6; Dusp6–/–, n=7). *p < 0.05 vs. WT, two-tailed unpaired Student’s t test.
Figure 3.
DUSP6 deficiency reduces neointima formation in response to arterial injury in mice. (a) wild-type (WT) and Dusp6–/– mice were subjected to femoral artery injury. Vessels were harvested 4 weeks later for Verhoeff’s staining for elastin layers. IEL, internal elastic lamina; EEL, external elastic lamina; neo, neointima. Arrows indicate IEL and EEL, respectively. Quantitative morphometric analysis of intimal and medial area and expressed as intima/media ratio (WT, n=7; Dusp6–/–, n=6). *p < 0.05 vs. WT, Two-tailed unpaired Student’s t test. (b) Serum-starved wild-type and Dusp6–/– VSMCs were treated with or without IL-1β. DUSP6 expression and proliferation were assessed by Western blotting and BrdU incorporation assays, respectively (n=3). *p < 0.05 vs. WT without IL-1β, #p < 0.05 vs. WT+IL-1β, Mann-Whitney U test. (c) WT (n=6) and Dusp6–/– mice (n=7) were subjected to femoral artery injury and vessels harvested 2 weeks later. Mice were injected with BrdU at 16-18 h and 1-2 h before harvest. Vessel sections were stained with Verhoeff’s staining for elastin layers (left column) and with BrdU antibody (brown) for proliferating cells (middle column). Right column, higher magnification of the box in the middle column, respectively. Dashed lines demarcate medial layer. med, media; neo, neointima; adv, adventitia. (d) Quantitation of intima/media ratio of WT (n=6) and Dusp6–/– (n=7) mice 2 weeks after injury. *p < 0.05 vs. WT, two-tailed unpaired Student’s t test. (e) BrdU-positive cells were quantified and expressed as % of total cells in the neointima or media (WT, n=6; Dusp6–/–, n=7). *p < 0.05 vs. WT, two-tailed unpaired Student’s t test.
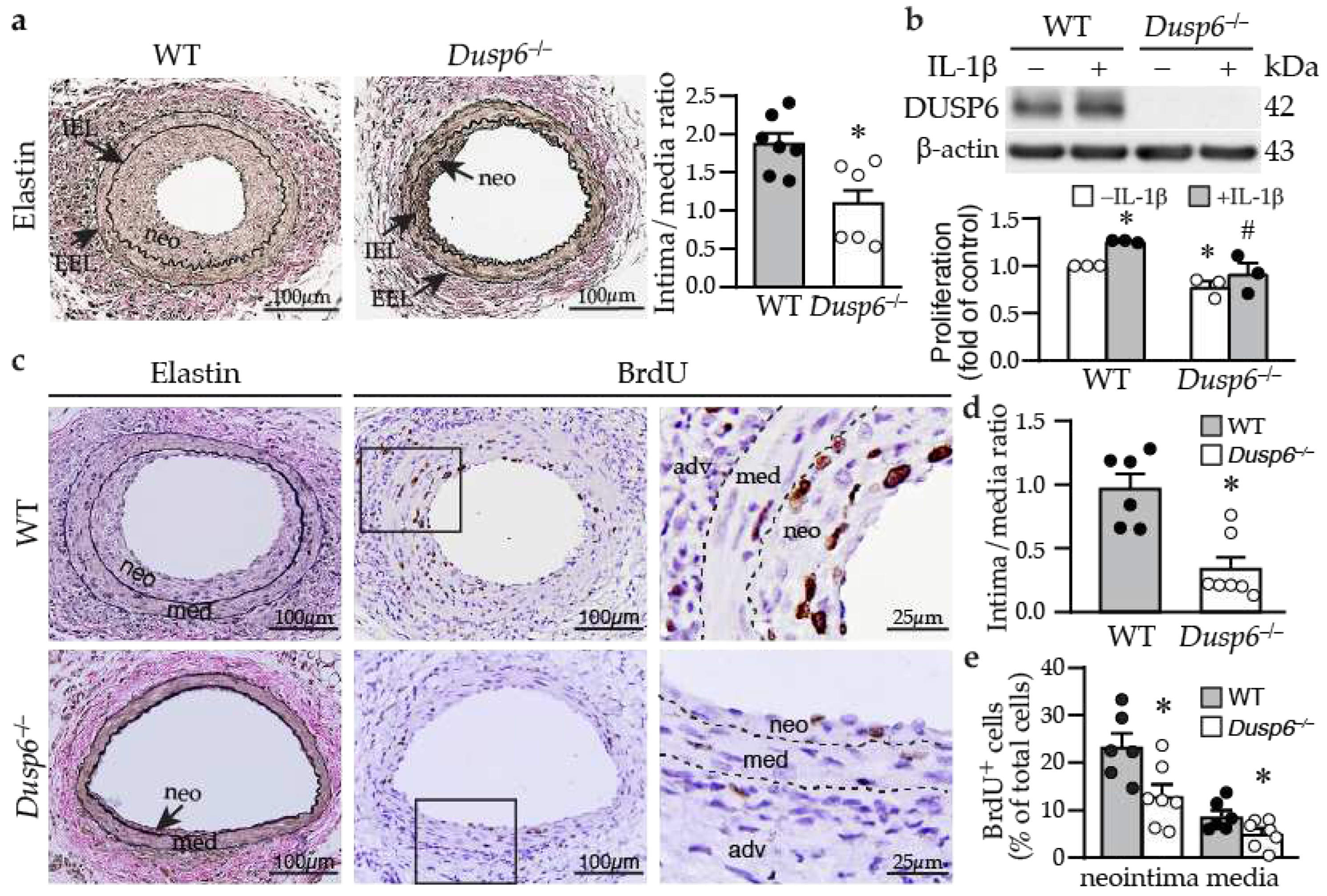
Figure 4.
ERK1/2 activation precedes and mediates DUSP6 induction and VSMC proliferation by IL-1β. (a) Serum-starved VSMCs were treated with IL-1β, and total proteins were isolated at different time points for Western blotting to detect ERK1/2 activation and DUSP6 expression. As a loading control, blots were subsequently hybridized with α-tubulin antibody. (b) Serum-starved wild-type (WT) and Dusp6–/– VSMCs were treated with IL-1β and proteins were prepared at different time points for Western blotting to detect phosphorylated, and total ERK1/2, and DUSP6 expression. α-Tubulin was used as a loading control. n=4. (c) Serum-starved VSMCs were pretreated with ERK1/2 inhibitor U0126 30 min prior to IL-1β treatment, and total proteins were isolated at different time points for Western blotting to detect ERK1/2 activation and DUSP6 expression. α-Tubulin was used as a loading control. n=4. (d) Serum-starved WT VSMCs were pretreated with different concentrations of U0126 prior to IL-1β stimulation. Proliferation was measured 24 h later. *p < 0.05 vs. vehicle without IL-1β, n=4 each, Mann-Whitney U test.
Figure 4.
ERK1/2 activation precedes and mediates DUSP6 induction and VSMC proliferation by IL-1β. (a) Serum-starved VSMCs were treated with IL-1β, and total proteins were isolated at different time points for Western blotting to detect ERK1/2 activation and DUSP6 expression. As a loading control, blots were subsequently hybridized with α-tubulin antibody. (b) Serum-starved wild-type (WT) and Dusp6–/– VSMCs were treated with IL-1β and proteins were prepared at different time points for Western blotting to detect phosphorylated, and total ERK1/2, and DUSP6 expression. α-Tubulin was used as a loading control. n=4. (c) Serum-starved VSMCs were pretreated with ERK1/2 inhibitor U0126 30 min prior to IL-1β treatment, and total proteins were isolated at different time points for Western blotting to detect ERK1/2 activation and DUSP6 expression. α-Tubulin was used as a loading control. n=4. (d) Serum-starved WT VSMCs were pretreated with different concentrations of U0126 prior to IL-1β stimulation. Proliferation was measured 24 h later. *p < 0.05 vs. vehicle without IL-1β, n=4 each, Mann-Whitney U test.
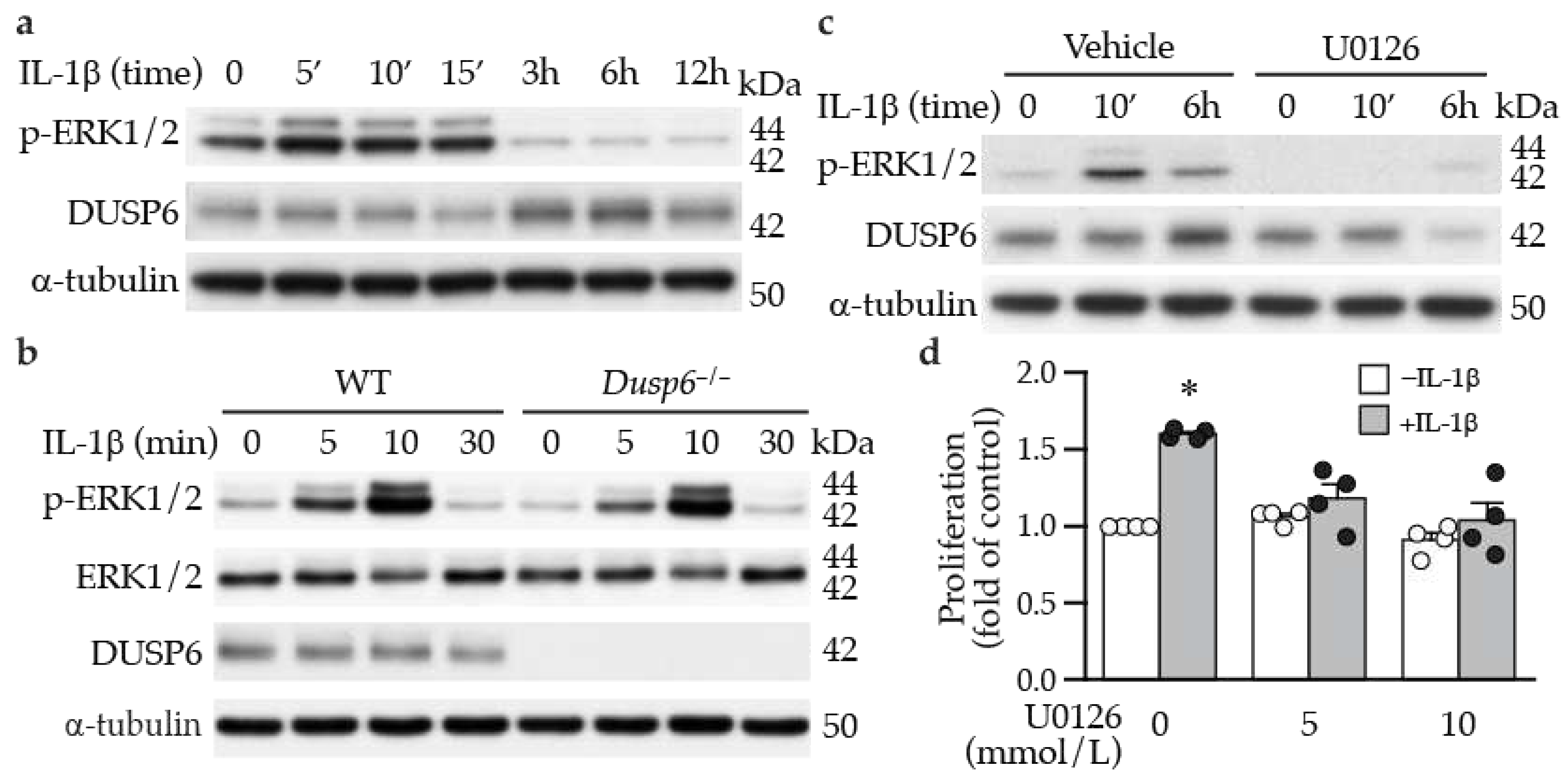
Figure 5.
DUSP6 regulates cell cycle inhibitor p27 levels in VSMCs. (a) Serum-starved wild-type (WT) and Dusp6–/– VSMCs were stimulated with or without IL-1β for 12 h and proteins were prepared for Western blotting to detect total p27, phospho-p27 at serine 10 (p-p27 (S10)), DUSP6, and β-actin as loading control. n=4. Expression levels of total p27 and p-p27 were quantified. n=4 each, *p < 0.05 vs. WT without IL-1β; #p < 0.05 vs. WT with IL-1β. Mann-Whitney U test. (b) WT VSMCs were transfected with siControl (siC) or siDusp6 (siD6) prior to treatment with or without IL-1β. Total p27, p-p27, and α-tubulin (α-tubu) as loading control. Representative blot from n=4 each. Protein levels were quantified. *p < 0.05 vs. siControl without IL-1β; #p < 0.05 vs. siControl with IL-1β. Mann-Whitney U test. (c) Serum-starved VSMCs were pretreated with U0126 prior to IL-1β stimulation for 12 h, and proteins were harvested for Western blot analysis to detect total p27, p-p27(S10), and α-tubulin as loading control. n=4. Levels of p27 and p-p27 were quantified. n=4 each, *p < 0.05 vs. vehicle without IL-1β; #p < 0.05 vs. vehicle with IL-1β. Mann-Whitney U test.
Figure 5.
DUSP6 regulates cell cycle inhibitor p27 levels in VSMCs. (a) Serum-starved wild-type (WT) and Dusp6–/– VSMCs were stimulated with or without IL-1β for 12 h and proteins were prepared for Western blotting to detect total p27, phospho-p27 at serine 10 (p-p27 (S10)), DUSP6, and β-actin as loading control. n=4. Expression levels of total p27 and p-p27 were quantified. n=4 each, *p < 0.05 vs. WT without IL-1β; #p < 0.05 vs. WT with IL-1β. Mann-Whitney U test. (b) WT VSMCs were transfected with siControl (siC) or siDusp6 (siD6) prior to treatment with or without IL-1β. Total p27, p-p27, and α-tubulin (α-tubu) as loading control. Representative blot from n=4 each. Protein levels were quantified. *p < 0.05 vs. siControl without IL-1β; #p < 0.05 vs. siControl with IL-1β. Mann-Whitney U test. (c) Serum-starved VSMCs were pretreated with U0126 prior to IL-1β stimulation for 12 h, and proteins were harvested for Western blot analysis to detect total p27, p-p27(S10), and α-tubulin as loading control. n=4. Levels of p27 and p-p27 were quantified. n=4 each, *p < 0.05 vs. vehicle without IL-1β; #p < 0.05 vs. vehicle with IL-1β. Mann-Whitney U test.
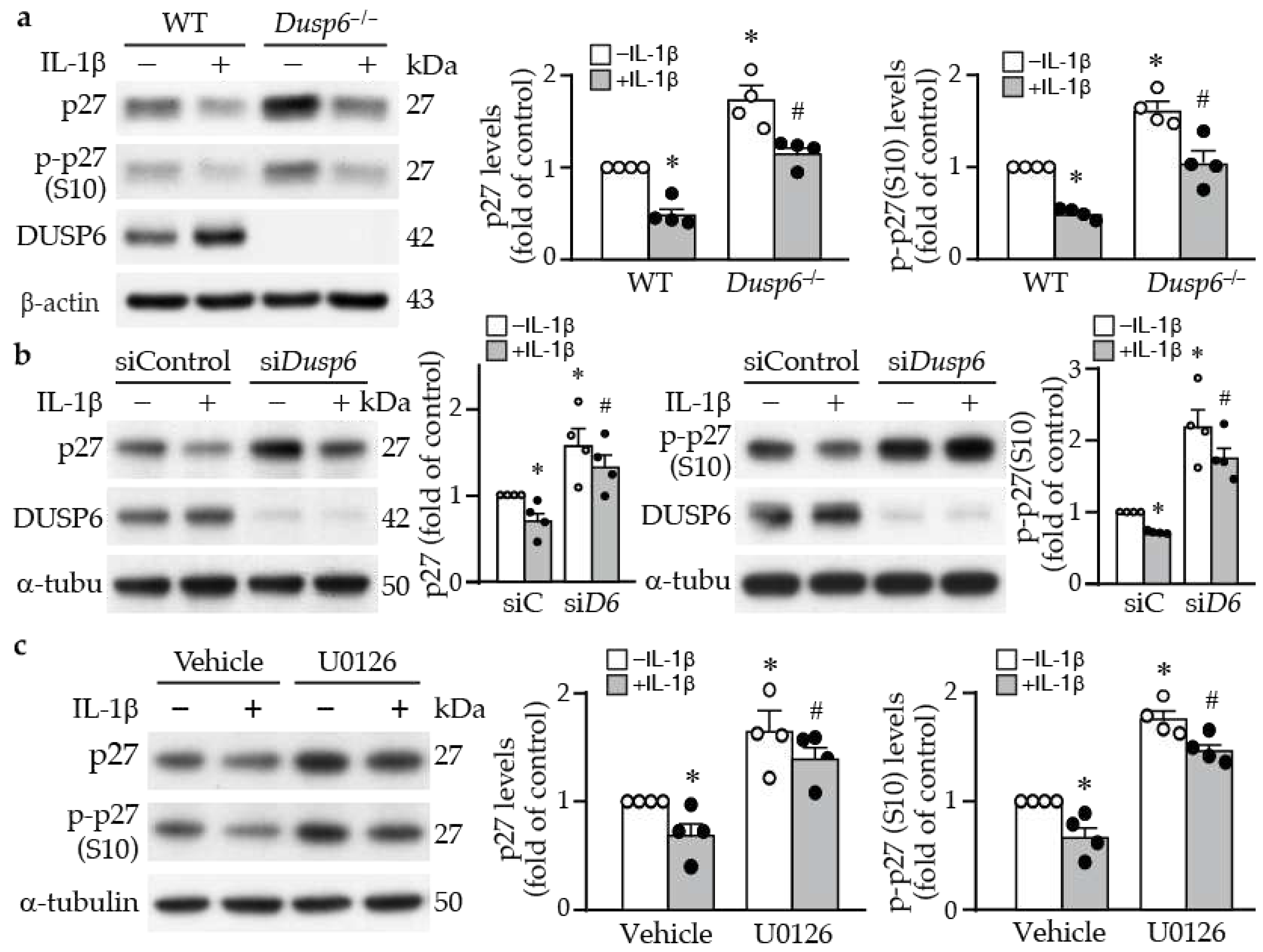
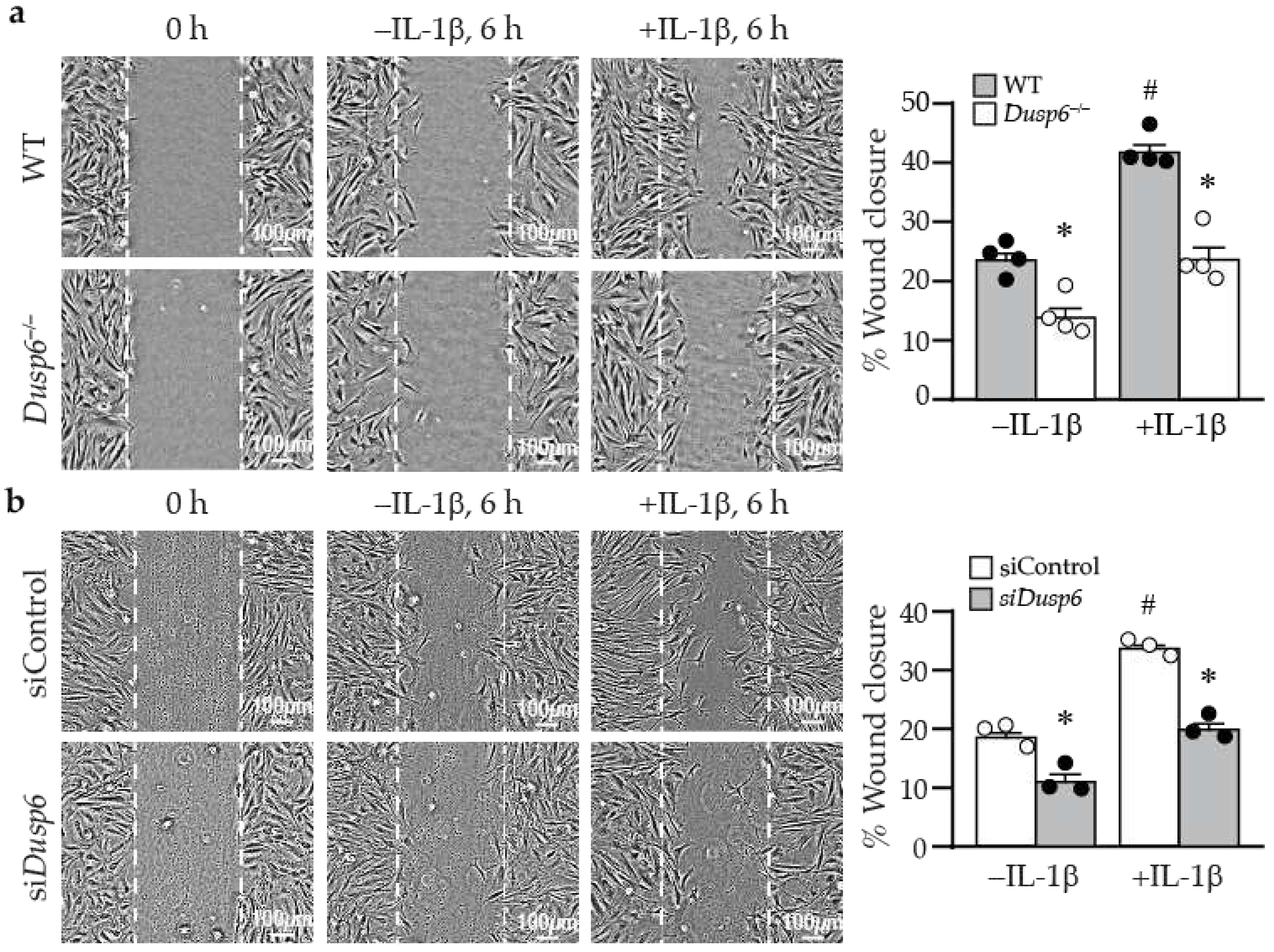
Figure 6.
DUSP6 deficiency abrogates basal and IL-1β-induced VSMC migration. (a) WT and Dusp6–/– VSMCs were wounded and then stimulated with or without IL-1β (10 ng/mL) for migration assays, and wound closure evaluated at 6 h after wounding. Dashed lines indicate initial wound margins. Quantitation of wound closure at 6 h (n=4 each group). *p < 0.05 vs. respective WT, #p < 0.05 vs. WT without IL-1β. One-way ANOVA followed by Tukey’s test. (b) WT VSMCs were transfected with siControl or siDusp6, wounded, stimulated with or without IL-1β, and wound closure was assessed. Quantitation of wound closure at 6 h (n=3 each group). *p < 0.05 vs. siControl in each group, #p < 0.05 vs. siControl without IL-1β, One-way ANOVA followed by Tukey’s test.
Figure 6.
DUSP6 deficiency abrogates basal and IL-1β-induced VSMC migration. (a) WT and Dusp6–/– VSMCs were wounded and then stimulated with or without IL-1β (10 ng/mL) for migration assays, and wound closure evaluated at 6 h after wounding. Dashed lines indicate initial wound margins. Quantitation of wound closure at 6 h (n=4 each group). *p < 0.05 vs. respective WT, #p < 0.05 vs. WT without IL-1β. One-way ANOVA followed by Tukey’s test. (b) WT VSMCs were transfected with siControl or siDusp6, wounded, stimulated with or without IL-1β, and wound closure was assessed. Quantitation of wound closure at 6 h (n=3 each group). *p < 0.05 vs. siControl in each group, #p < 0.05 vs. siControl without IL-1β, One-way ANOVA followed by Tukey’s test.
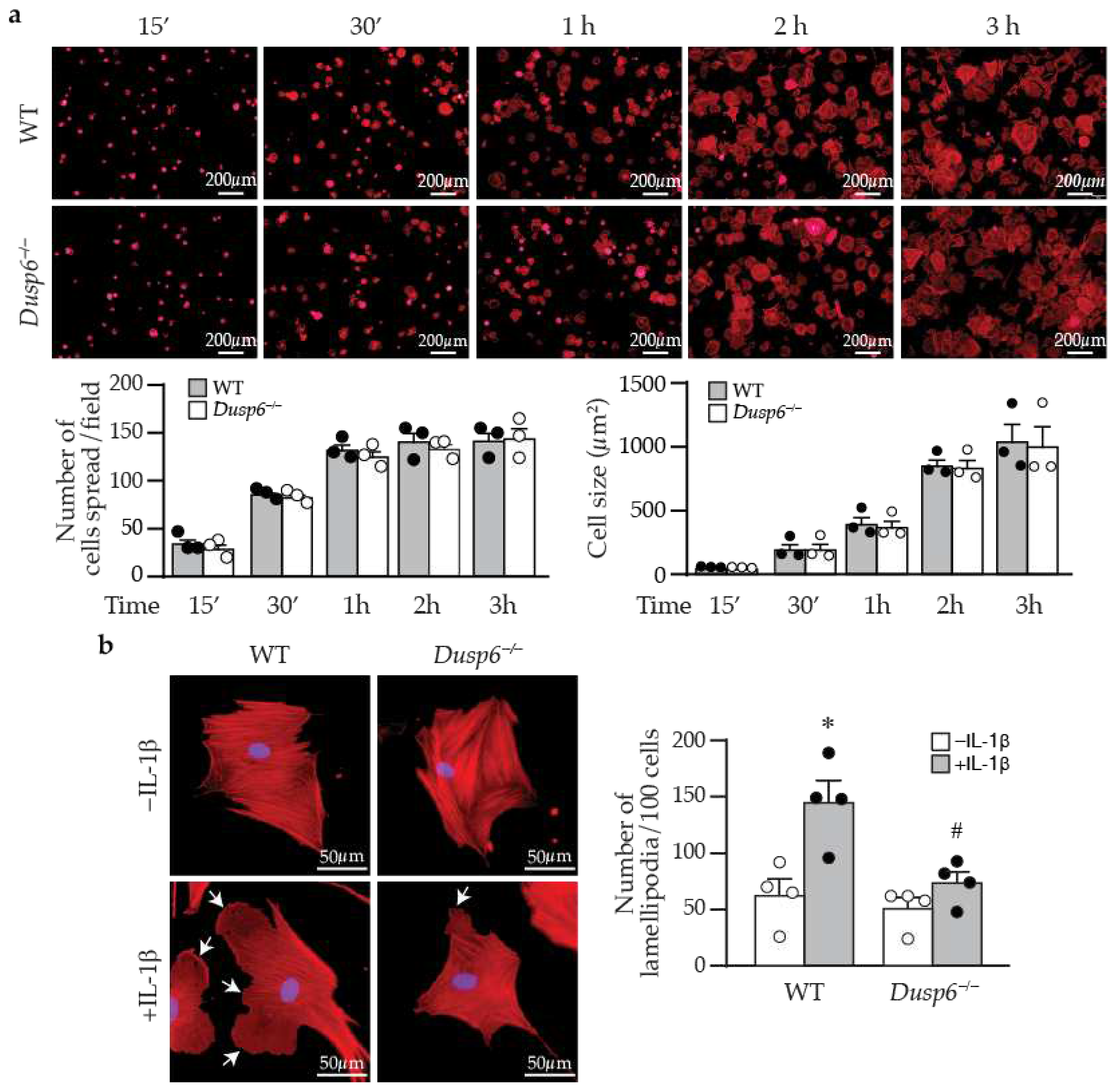
Figure 7.
DUSP6 deficiency inhibits lamellipodia formation after IL-1β stimulation in VSMCs. (a) Wild-type or Dusp6–/– VSMCs were plated, fixed, and stained with phalloidin (red) at indicated time points. Images were captured with florescence microscope Olympus IX71, 40X magnification, representative images from 3 independent experiments are shown. Number of cells attached and spread per field was quantified. Cell size was measured with ImageJ and expressed as µm2/cell. (b) WT and Dusp6–/– VSMCs were serum-starved prior to stimulation with or without IL-1β for 60 min. Phalloidin staining (red) was performed to assess lamellipodia formation. Arrows indicate lamellipodia. Lamellipodia were counted in 100 cells and expressed as the number of lamellipodia per 100 cells. n=4 independent experiments. *p < 0.05 vs. WT without IL-1β, #p < 0.05 vs. WT with IL-1β, One-way ANOVA followed by Sidak’s test.
Figure 7.
DUSP6 deficiency inhibits lamellipodia formation after IL-1β stimulation in VSMCs. (a) Wild-type or Dusp6–/– VSMCs were plated, fixed, and stained with phalloidin (red) at indicated time points. Images were captured with florescence microscope Olympus IX71, 40X magnification, representative images from 3 independent experiments are shown. Number of cells attached and spread per field was quantified. Cell size was measured with ImageJ and expressed as µm2/cell. (b) WT and Dusp6–/– VSMCs were serum-starved prior to stimulation with or without IL-1β for 60 min. Phalloidin staining (red) was performed to assess lamellipodia formation. Arrows indicate lamellipodia. Lamellipodia were counted in 100 cells and expressed as the number of lamellipodia per 100 cells. n=4 independent experiments. *p < 0.05 vs. WT without IL-1β, #p < 0.05 vs. WT with IL-1β, One-way ANOVA followed by Sidak’s test.
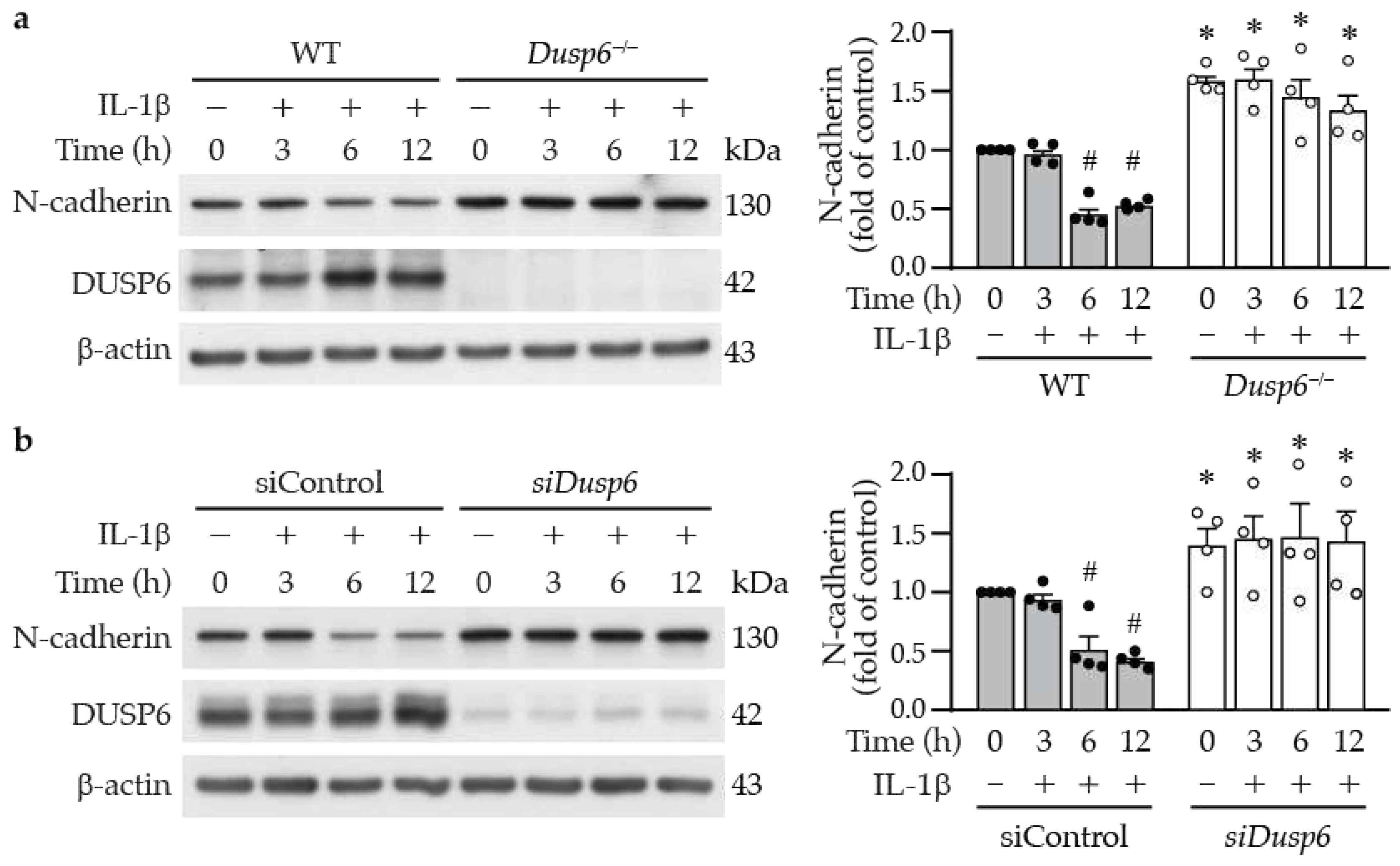
Figure 8.
Lack of DUSP6 increases cell-cell adhesion molecule N-cadherin in VSMCs. (a) Serum-starved WT or Dusp6–/– VSMCs were treated with IL-1β for different time periods and proteins were prepared for Western blotting to detect N-cadherin, DUSP6, and β-actin as loading control. N-cadherin levels were quantified, n=4. *p < 0.05 vs. WT at respective time points, #p < 0.05 vs. WT without IL-1β at 0 h, Mann-Whitney U test. (b) WT VSMCs were transfected with siControl or siDusp6, serum starved, and treated with IL-1β and then analyzed as in (a). N-cadherin levels were quantitated. n=4. *p < 0.05 vs. siControl at each time point, #p < 0.05 vs. siControl without IL-1β at 0 h, Mann-Whitney U test.
Figure 8.
Lack of DUSP6 increases cell-cell adhesion molecule N-cadherin in VSMCs. (a) Serum-starved WT or Dusp6–/– VSMCs were treated with IL-1β for different time periods and proteins were prepared for Western blotting to detect N-cadherin, DUSP6, and β-actin as loading control. N-cadherin levels were quantified, n=4. *p < 0.05 vs. WT at respective time points, #p < 0.05 vs. WT without IL-1β at 0 h, Mann-Whitney U test. (b) WT VSMCs were transfected with siControl or siDusp6, serum starved, and treated with IL-1β and then analyzed as in (a). N-cadherin levels were quantitated. n=4. *p < 0.05 vs. siControl at each time point, #p < 0.05 vs. siControl without IL-1β at 0 h, Mann-Whitney U test.
Figure 9.
DUSP6 deficiency attenuates arterial injury-induced intimal hyperplasia in mice. Arterial injury such as guide wire denudation of endothelium releases inflammatory cytokines including IL-1β at the injured site. IL-1β activates ERK1/2 pathways, which induces DUSP6 expression in VSMCs, and in turn decreases p27 and N-cadherin levels, leading to enhanced cellular proliferation and migration, and eventual intimal hyperplasia after injury. On the other hand, lack of DUSP6 increases baseline levels of p27 and N-cadherin in VSMCs. Furthermore, lack of DUSP6 prevents IL-1β-decreased p27 and N-cadherin, resulting in reduced VSMC proliferation and migration, and consequently mitigating neointima formation. Created with Biorender.com.
Figure 9.
DUSP6 deficiency attenuates arterial injury-induced intimal hyperplasia in mice. Arterial injury such as guide wire denudation of endothelium releases inflammatory cytokines including IL-1β at the injured site. IL-1β activates ERK1/2 pathways, which induces DUSP6 expression in VSMCs, and in turn decreases p27 and N-cadherin levels, leading to enhanced cellular proliferation and migration, and eventual intimal hyperplasia after injury. On the other hand, lack of DUSP6 increases baseline levels of p27 and N-cadherin in VSMCs. Furthermore, lack of DUSP6 prevents IL-1β-decreased p27 and N-cadherin, resulting in reduced VSMC proliferation and migration, and consequently mitigating neointima formation. Created with Biorender.com.