1. Introduction
The Philippines, an archipelagic country known for its rich biodiversity, stands proudly as one of the 18 megadiverse nations on Earth [
1]. This designation signifies that it harbors over two-thirds of the world's biodiversity resources, which play a crucial role in supporting human well-being and survival while maintaining ecosystem stability [
2,
3]. Biodiversity generously provides us with essential resources like food, water, raw materials, and clean air. Moreover, it diligently regulates climate and protects us from natural disasters [
4]. Ecologically speaking, biodiversity enables vital processes such as pollination, nutrient cycling, water filtration, and erosion control – all working together to create balanced ecosystems [
5].
Unfortunately, biodiversity has long been facing numerous threats that jeopardize its existence and the critical services it provides. Climate change, along with many undesirable human activities such as deforestation, habitat destruction, land use change, and overexploitation primarily drive biodiversity loss, globally [
6]. Due to these, scientists were able to identify biodiversity hotspots which contain very high rate of endemism and drastic loss of vegetation and habitat that threatens various key biodiversity species [
7]. At present, there are already 36 biodiversity hotspots, including the Philippines [
8]. This signifies the need for immediate planning and implementation of strategies to prevent total biodiversity loss.
In the Philippines, various conservation and rehabilitation efforts are continuously implemented. The establishment and monitoring of protected zones under the National Integrated Protected Areas Systems (NIPAS) Act is considered one of the most important tools in conserving the country’s key biodiversity resources as recommended by the Convention on Biological Diversity [
9]. Other conservation and rehabilitation programs such as the National Greening program under the Executive Order No. 26 [
10], community-based forest management under the Executive Order No. 263 [
11], and sustainable ecotourism [
12], among others, are recognized to greatly contribute to biodiversity conservation while educating the people about its values and services.
However, there were critical issues in some rehabilitation and conservation programs. One of these is the unsuitable choice of plant species to rehabilitate a degraded or disturbed area. Several efforts in the past used exotic and invasive species such as
Gmelina arborea Roxb. and
Swietenia macrophylla King [
13] in many greening activities. Some used native species but there was lack of pre-assessment on the site-species relationship, thus, introducing the natives to inappropriate habitat hindering their successful growth and survival [
14]. This is where the importance of plant inventory and assessments comes in. The data and findings yielded by these studies provide essential understanding on the population structure, composition, and ecology of an area and its resources beneficial in recovery planning such as biodiversity conservation and habitat rehabilitation [
15].
This current study aims to contribute to the conservation of Philippine biodiversity by assessing the tree diversity in the municipality of San Luis in the province of Aurora. The province is a part of the Sierra Madre Mountain Range, the longest mountain range in the country, which is considered a highly important area in terms of valuable ecological resources distributed among its 68 protected areas [
16,
17]. Furthermore, there is a very few studies about the plant composition and diversity in the province which only covers the tree species in the municipalities of Baler [
18] and Dipaculao [
19], as well as the diversity of ferns in the municipalities of Maria Aurora [
20] and Baler [
21]. Hence, this study will pioneer the assessment of plants in the municipality of San Luis, beneficial in identifying the area’s key biodiversity resources such as the endemic, native, and threatened species which is a crucial step in biodiversity conservation.
2. Materials and Methods
2.1. Study Site
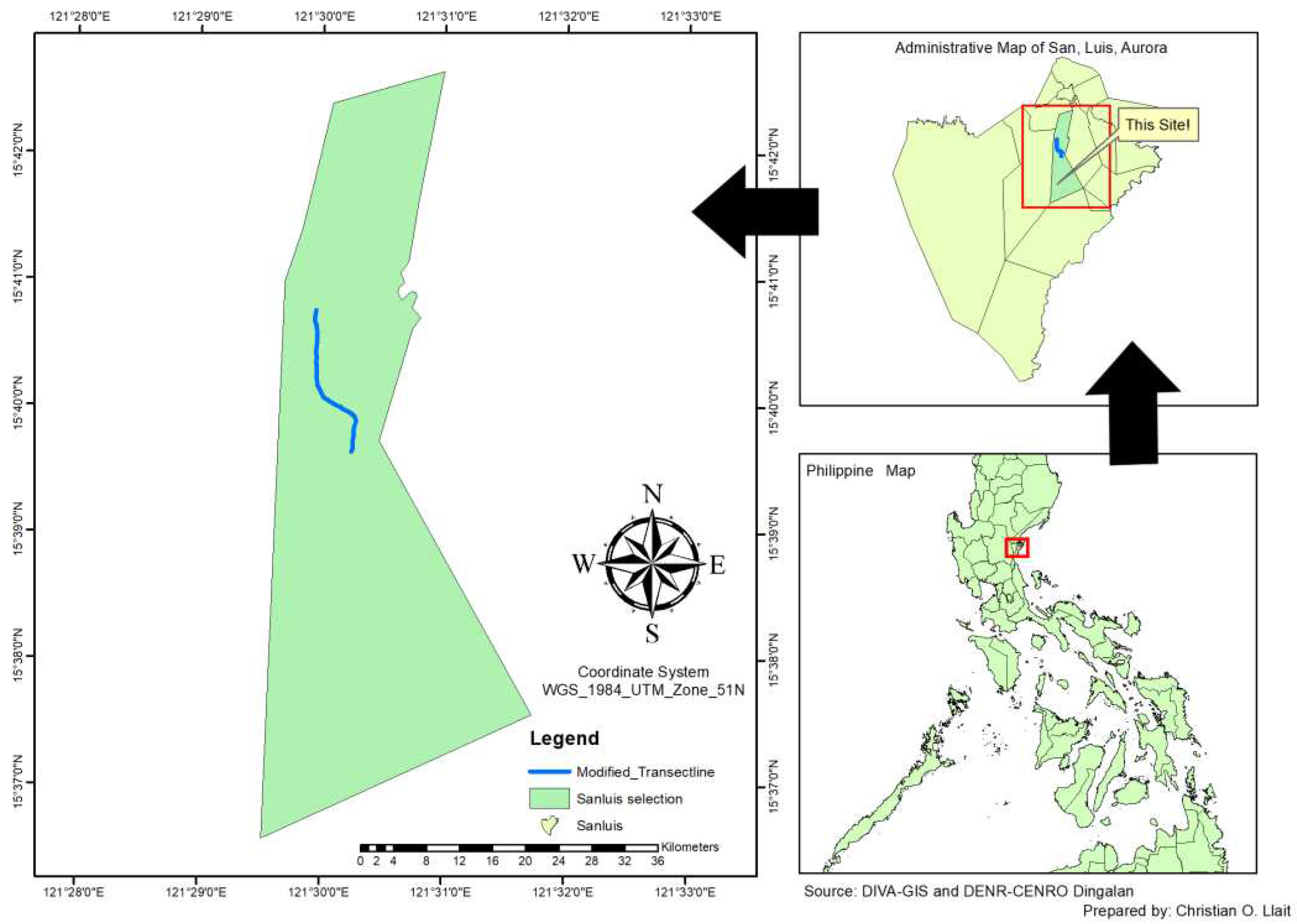
The study was conducted in April 2023 in Barangay L. Pimentel in the municipality of San Luis, province of Aurora situated at approximately 15˚41’2.94” N and 121°30'1.23" E (
Figure 1). The barangay is composed of residential, agricultural, and mountainous forest lands. Specifically, the survey was done in the mountainous forest lands which is a portion of the Sierra Madre Mountain Range which the backbone of Luzon Island being a protector and barrier from typhoons coming from the Pacific Ocean [
22]. The survey area had a moderately steep topography with elevations ranging from 273 to 581 masl. Climate-wise, the municipality has an average of 28°C during daytime and 24°C during nighttime as well as an 8 rain days and precipitation of around 300 mm during the month of April [
23].
2.2. Survey and Mapping of Tree Species
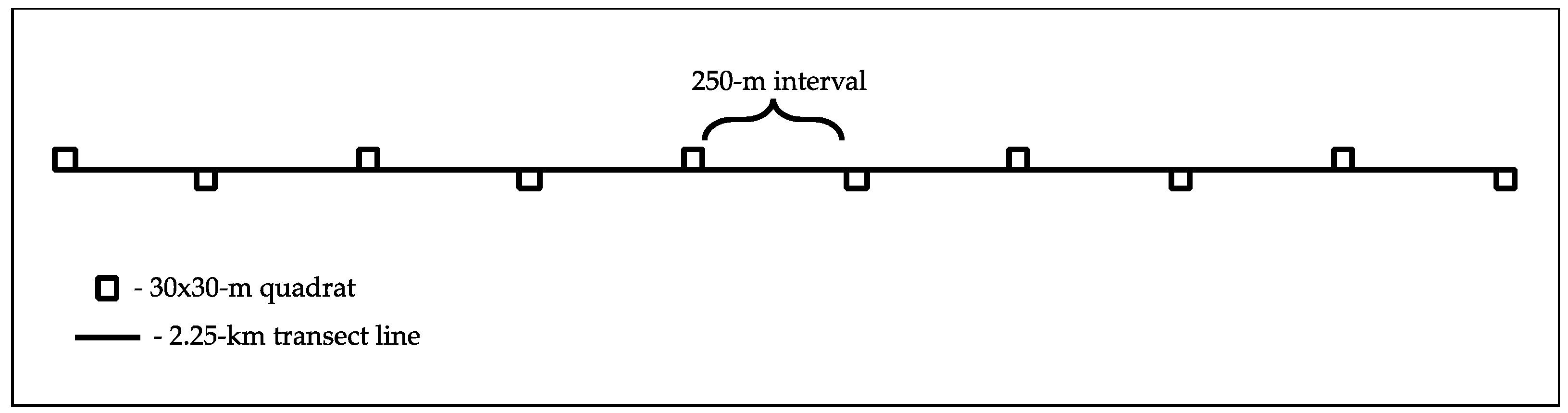
Inventory of tree species were done along a 2.25-km transect line with 10 30by30-m quadrats established at every 250-m point (
Figure 2). The transect line was established following the trail while the quadrats were positioned alternately at the left and right of the transect line with an approximate distance of 5 meters away from the trail. The total coverage of the quadrats was 9000m
2. The use of transect in conducting this plant inventory was used to ensure that the quadrats were evenly distributed throughout the forest stand [
24].
After the establishment of the transect and quadrats, the plant survey was done. Trees with a diameter at breast height (DBH) of at least 10 cm were included in the study following DBH cut-off of many tree species inventory in the Philippines [
25,
26]. Plant identities were determined on the field using morphological characteristics. For individuals that were not identified on the field, photos were taken for further verification. References and databases such as Co’s Digital Flora of the Philippines [
27] and Revised Lexicon of Philippine Trees [
28] were used in verifying plant identities. Finally, the accepted scientific names of plants were determined using the Plants of the World Online database of the Kew Royal Botanic Gardens [
29]. Significant ecological status (i.e., indigeneity, endemism, conservation status) of all species were also assessed. Indigeneity and endemism were obtained from the Co’s Digital Flora of the Philippines [
27]. Meanwhile, conservation statuses were determined using the IUCN Red List of Threatened Species [
30] for the global scale, and DAO 2017-11 or the Updated National Checklist of Threatened Plants and their Categories [
31] for the national scale.
Mapping was also done to visually present the location of each tree individual which will also serve as basis for future implementation of targeted biodiversity conservation and management measures. Initially, a locus map was used to record the location of the transect line and quadrats. Then, geographic coordinates of each tree were recorded and encoded in Microsoft Excel. Geographic coordinates were then converted into the Universal Transverse Mercator (UTM) format before feeding it to the ArcGIS software. After that, the locations of all trees were plotted in the map. Lastly, final editing was done to produce the final copy of the map in .jpeg format.
2.3. Data Analysis and Interpretation
2.3.1. Species Richness, Abundance, and Importance Values
Species richness, abundance, and importance values were either counted or calculated to discover the species composition in the area. Species abundance refers to the number of individuals of a species in an area [
32] while species richness is the number of species present [
33]. Hence, the number of species and its individuals were counted to determine the species richness and abundance. Lastly, importance values (IVs) serve as an index to measure how dominant a certain species is in a forest area through the relative values of its abundance, frequency, and dominance [
34]. Thus, IVS were computed using the following formulas [
35]:
2.3.2. Diversity Indices
Biological diversity can be quantified using mathematical functions known as the diversity indices [
36]. In this study, Shannon Weiner (H’) and Simpson evenness (E) were the species diversity indices computed through the Paleontological Statistics (PAST) software. To easily interpret the results, the Fernando Biodiversity Scale was utilized following many diversity studies in the country [
37,
38] (
Table 1).
2.3.3. Correlation Analysis
Pearson correlation analysis was used to explore underlying relationship (i.e., monotonic association) among important variables namely, elevation, species richness, abundance, Shannon Weiner, and Simpson Evenness. This was computed at significance level of
p < .05 through JASP v. 0.16.1, an open-source statistical software package. Results were interpreted using the computed correlation coefficient values (
r-values) and their associated
p-values, as well as the conventional approach in interpreting
r-values, contextualized as direct or inverse relationship [
39] (
Table 2).
3. Results and Discussion
3.1. Tree Species Composition
The study recorded a total of 148 individuals of 38 morphospecies of trees from 20 families and 28 genera. In terms of the families, Dipterocarpaceae and Moraceae were the most speciose with 7 and 5 species, respectively. The most abundant families were Euphorbiaceae, Dipterocarpaceae, and Moraceae with 29, 28, and 21 individuals, respectively. These families are abundant in the Philippines especially in tropical lowland evergreen forests that are dominated by dipterocarps [
40]. Sadly, dipterocarps are among the most threatened plant species in the Philippines and in Southeast Asia due to deforestation and its history of their timbers being massively exported in the past [
41,
42]. Species-wise,
Macaranga stonei Whitmore was the most abundant followed by
Parashorea malaanonan (Blanco) Merr. with 24 and 9 individuals, respectively.
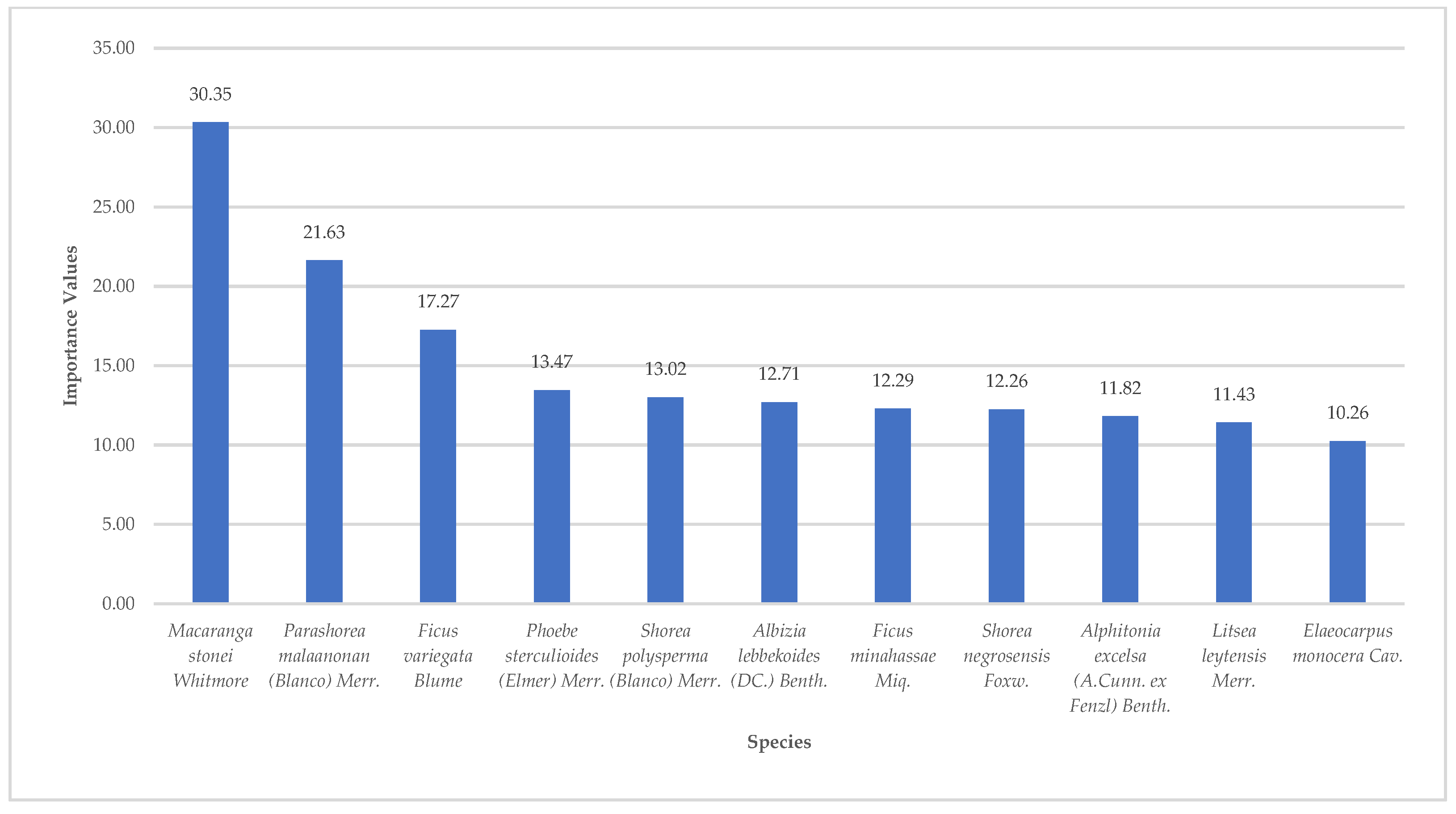
The importance values computation also revealed significant findings in terms of the species composition. Eleven (11) species had individual IVs of more than 10 (
Figure 3). In total, these 11 species contributed to 55.50% of the total IV of all species in the area. Among them,
M. stonei had the highest IV of 30.35 equivalent to 10.11% of the total IV of all the species recorded followed by
Parashorea malaanonan (Blanco) Merr with 21.63 (7.21%).
M. stonei’s high IV was contributed by its high abundance of 24, occurrence in 6 plots out of the total 10 plots, and a total basal area of 136.73m
2.
M. stonei is an Aurora province endemic and critically endangered plant species belonging to the family Euphorbiaceae [
27,
30]. This keystone species lacks focus in terms of research, thus, dictating the need to study the species more and include it as one of the top priorities for conservation being a species restricted to the province of Aurora only.
The surveyed forest was also found to be home for ecologically important species namely, the native, endemic, and threatened species. Out of the 38 species found, 33 (86.84%) were natives while five were exotics (with one invasive
Gmelina arborea Roxb. ex Sm.). The native species were composed of 12 endemics, five IUCN threatened species, and 9 Philippine nationally threatened species. Specifically, there were one critically endangered, two endangered, and two vulnerable species found in the IUCN. Furthermore, there were two endangered, six vulnerable, and one other threatened species found in DAO 2017-11 or the Philippine Red List. The most notable among the Philippine endemic species were the IUCN critically endangered
M. stonei and the IUCN vulnerable and DAO 2017-11 endangered
Hopea acuminata Merr, and the IUCN endangered and DAO 2017-11 vulnerable Philippine national tree
Pterocarpus indicus Willd. The presence of the critically important plants in the area dictates the need for immediate action to conserve, protect, and even spread their population. It is emphasized that these species, particularly the endemics, have higher probabilities of extinction because of their narrow and restricted habitat than widespread species [
43]. The native and endemic plant species also provide suitable habitat and enough food source for native and endemic fauna species [
44]. In fact, we were able to witness a couple of the Philippine endemic Luzon Rufous Hornbill (
Buceros hydrocorax Linnaeus
) during the survey. However, the presence of invasive species like the
G. arborea adds pressure to the survival and propagation of the native and endemic flora and fauna species, due to the aggressive nature of most invasive plants [
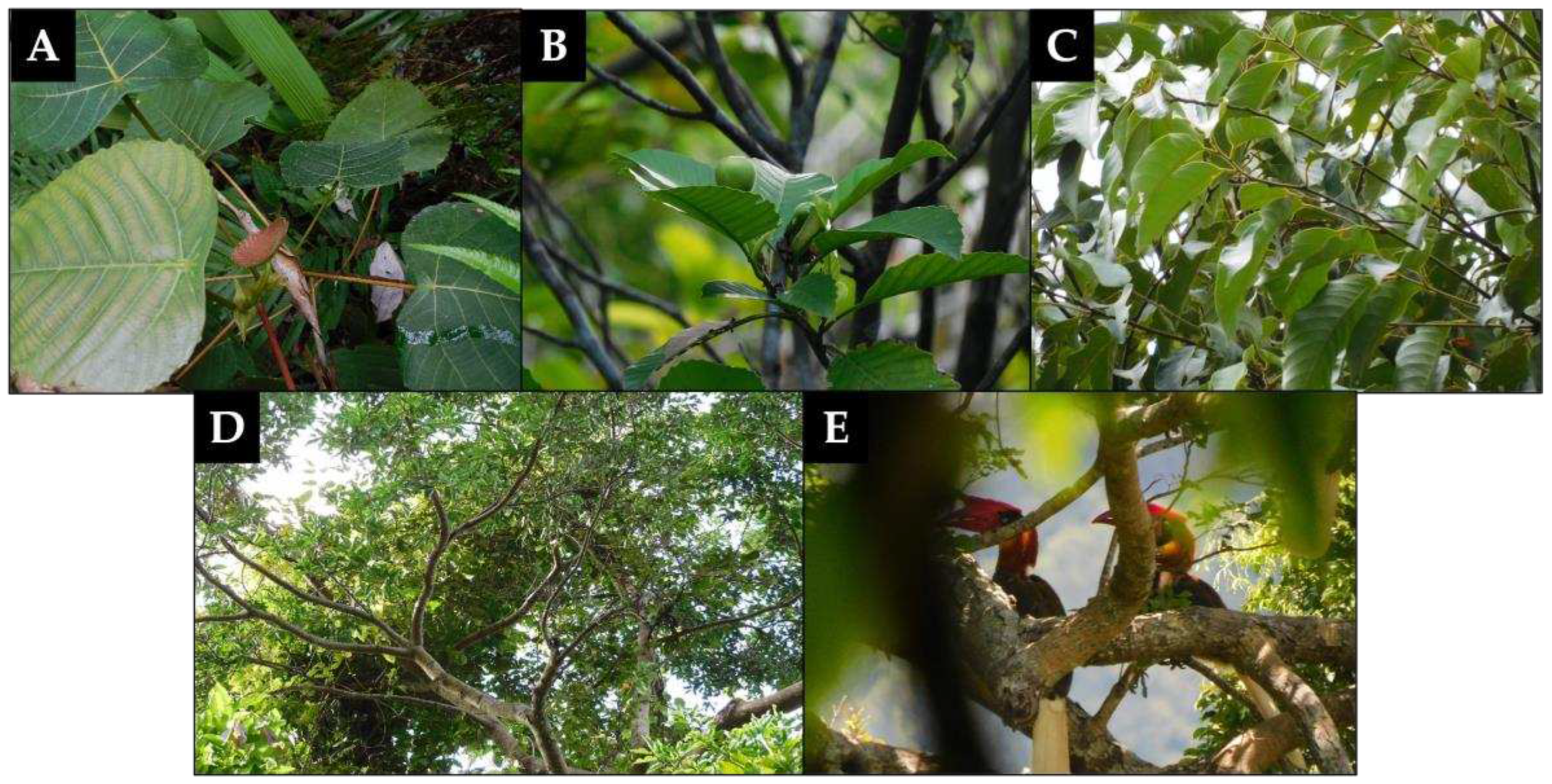
45]. Shown in
Figure 4 are actual representative photos of some critically important plant species in the area and an image of
B. hydrocorax individuals.
Table 3.
Taxonomic list of native species recorded with their corresponding endemism and conservation statuses.
Table 3.
Taxonomic list of native species recorded with their corresponding endemism and conservation statuses.
| Family |
Species |
Endemism1
|
Conservation Status2
|
| IUCN Red List |
DAO 2017-11 |
| Anacardiaceae |
Koordersiodendron pinnatum (Blanco) Merr. |
NE |
ND |
OTS |
| Brownlowiaceae |
Diplodiscus paniculatus Turcz. |
PE |
LC |
ND |
| Cannabaceae |
Celtis philippensis Blanco |
NE |
LC |
ND |
| Dilleniaceae |
Dillenia philippinensis Rolfe |
PE |
NT |
ND |
| Dilleniaceae |
Tetracera scandens (Linn.) Merr. |
NE |
ND |
ND |
| Dipterocarpaceae |
Dipterocarpus grandiflorus (Blanco) |
NE |
EN |
VU |
| Dipterocarpaceae |
Hopea acuminata Merr. |
PE |
VU |
EN |
| Dipterocarpaceae |
Parashorea malaanonan (Blanco) Merr. |
NE |
LC |
ND |
| Dipterocarpaceae |
Shorea contorta Vidal |
PE |
LC |
VU |
| Dipterocarpaceae |
Shorea negrosensis Foxw. |
PE |
LC |
VU |
| Dipterocarpaceae |
Shorea polysperma (Blanco) Merr. |
PE |
LC |
VU |
| Dipterocarpaceae |
Shorea squamata (Turcz.) Benth. & Hook. |
PE |
LC |
ND |
| Elaeocarpaceae |
Elaeocarpus cumingii Turcz. |
NE |
LC |
ND |
| Elaeocarpaceae |
Elaeocarpus monocera Cav. |
PE |
ND |
ND |
| Euphorbiaceae |
Macaranga grandifolia (Blanco) Merr. |
NE |
VU |
ND |
| Euphorbiaceae |
Macaranga stonei Whitmore |
PE |
CR |
ND |
| Euphorbiaceae |
Macaranga tanarius (L.) Muell. Arg. |
NE |
LC |
ND |
| Euphorbiaceae |
Mallotus paniculatus (Lam.) Müll.Arg. |
NE |
LC |
ND |
| Fabaceae |
Albizia lebbekoides (DC.) Benth. |
NE |
LC |
ND |
| Fabaceae |
Pterocarpus indicus Willd. |
NE |
EN |
VU |
| Hypericaceae |
Cratoxylum sumatranum Blume |
NE |
LC |
ND |
| Lauraceae |
Litsea leytensis Merr. |
PE |
NT |
EN |
| Lauraceae |
Phoebe sterculioides (Elmer) Merr. |
PE |
LC |
ND |
| Meliaceae |
Aglaia luzoniensis (Vidal) Merr. & Rolfe |
NE |
NT |
ND |
| Moraceae |
Artocarpus blancoi (Elmer) Merr. |
PE |
LC |
ND |
| Moraceae |
Ficus minahassae Miq. |
NE |
LC |
ND |
| Moraceae |
Ficus nota (Blanco) Merr. |
NE |
LC |
ND |
| Moraceae |
Ficus variegata Blume |
NE |
LC |
ND |
| Myrtaceae |
Syzygium nitidum Benth. |
NE |
ND |
VU |
| Myrtaceae |
Syzygium tripinnatum (Blanco) Merr. |
NE |
ND |
ND |
| Rhamnaceae |
Alphitonia excelsa (A.Cunn. ex Fenzl) Benth. |
NE |
LC |
ND |
| Sterculiaceae |
Sterculia ceramica R.Br. |
NE |
ND |
ND |
| Urticaceae |
Leucosyke capitellata (Poir.) Wedd. |
NE |
LC |
ND |
3.2. Tree Species Diversity
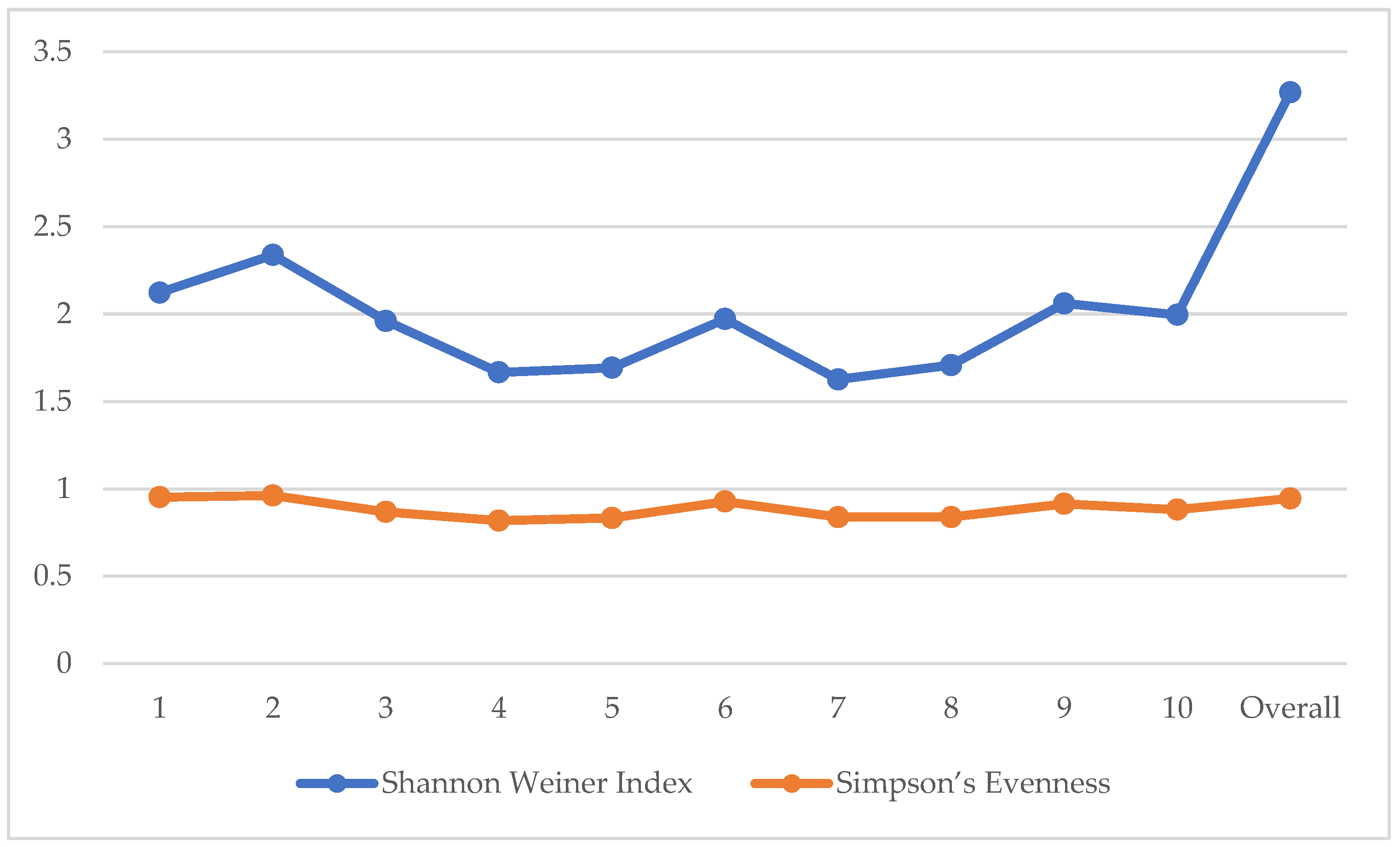
The diversity indices of the secondary forest in San Luis are presented in
Figure 5. The Shannon Weiner index values per quadrat ranged from 1.626 to 2.3384 interpreted as very low to low based on Fernando Biodiversity Scale. In terms of Simpson’s Evenness, values ranged from 0.8182 to 0.9619 interpreted as very high. Quadrat 2 had the highest diversity (H’ = 2.384 and E = 0.9619). Overall, the study area had a high Shannon Weiner index (H’ = 3.269) and a very high Simpson Evenness index (E = 0.9453) which means that the trees in the area were relatively diverse and had a considerably even distribution of individuals among species. In most ecological studies in the Philippines, H’ values generally range from 1.5 to 3.5 wherein higher values dictate higher species diversity [
46]. The overall H’ value of the present study falls within this range interpreted as high which is possibly attributed by a variety of native and endemic species that still thrives therewith. This is comparable with some studies in the Philippines such as in a lowland forest in Agusan del Sur (H’ = 3.32, E = 0.52) [
47], in a secondary forest in Benguet (H’ = 2.40) [
46], and in a secondary forest in Pampanga (H’ = 2.2807, E = 0.8549) [
48], which were all categorized as low to moderate diversity based on Shannon Weiner index. Similarly, these study sites were either under the management of upland communities or near their residential or agricultural sites. In contrast, the values are lower than the studies in a private mountainous forest in Baler, Aurora (H’ = 4.096; E = 0.9735) [
18], in Quezon Protected Landscape (H’ = 3.90, E = 0.81) [
49], and in Mt. Makiling Forest Reserve (H’ = 3.50, E = 0.91) [
50]. The common characteristics that possibly caused these high values were their classifications as a private property with strict monitoring and considerably high protection for the site in the first study and being classified as protected areas under the law of the second and third study sites, dictating a monitoring and protection activities implementation of the government.
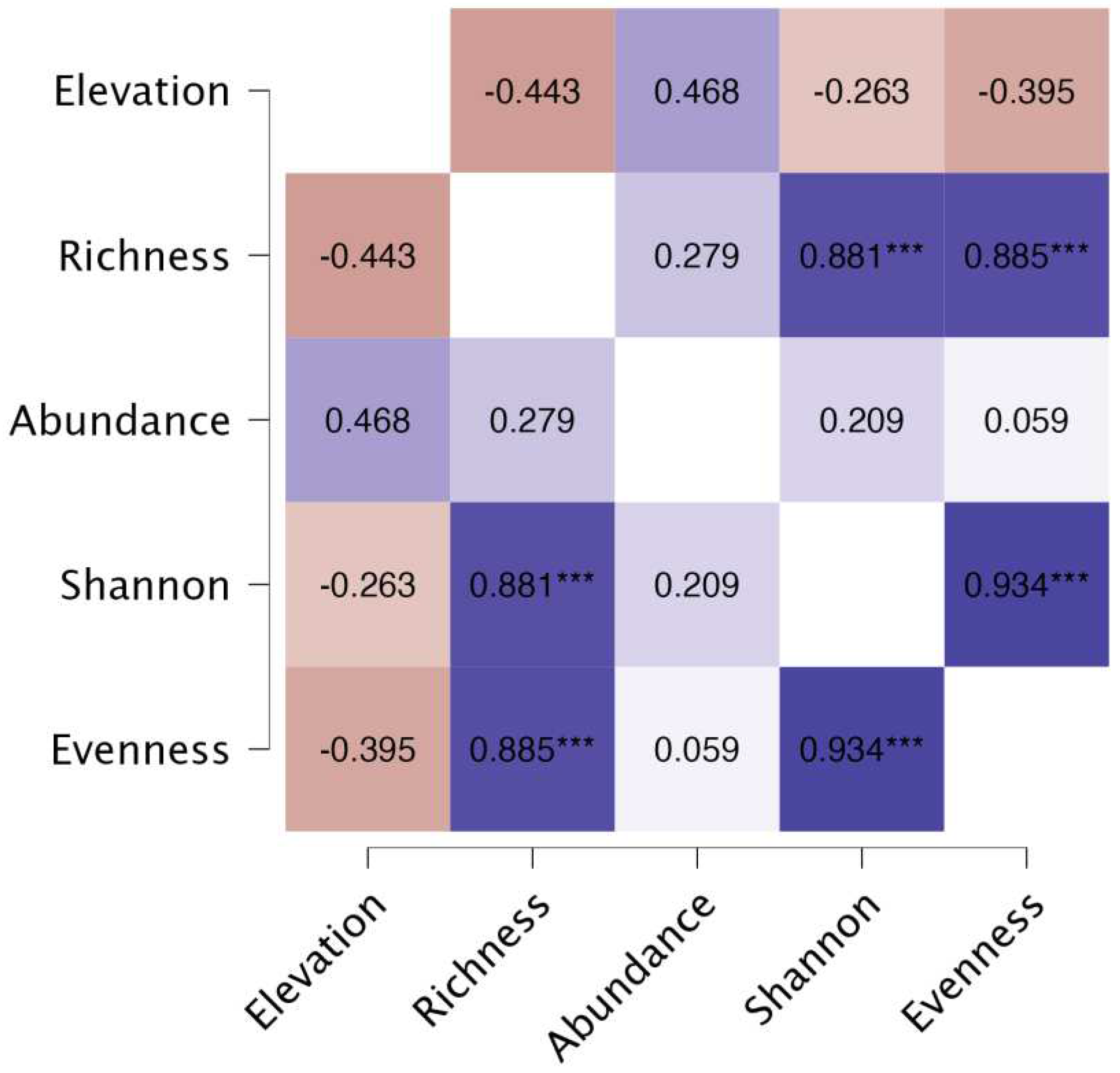
3.3. Correlation among Parameters
The study also tested the correlation among elevation, species richness, abundance, Shannon Weiner, and Simpson’s Evenness values. As a result, significant correlational relationships were only observed for the following: a) Species Richness and Shannon Weiner (
r = 0.881,
p < .001), b) Species Richness and Simpson’s Evenness (
r = 0.885,
p < .001), and c) Simpson’s Evenness and Shannon Weiner (
r = 0.934,
p < .001) (
Figure 6). Based on the
r values, there was a strong positive correlation between species richness and Shannon Weiner index as well as between species richness and Simpson’s Evenness supported by a very high significance value of
p that is less than 0.001. This relationship suggests that as species richness increases, the values of Shannon Weiner and Simpson’s Evenness indices also tend to increase. Thus, this observation indicates that having a greater variety of species can lead to a higher diversity as measured by the mentioned indices. Furthermore, there was a very strong positive correlation found between Simpson’s Evenness index and Shannon Weiner index based on the obtained
r value backed up by a very high statistical significance with
p < .001. This indicates that as the value of Shannon Weiner index increase, the value of Simpson’s Evenness also tends to increase. The findings are corroborated by the study of DeJong which also found a very strong correlation among species richness, Shannon Weiner index, and Simpson’s Evenness index with correlation coefficients of more than 0.96 [
51]. In essence, these results are beneficial in understanding the dynamics of an ecosystem which can be a foundation for implementing management and rehabilitation strategies in different areas with a goal of improving biodiversity.
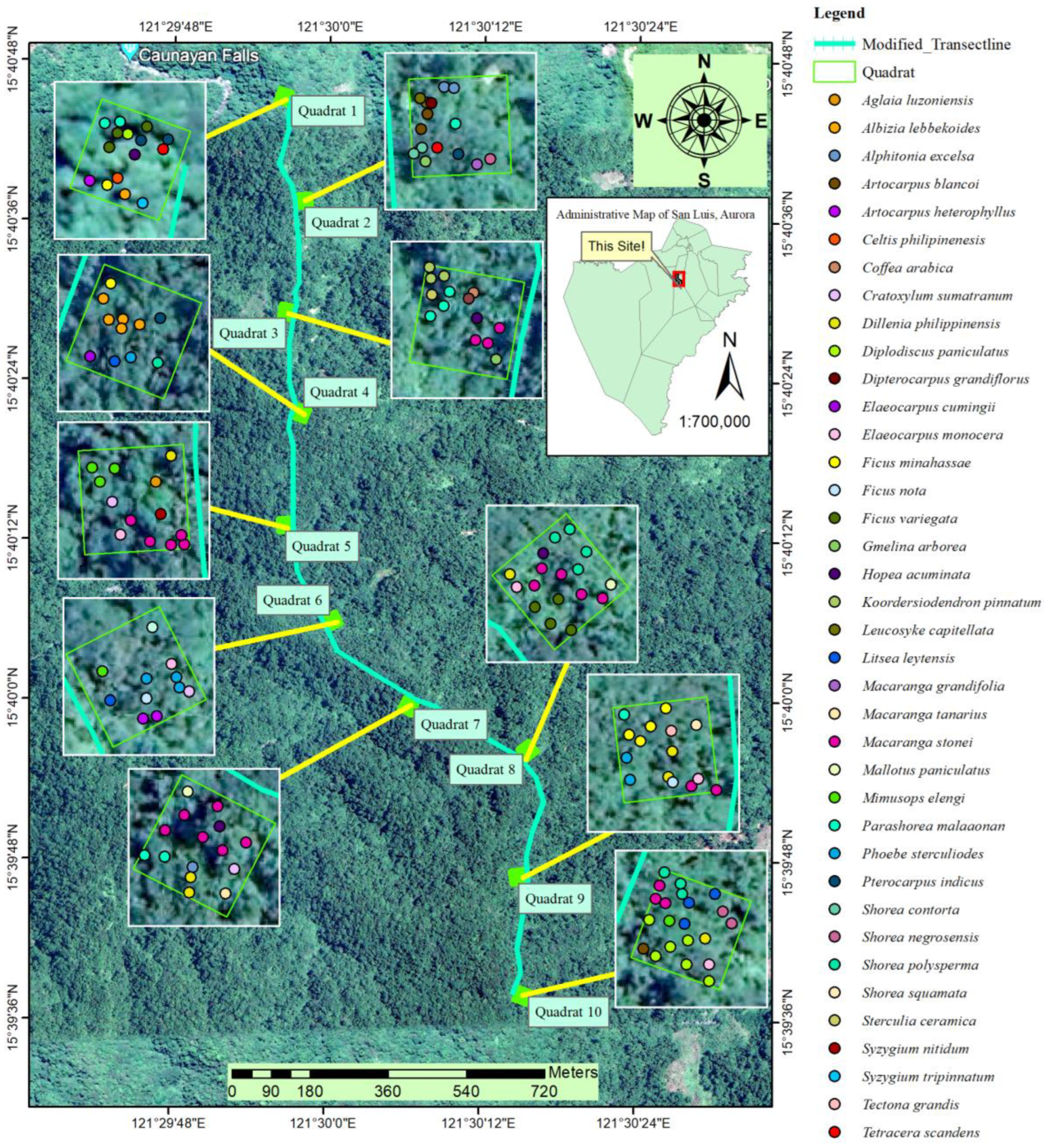
3.3. Spatial Distribution of Trees
Shown in
Figure 4 is the spatial distribution of trees across sampling plots in the secondary forest of San Luis in Aurora, Philippines. This map shows the approximate position of each tree individual, represented by colored dots, based on the recorded coordinates. Mapping the spatial distribution of trees is a crucial element in devising strategies for sustainable management and conservation of natural resources [
52]. For instance, locating the trees can help us identify areas with possible source of mother trees of the targeted species that we aim to propagate [
53]. Furthermore, distribution maps can visually present areas needing attention and immediate measures such as in the case of our study, the presence of the invasive
G. arborea pose threat to the native biodiversity. Knowing the location of its recorded individual will allow the forest managers to perform targeted measures in managing specific portions of the area where invasion issues arise [
45]. Lastly, we can identify micro-biodiversity hotspot among the sampling plots in the study area by determining the number of critically important species [
54].
4. Conclusions and Implications
The study yielded valuable findings and insights regarding the species composition and diversity of the secondary forest in San Luis, Aurora. Overall, the area had a relatively high diversity and significant conservation signified by the recorded 148 individuals of 38 morphospecies belonging to 20 families and 28 genera of trees with 33 natives, 12 endemics, five IUCN threatened, and nine Philippine threatened species. Furthermore, diversity was found to be high for Shannon Weiner index (H’ = 3.269) and very high for Simpson’s Evenness index (E =0.9453). Significant correlational relationships were also found among species richness, Shannon Weiner index, and Simpson’s Evenness index. Lastly, tree individuals were mapped to serve as a guide for targeted conservation measures. These findings are critical in the following applications for the conservation of native, endemic, and threatened species:
The presence of many native, endemic, and threatened species underscores the immediate need to prioritize the conservation of these species through the aid of the map produced in locating the micro-biodiversity hotspots in the area. Furthermore, many endemic species lack scientific studies highlighting the need to conduct focused studies to explore the ecology and distribution of these critically important species. Furthermore, this can serve as a basis for the Department of Environment and Natural Resources to include the forest as one of the high conservation priorities or to expanded protected areas to cover the area surveyed.
The relatively high diversity values and even distribution of plants calculated for the area somehow indicates a relatively healthy ecosystem. Thus, dictating the need intensified law enforcement to protect the remaining forests that serve as habitat for the native and endemic wildlife such as the Buceros hydrocorax Linnaeus.
The presence of introduced and invasive species such as Gmelina arborea Roxb. pose a very significant threat to the local native biodiversity. A targeted and participatory invasive species management is needed to control and eventually eradicate the impact of invasive plants in the ecosystem.
All the implications and conservation strategies discussed above will need the participation of locals and other stakeholders being an area adjacent to residential communities. Thus, information and educational campaigns as well as participatory approach in implementing conservation strategies are ideal tools to ensure a more effective biodiversity conservation and protection.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not Applicable.
Acknowledgments
The author would like to thank for the great contribution of the following individual Diomedes Llait, Diadema Llait, Cherie Win L. Sanchez, and John Paul Llait for financial and moral guidance. The author also would like to extend gratitude to the faculty of the Department of Forestry, Tarlac Agricultural University For. Christian Arvin M. Arce and For. Mark Anthony L. Bagsit for the assistance during the conduct of the research. The author also greatly appreciated the Local Government Unit of Barangay L. Pimentel, San Luis, Aurora for allowing to conduct the research on their vicinity. Finally, a sincere thanks is given to the faculty of School of Graduate Studies of Aurora State College of Technology especially For. Mary Jane Aragon-Marigmen, Dr. RB Gallego, For. Michelle Resueño, Dr. Jay Amon, For. Maria Cristina Cañada, and For. Afed Daiwey, for their untiring support and advise.
Conflicts of Interest
The author declares no conflict of interest.
References
- Vidallon, S. L., & Arriola, A. H. (2023). A preliminary checklist of Rubiaceae in Mt. Mariveles, Bataan, Philippines. Biodiversity, 1-10. [CrossRef]
- Davids, S., & Mavumengwana, Z. (2020). Stocktaking SA’s natural treasures. Veld & Flora, 106(1), 34-37.
- Li, F., Altermatt, F., Yang, J., An, S., Li, A., & Zhang, X. (2020). Human activities' fingerprint on multitrophic biodiversity and ecosystem functions across a major river catchment in China. Global change biology, 26(12), 6867-6879. [CrossRef]
- Ansari, N. A., Agus, C., & Nunoo, E. K. (2021). Foundations of ‘SDG15–LIFE on Land’: Earth, Forests and Biodiversity. In SDG15–Life on Land: Towards Effective Biodiversity Management (pp. 7-48). Emerald Publishing Limited.
- Sahu, D., Sahu, J. K., Kumar, V., & Gupta, P. (2023). Role of Floriculture in Promoting Biodiversity and Enhancing Ecosystems: A Comprehensive Review. International Journal of Environment and Climate Change, 13(9), 2077-2084. [CrossRef]
- Caro, T., Rowe, Z., Berger, J., Wholey, P., & Dobson, A. (2022). An inconvenient misconception: Climate change is not the principal driver of biodiversity loss. Conservation Letters, 15(3), e12868. [CrossRef]
- Jepson, P., & Canney, S. (2001). Biodiversity hotspots: hot for what?. Global Ecology and Biogeography, 10(3), 225-227. [CrossRef]
- Cunningham, C., & Beazley, K. F. (2018). Changes in human population density and protected areas in terrestrial global biodiversity hotspots, 1995–2015. Land, 7(4), 136. 10.3390/land7040136.
- Hind, E. J., Hiponia, M. C., & Gray, T. S. (2010). From community-based to centralised national management—A wrong turning for the governance of the marine protected area in Apo Island, Philippines?. Marine policy, 34(1), 54-62. [CrossRef]
- Gregorio, N., Herbohn, J., Harrison, S., Pasa, A., & Ferraren, A. (2017). Regulating the quality of seedlings for forest restoration: Lessons from the National Greening Program in the Philippines. Small-scale forestry, 16, 83-102. [CrossRef]
- Lasco, R. D., & Pulhin, J. M. (2006). Environmental impacts of community-based forest management in the Philippines. International Journal of Environment and Sustainable Development, 5(1), 46-56. [CrossRef]
- Catibog-Sinha, C. (2010). Biodiversity conservation and sustainable tourism: Philippine initiatives. Journal of Heritage Tourism, 5(4), 297-309. [CrossRef]
- Mangaoang, E. O., & Pasa, A. E. (2003). Preferred native tree species for smallholder forestry in Leyte. Annals of Tropical Research, 25(1), 25-30.
- Navarrete, I. A., Peque, D. P., & Macabuhay, M. D. (2018). Soil information as a reforestation decision-making tool and its implication for forest management in the Philippines. Environmental Resources Use and Challenges in Contemporary Southeast Asia: Tropical Ecosystems in Transition, 97-116.
- Appiah, M. (2013). Tree population inventory, diversity and degradation analysis of a tropical dry deciduous forest in Afram Plains, Ghana. Forest Ecology and Management, 295, 145-154. [CrossRef]
- Coracero, E. E., & Malabrigo Jr, P. (2020). Carbon storage potential of the tree species along the ultramafic forest in Sitio Dicasalarin, Barangay Zabali, Baler, Aurora, Philippines. AIMS Environmental Science, 7(6), 589-601.
- Forest Foundation Philippines. (2022). Sierra Madre Mountain Range: Backbone of Luzon. https://www.forestfoundation.ph/wp-content/uploads/2022/04/Sierra-Madre-Mountain-Range_Landscape-Profile.pdf.
- Coracero, E. E., & Malabrigo Jr, P. L. (2020). Diversity Assessment of Tree Species in Sitio Dicasalarin, Barangay Zabali, Baler, Aurora, Philippines. Open Journal of Ecology, 10(11), 717-728.
- Bambalan, J. M, Palapal, I. K. S., Guleng, R. V., Coracero, E. E., Gallego, R. J., & Suniega, M. J. S. (2022). Tree diversity and carbon stock in North Poblacion and South Poblacion (Dipaculao, Aurora, Philippines). Theoretical and Applied Ecology, 2, 198-208. [CrossRef]
- Balberona, A. N., Noveno, J. J., Angeles, M. G. B., Santos, R. I., Cachin, E. J. D. J., & Cruz, K. G. J. (2018). Ethnomedicinal plants utilized by the ilongot-eǵongot community of Bayanihan, Maria Aurora, Aurora, Philippines. International Journal of Agricultural Technology, 14(2), 145-159.
- Barrogo, K. N., Delos Santos, M. P., Montes, A. A. T., Quiben, A. D., Rotaquio Jr, E. L., & Valete, E. J. P. (2021). Fern and fern allies as non-timber forest product in Baler, Aurora, Philippines. International Journal of Agricultural Technology, 17(2), 423-432.
- Coracero, E. E., Malabrigo, P. J. L., Bambalan, J. M., Palapal, I. K. S., Guleng, R. V., Gallego, R. J., & Suniega, M. J. A. (2022). Diversity, Species Composition, and Carbon Stock Assessment of Trees in Aurora, Philippines: Variations between Preserved and Developed Ecosystems. Environmental Sciences Proceedings, 22(1), 1-5.
- World Weather Online. San Luis Annual Weather Averages [Internet]. 2023. Available online: https://www.worldweatheronline.com/san-luis-weather-averages/aurora/ph.aspx (accessed on 5 July 2023).
- USDA. (2003). Multiparty Monitoring and Assessment Guidelines for Community Based Forest Restoration in Southwestern Ponderosa Pine Forests. US Department of Agriculture, Forest Service, Southwestern Region. 94 pp.
- Coritico, F. P., Lagunday, N. E., Galindon, J. M. M., Tandang, D. N., & Amoroso, V. B. (2020). Diversity of trees and structure of forest habitat types in Mt. Tago Range, Mindanao, Philippines. Philippine Journal of Systematic Biology, 14(3), 1-11.
- Malabrigo, P., Tobias, A., Eduarte, G., Terbio, L., Hernandez, J., & Umali, A. G. (2022). Tree diversity and stand structure of a 2-hectare Permanent Biodiversity Monitoring Area (PBMA) in Mts. Iglit-Baco National Park, Mindoro Island, Philippines. Ecosystems and Development Journal, 12(1), 83-94.
- Pelser, P. B., Barcelona, J. F., & Nickrent, D. L. (2011 onwards). Co’s Digital Flora of the Philippines. URL: http://www. philippineplants. org. Accessed: 1 May, 2023.
- Rojo, J. P. (1999). Revised lexicon of Philippine trees. Forest Products Research and Development Institute, Department of Science and Technology.
- POWO. (2023). Plants of the World Online. https://powo.science.kew.org/.
- IUCN. (2023). The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org.
- DENR. (2017). DAO 2017-11. Department of Environment and Natural Resources, Philippines.
- He, F., & Gaston, K. J. (2000). Estimating species abundance from occurrence. The American Naturalist, 156(5), 553-559.
- Chao, A., & Chiu, C. H. (2016). Species richness: estimation and comparison. Wiley StatsRef: statistics reference online, 1, 26.
- Ismail, M. H., Zaki, P. H., Fuad, M. F. A., & Jemali, N. J. N. (2017). Analysis of importance value index of unlogged and logged peat swamp forest in Nenasi Forest Reserve, Peninsular Malaysia. International Journal of Bonorowo Wetlands, 7(2), 74-78.
- Hernandez, J., Umali, A. G., & Malabrigo, P. (2021). Floristic diversity assessment of Caramoan National Park, Camarines Sur, Philippines. Ecosystems and Development Journal, 11(1 and 2), 73-81.
- Daly, A. J., Baetens, J. M., & De Baets, B. (2018). Ecological diversity: measuring the unmeasurable. Mathematics, 6(7), 119. [CrossRef]
- Pampolina, N. M., Coracero, E. E., Eco, K. O., & Tingson, K. N. (2022). Floristic Composition, Diversity, and Ecology for Conservation of Lower Agno Watershed Forest Reserve, Mountain Province, Philippines. Asian Journal of Biodiversity, 13(1).
- De Villa, K. R., & Lagat, R. D. (2023). Species Diversity and Habitat Association of Ferns and Lycophytes in Mts. Palay-Palay Mataas na Gulod Protected Landscape. In Plant Diversity in Biocultural Landscapes (pp. 135-161). Singapore: Springer Nature Singapore.
- Schober, P., Boer, C., & Schwarte, L. A. (2018). Correlation coefficients: appropriate use and interpretation. Anesthesia & analgesia, 126(5), 1763-1768. [CrossRef]
- Salvaña, F. R. P., Lopez, C. K. C., Mangaoang, C. C., & Bretaña, B. L. P. (2019). Diversity and community structure of trees in two forest types in Mt. Apo Natural Park (MANP), Philippines. Biodiversitas Journal of Biological Diversity, 20(7), 1794-1801. [CrossRef]
- Langenberger, G. (2006). Habitat distribution of dipterocarp species in the Leyte Cordillera: an indicator for species - site suitability in local reforestation programs. Ann. For. Sci., 63, 149-156. [CrossRef]
- Pang, S. E., De Alban, J. D. T., & Webb, E. L. (2021). Effects of climate change and land cover on the distributions of a critical tree family in the Philippines. Scientific Reports, 11(1), 276. [CrossRef]
- Volis, S., Belolipov, I. V., Asatulloev, T., & Turgunov, M. (2023). Role of endemism and other factors in determining the introduction success of rare and threatened species in Tashkent Botanical Garden. Journal of Zoological and Botanical Gardens, 4(2), 325-334. [CrossRef]
- Manchester, S. J., & Bullock, J. M. (2000). The impacts of non-native species on UK biodiversity and the effectiveness of control. Journal of Applied Ecology, 37(5), 845-864. [CrossRef]
- Coracero, E. E. (2023). Distribution and Management of the Invasive Swietenia macrophylla King (Meliaceae) at the Foot of a Protected Area in Luzon Island, Philippines. Journal of Zoological and Botanical Gardens, 4(3), 637-647. [CrossRef]
- Batani, R. S., Basbas Jr, A. V., Loncio, R. S., & Napaldet, J. T. (2023). Floral diversity in a secondary forest managed by indigenous community: the case of Mt. Kili-kili in Benguet, Cordillera Central Range, Northern Philippines. Biodiversity, 1-19.
- Lleno, J. V., Ligalig, R. J., Sarmiento, R. T., & Along, A. A. (2023). Tree diversity, composition, and stand structure of lowland tropical forest in Prosperidad, Agusan del Sur, Philippines. Journal of Survey in Fisheries Sciences, 10(1S), 4810-4830.
- Mancera, J. P., Ragragio, E. M., Su, G. L. S., & Rubite, R. R. (2013). Plant community structure of a secondary forest at Barangay Camias, Porac, Pampanga, The Philippines. Philippine Journal of Science, 142(3), 135-143. [CrossRef]
- Carig, E. T., & Manuel, R. P. (2021). Tree Diversity and Timber Resources Assessment in Secondary Forests of Quirino Forest Landscape Project, Philippines. Asian Journal of Biodiversity, 12(1), 36-54.
- Castillo, M. L., Castillo, L. A., Canceran, M. S., Gonzalvo, K. J. P., Barua, L. D., Alegre, A. C., Barredo-Parducho, V. O., Gestiada, E., Breva, R., & Bantayan, N. C. (2018). Distribution, diversity and spatial analysis of tree species in a long-term ecological research plot in Molawin-Dampalit Watershed, Mount Makiling Forest Reserve. Asian Journal of Biodiversity, 9(1), 12-36. [CrossRef]
- DeJong, T. M. (1975). A comparison of three diversity indices based on their components of richness and evenness. Oikos, 26(2), 222-227. [CrossRef]
- Pu, R. (2021). Mapping tree species using advanced remote sensing technologies: a state-of-the-art review and perspective. Journal of remote sensing. [CrossRef]
- Engay-Gutierrez, K., Dimailig, E., & Yacon, J. (2022). Plus and Mother Trees in Mt. Banahaw de Lucban, Quezon, Philippines. Journal of Environmental Science and Management, 25(2).
- Harris, G. M., Jenkins, C. N., & Pimm, S. L. (2005). Refining biodiversity conservation priorities. Conservation Biology, 19(6), 1957-1968. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).