Submitted:
29 January 2024
Posted:
30 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
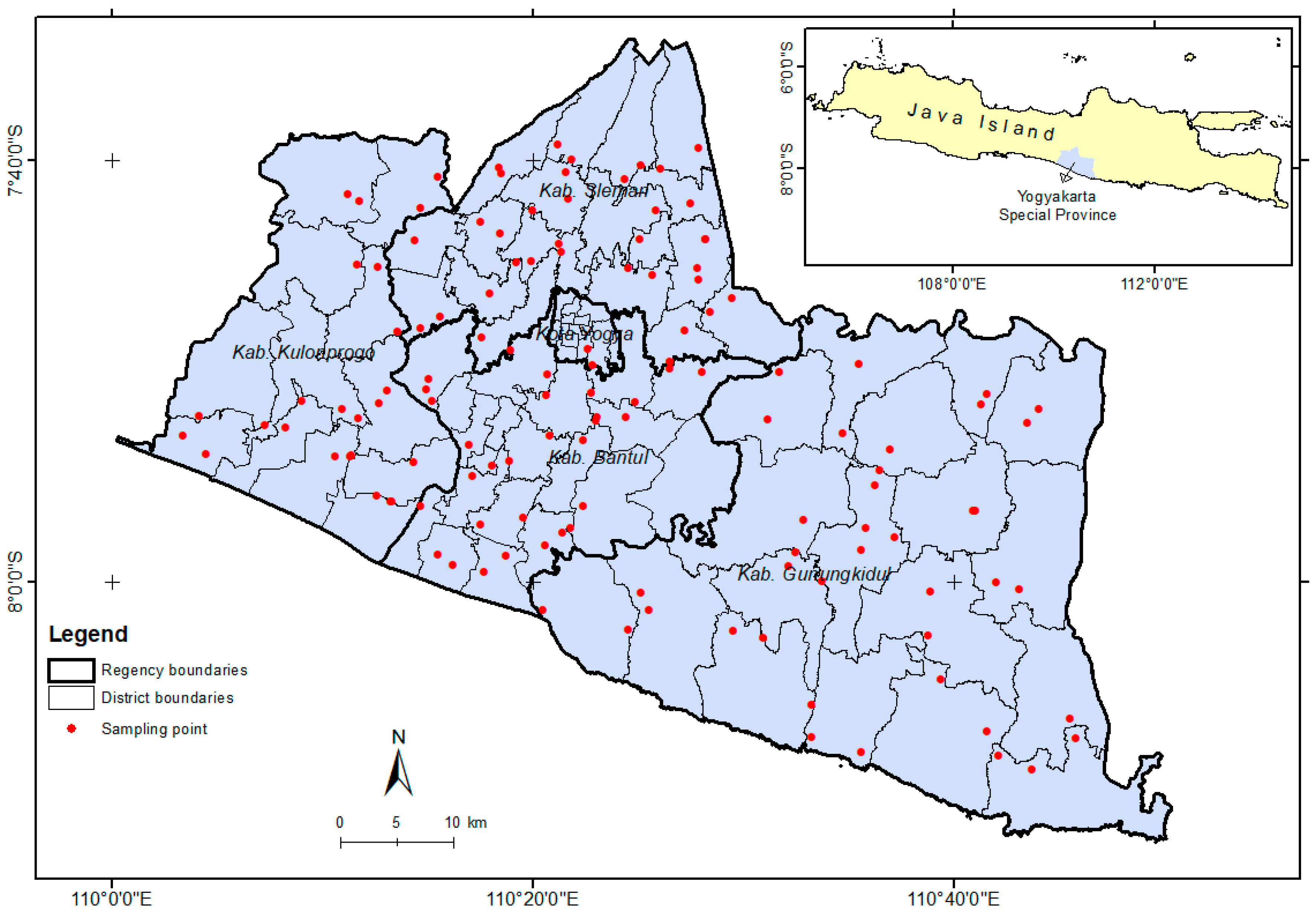
2.1. Sampling Location Determination
2.2. Sampling
2.3. Parasitoid Identification
2.4. Data Analysis
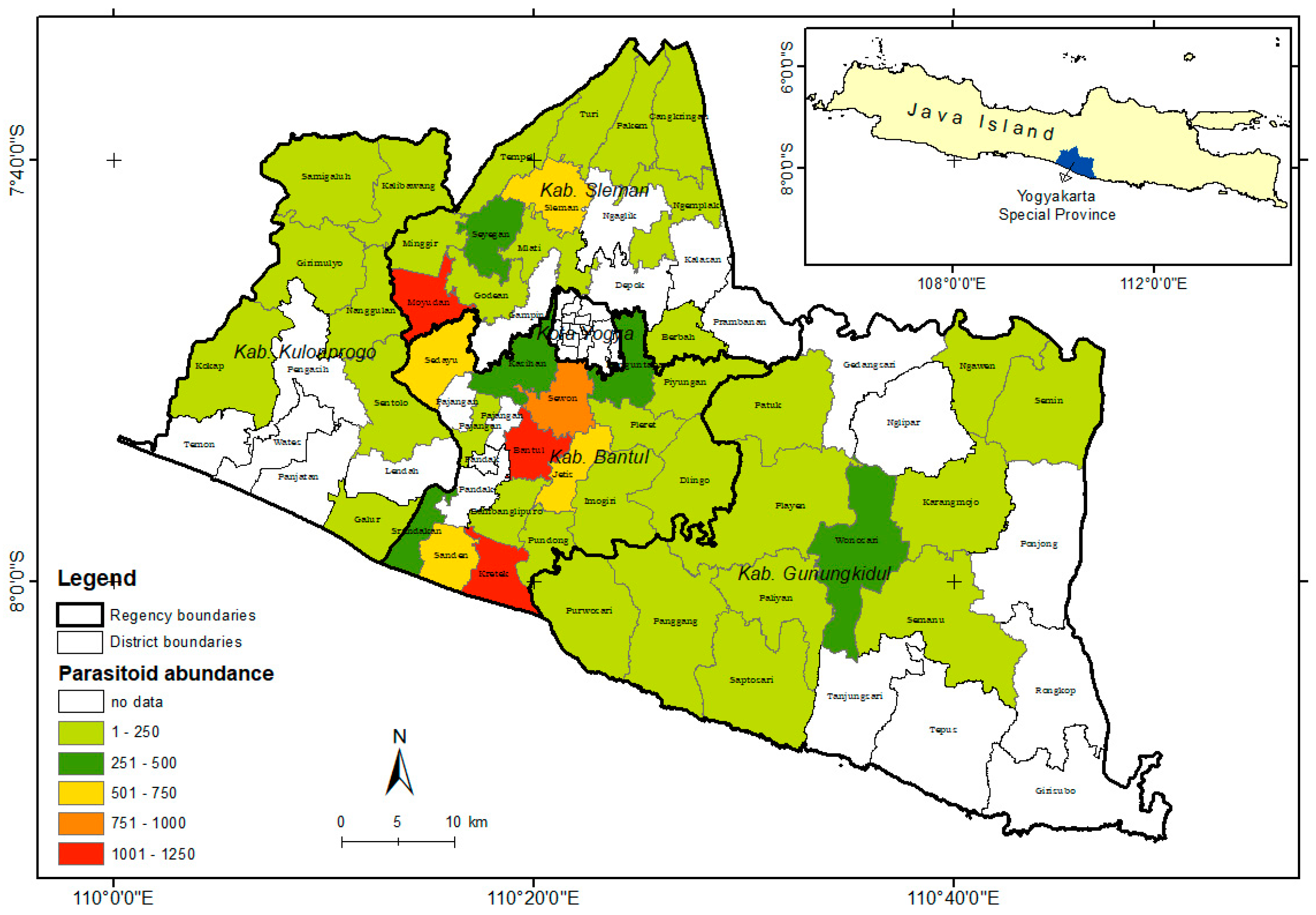
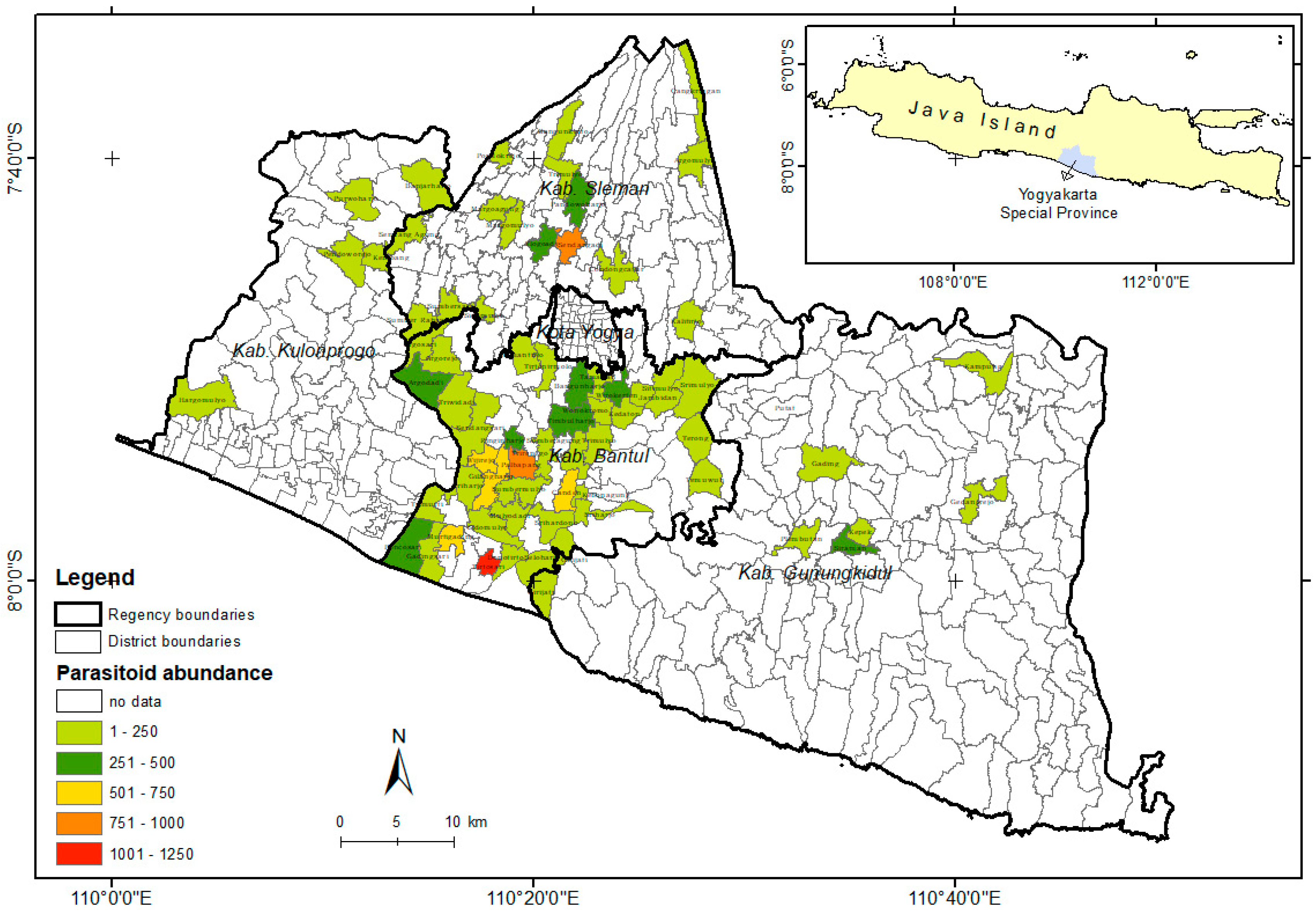
3. Results
| District | Number of S. frugiperda (Mean ± S.E.) |
Parasitism rate (%) (Mean ± S.E.) |
N |
| Bantul | 1638.60 ± 364.99 b | 22.95 ± 5.18 ab | 17 |
| Gunung Kidul | 649.27 ± 281.13 a | 13.54 ± 6.69 a | 10 |
| Kulonprogo | 108.28 ± 41.57 ab | 54.68 ± 16.46 b | 7 |
| Sleman | 140.11 ± 27.55 ab | 23.46 ± 7.45 ab | 12 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, M.P.; Clusella-Trullas, S.; Terblanche, J.S.; Richardson, D.M. Drivers, impacts, mechanisms and adaptation in insect invasions. Biological Invasion 2016, 18, 883–891. [Google Scholar] [CrossRef]
- Rizali, A.; Oktaviyani, O.; Putri, S.; Doananda, M.; Linggani, A. Invasion of fall armyworm Spodoptera frugiperda, a new invasive pest, alters native herbivore attack intensity and natural enemy diversity. Biodiversitas Journal of Biological Diversity 2021, 22, 3482–3488. [Google Scholar] [CrossRef]
- Nonci, N.; Kalqutny, S.H.; Muis, A.; Azrai, M.; Aqil, M. Pengenalan Fall Armyworm (Spodoptera frugiperda JE Smith) Hama Baru pada Tanaman Jagung di Indonesia (in Indonesian); Balai Penelitian Tanaman Serealia: 2019.
- Girsang, S.S.; Nurzannah, S.E.; Girsang, M.A.; Effendi, R. The distribution and impact of fall army worm (Spodoptera frugiperda) on maize production in North Sumatera. In Proceedings of the International Conference on Sustainable Cereals and Crops Production Systems in the Tropics Makassar, Indonesia, 2020; p. 012099. [CrossRef]
- Maharani, Y.; Dewi, V.K.; Puspasari, L.T.; Rizkie, L.; Hidayat, Y.; Dono, D. Cases of fall army worm Spodoptera frugiperda JE Smith (Lepidoptera: Noctuidae) attack on maize in Bandung, Garut and Sumedang district, West Java. CROPSAVER-Journal of Plant Protection 2019, 2, 38–46. [Google Scholar] [CrossRef]
- Subiono, T. Preferences of Spodoptera frugiperda (Lepidoptera: Noctuidae) in several feed sources (in Indonesian). Jurnal Agroekoteknologi Tropika Lembab 2020, 2, 130–134. [Google Scholar] [CrossRef]
- Noer, H. Population and attack rate Spodoptera frugiperda on corn plants in Tulo Village, Sigi Regency (in Indonesian). Jurnal Agrotech 2020, 10, 66–68. [Google Scholar] [CrossRef]
- Kumela, T.; Simiyu, J.; Sisay, B.; Likhayo, P.; Mendesil, E.; Gohole, L.; Tefera, T. Farmers’ knowledge, perceptions, and management practices of the new invasive pest, fall armyworm (Spodoptera frugiperda) in Ethiopia and Kenya. International Journal of Pest Management 2018, 65, 1–9. [Google Scholar] [CrossRef]
- Baudron, F.; Zaman-Allah, M.A.; Chaipa, I.; Chari, N.; Chinwada, P. Understanding the factors influencing fall armyworm (Spodoptera frugiperda JE Smith) damage in African smallholder maize fields and quantifying its impact on yield. A case study in Eastern Zimbabwe. Crop Protection 2019, 120, 141–150. [Google Scholar] [CrossRef]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J. Fall armyworm: impacts and implications for Africa. Outlooks on Pest Management 2017, 28, 196–201. [Google Scholar] [CrossRef]
- Lubis, A.A.N.; Anwar, R.; Soekarno, B.P.; Istiaji, B.; Dewi, S.; Herawati, D. Coray wood corn (Spodoptera frugiperda) caterpillars in corn crops in Petir Village, Daramaga Sub-District, Bogor District, and its control potential using Metarizhium rileyi (in Indonesian). Jurnal Pusat Inovasi Masyarakat 2020, 2, 931–939. [Google Scholar]
- Song, Y.; Yang, X.; Li, H.; Wu, K. The invasive Spodoptera frugiperda (JE Smith) has displaced Ostrinia furnacalis (Guenée) as the dominant maize pest in the border area of southwestern China. Pest Management Science 2023, 79, 3354–3363. [Google Scholar] [CrossRef] [PubMed]
- Kenis, M. Prospects for classical biological control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in invaded areas using parasitoids from the Americas. Journal of Economic Entomology 2023, 116, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.E. Natural Enemies: An Introduction to Biological Control; Cambridge University Press: 2012. [CrossRef]
- Caltagirone, L.E.; Doutt, R.L. The history of the vedalia beetle importation to California and its impact on the development of biological control. Annual Review of Entomology 1989, 34, 1–16. [Google Scholar] [CrossRef]
- Winston, R.; Schwarzländer, M.; Hinz, H.L.; Day, M.D.; Cock, M.J.; Julien, M. Biological control of weeds: A world catalogue of agents and their target weeds; USDA Forest Service, Forest Health Technology Enterprise Team: 2014.
- USAID. Environmental Assessment for Implementation of Biological Control for The Leucaena Psyllid in Asia and Africa: Case Studies from India, Indonesia, Laos, Malaysia, Nepal, the Philippines and Thailand; Center for the Environment: Washington DC, 1995. [Google Scholar]
- Wyckhuys, K.A.G.; Rauf, A.; Ketelaar, J. Parasitoids introduced into Indonesia: part of a region-wide campaign to tackle emerging cassava pests and diseases. Biocontrol News and Information 2014, 35, 35N–37N. [Google Scholar]
- Tjitrosemito, S. The establishment of Procecidochares connexa in West Java, Indonesia: a biological control agent of Chromolaena odorata. Biotropia - The Southeast Asian Journal of Tropical Biology 1999, 12, 19–24. [Google Scholar] [CrossRef]
- Buchori, D.; Rizali, A.; Lukvitasari, L.; Triwidodo, H. Insect communities associated with siam weed: evaluation after three decades of Cecidochares connexa release as biocontrol agent. Diversity 2020, 12, 344. [Google Scholar] [CrossRef]
- Lukvitasari, L.; Triwidodo, H.; Rizali, A.; Buchori, D. Effects of locations on gall fly Cecidochares connexa (Macquart) attacks on invasive exotic plant Chromolaena odorata (L.) King & Robinson and its interaction with local insect communities (in Indonesian). Jurnal Entomologi Indonesia 2021, 18, 127–139. [Google Scholar] [CrossRef]
- McFadyen, R.E.C.; Desmier de Chenon, R.; Sipayung, A. Biology and host specificity of the Chromolaena stem gall fly, Cecidochares connexa (Macquart)(Diptera: Tephritidae). Australian Journal of Entomology 2003, 42, 294–297. [Google Scholar] [CrossRef]
- Safi’i, I. Parasitisasi pada Cecidochares connexa di beberapa tempat di Jawa Barat. Undergraduate Thesis, IPB University, Bogor, 2006. [Google Scholar]
- Rauf, A. Liriomyza: hama pendatang baru di Indonesia (in Indonesia). Buletin HPT 1995, 8, 46–48. [Google Scholar]
- Rauf, A.; Shepard, B.M.; Johnson, M.W. Leafminers in vegetables, ornamental plants and weeds in Indonesia: surveys of host crops, species composition and parasitoids. International Journal of Pest Management 2000, 46, 257–266. [Google Scholar] [CrossRef]
- Tapahillah, T. Survei lalat pengorok daun Liriomyza spp. (Diptera: Agromyzidae) dan parasitoidnya pada berbagai tumbuhan inang dan ketinggian tempat di Jawa Barat. Undergraduate Thesis, IPB University, Bogor, 2002. [Google Scholar]
- Hokkanen, H.M.; Pimentel, D. New associations in biological control: theory and practice. The Canadian Entomologist 1989, 121, 829–840. [Google Scholar] [CrossRef]
- Trisyono, Y.A.; Suputa, S.; Aryuwandari, V.E.F.; Hartaman, M.; Jumari, J. Occurrence of heavy infestation by the fall armyworm Spodoptera frugiperda, a new alien invasive pest, in corn Lampung Indonesia. Jurnal Perlindungan Tanaman Indonesia 2019, 23, 156–160. [Google Scholar] [CrossRef]
- Megasari, D.; Putra, I.L.I.; Martina, N.D.; Wulanda, A.; Khotimah, K. Biology of Spodoptera frugiperda JE Smith on some types of feed in the laboratory (in Indonesian). Agrovigor: Jurnal Agroekoteknologi 2022, 15, 63–67. [Google Scholar] [CrossRef]
- Putra, I.L.I.; Martina, N.D. Life cycle of Spodoptera frugiperda with feeding kale and leeks in laboratory (in Indonesian). Jurnal Ilmu Pertanian Indonesia 2021, 26, 386–391. [Google Scholar] [CrossRef]
- Nurkomar, I.; Trisnawati, D.W.; Fahmi, F.; Buchori, D. Survival, development, and fecundity of Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) on various host plant species and their implication for the pest management. Insects 2023, 14, 629. [Google Scholar] [CrossRef]
- Sari, A.; Buchori, D.; Nurkomar, I. The potential of Telenomus remus Nixon (Hymenoptera: Scelinoidae) as biocontrol agent for the new fall armyworm S. frugiperda (Lepidoptera: Noctuidae) in Indonesia. Planta Tropika 2020, 8, 69–74. [Google Scholar] [CrossRef]
- Putra, I.L.I.; Wati, C.D.N.S. Parasicity level of Telenomus sp. parasitoid against Spodoptera frugiperda JE Smith eggs in the laboratory. Journal of Natural Sciences and Mathematics Research 2020, 6, 73–77. [Google Scholar] [CrossRef]
- Polem, E.S.N. Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae): Serangan dan Parasitoidnya di Nagan Raya, Aceh (in Indonesian). Undergraduate Thesis, IPB University, Bogor, 2021. [Google Scholar]
- Rongkok, H.T.; Pasaru, F. Identification of paracitoids of Spodoptera frugiperda (Lepidoptera: Noctuidae) and its paracity levels in farmer-owned corn plantation in District Sigi and in District Donggala (in indonesian). Jurnal Agrotekbis 2021, 9, 972–978. [Google Scholar]
- Minarni, E.W. Eksplorasi musuh alami hama Spodoptera frugiperda pada pertanaman jagung di lahan kelompok tani raden Kelurahan Pabuwaran Kecamatan Purwokerto Utara Kabupaten Banyumas (in Indonesia). In Proceedings of the Prosiding Seminar Nasional LPPM Unsoed; 2023; pp. 139–147. [Google Scholar]
- Sari, W.; Nelly, N. Natural enemies of Spodoptera frugiperda JE Smith (Lepidoptera: Noctuidae) on corn plants in West Sumatera. In Proceedings of the 2nd Agrifood System International Conference Padang, Indonesia; 2023; p. 012045. [Google Scholar] [CrossRef]
- Supeno, B.; Tarmizi, T.; Meidiwarman, M.; Haryanto, H. Keragaman parasitoid yang berasosiasi dengan telur hama baru Spodoptera frugiperda di Pulau Lombok (in Indonesian). Prosiding SAINTEK 2021, 3, 418–423. [Google Scholar]
- Suroto, A.; Soesanto, L.; Bahrudin, M. Attack rate and natural enemies of Spodoptera frugiperda JE Smith on corn plants in five districts in Banyumas regency (in Indonesian). Proceedings Series on Physical & Formal Sciences 2021, 2, 44–49. [Google Scholar] [CrossRef]
- Tarigan, S.; Maryana, N.; Mubin, N. Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae): Attacks and their natural enemies on corn plantations in Munte Village, Munte Sub-district, Karo District, North Sumatera. In Proceedings of the International Conference on Modern and Sustainable Agriculture 2022; 2023; p. 012030. [Google Scholar] [CrossRef]
- Tawakkal, M.; Buchori, D.; Maryana, N. New association between Spodoptera frugiperda JE Smith (Lepidoptera: Noctuidae) and native natural enemies: Bioprospection of native natural enemies as biological control agents. In Proceedings of the IOP Conference Series: Earth and Environmental Science; 2021; p. 012030. [Google Scholar] [CrossRef]
- Wahyuningsih, R.D.; Harjaka, T.; Suputa, S.; Trisyono, Y.A. Parasitization levels of Spodoptera frugiperda eggs (Smith)(Lepidoptera: Noctuidae) in three different corn ecosystems in East Java. Jurnal Perlindungan Tanaman Indonesia 2022, 26, 28–39. [Google Scholar] [CrossRef]
- Waruwu, A.; Tobing, M.C.; Siregar, A.Z. Ekplorasi parasitoid telur Spodoptera frugiperda JE Smith (Lepidoptera: Noctuidae) pada tanaman jagung di Desa Purwobinangun Kec. Sei Bingai, Kab. Langkat (in Indonesian). Jurnal Online Agroteknologi 2023, 11, 10–18. [Google Scholar] [CrossRef]
- Nurkomar, I.; Putra, I.; Trisnawati, D.; Saman, M.; Pangestu, R.; Triyono, A. The existence and population dynamic of new fall armyworm species Spodoptera frugiperda JE Smith (Lepidoptera: Noctuidae) in Yogyakarta, Indonesia. In Proceedings of the The 3rd International COnference on Sustainable Agriculture, Yogyakarta, Indonesia, 2021; p. 012023. [CrossRef]
- Agboyi, L.K.; Mensah, S.A.; Clottey, V.A.; Beseh, P.; Glikpo, R.; Rwomushana, I.; Day, R.; Kenis, M. Evidence of leaf consumption rate decrease in fall armyworm, Spodoptera frugiperda, larvae parasitized by Coccygidium luteum. Insects 2019, 10, 410. [Google Scholar] [CrossRef]
- Shylesha, A.; Jalali, S.; Gupta, A.; Varshney, R.; Venkatesan, T.; Shetty, P.; Ojha, R.; Ganiger, P.C.; Navik, O.; Subaharan, K. Studies on new invasive pest Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae) and its natural enemies. Journal of Biological control 2018, 32, 1–7. [Google Scholar] [CrossRef]
- Wei, Y.-W.; Zhou, Y.-B.; Zou, Q.-C.; Sheng, M.-L. A new species of Campoletis Förster (Hymenoptera, Ichneumonidae) with a key to species known from China, Japan and South Korea. ZooKeys 2020, 1004, 99–108. [Google Scholar] [CrossRef]
- RCoreTeam. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, 2015. [Google Scholar]
- DinasPerindustrianPerdagangandanKoperasiKabupatenBantul. Prospektus Investasi Sektor Pangan Kabupaten Bantul (in Indonesian). Available online: https://dpmptsp.bantulkab.go.id/web/potensi_investasi/detail/19-sektor-pangan (accessed on 12 September).
- Sunari, A.A.A.A.S.; Sumiartha, I.K.; Supartha, I.W.; Mahaputra, I.F.; Yudha, I.K.W.; Utama, I.W.E.K.; Wiradana, P.A. Potential yield reduction of sweet and glutinous corn varieties damaged by the invasive pest Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae): Field trial scale. In Proceedings of the 2nd International Conference on Education and Technology (ICETECH 2021); 2022; pp. 14–19. [Google Scholar] [CrossRef]
- Kishinevsky, M.; Keasar, T.; Bar-Massada, A. Parasitoid abundance on plants: Effects of host abundance, plant species, and plant flowering state. Arthropod-Plant Interactions 2017, 11, 155–161. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P. Elton, CS 1958: The ecology of invasions by animals and plants. London: Methuen. Progress in Physical Geography 2007, 31, 659–666. [Google Scholar] [CrossRef]
- Jindal, J.; Sharma, K.P.; Shera, P.S.; Cheema, H.K. Native Parasitoids of Fall Army Worm Spodoptera frugiperda (J E Smith) in Maize. Indian Journal of Entomology 2022, 84, 865–867. [Google Scholar] [CrossRef]
- Pu’u, Y.M.; Mutiara, C. Attacks of invasive pest Spodoptera frugiperda (J. E. Smith) (Lepidoptera:Noctuidae) on corn plantation in Ende District Flores, Indonesia [in Indonesian]. Jurnal Entomologi Indonesia 2021, 18, 153–153. [Google Scholar] [CrossRef]
- Tawakkal, M.; Buchori, D.; Maryana, N. New association between Spodoptera frugiperda JE Smith (Lepidoptera: Noctuidae) and native natural enemies: Bioprospection of native natural enemies as biological control agents. In Proceedings of the 2nd International Symposium on Transdisciplinarity Approach for Knowledge Co-Creation in Sustainability - Understanding Complexity and Transdisciplinarity for Environmental Sustainability Bogor, Indonesia; 2021; p. 012030. [Google Scholar] [CrossRef]
- Salazar-Mendoza, P.; Peralta-Aragón, I.; Romero-Rivas, L.; Salamanca, J.; Rodriguez-Saona, C. The abundance and diversity of fruit flies and their parasitoids change with elevation in guava orchards in a tropical Andean forest of Peru, independent of seasonality. Plos one 2021, 16, e0250731. [Google Scholar] [CrossRef]
- Syahidah, T.; Prasetyo, L.; Buchori, D. Landscape structure and parasitoid diversity as measures of sustainable landscape. In Proceedings of the IOP Conference Series: Earth and Environmental Science; 2019; p. 012012. [Google Scholar] [CrossRef]
- Schoeninger, K.; Souza, J.L.; Krug, C.; Oliveira, M.L. Diversity of parasitoid wasps in conventional and organic guarana (Paullinia cupana var. sorbilis) cultivation areas in the Brazilian Amazon. Acta Amazonica 2019, 49, 283–293. [Google Scholar] [CrossRef]
- Abang, A.; Nanga, S.; Fotso Kuate, A.; Kouebou, C.; Suh, C.; Masso, C.; Saethre, M.; Fiaboe, K. Natural Enemies of Fall Armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) in Different Agro-Ecologies. Insects 2021, 12, 509. Insect 2021, 12, 509. [Google Scholar] [CrossRef]
- Sharanabasappa, S.; Kalleshwaraswamy, C.; Poorani, J.; Maruthi, M.; Pavithra, H.; Diraviam, J. Natural enemies of Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), a recent invasive pest on maize in South India. The Florida Entomologist 2019, 102, 619–623. [Google Scholar] [CrossRef]
- Kenis, M.; Du Plessis, H.; Van den Berg, J.; Ba, M.N.; Goergen, G.; Kwadjo, K.E.; Baoua, I.; Tefera, T.; Buddie, A.; Cafà, G. Telenomus remus, a candidate parasitoid for the biological control of Spodoptera frugiperda in Africa, is already present on the continent. Insects 2019, 10, 92. [Google Scholar] [CrossRef]
- Liao, Y.-L.; Yang, B.; Xu, M.-F.; Lin, W.; Wang, D.-S.; Chen, K.-W.; Chen, H.-Y. First report of Telenomus remus parasitizing Spodoptera frugiperda and its field parasitism in southern China. Journal of Hymenoptera Research 2019, 73, 95–102. [Google Scholar] [CrossRef]
- Sari, A.; Nurkomar, I.; Buchori, D. Comparison of Spodoptera frugiperda parasitoid performance under laboratory conditions. In Proceedings of the 3rd International Symposium on Transdisciplinary Approach for Knowledge Co-Creation in Sustainability, Bogor, Indonesia, 2023; p. 012031. [CrossRef]
- Junaedi, E.; Yunus, M.; Hasriyanty, H. Parasitoids and it is parasitisme on white rice stem borer (Scirpophaga innotata Walker) in two different altitudes of rice fields (Oryza sativa l.) in District of Sigi (in Indonesian). e-Journal Agrotekbis 2016, 4, 280–287. [Google Scholar]
- Legault, S.; Hébert, C.; Blais, J.; Berthiaume, R.; Bauce, E.; Brodeur, J. Seasonal ecology and thermal constraints of Telenomus spp.(Hymenoptera: Scelionidae), egg parasitoids of the hemlock looper (Lepidoptera: Geometridae). Environmental Entomology 2012, 41, 1290–1301. [Google Scholar] [CrossRef]
- Goulart, M.M.P.; Bueno, A.d.F.; Bueno, R.C.O.d.F.; Diniz, A.F. Host preference of the egg parasitoids Telenomus remus and Trichogramma pretiosum in laboratory. Revista Brasileira de Entomologia 2011, 55, 129–133. [Google Scholar] [CrossRef]
- Hernandez, D.; Ferrer, F.; Linares, B. Introducción de Telenomus remus Nixon (Hym.: Scelionidae) para controlar Spodoptera frugiperda (Lep.: Noctuidae) en Yaritagua-Venezuela. Agronomía Tropical 1989, 39, 45–61. [Google Scholar]
- Cave, R.D. Biology, ecology and use in pest management of Telenomus remus. Biocontrol News and Information 2000, 21, 21N–26N. [Google Scholar]
- Colmenarez, Y.C.; Babendreier, D.; Ferrer Wurst, F.R.; Vásquez-Freytez, C.L.; de Freitas Bueno, A. The use of Telenomus remus (Nixon, 1937)(Hymenoptera: Scelionidae) in the management of Spodoptera spp.: potential, challenges and major benefits. CABI Agriculture and Bioscience 2022, 3, 1–13. [Google Scholar] [CrossRef]
- Copeland, R. Fall armyworm natural enemy of the day: Telenomus remus (Hymenoptera: Scelionidae). Available online: http://www.icipe.org/content/fall-armyworm-natural-enemy-day-telenomus-remus (accessed on 12 September).
- Chandler, A.C.; Read, C. Introduction to Parasitology; John Willey and Sons: New York, USA, 1961. [Google Scholar]
- Pimentel, D. Introducing parasites and predators to control native pests. The Canadian Entomologist 1963, 95, 785–792. [Google Scholar] [CrossRef]
- Polaszek, A.; Kimani, S.W. Telenomus species (Hymenopetra: Scelionidae) attacking eggs of pyralid pests (Lepidoptera) in Africa: A review and guide to identification. Bulletin of Entomological Research 1990, 80, 57–71. [Google Scholar] [CrossRef]
- Kalyanasundaram, M.; Kamala, I.M. Parasitoids. In Ecofriendly Pest Management for Food Security; 2016; pp. 109-138. [CrossRef]
- Buhl, P.N. Species of Platygastrinae and Sceliotrachelinae from rainforest canopies in Tanzania, with keys to the Afrotropical species of Amblyaspis, Inostemma, Leptacis, Platygaster and Synopeas (Hymenoptera, Platygastridae). Tijdschrift voor Entomologie 2011, 154, 75–126. [Google Scholar] [CrossRef]
- Buhl, P.N. Key to Platygaster (Hymenoptera: Platygastridae) from Denmark, with descriptions of new species. Steenstrupia 2006, 29, 127–167. [Google Scholar]
- McCutcheon, G.S.; Salley, W.; Turnipseed, S. Biology of Apanteles ruficrus, an imported parasitoid of Pseudoplusia includens, Trichoplusia ni, and Spodoptera frugiperda (Lepidoptera: Noctuidae). Environmental Entomology 1983, 12, 1055–1058. [Google Scholar] [CrossRef]
- Rajapakse, R.H.; Ashley, T.R.; Waddill, V.H. Biology and host acceptance of Microplitis manilae (Hymenoptera: Braconidae) raised on fall armyworm larvae Spodoptera frugiperda (Lepidoptera: Noctuidae). Florida Entomologist 1985, 68, 653–657. [Google Scholar] [CrossRef]
- Alam, M. Attempts at the biological control of major insect pests of maize in Barbados, WI. Proceeding of the Caribbean Food Crops Society 1978, 15, 127–135. [Google Scholar] [CrossRef]
- Whitfield, J.B.; Austin, A.D.; Fernandez-Triana, J.L. Systematics, biology, and evolution of microgastrine parasitoid wasps. Annual Review of Entomology 2018, 63, 389–406. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Meagher, R.L. The Spodoptera frugiperda host strains: What they are and why they matter for understanding and controlling this global agricultural pest. Journal of Economic Entomology 2022, 115, 1729–1743. [Google Scholar] [CrossRef]
- Gross, H.R. Little-known fly-promising biocontrol weapon. Agricultural Research 1985, 33, 12. [Google Scholar]
- Deshmukh, S.S.; Kiran, S.; Naskar, A.; Pradeep, P.; Kalleshwaraswamy, C.; Sharath, K. First record of a parasitoid, Megaselia (M) scalaris (Diptera: Phoridae) of fall armyworm, Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae) from India. Egyptian Journal of Biological Pest Control 2021, 31, 94. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Q.; Xiang, L.; Gu, R.; Wu, Y.; Zhang, Y.; Bai, X.; Niu, X.; Li, T.; Wei, J. First report on Megaselia scalaris Loew (Diptera: Phoridae) infestation of the invasive pest Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in China. Insects 2021, 12, 65. [Google Scholar] [CrossRef]
- Ruíz-Nájera, R.E.; Molina-Ochoa, J.; Carpenter, J.E.; Espinosa-Moreno, J.A.; Ruíz-Nájera, J.A.; Lezama-Gutierrez, R.; Foster, J.E. Survey for hymenopteran and dipteran parasitoids of the fall armyworm (Lepidoptera: Noctuidae) in Chiapas, Mexico. Journal of Agricultural and Urban Entomology 2007, 24, 35–42. [Google Scholar] [CrossRef]
- Disney, R.H.L. Natural history of the scuttle fly, Megaselia scalaris. Annuual Review of Entomology 2008, 53, 39–60. [Google Scholar] [CrossRef]
- Daif, M.K.; Mosallam, A.; Ebrahim, A. The scuttle fly, Megaselia scalaris (Loew, 1866)(Diptera: Phoridae): A new threat on laboratory aass production of fruit flies. Journal of Plant Protection and Pathology 2023, 14, 109–113. [Google Scholar] [CrossRef]
- Rajan, R.; Shamsudeen, R. First record of Megaselia scalaris (Diptera: Phoridae) as a parasitoid of Thyas coronata (Lepidoptera: Erebidae). Indian Journal of Entomology 2023, 1–3. [Google Scholar] [CrossRef]
- Acevedo-Alcalá, A.; Lomeli-Flores, J.R.; Rodríguez-Leyva, E.; Rodríguez-Rodríguez, S.E.; Ortiz-Andrade, E. Does Megaselia scalaris (Diptera: Phoridae) have potential as a biological control agent of fall armyworm? Florida Entomologist 2023, 106, 56–58. [Google Scholar] [CrossRef]
- Wibowo, L. The abundance and diversity of the parasitoid of banana leaf skipper pest (Erionota thrax L.) in South Lampung Regency (in Indonesian). Jurnal Hama dan Penyakit Tumbuhan Tropika 2015, 15, 26–32. [Google Scholar] [CrossRef]
- Fawzi, F.S.; Iman, I.a.-S.; Adil, A.H.M., Abd al-Salam. Survey and distribution density of genus Brachymeria species (Hymenoptera: Chalcididae) in Egypt. Egyptian Journal of Plant Protection Research Institute 2020, 3, 415-432.
- Agboyi, L.K.; Goergen, G.; Beseh, P.; Mensah, S.A.; Clottey, V.A.; Glikpo, R.; Buddie, A.; Cafà, G.; Offord, L.; Day, R. Parasitoid complex of fall armyworm, Spodoptera frugiperda, in Ghana and Benin. Insects 2020, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Buchori, D.; Herawati, E.; Sari, A. The effectiveness of Telenomus remus (Nixon)(Hymenoptera: Scelionidae) for controlling welsh onion pest Spodoptera exigua Hübner (Lepidoptera: Noctuidae) (in indonesian). Jurnal Entomologi Indonesia 2008, 5, 81–95. [Google Scholar] [CrossRef]
- Ratna, E.S. Efficiency of parasitization on larval host, Spodoptera litura (F.), by an endoparasitoid Snellenius (=Microplitis) manilae Ashmead in the laboratory (in Indonesian). Jurnal Hama dan Penyakit Tumbuhan Tropika 2008, 8, 8–16. [Google Scholar] [CrossRef]
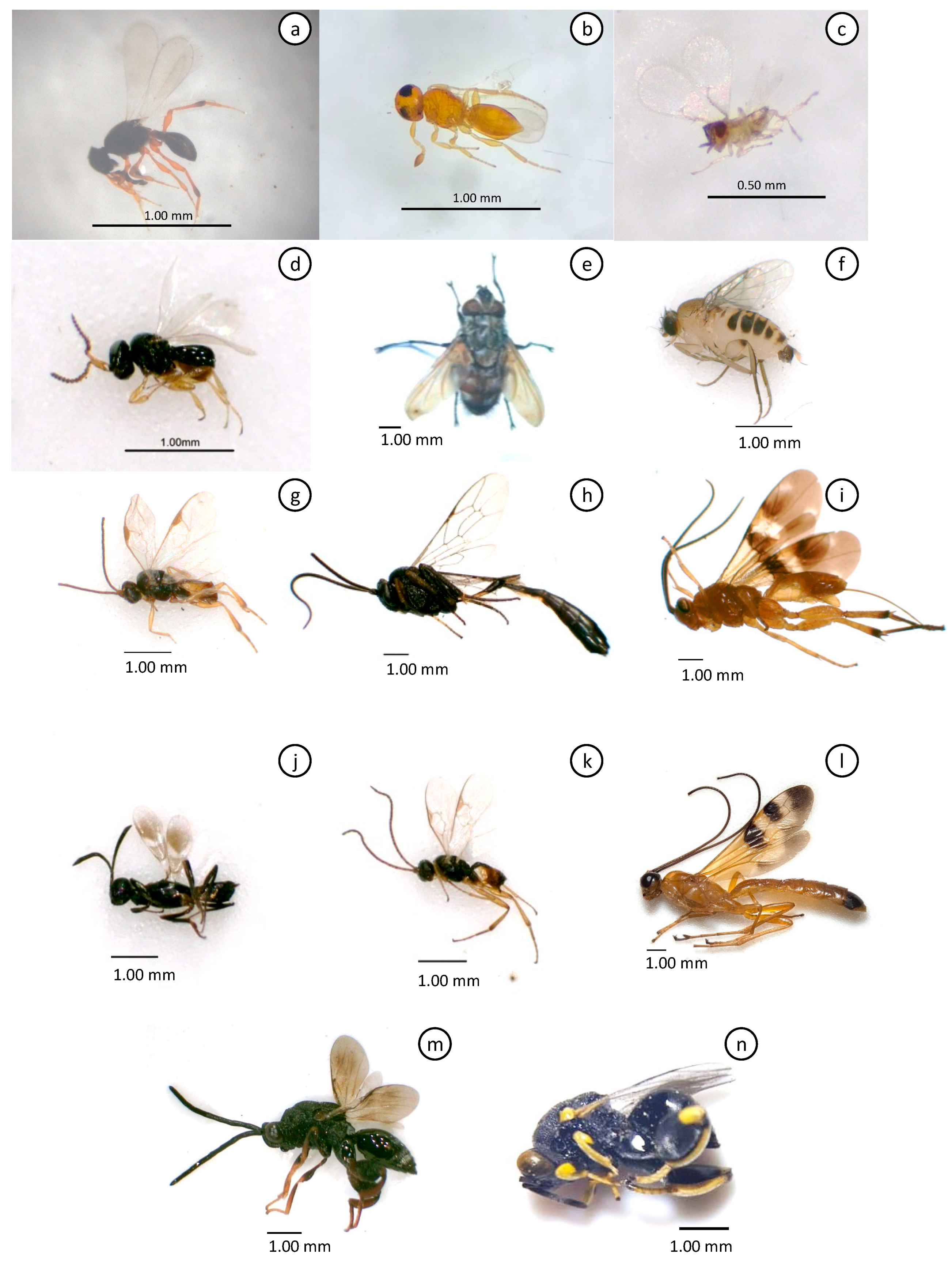
| Order | Family | Species | Parasitism rate (Mean, %) | |||
| Bantul | Gunung Kidul |
Kulon progo |
Sleman | |||
| Egg parasitoid | ||||||
| Hymenoptera | Platygasteridae | Platygasteridae.sp01 + | 1.46 | |||
| Platygasteridae | Platygasteridae.sp02 + | 42.00 | ||||
| Platygasteridae | Telenomus remus• | 37.29 | 14.74 | 71.97 | 33.23 | |
| Trichogrammatidae | Trichogramma sp. | 5.78 | ||||
| Larval parasitoid | ||||||
| Hymenoptera | Braconidae | Cotesia sp. • | 11.89 | 16.67 | 5.02 | |
| Ichneumonidae | Campoletis sp. • | 1.61 | ||||
| Braconidae | Coccygidium sp. * | 5.56 | ||||
| Eupelmidae | Eupelmidae.sp01 + | 6.25 | ||||
| Braconidae | Microplitis sp. • | 39.70 | 61.29 | |||
| Braconidae | Stenobracon sp. * | 11.54 | ||||
| Diptera | Tachinidae | Archytas marmoratus | 4.03 | |||
| Phoridae | Megaselia scalaris | 3.70 | ||||
| Pupal parasitoid | ||||||
| Hymenoptera | Chalcididae | Brachymeria femorata• | 6.25 | |||
| Chalcididae | Brachymeria lasusi• | 6.82 | ||||
| Ichneumonidae | Charops sp. • | 1.04 | 4.02 | |||
| Order | Family | Species | Abundance | |||
| Bantul | Gunung Kidul |
Kulon progo |
Sleman | |||
| Egg parasitoid | ||||||
| Hymenoptera | Platygasteridae | Platygasteridae.sp01 | 49 | |||
| Platygasteridae | Platygasteridae.sp02 | 21 | ||||
| Platygasteridae | Telenomus remus | 8536 | 831 | 466 | 2324 | |
| Trichogrammatidae | Trichogramma sp. | 1988 | ||||
| Larval parasitoid | ||||||
| Hymenoptera | Braconidae | Cotesia sp. | 27 | 12 | 110 | |
| Ichneumonidae | Campoletis sp. | 1 | 1 | |||
| Braconidae | Coccygidium sp. | 1 | 1 | |||
| Eupelmidae | Eupelmidae.sp01 | 1 | ||||
| Braconidae | Microplitis sp. | 15 | 19 | |||
| Braconidae | Stenobracon sp. | 3 | ||||
| Diptera | Tachinidae | Archytas marmoratus | 2 | |||
| Phoridae | Megaselia scalaris | 5 | ||||
| Pupal parasitoid | ||||||
| Hymenoptera | Chalcididae | Brachymeria femorata | 5 | |||
| Chalcididae | Brachymeria lasusi | 3 | ||||
| Ichneumonidae | Charops sp. | 1 | 2 | |||
| Species richness | 8 | 6 | 3 | 7 | ||
| Total abundance | 8753 | 924 | 479 | 2478 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





