1. Introduction
The introduction should briefly place the study in a broad context and highlight why it is important. It should define the purpose of the work and its significance. The current state of the research field should be carefully reviewed and key publications cited. Please highlight controversial and diverging hypotheses when necessary. Finally, briefly mention the main aim of the work and highlight the principal conclusions. As far as possible, please keep the introduction comprehensible to scientists outside your particular field of research. References should be numbered in order of appearance and indicated by a numeral or numerals in square brackets—e.g., [
1] or [
2,
3], or [
4,
5,
6]. See the end of the document for further details on references.
At present, heap bioleaching has a very broad application prospect in utilizing the low-grade sulfide ores with low-cost [
1,
2,
3,
4,
5,
6]. However, the low oxygen concentration in ore heaps seriously restricts the development of this technology. Heap leaching is a complex system with multiphase and multiple mechanic fields. The coordination of solid, liquid, gas, bacteria and heat act synergistically to ensure an effective leaching of low-grade ores [
7,
8]. The key role of forced aeration is to provide oxidant O
2 for ore chemical reaction and promote the chemical reaction between minerals and leaching solution. The artificial airflow adjusts the temperature and oxygen concentration distribution of ore heap and provides necessary O
2 and carbon source for the growth of involved microorganisms [
9].
However, due to the long period of bioleaching process and the huge volume of heaps, airflow is difficult to infiltrate the heap by natural convection [
10]. This will seriously inhibit the reproduction of acidophilic and oxygen-consuming autotrophic microorganisms and ore leaching. Casas et al [
11] found that oxygen failed to diffuse into 5 m of the heap only by natural diffusion. In the heap leaching process of Aitik copper mine, the oxygen concentration decreased 75% from heap surface to 12 m below [
12]. Hector et al [
13] believed that the oxygen concentration at different heap depths increases with the increase of aeration rate, and oxygen concentration distribution at different heights can be regarded as a function only related to heap height during leaching. Giralambone copper mine implemented forced aeration into the heap for the first time in commercial bioleaching, which provided O
2 and CO
2 for the growth and reproduction of bacteria in the heap and accelerated the leaching process of copper sulfides [
14]. In general, the oxygen concentration is prone to be a limiting factor for mineral bioleaching [
15].
Oxygen is an essential participant in the bioleaching process [
16,
17,
18,
19,
20]. Both the oxidation reaction in the leaching process and the growth and reproduction of leaching microorganisms are amenable to oxygen and carbon dioxide. Therefore, the oxygen concentration in the reactor has become an important factor restricting the leaching reaction rate of sulfide minerals. Oxygen enters the heap mainly through two ways [
21,
22]: (1) Molecular diffusion. Oxygen molecules dissolve into the solution by diffusion and enter the heap with the solution seepage. Besides, for unsaturated seepage, the gas phase in ore heap is continuous, resulting in an oxygen flow within the heap by diffusion; (2) Air convection: Air enters the ore heap through convection due to air pressure difference, and oxygen enters the heap with the airflow. Thus, the oxygen transmission speed is faster under the action of this way. Artificial aeration is an effective and economic technology to improve oxygen supply. However, there are some problems such as low effective air volume rate, high power consumption and high aeration cost [
23]. Therefore, forced aeration and determination of aeration rate become key issues with regard to successful application of microbial leaching [
24].
The objective of this work is to understand the relationship between aeration rate and enhanced leaching behavior of low-grade copper sulfide ores. Firstly, At. ferrooxidans strain was sampled from acidic pit water of Zijinshan Copper Mine. After separation, enrichment and domestication, this acidophilic mesophilic bacterium suitable for copper sulfide leaching was obtained. Secondly, the spray mode and high leaching temperature environment of Zijinshan copper mine were simulated, with aeration rate and temperature as variables. Furthermore, airtight column bioleaching of copper sulfides were carried out. Finally, the leaching behavior of copper sulfide with different aeration rate in high temperature environment was discussed by analyzing the variation laws of pH, Eh (redox potential of solution), ore particle sizes and heap height, TFe concentration (total iron ion concentration) and Cu leaching rate of leaching solution throughout the bioleaching experiments. This work reasonably offers a reference for the formulation of aeration system in heap leaching of low-grade copper sulfide ores.
2. Materials and Methods
2.1. Minerals and reagents
The copper sulfide ores used in the experiments were obtained from Zijinshan copper mine in Fujian, China. The ore sample was crushed into particles less than 30 mm by Jaw Crusher ( China Chenggong Mining Equipment and Instrument Co., Ltd. ), and the particle size was screened to-30 mm by vibrating screen. According to the proportion of-5 mm particles to 30 %, 5 ~ 10 mm particles to 20 %, 10 ~ 20 mm particles to 20 %, 20 ~ 30 mm particles to 30 %, the particle gradation of C1 ~ C6 group was configured. Pyrite was the main metal mineral in the ore, followed by chalcocite, covellite and chalcopyrite. Panalytical Axios X-ray Fluorescence Spectrometer was used to analyze the element composition and copper phase composition of raw ores. The mass proportion of each metal mineral was shown in
Table 1. The total copper grade of the ores was 0.325%. The secondary copper sulfides accounted for 86.7%, and the primary copper sulfide was only 4.23%. Metal minerals were produced in aggregates, mostly in vein, veinlet, banded and patchy structures, which were easy to be exposed or dissociated. The mineralogical characteristics were conducive to bioleaching. The element analysis and copper phase analysis of raw ore are shown in
Table 2 and
Table 3.
The reagents used in 9K medium preparation were purchased from Aladdin Industrial, China. Pure water, prepared by passing deionized water through a laboratory water-purification system, was used in the experiments.
2.2. Leaching microorganisms
Microorganisms used in leaching experiments were At. ferrooxidans sampled from Zijinshan copper mine, China. Modified 9K medium was used to culture these microorganisms. The specific production method of the Modified 9K medium is as follows: Liquid A: 44.3 g FeSO
4 · 7H
2O is fully dissolved in 200 mL deionized water; solution B : 0.5 g K
2HPO
4, 0.1 g KCL, 3 g ( NH
4 )
2SO
4, 0.5 g MgSO
4 · 7H
2O, 0.01 g Ca ( NO
3 )
2 and 44.2 g FeSO
4 · 7H
2O were fully dissolved in 800 mL deionized water. Liquid C: Concentrated sulfuric acid and deionized water were mixed in equal volume (1 : 1 H
2SO
4) [
25]. The pH value of B solution was adjusted to 2.0 by adding C solution to B solution, and the filter membrane (pore size 0.22 μm ) was used to remove bacteria. The liquid A was evenly mixed with the liquid B after sterilization.
Leaching microorganisms were cultured in 250 mL conical flasks after high temperature sterilization by following steps. Firstly, 10 mL microorganism-bearing solution were extracted to 90 mL sterilized culture medium, and the pH of medium were adjusted to 1.8 with 1:1 H2SO4. The prepared culture medium was placed into a thermostatic oscillator at 30 ℃ and a rotary rate of 150 r/min. The microorganisms reached its stationary phase once the solution colour turned tawny from light blue and precipitation generated.
Secondly, At. ferrooxidans with highest Fe
2+ transformation rate was selected for subsequent enrichment for another three generations. A total of six transition culturing were sequentially carried out for enriched microorganisms with an inoculation rate of 10%, 9%, 8%, 7%, 6% and 5%, respectively. Lastly, a relatively pure microorganism was therefore obtained. Compared with the appearance and growth characteristics of bacteria in the study of Huang et al [
26], At. ferrooxidans dominated the bacterial communities.
2.3. Experimental
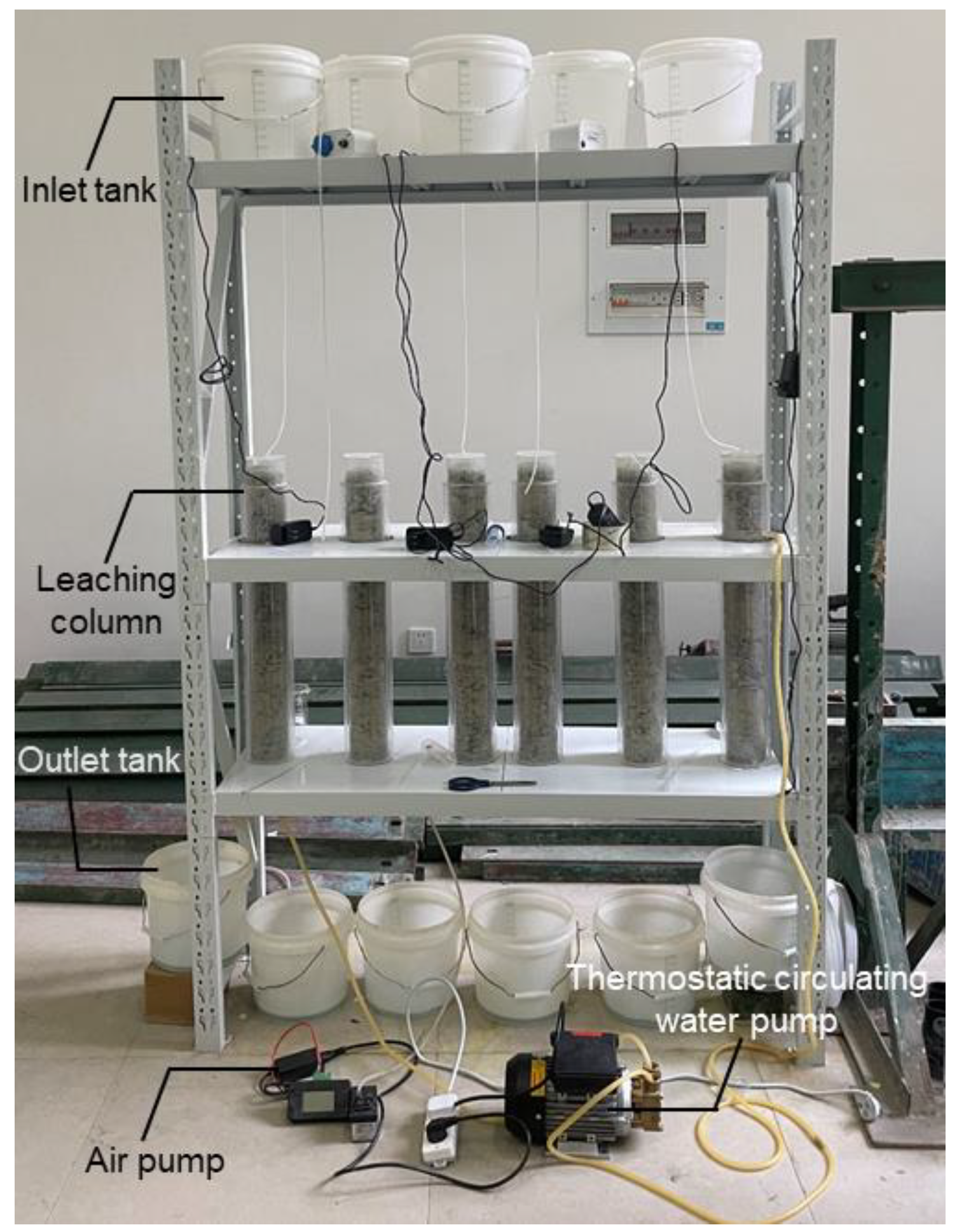
Bioleaching system consisted of six columns (C1~C6), 10 L solution inlet tanks, 10 L solution outlet tanks, rubber hoses and air pumps (
Figure 1). Each column was designed in bilayer structure with acrylic glasses and 650 mm in length, 110 mm in external diameter, 90 mm in internal diameter. The uppermost 50 mm was placed with 5 mm anticorrosion glass bead as filter layer. The middle 500 mm was charged with ore particles, where particles with diameters of –5 mm, 5~10 mm, 10~20 mm and 20~30 mm approximately accounted for 30%, 20%, 20% and 30%, respectively. The bottommost 100 mm was designed as gas stationary chamber, sidewall of which was connected to air pump and base was connected to outlet tank with rubber hose. The column top was sealed with an acrylic lid to maintain an airtight environment. The inlet tank and the column were also connected by rubber hose.
2.4. Measurements
The initial bacteria inoculation was 15% (8.5×105/mL), volume of leaching solution in inlet tank was 6 L, solution pH was 2.2 and irrigation rate was 4 mL/min. Irrigation of leaching solution was proceeded by using on-off irrigation, i.e., 3 irrigation days with 1 idle day in initial phase, and 2 irrigation days and 2 idle days intervals in later phase. Column C1 was control group and thus conducted without aeration, while columns C2~C6 were aerated for 2 h every 2 days. The forced aeration rates in columns C2~C6 were controlled by micro air pumps at 60 L/h, 95 L/h, 110 L/h, 120 L/h and 150 L/h, respectively. The constant temperature was realized by controlling water temperature of acrylic interlayers with circulating water pumps. The temperature of leaching system was kept at 30 ℃ before day 8, 35 ℃ at day 9~25, 40 ℃ at day 25~46, 45 ℃ at day 46~68.
3. Results
3.1. Evolution of pH and Eh in pregnant leach solutions
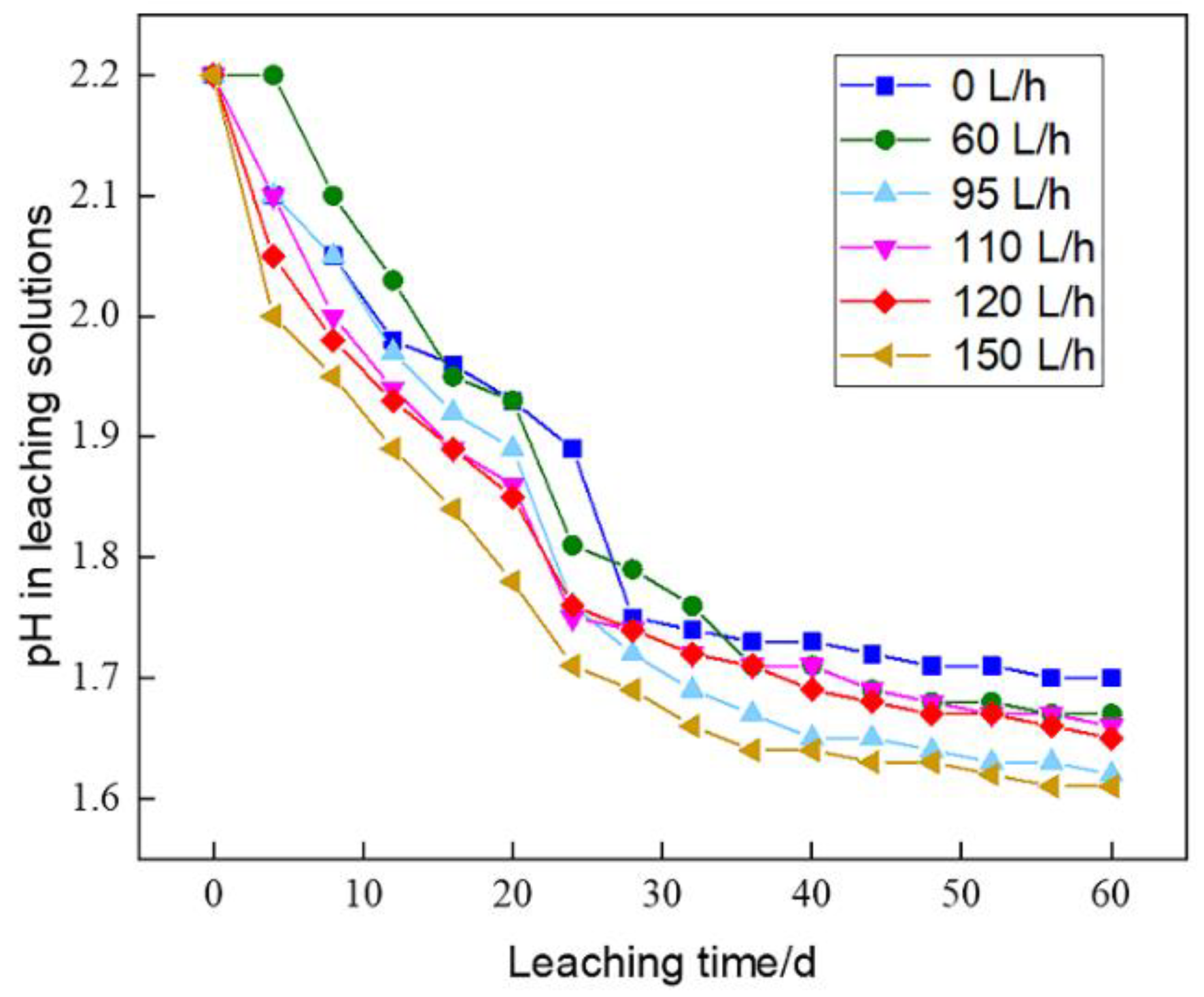
The pH evolutions of pregnant leach solutions in C1~C6 during microbial leaching (
Figure 2) showed that pH decreased at a faster rate in the early and intermediate stages of leaching, while it remained a relatively stable rate with slight decrease. The temperature of bioleaching system increased to above 40 ℃ in the middle and late stages of leaching, when leaching bacteria were inhibited in growth activity, thus the chemical reaction between minerals and the leaching solution entered a stable period and therefore the rate of acid consumption decreased.
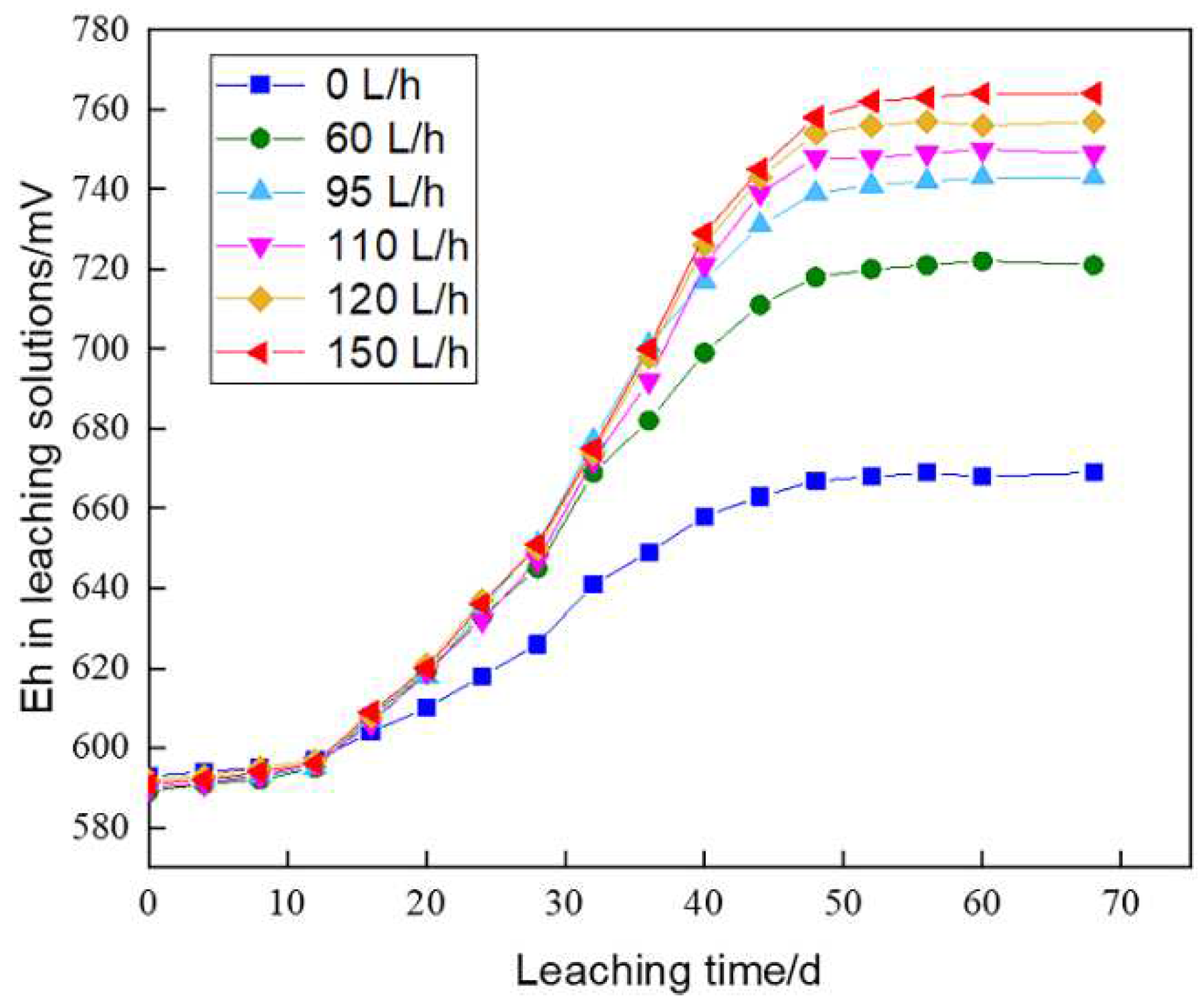
The redox potential (Eh) during bioleaching showed a slow increase in the initial stage, a sharp increase in the intermediate stage, and a steady increase in the late stage, with the final Eh remained in the range of 721-764 mV (
Figure 3). According to the Nernst equation, Eh mainly depended on the ratio of Fe
3+/Fe
2+ in the solution, which was related to the number of bacteria and the oxidative capacity.
3.2. Particle size distribution and settlement of heaps
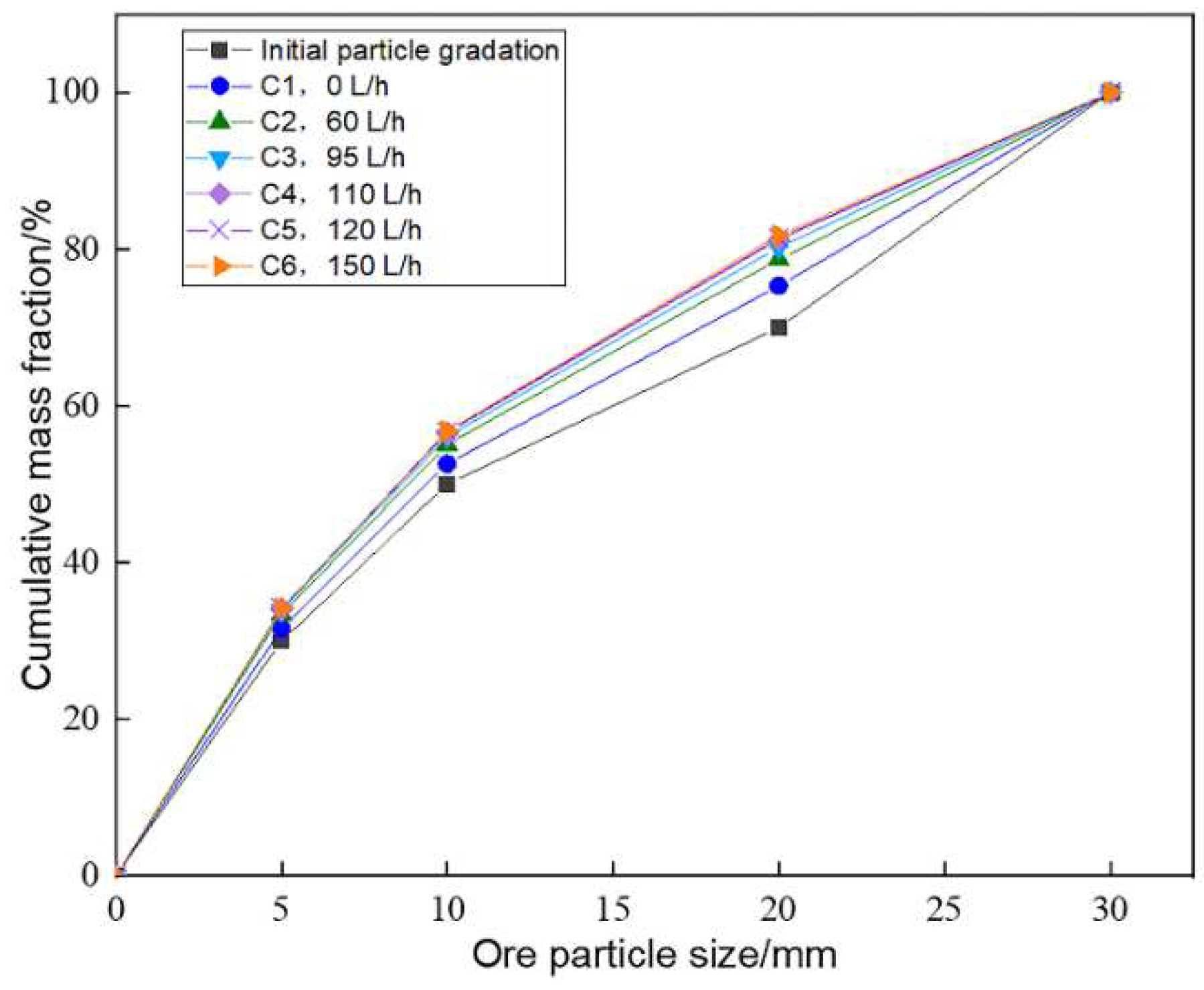
Compared to the initial size distribution of ore particles, size distributions of six columns changed significantly after leaching (
Figure 4). It mainly reflected in the decrease of coarse particle (20~30 mm), and the increase content of fine particles (-5 mm) and medium particles (5~20 mm). The higher the aeration rate, the greater the increase of fine and medium particle content.
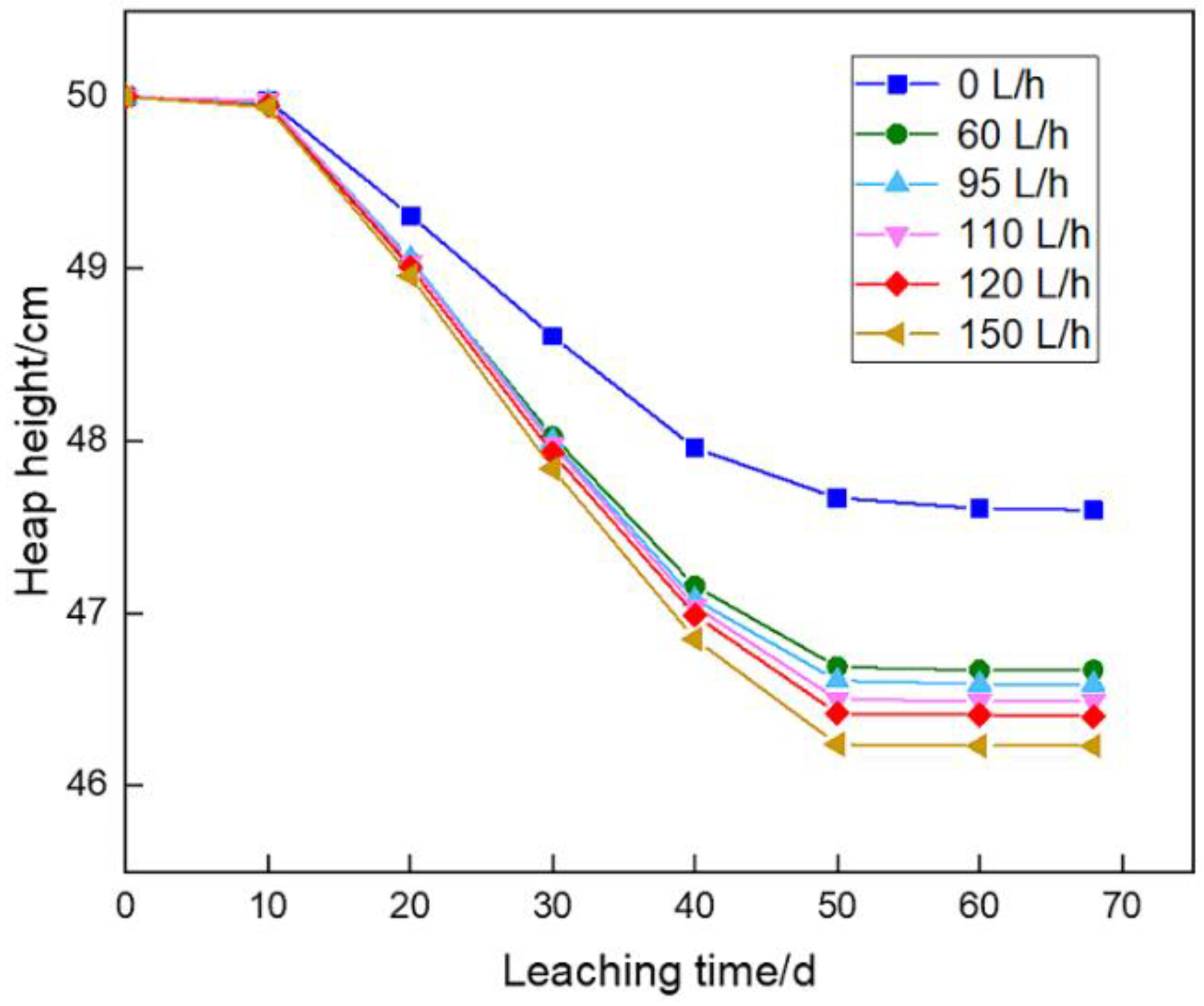
The variation of heap height during bioleaching showed that heap height decreased rapidly in the early leaching stage, while it changed slightly afterwards in the presence of biochemical reaction (
Figure 5). A part of argillaceous particles were gradually dissolved by acid or flushed out of the heap by leaching solution. When fine particles were infiltrated by the solution, a bonding water layer was formed on particle surface. Under the combined action of electric double layer, ion layer and solution surface tension, the attraction between the particles increased and thus the spacing decreased gradually.
3.3. Evolution of leaching bacterial cell numbers
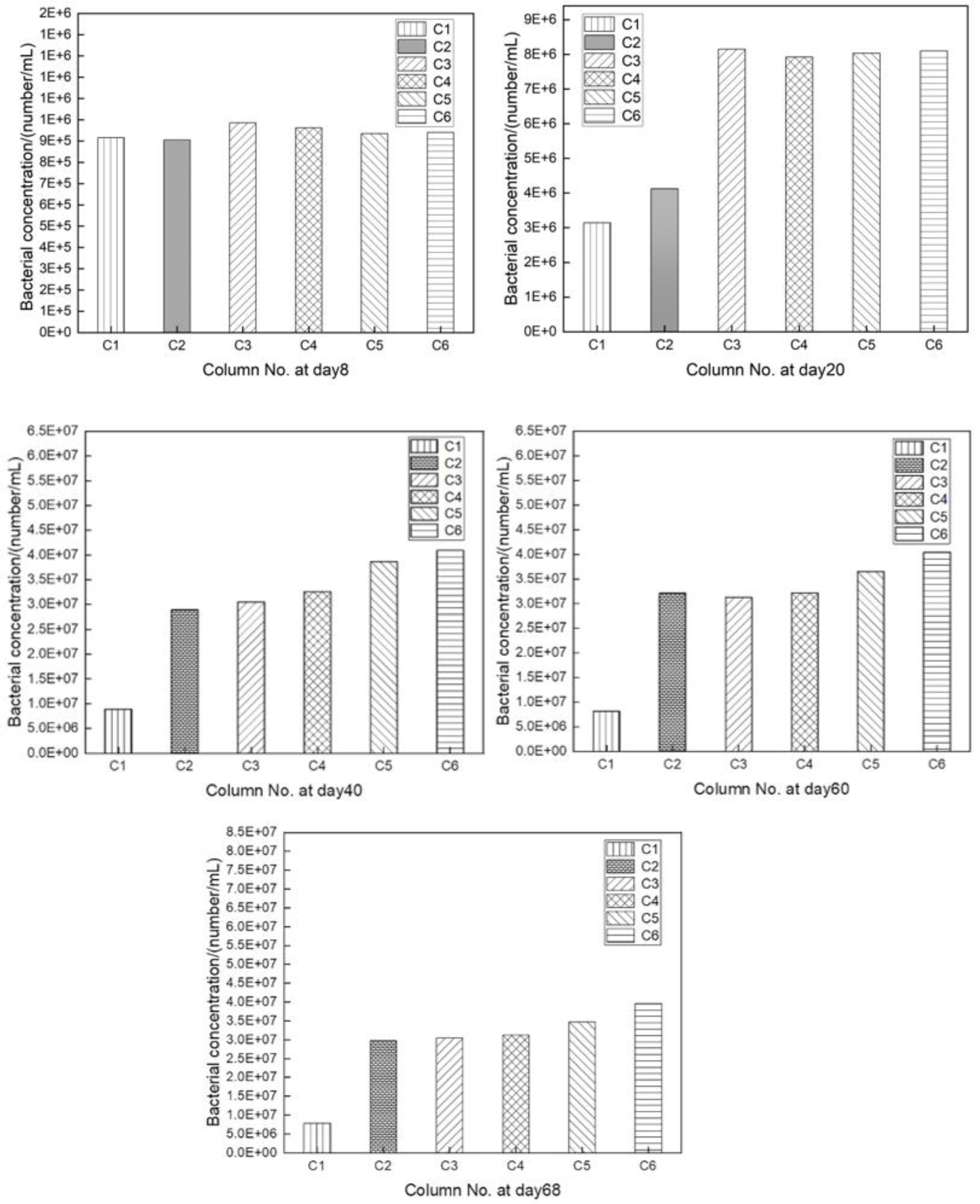
Bacterial cell numbers of C1~C6 in pregnant leach solutions were close and relatively stable at 9×105~1×106/mL in the early leaching stage (
Figure 6). The highest microorganism cell numbers in C3 was observed when the aeration rate was 95 L/h. After leaching for 20 days, microorganism cell numbers in columns 3~6 (aeration rate ≥95 L/h) remained stable at 7×106/mL and above, which were higher than those in C1 and C2. As microbial leaching proceeded, the temperature of the leaching system gradually exceeded 40 ℃, and the bacterial growth was prone to be inhibited.
Figure 6 also showed that the microorganism cell numbers in columns 3~6 accordingly increased with the increase of aeration rates. It indicated that aeration rate had a significant effect on the growth and reproduction of leaching bacteria [
28,
29].
3.4. Evolution of TFe concentrations in pregnant leach solutions
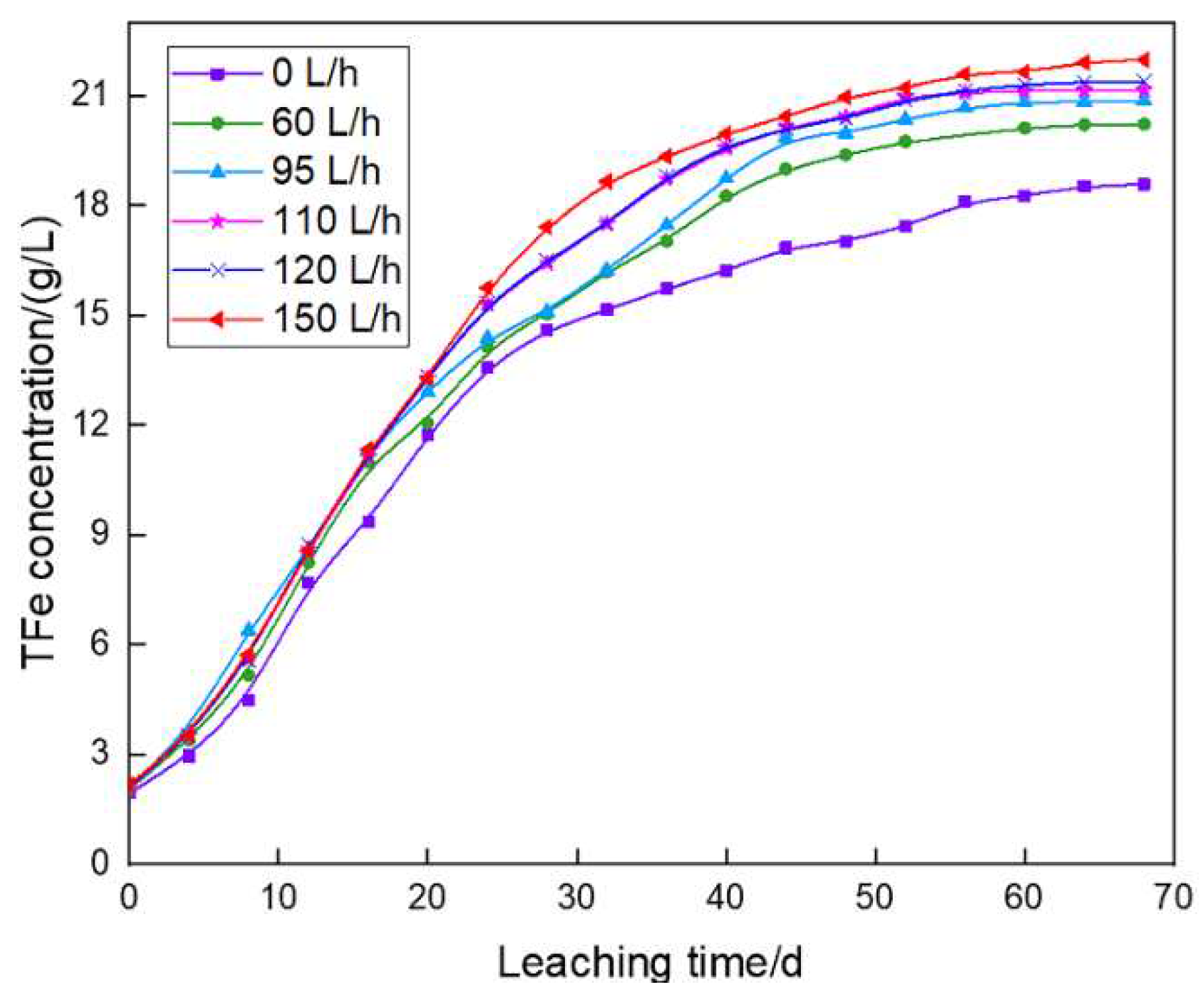
The evolution rules of TFe concentrations in the pregnant leach solutions showed that, in the early and intermediate leaching stages, TFe concentrations in pregnant leach solutions of six columns increased continuously, and reached its highest leaching rate in C3 when aeration rate was 95 L/h. Until the end of leaching, the increasing temperature led to the inhibition of bacterial oxidation capacity, and thus the increase rate in TFe concentration in each column slowed down and remained relatively stable. In general, the evolution rules of TFe concentrations in pregnant leach solutions were basically the same with or without aeration.
The TFe leaching rates of C1~C6 groups were 46.96%, 50.5%, 52.2%, 53.11%, 53.91% and 55%, respectively.
3.5. Evolution of Cu leaching rate
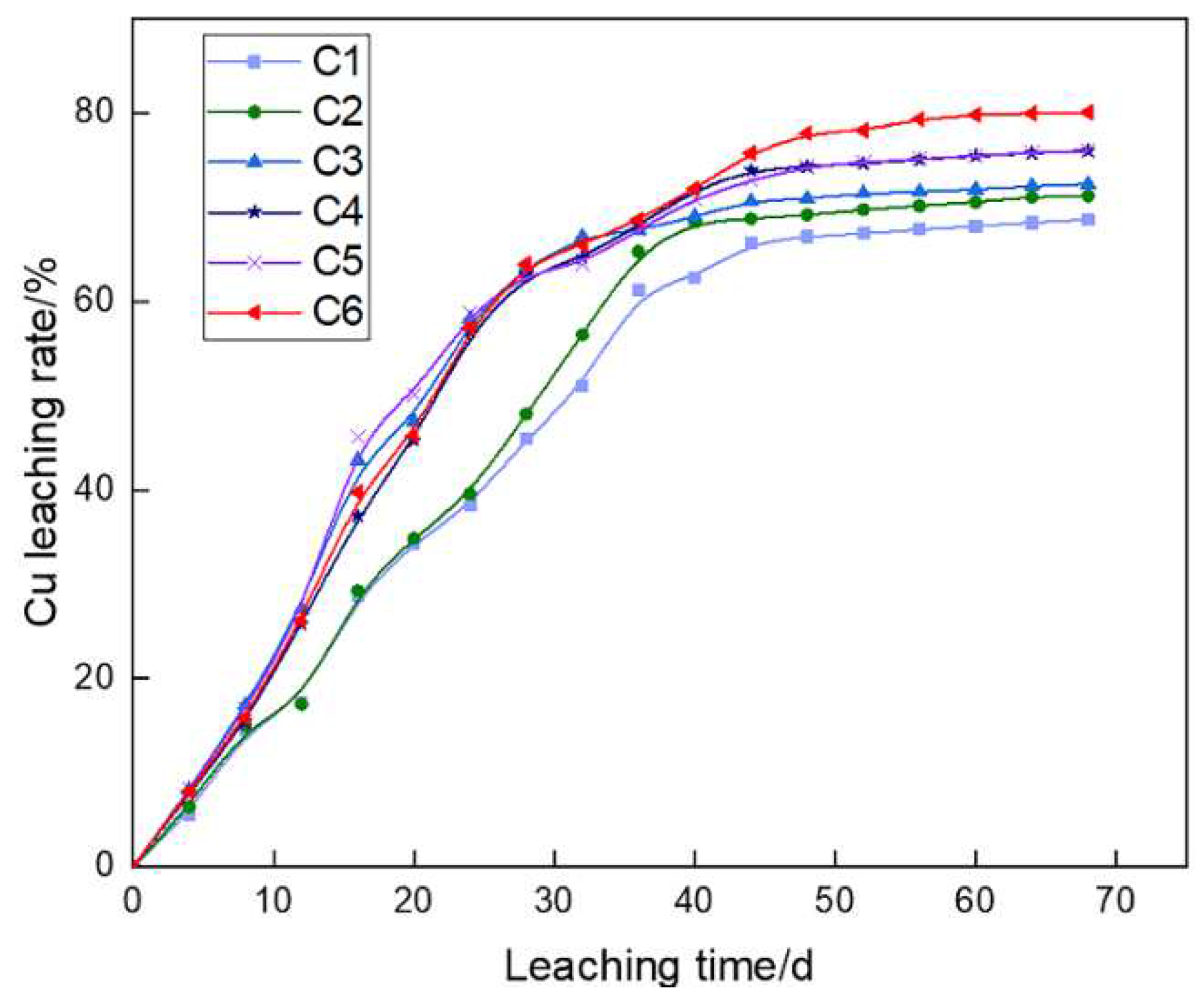
The evolutions of Cu leaching rates in C1~C6 showed that Cu leaching in all six experimental groups sequentially experienced a relatively slow growth, rapid growth and relatively stable process (
Figure 8). Cu recovery rate was higher when aeration rates were greater than 95 L/h than those without aeration or with insufficient aeration. Besides, the final Cu
2+ concentrations in the pregnant leach solution under aeration 60~150 L/h were 1.411, 1.432, 1.498, 1.491 and 1.583 g/L, respectively, which were higher than that without aeration (1.345 g/L). The calculation results of liquid-meter leaching rate method show that when the aeration rate was 150 L/h, the final Cu leaching rate was 80.1%, which was 11.4% higher than that without aeration (68.7%).
4. Discussion
4.1. Discussion on the evolution of pH and Eh in pregnant leach solutions
Though initial pH values were set as the same, the greater the aeration rate, the greater pH decreased in early stage and the lower the final pH values of the pregnant leach solution. An increase in aeration rate led to an increase of oxygen content in the leaching column. Since the biochemical reaction between minerals and leaching solution used O
2 as the oxidant, higher oxygen content was beneficial for bacterial oxidation activity and minerals dissolution [
8].
The leaching of sulfide minerals was an acid-producing process, hence the aeration rate was the prominent factor affecting pH evolution. The pH values of the pregnant leach solution were also influenced by gangue contents within ores. The main gangue minerals were alunite, quartz and dickite, which were resistant to acid and consumed rarely acid. Therefore, the pH values decreased sequentially with the increase of aeration rate in the same leaching cycle.
In the early stage, bacterial ability of oxidizing Fe2+ to Fe3+ was limited due to their low inoculum. In the intermediate stage, the bacterial growth gradually entered the logarithmic growth phase, which greatly increased the dissolution rate of sulfide ores and the growth rate of Fe3+ concentration, and therefore resulted in a sharp rise in Eh. In the late stage, however, the bacteria entered the stable or decaying period, and the easily leachable sulfide minerals had been leached, thus the Fe3+/Fe2+ ratio was relatively stable.
4.2. Discussion on the particle size distribution and settlement of heaps
Throughout the leaching process, because the leaching solution continuously infiltrated into the heap by gravity, and the ore particles reacted with the leaching solution to be continuously dissolved and eroded, resulting in the reduction of the particle size of coarse particles. Under natural ventilation conditions, the pore structure of the heap is deteriorating due to physical hardening and chemical precipitation. The deterioration of the porosity of the heap will greatly reduce the penetration effect of the solution and even cause the interruption of leaching. Forced ventilation can effectively improve the structural parameters such as pore shape, size and connectivity of the ore heap. On the one hand, ventilation can promote the interaction of gas, liquid and solid phases, break the original structural balance and force balance, so as to improve the cross-sectional area of solution seepage. On the other hand, bubbles and bubble groups are generated during forced ventilation. When the bubble group breaks up in the reactor, a complex environment of local high temperature, high pressure and high speed airflow is generated, and an airflow shock wave is formed in the local area. This vibration reduces the internal friction between the particles and promotes the fine particles enriched and deposited in the pore throat to jump and migrate, thus improving the connectivity of the pores [
30]. Aeration improved the porosity distribution of the heap, aggravated the dissolution behavior of ore particles and leaching solution, and therefore increased the content of fine particles. However, under the condition of similar aeration rate, it was difficult to compare the effect of aeration rate on size distribution for those columns with similar size distribution (C3~C6).
4.3. Discussion on the evolution of leaching bacterial cell numbers
In the early leaching stage, bacterial growth was slow and relatively stable due to their adaptation to the leaching environment. The catalytic oxidation of elemental sulfur by
At. ferrooxidans was an acid-producing process, and therefore of pH evolution was negatively correlated with the evolution of bacterial cell numbers [
31]. During bacteria’s retard phase, pH decreased slowly and then decreased rapidly as the metabolism acceleration of
At. ferrooxidans. After a certain adaptation, bacteria entered the logarithmic growth phase. Since the leaching system supplied sufficient nutrients, O
2 and carbon sources for bacterial growth, leaching microorganisms enabled a high activity and a rapid proliferation. When the aeration rate>95 L/h in the intermediate and late stages, the bacterial cell numbers was higher than 3×10
7/mL, and the bacterial oxidation capacity was robust at this time. However, bacterial in this phase was relatively stable, indicating that the bacterial growth entered a stable period. On day 68, the temperature of the leaching system reached 45 ℃, at which time the bacterial cell numbers of all test groups decreased. It indicated that the bacterial growth entered an apoptotic phase and the metabolic activity decreased significantly, even leading to bacterial death.
During microbial leaching of copper sulfide ores, the intermediate product elemental sulfur S was produced, which was rhombohedral cyclic sulfur S
8 at room temperature. S
8 was insoluble in water but easily deposited on the mineral surface to act as a passivation layer [
32]. However,
At. ferrooxidans used elemental sulfur as an energy source and O
2 as an electron acceptor to oxidize inorganic reduced sulfur-containing compounds using dissolved oxygen and oxidized cyclic sulfur S
8 to sulfuric acid. The airflow shock wave formed by forced aeration promoted the chemical precipitate or physical clogging, and thereafter enhanced the diffusion of air in the heap. In this case, aeration increased the dissolved oxygen concentration and carbon dioxide content in the leach solution, thus increased the number and activity of bacteria and accelerated the oxidation of inorganic reduced sulfur-containing compounds.
4.4. Discussion on the evolution of TFe concentrations in pregnant leach solutions
The TFe leaching rates of six groups were not high due to the leaching bacteria in the experiments were mainly
At. ferrooxidans. The metabolic activity of the bacteria decreased significantly in high temperature environment above 40 ℃, and the bacterial growth was seriously inhibited at 45 ℃ or higher [
33]. In the early leaching stage, the temperature of column leaching system was lower than 40 ℃. At this time, the leaching bacteria still maintained high activity and oxidation capacity, and continuously promoted the dissolution of pyrite. Therefore, the TFe leaching rate in this stage was faster. The temperature at the end of leaching reached 40~45 ℃, and the bacterial growth was inhibited. However, the temperature improvement caused by forced aeration reduced the temperature of C2~C6, which improved the TFe leaching rate.
4.5. Discussion on the evolution of Cu leaching rate
In the early leaching stage, aeration rate was designed with an optimal value of 95 L/h for column bioleaching, at which Cu leaching rate was much higher than other columns with lower temperature. Besides, the temperature of leaching system continuously increased and even reached 40~45 ℃ in late stage. Forced aeration effectively reduced the temperature in heaps, so that the leaching microorganisms were in a more suitable growth environment and always maintain high oxidation activity. Therefore, when the temperature of the late leaching system increased and the aeration rate reached the maximum value of 150 L/h, the final Cu leaching rate was as high as 80.1%.
In addition to improving the activity and quantity of leaching microorganisms and TFe concentration, forced aeration was obviously conducive to the bioleaching of copper ore. On day 24, Cu leaching rates without aeration and with aeration rate of 60 L/h were 38.4% and 39.6% respectively, while the aeration rate was more than 95 L/h, the Cu leaching rate reached higher than 58.1%. Thus, when the aeration rate was far greater than the optimal design value of early leaching stage, the final Cu leaching rate could also be significantly improved.
5. Conclusion
1. Leaching bacteria grew slowly in the initial leaching stage. When the aeration rate was 95 L/h, the bacterial cell numbers remained the highest, indicating that the aeration intensity in column C3 met the oxygen requirements for bacterial growth. When leaching system temperature gradually exceeded 40 ℃, the growth of bacteria was prone to be inhibited. However, the bacterial cell numbers of column 3~6 increased successively with the increase of aeration rate, indicating that the aeration rate had a significant effect on the leaching bacteria growth.
2. After leaching, the heaps settled under the microbial assisted biochemical reaction between minerals and leaching solution. The coarse particles content in heaps decreased significantly, while the fine particles and medium-fine particles content increased. Aeration ameliorated the pore distribution of heaps and aggravated the dissolution behavior of ore particles and leaching solution. The higher the aeration rate, the greater the increase of medium-fine particles content.
3. The final total Fe concentration in pregnant leach solution increased with the increase of aeration rate. Bacterial growth law was basically the same as that of total Fe concentration in pregnant leach solution.
4. At the initial stage of leaching, Cu leaching rate was relatively slow, and there was little difference in Cu leaching rate between C1 (without aeration) and C2 (aeration rate was 60 L/h). When the aeration rate was 150 L/h, the Cu leaching rate was 80.1%, which was 11.4% higher than that without aeration. It indicated that forced aeration effectively lowered the heaps temperature, facilitated the growth of leaching microorganisms with a more suitable temperature range, and thus accelerated the leaching of low-grade copper sulfide ores.
Author Contributions
Conceptualization, Mingqing Huang and Ming Zhang; methodology, Mingqing Huang; software, Ming Zhang; validation, Zhaolan Li; formal analysis, Ming Zhang and Zhaolan Li; investigation, Mingqing Huang; resources, Mingqing Huang; data curation, Ming Zhang and Zhaolan Li; writing—original draft preparation, Mingqing Huang, Zhaolan Li, Ming Zhang and Weicheng Wang; writing—review and editing, Zhaolan Li and Ming Zhang; visualization Zhaolan Li and Ming Zhang; supervision, Mingqing Huang; project administration, Mingqing Huang; funding acquisition, the National Natural Science Foundation of China and the Natural Science Foundation of Fujian Province, China. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number “No. 51804079”, the Natural Science Foundation of Fujian Province, China, grant number “No. 2019J05039”.
Data Availability Statement
Not applicable.
Acknowledgments
All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
We have no conflicts of interest.
References
- Mokmeli, M.; Parizi, M.T. Low-grade chalcopyrite ore, heap leaching or smelting recovery route? Hydrometallurgy 2022, 211. [Google Scholar] [CrossRef]
- Seredkin, M.; Zabolotsky, A.; Jeffress, G. In situ recovery, an alternative to conventional methods of mining: Exploration, resource estimation, environmental issues, project evaluation and economics. Ore Geol. Rev 2016, 79, 500–514. [Google Scholar] [CrossRef]
- Ilankoon, I.M.S.K.; Tang, Y.; Ghorbani, Y.; et al. The current state and future directions of percolation leaching in the Chinese mining industry: Challenges and opportunities. Miner. Eng 2018, 125, 206–222. [Google Scholar] [CrossRef]
- Xie, H.; Ju, Y.; Gao, F.; Gao, M.; Zhang, R. Groundbreaking theoretical and technical conceptualization of fluidized mining of deep underground solid mineral resources. Tunn. Undergr 2017, 67, 68–70. [Google Scholar] [CrossRef]
- Faramarzi, M.A.; Mogharabi-Manzari, M.; Brandl, H. Bioleaching of metals from wastes and low-grade sources by HCN-forming microorganisms. Hydrometallurgy 2020, 191. [Google Scholar] [CrossRef]
- Miranda, I.T.P.; Moletta, J.; Pedroso, B.; Pilatti, L.A.; Picinin, C.T. A Review on Green Technology Practices at BRICS Countries: Brazil, Russia, India, China, and South Africa. SAGE Open 2021, 11. [Google Scholar] [CrossRef]
- Muravyov, M.; Panyushkina, A.; Bulaev, A.; Fomchenko, N. Biobeneficiation of bulk copper-zinc and copper-nickel concentrates at different temperatures. Miner. Eng 2021, 170. [Google Scholar] [CrossRef]
- Wang, L.; Yin, S.; Deng, B. Understanding the Effect of Stepwise Irrigation on Liquid Holdup and Hysteresis Behavior of Unsaturated Ore Heap. Minerals 2021, 11. [Google Scholar] [CrossRef]
- Wang, X.; Ma, L.; Wu, J.; Xiao, Y.; Tao, J.; Liu, X. Effective bioleaching of low-grade copper ores: Insights from microbial cross experiments. Bioresour Technol 2020, 308. [Google Scholar] [CrossRef]
- Chen, W.; Yin, S.; Ilankoon, I.M.S.K. Effects of forced aeration on community dynamics of free and attached bacteria in copper sulphide ore bioleaching. Int. J. Miner. Metall 2022, 29, 59–69. [Google Scholar] [CrossRef]
- Casas, J.M.; Vargas, T.; Martinez, J.; Moreno, L. Bioleaching model of a copper-sulfide ore bed in heap and dump configurations. Metall. Mater. Trans B 1998, 29, 899–909. [Google Scholar] [CrossRef]
- Linklater, C.M.; Sinclair, D.J.; Brown, P.L. Coupled chemistry and transport modelling of sulphidic waste rock dumps at the Aitik mine site, Sweden. Applied Geochemistry 2005, 20, 275–293. [Google Scholar] [CrossRef]
- Lizama, H.M. Copper bioleaching behaviour in an aerated heap. Int. J. Miner 2001, 62, 257–269. [Google Scholar] [CrossRef]
- Rawlings, D.E.; Dew, D.; du Plessis, C. Biomineralization of metal-containing ores and concentrates. Trends Biotechnol 2003, 21, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Kalka, H.; Märten, H. Potential Bioleaching Effects in In Situ Recovery Applications. Solid State Phenomena 2017, 262, 456–460. [Google Scholar] [CrossRef]
- Third, K.A.; Cord-Ruwisch, R.; Watling, H.R. Control of the redox potential by oxygen limitation improves bacterial leaching of chalcopyrite. Biotechnol Bioeng 2002, 78, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Domic, E.M. A Review of the Development and Current Status of Copper Bioleaching Operations in Chile: 25 Years of Successful Commercial Implementation. In: Rawlings DE, Johnson DB, eds. Biomining. Springer Berlin Heidelberg; 2007, 81-95. [CrossRef]
- Huang, M.-q.; Wu, A.-x. Numerical analysis of aerated heap bioleaching with variable irrigation and aeration combinations. J. Cent. South. Univ 2020, 27, 1432–1442. [Google Scholar] [CrossRef]
- Wu, A.-x.; Yao, G.-h.; Huang, M.-q. Influence factors of permeability during heap leaching of complex copper oxide ore. Adv. Mat. Res 2012, 347-353, 1037–1043. [CrossRef]
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides — A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Meng, Y.; Li, Z. Experimental study on diffusion property of methane gas in coal and its influencing factors. Fuel 2016, 185, 219–228. [Google Scholar] [CrossRef]
- Blight Geoffrey, E. Flow of Air through Soils. J. Soil Mech. & Found 1971, 97, 607–624. [Google Scholar] [CrossRef]
- Batty, J.D.; Rorke, G.V. Development and commercial demonstration of the BioCOP™ thermophile process. Hydrometallurgy 2006, 83, 83–89. [Google Scholar] [CrossRef]
- Brierley, C.L. Biohydrometallurgical prospects. Hydrometallurgy 2010, 104, 324–328. [Google Scholar] [CrossRef]
- Xiong, H.; Guo, R. Effects of chloride acclimation on iron oxyhydroxides and cell morphology during cultivation of Acidithiobacillus ferrooxidans. Environ Sci Technol 2011, 45, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Q.; Zhang, M.; Zhan, S.L.; Chen, L.; Xue, Z.L. Saturated Dissolved Oxygen Concentration in in situ Fragmentation Bioleaching of Copper Sulfide Ores. Front. Microbio. 2022, 13. [Google Scholar] [CrossRef]
- Yahya, A.; Johnson, D.B. Bioleaching of pyrite at low pH and low redox potentials by novel mesophilic Gram-positive bacteria. Hydrometallurgy 2002, 63, 181–188. [Google Scholar] [CrossRef]
- Mandl, M.; Pakostova, E.; Poskerova, L. Critical values of the volumetric oxygen transfer coefficient and oxygen concentration that prevent oxygen limitation in ferrous iron and elemental sulfur oxidation by Acidithiobacillus ferrooxidans. Hydrometallurgy 2014, 150, 276–280. [Google Scholar] [CrossRef]
- Mandl, M.; Pakostova, E.; Poskerova, L. Minimum Aeration in Acidithiobacillus ferrooxidans Cultures Required to Maintain Substrate Oxidation without Oxygen Limitation. Adv. Mat. Res 2013, 2749, 414–417. [Google Scholar] [CrossRef]
- Huang, M.Q. Gas seepage law and ventilation enhanced leaching mechanism of bio-heap leaching of copper sulfide ore. Doctor. Beijing University of Science and Technology, Beijing, 2016.
- Ceskova, P.; Mandl, M.; Helanova, S.; Kasparovska, J. Kinetic studies on elemental sulfur oxidation by Acidithiobacillus ferrooxidans: sulfur limitation and activity of free and adsorbed bacteria. Biotechnol. Bioeng 2002, 78, 24–30. [Google Scholar] [CrossRef]
- Harmer, S.L.; Thomas, J.E.; Fornasiero, D.; Gerson, A.R. The evolution of surface layers formed during chalcopyrite leaching. Geochim. Cosmochim. Acta 2006, 70, 4392–4402. [Google Scholar] [CrossRef]
- Jia, Y.; Tan, Q.; Sun, H.; Zhang, Y.; Gao, H.; Ruan, R. Sulfide mineral dissolution microbes: Community structure and function in industrial bioleaching heaps. Green Energy Environ 2019, 4, 29–37. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).