Submitted:
30 October 2023
Posted:
31 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
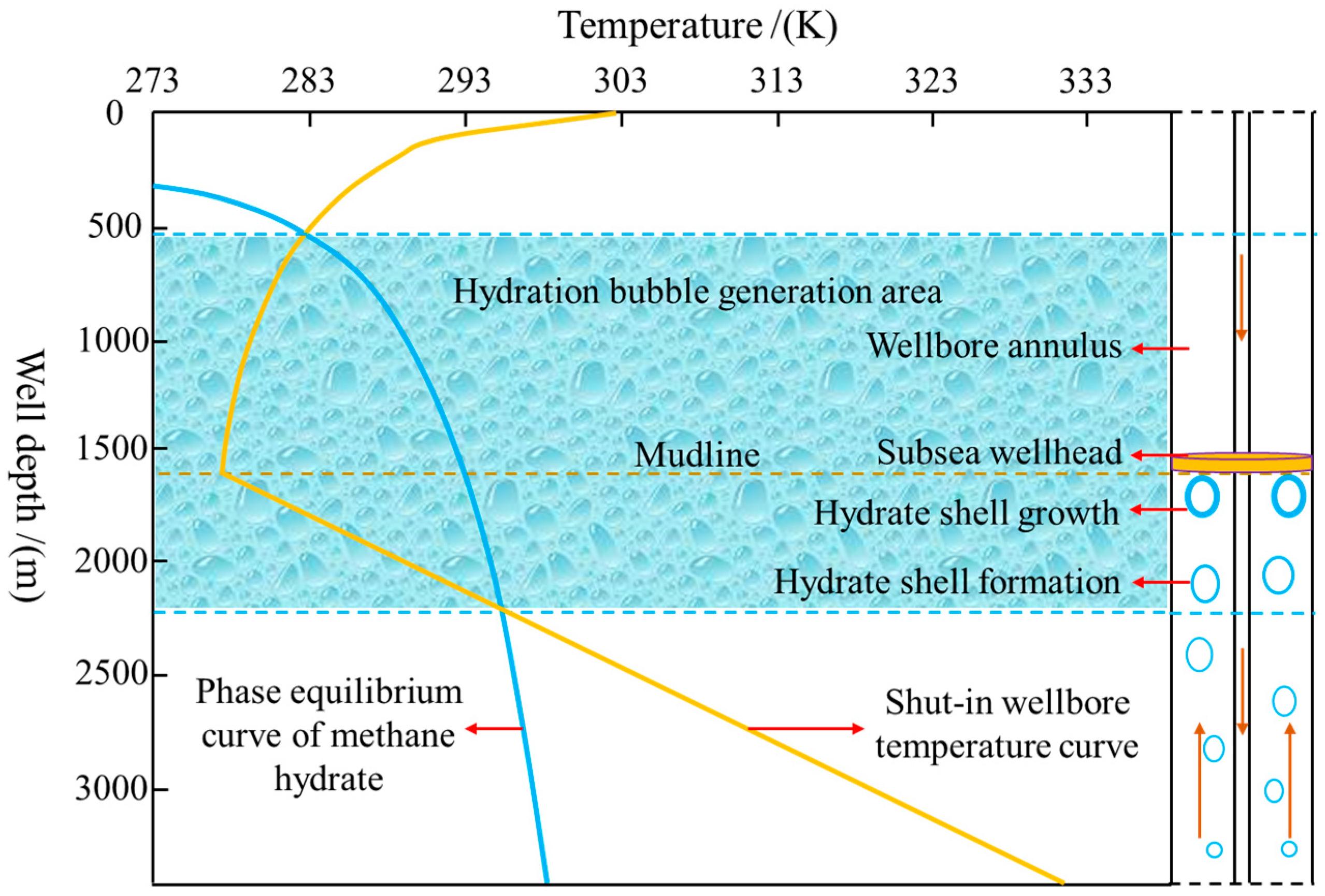
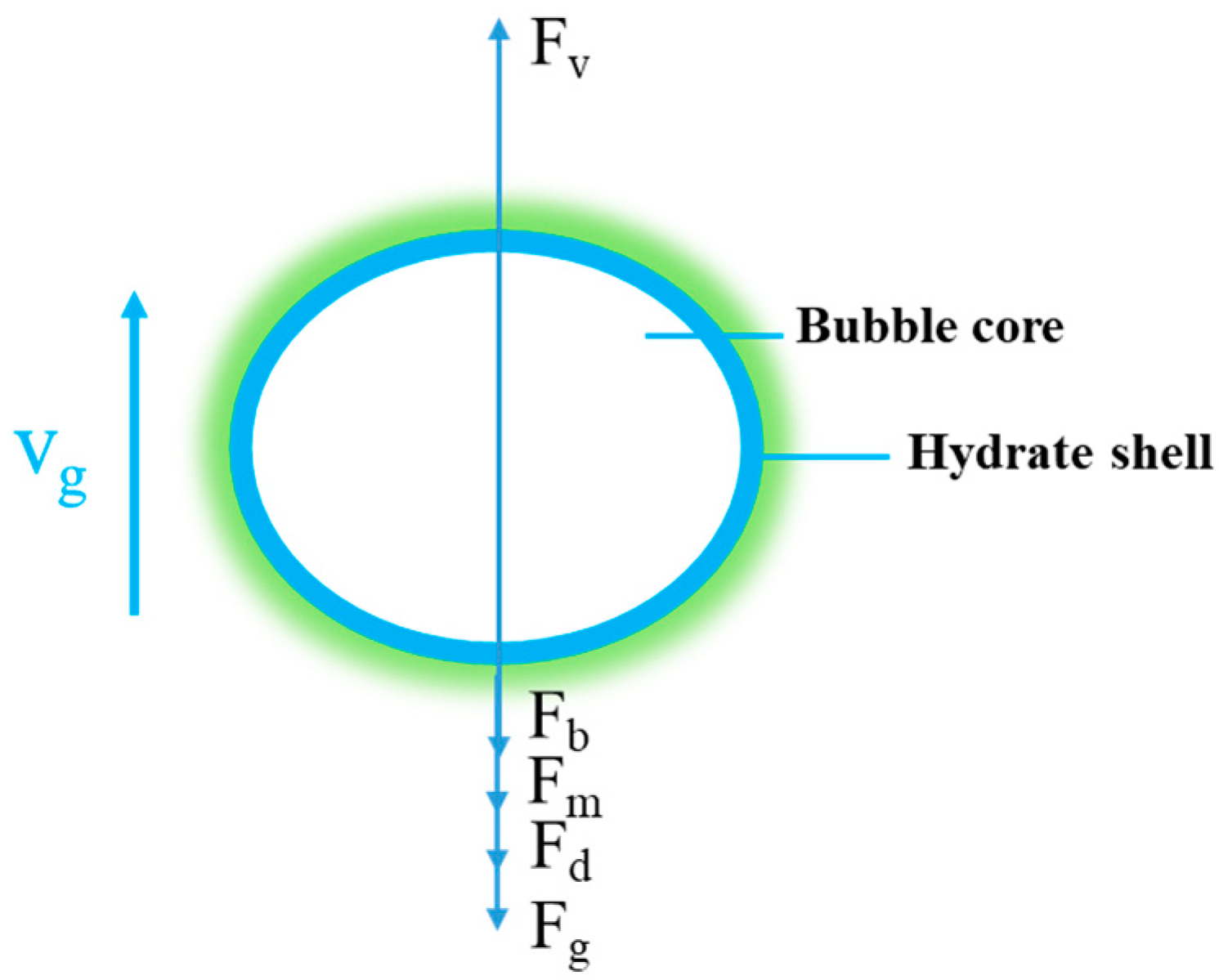
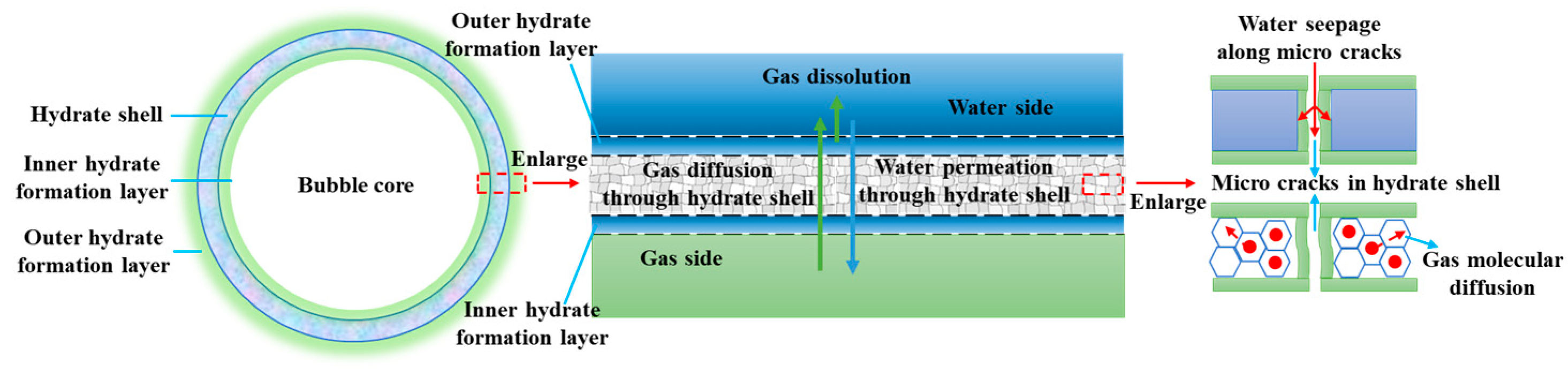
2. Model description

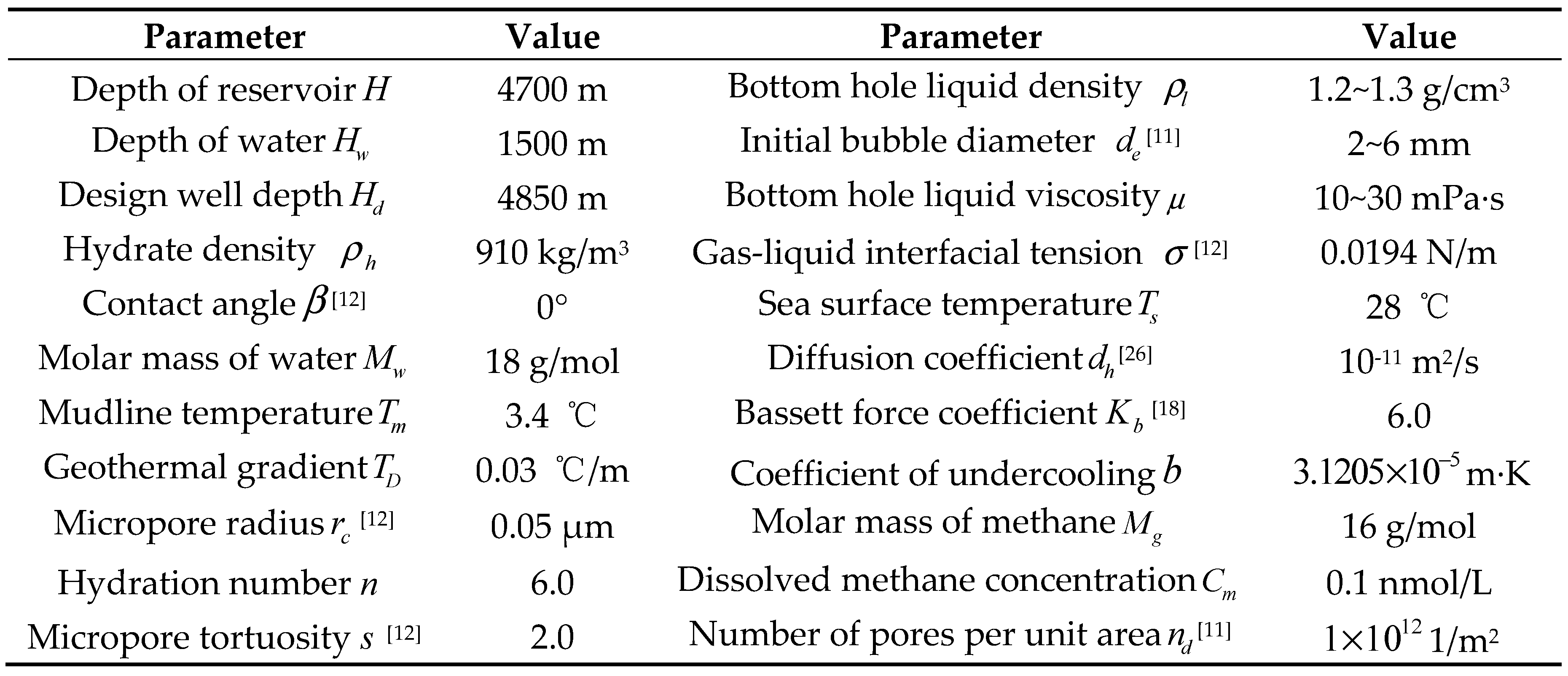
3. Determination of key model parameters
3.1. Dynamic growth thickness of hydrate shell
3.2. Bubble dynamic equivalent radius
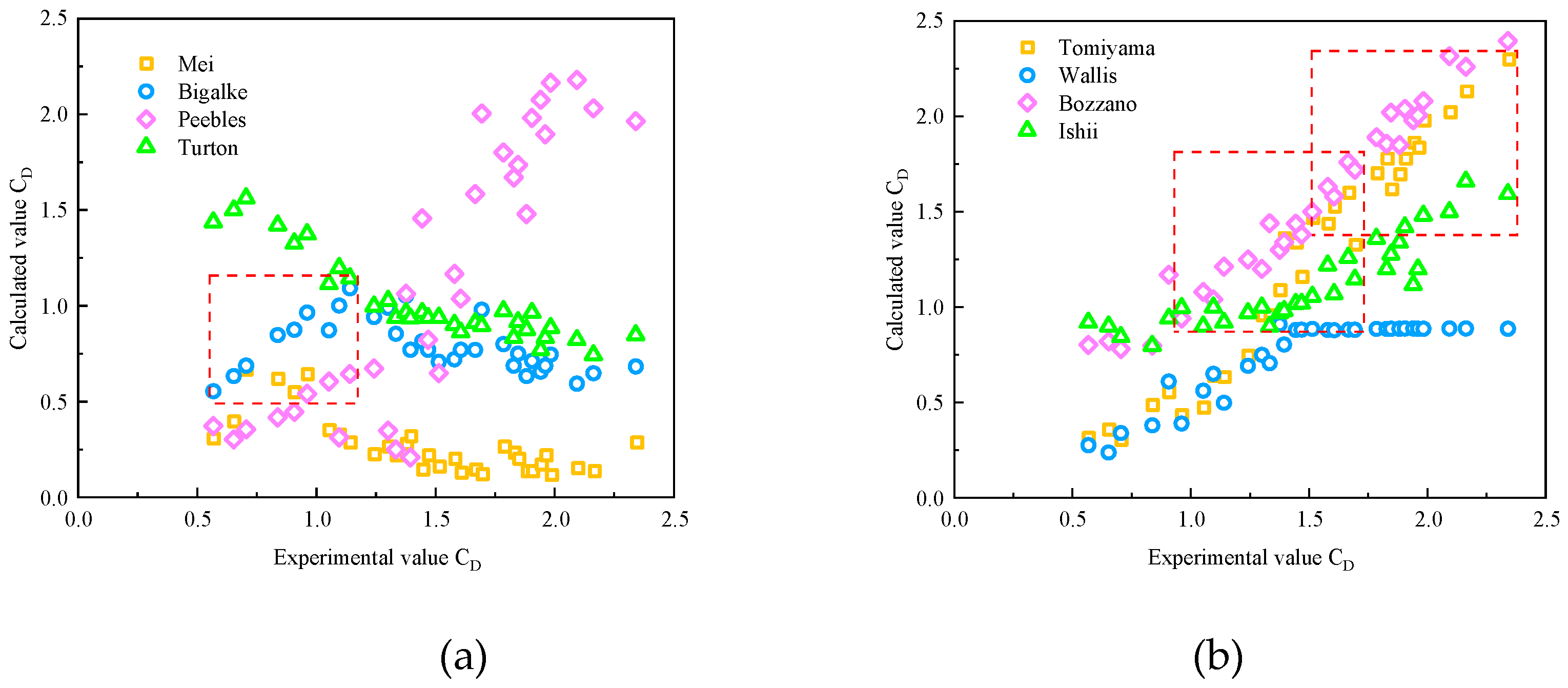
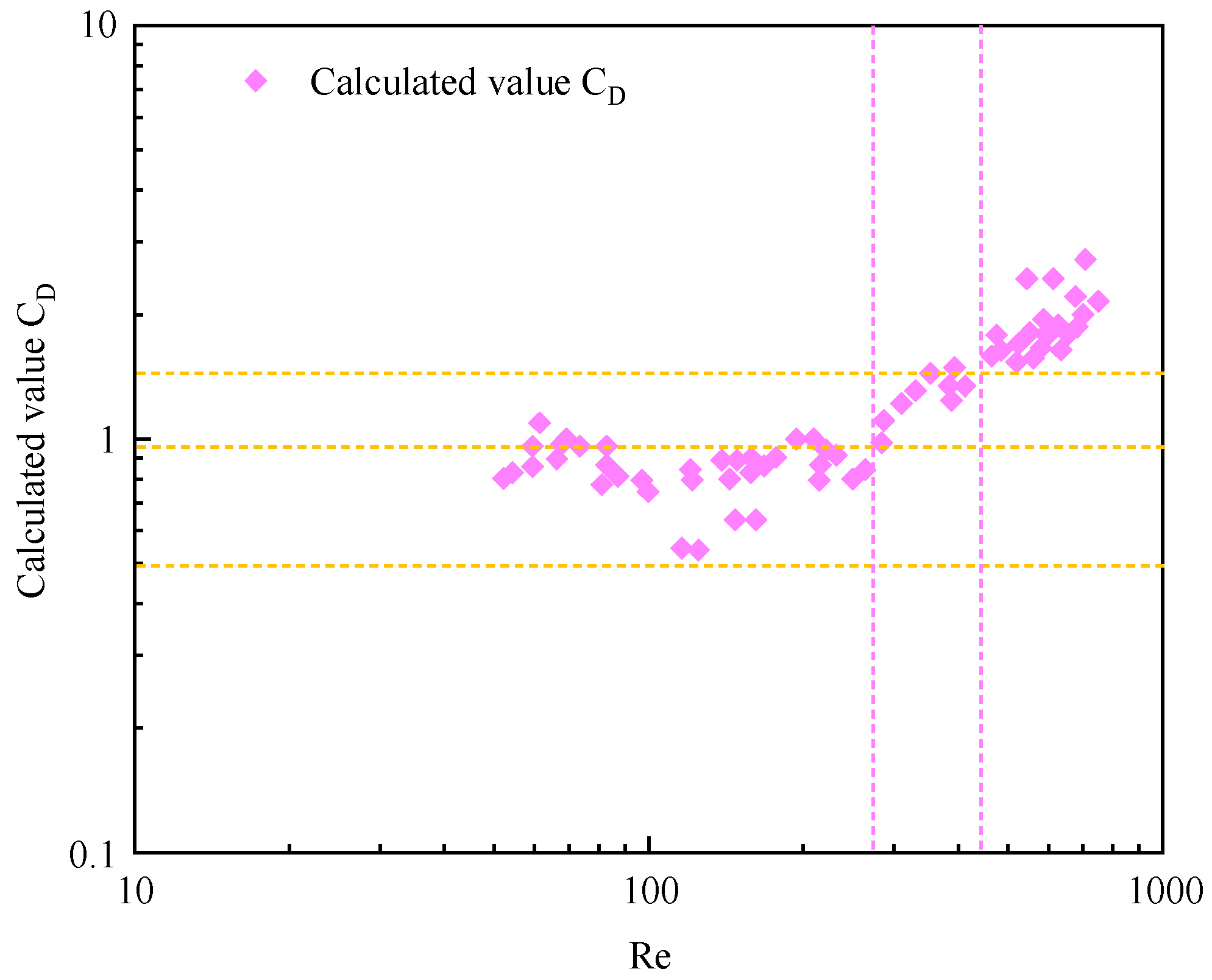
3.3. Drag coefficient during bubble migration
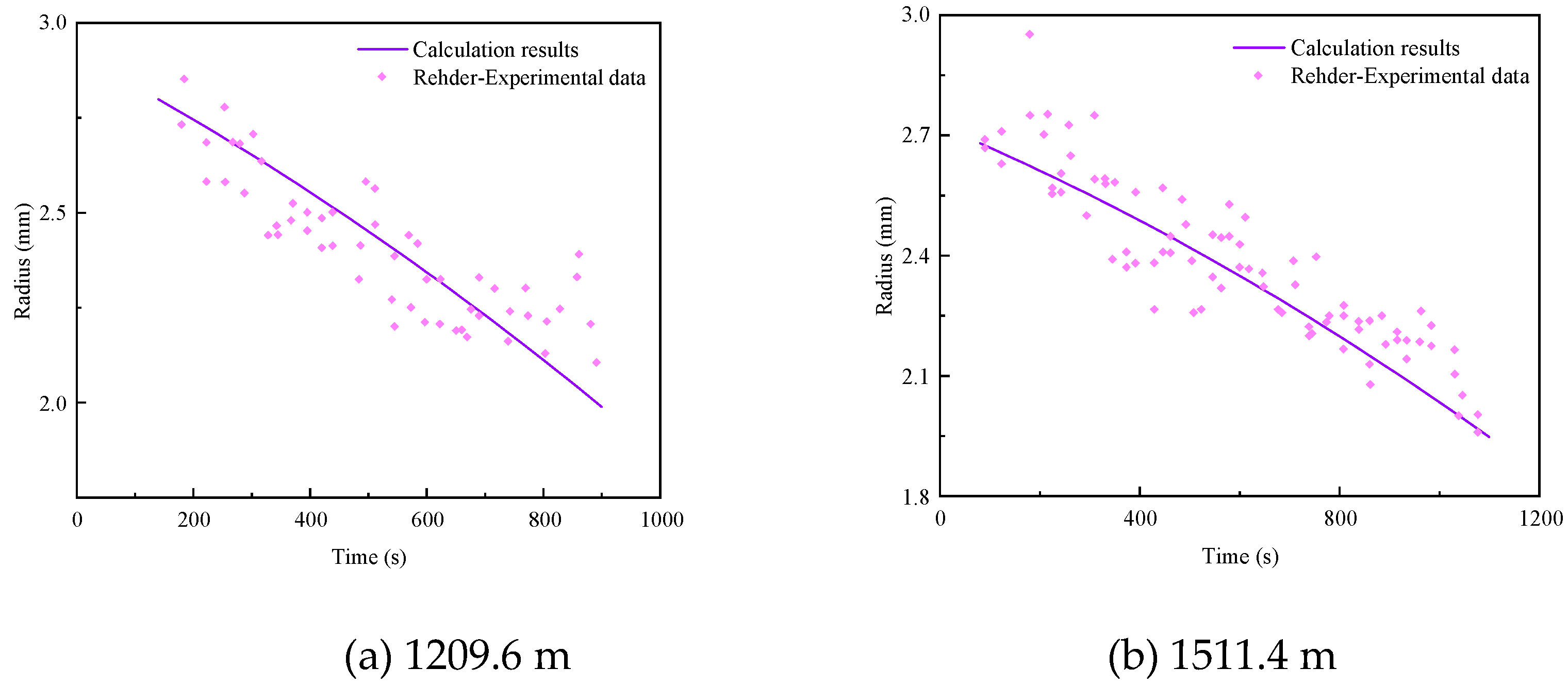
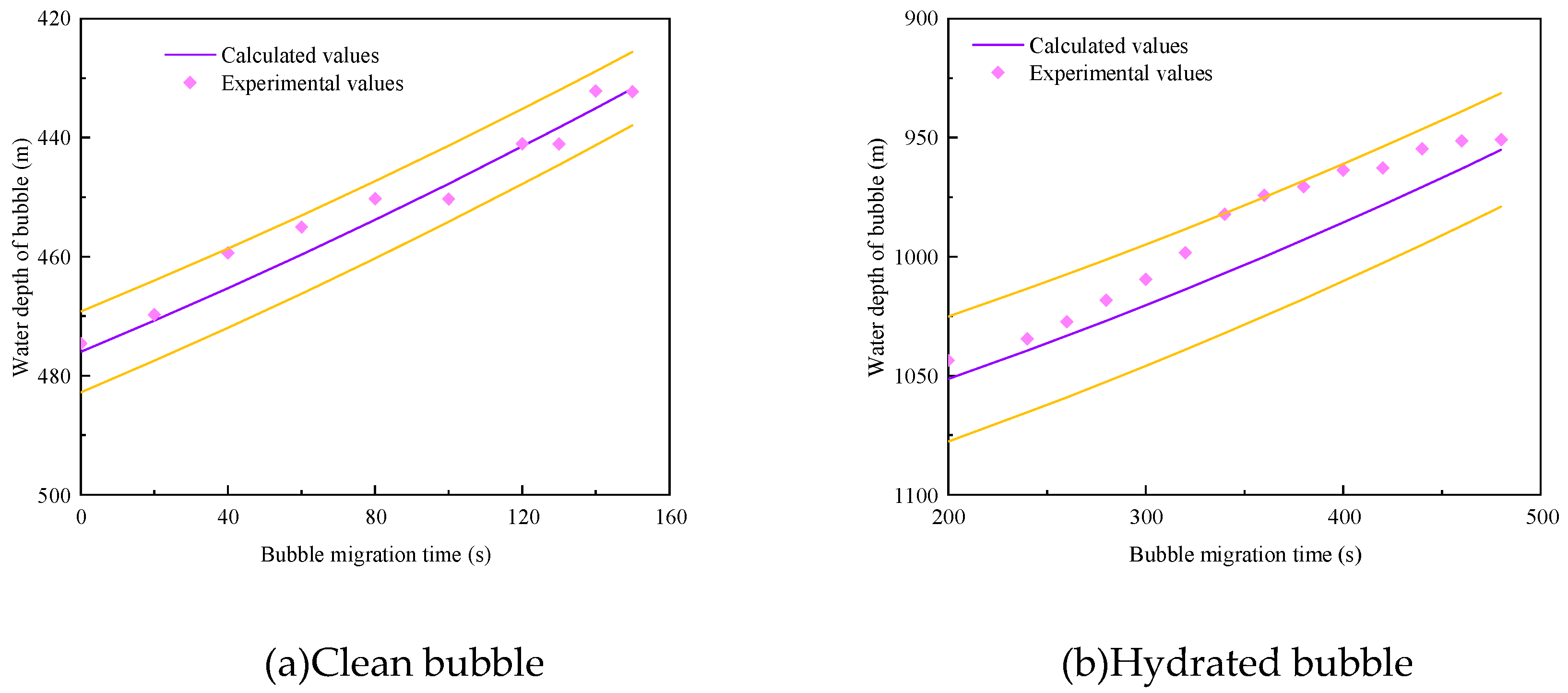
3.4. Model Verification
4. Discussion
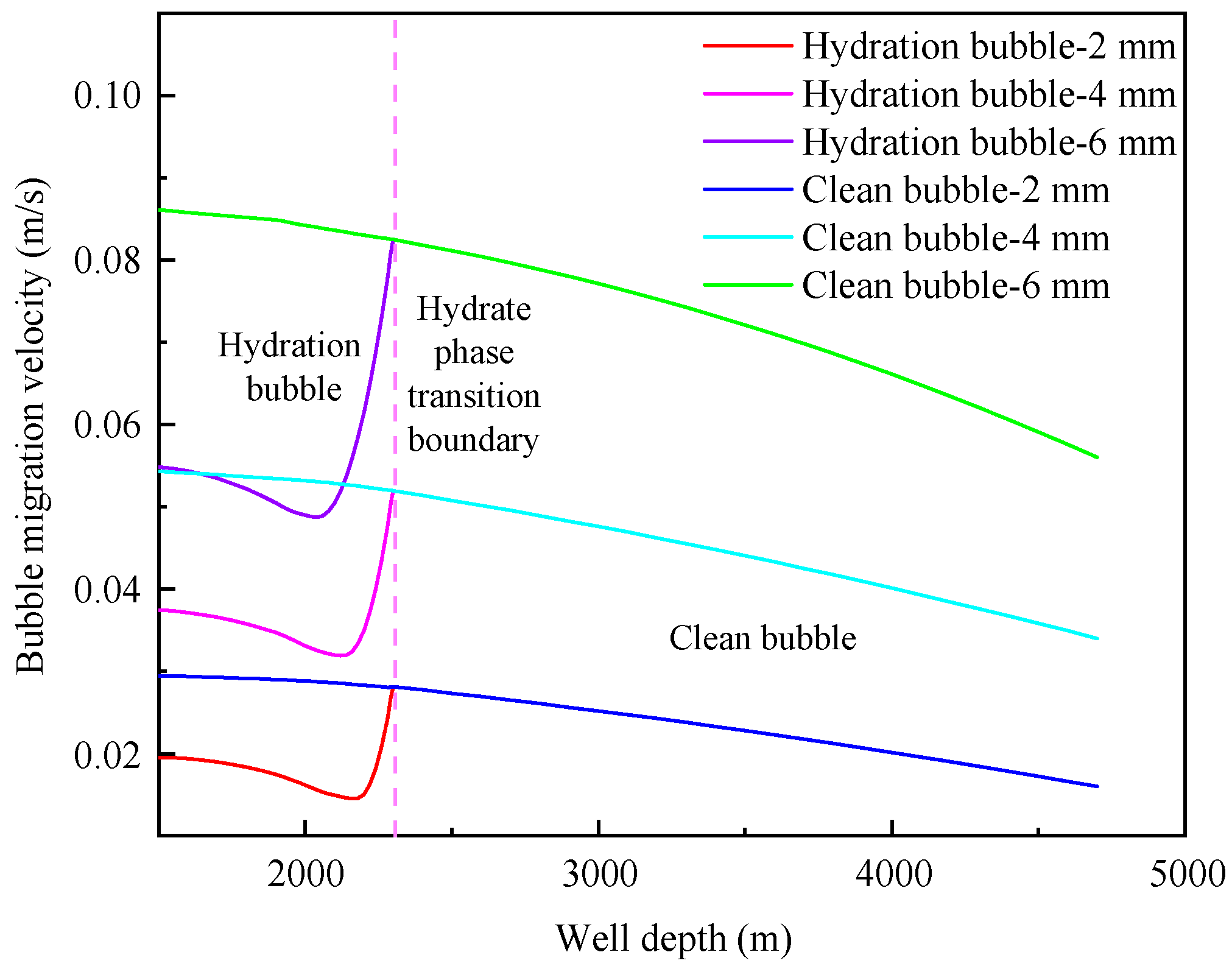
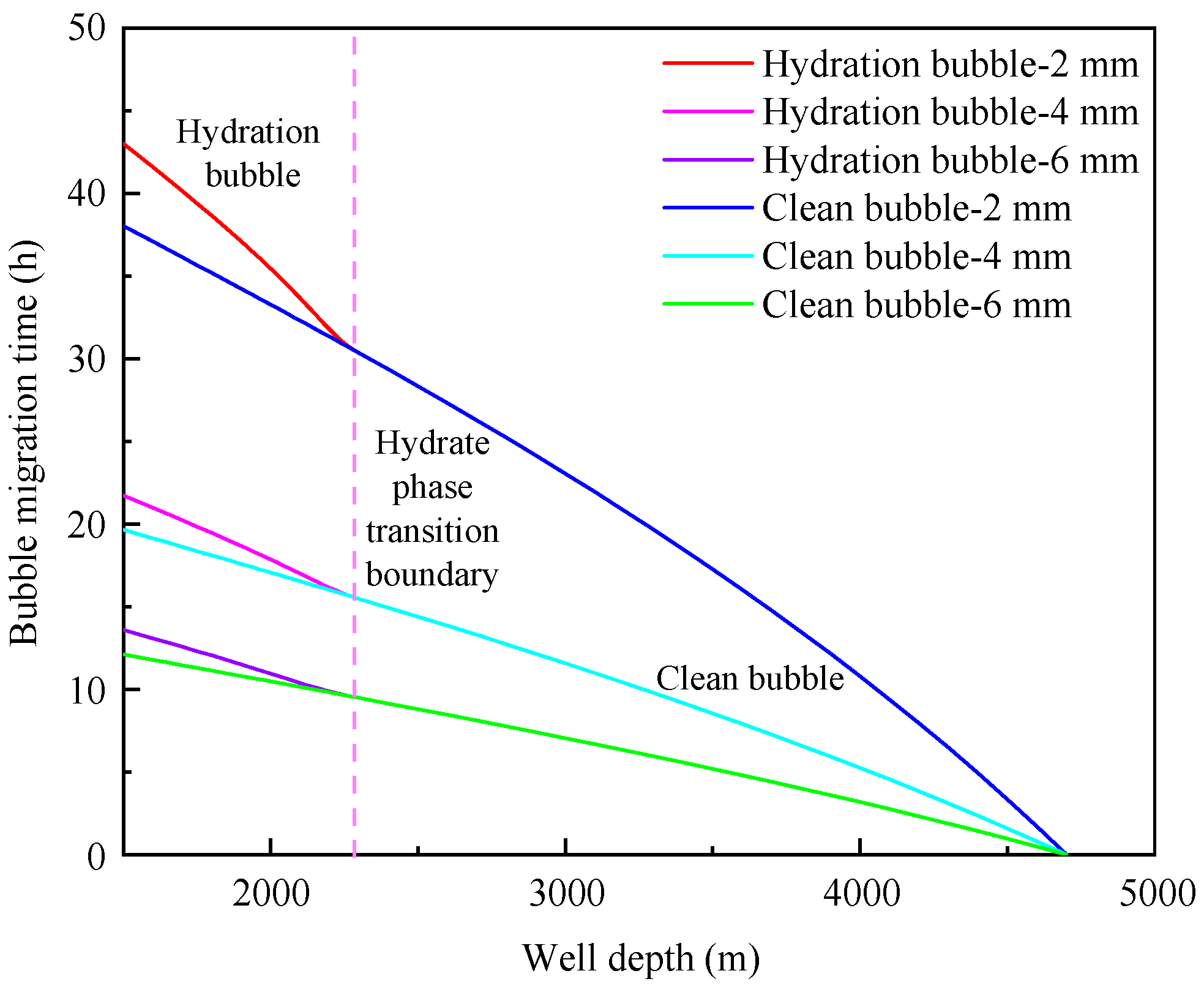
4.1. Influence of initial bubble size
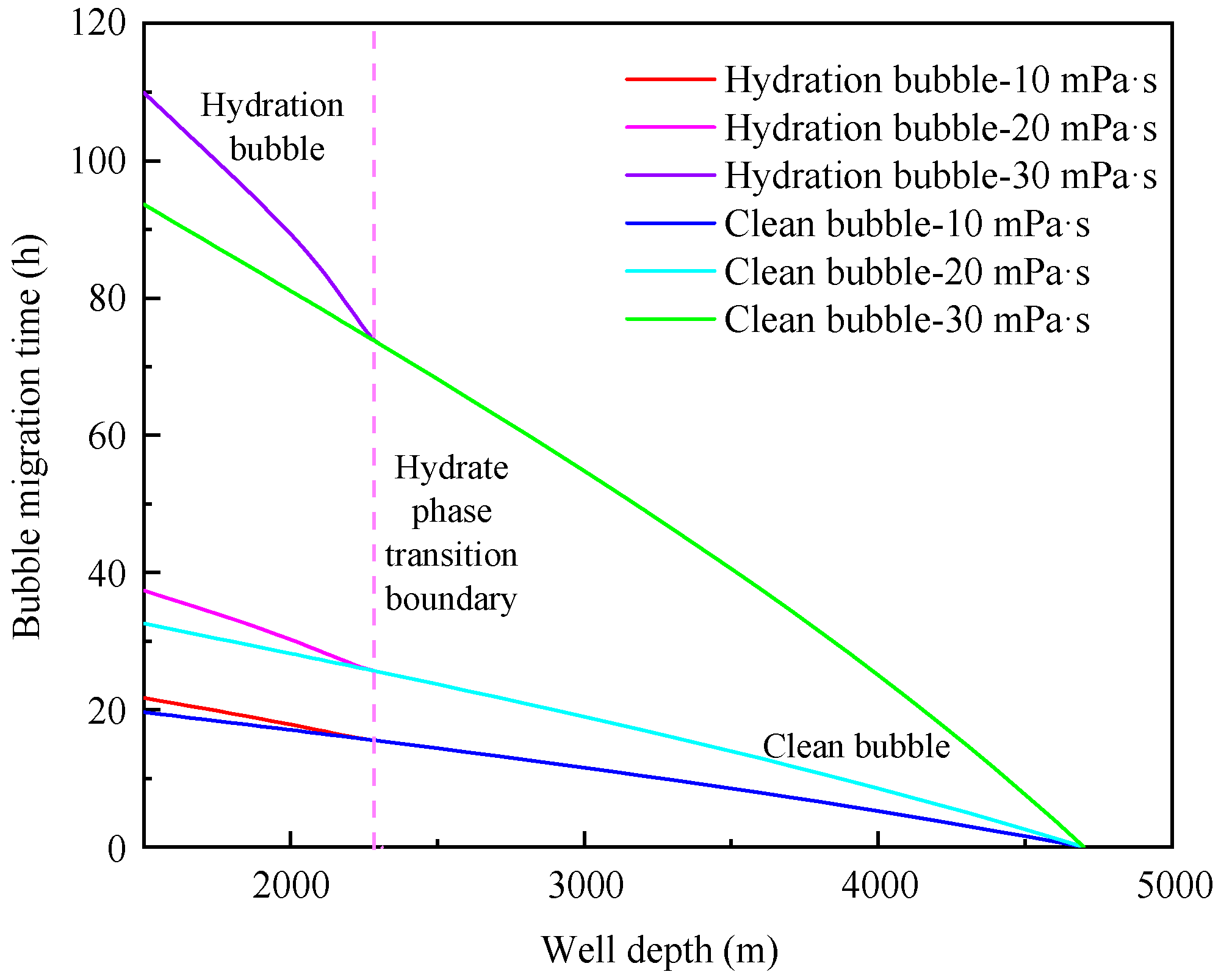
4.2. Influence of annular fluid viscosity
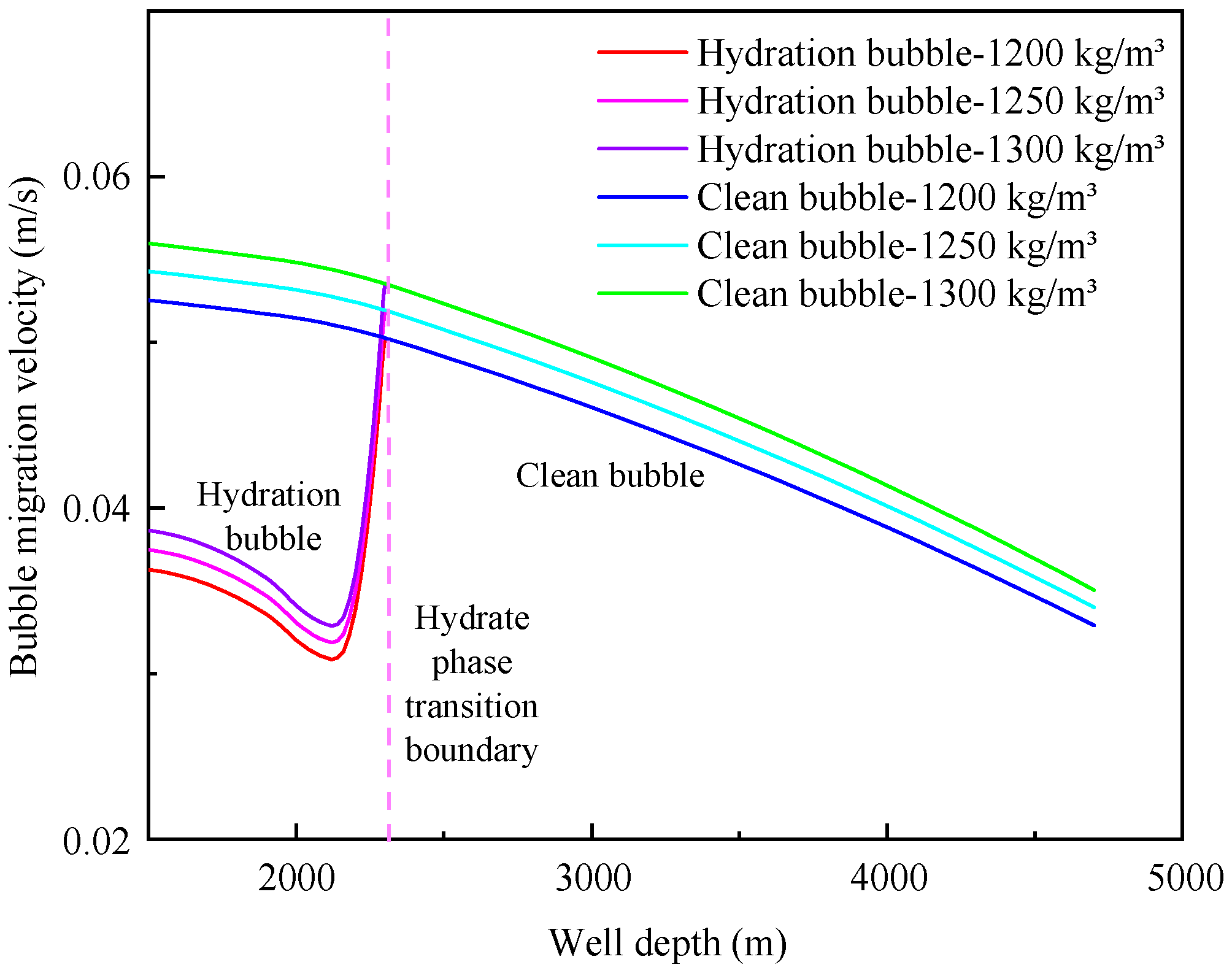
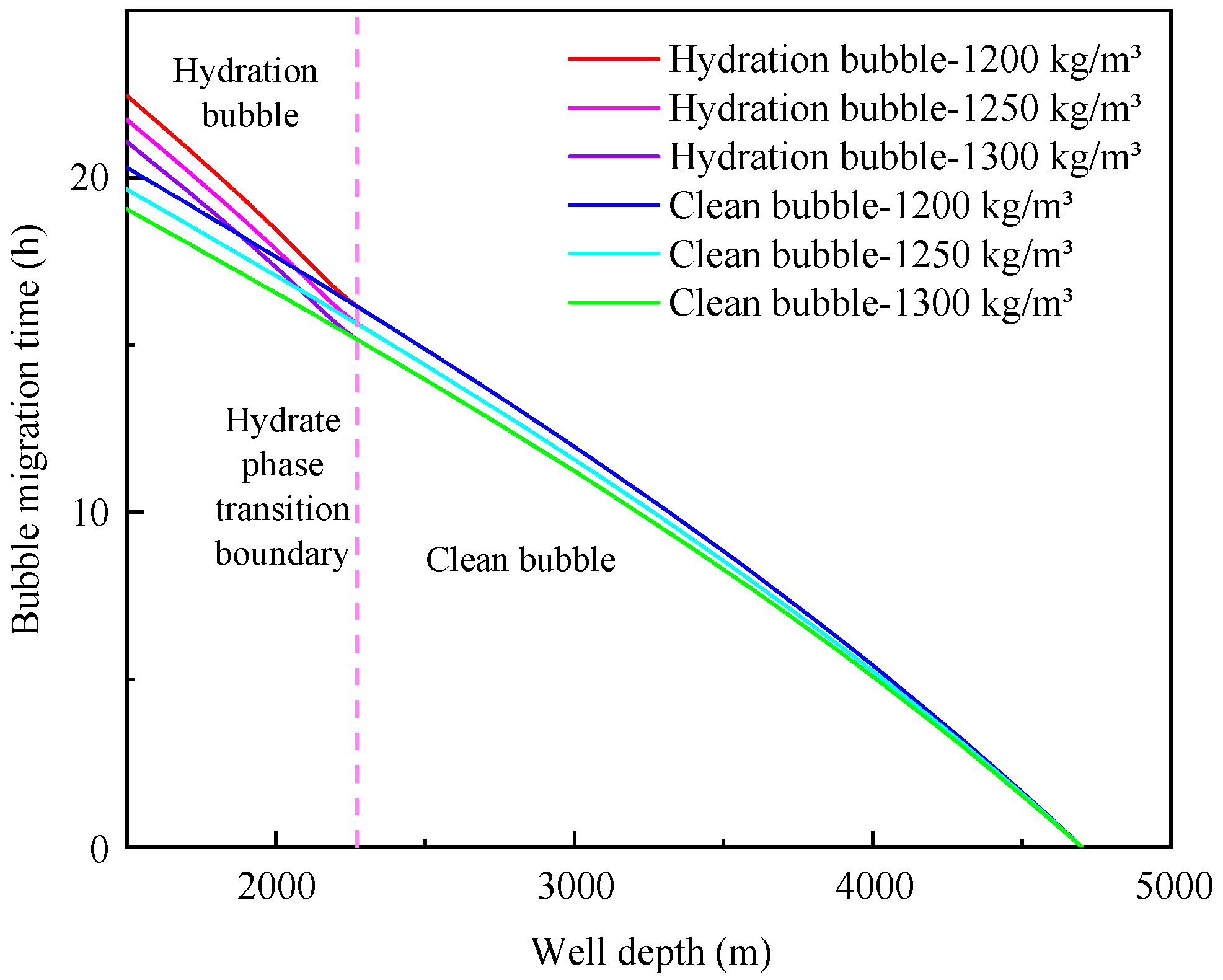
4.3. Influence of annulus fluid density
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Y H Mori. Clathrate hydrate formation at the interface between liquid CO2 and water phases—a review of rival models characterizing “hydrate films”, Energy Convers. Manage. 1998, 39 (15) :1537-1557.
- Gao Y, Ye C, Zhao X, et al. Risk analysis on the blowout in deepwater drilling when encountering hydrate-bearing reservoir[J]. Ocean Engineering, 2018, 170:1-5.
- Sun B, Pan S, Zhang J, et al. A Dynamic Model for Predicting the Geometry of Bubble Entrapped in Yield Stress Fluid[J]. Chemical Engineering Journal, 2019, 391:123569.
- Li C, Huang T. Simulation of gas bubbles with gas hydrates rising in deep water[J]. Ocean Engineering, 2016, 112:16-24.
- Joseph D. D. Rise velocity of a spherical cap bubble[J]. Journal of Fluid Mechanics, 2003, 488: 213-223.
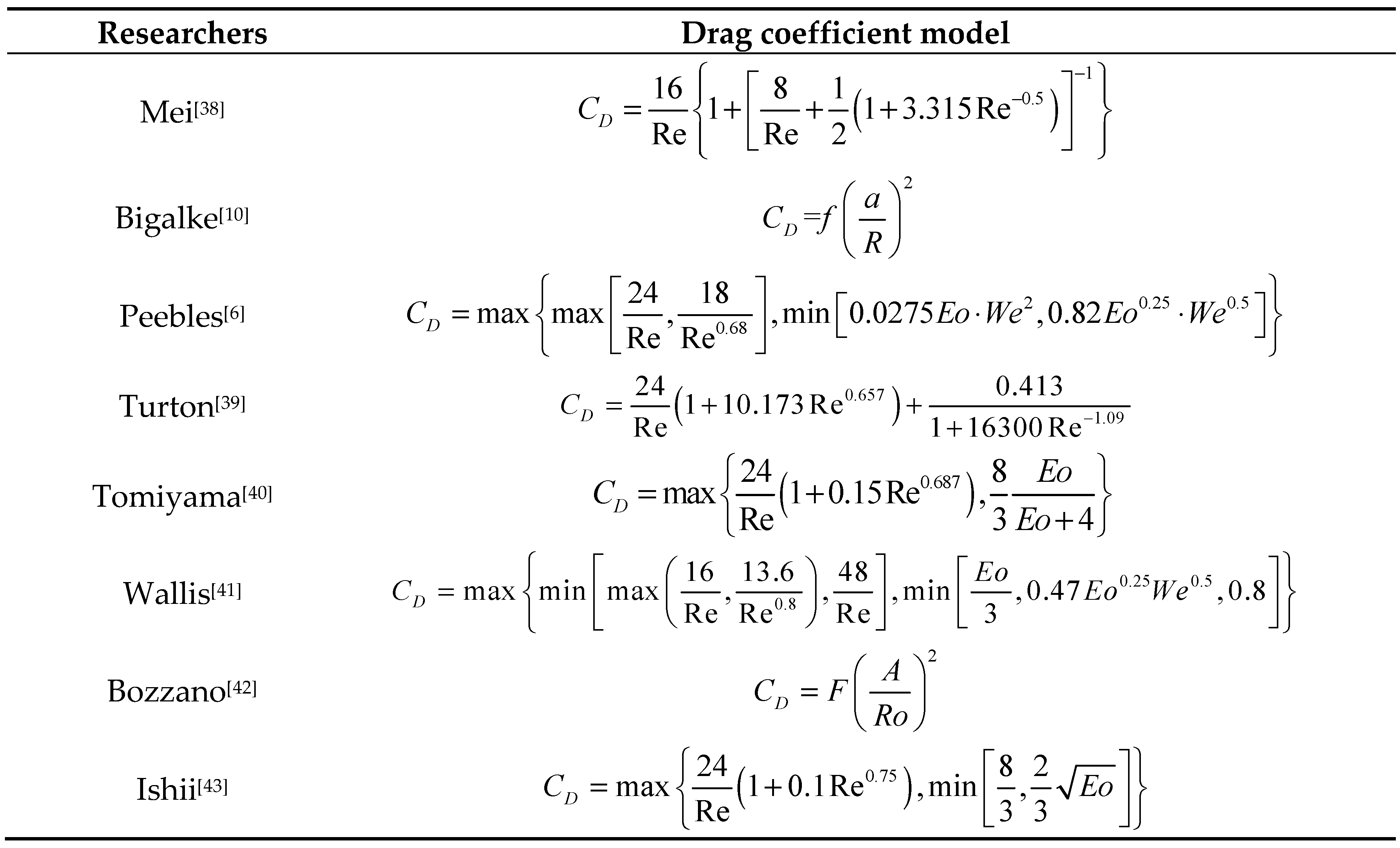
- Peebles F N, Garber H J. Studies on the motion of gas bubbles in liquids[J]. Chemical Engineering Progress, 1953, 49(2): 88-97.
- Collins R. The effect of a containing cylindrical boundary on the velocity of a large gas bubble in a liquid[J]. Journal of Fluid Mechanics, 1967, 28(01): 97-112.
- Margaritis, Bokkel. Bubble rise velocities and drag coefficients in non-Newtonian polysaccharide solutions[J]. Biotechnology and Bioengineering, 1999, 64(3): 257-266.
- Taylor C J, Miller K T, Koh C A, et al. Macroscopic Investigation of Hydrate Film Growth at the Hydrocarbon/Water Interface. Chem. Eng. Sci. 2007, 62, 6524-6533.
- Bigalke N K, Enstad L I, Rehder G, et al. Terminal velocities of pure and hydrate coated CO2 droplets and CH4 bubbles rising in a simulated oceanic environment[J]. Deep-Sea Research, 2010, 57: 1102-1110.
- Sun X, Sun B, Wang Z, et al. A new model for hydrodynamics and mass transfer of hydrated bubble rising in deep water[J]. Chemical Engineering Science, 2017, 173:168-178.
- Liu Z, Li H, Chen L, et al. A new model of and insight into hydrate film lateral growth along the gas-liquid interface considering natural convection heat transfer[J]. Energy & Fuels, 2018, 32(2): 2053-2063.
- Sun B, Guo Y, Wang Z, et al. Experimental study on the drag coefficient of single bubbles rising in static non-Newtonian fluids in wellbore[J]. Journal of Natural Gas Science and Engineering, 2015, 26:867-872.
- Wei H, Du Q, Cao B, et al. The Ascending Law of Gas Bubbles in a Wellbore Considering the Phase Change of Natural Gas Hydrates during Deepwater Well Shut-in [J]. Petroleum Drilling Techniques, 2019, 047(002):42-49.
- D. Sikorski, H. Tabuteau, and J. R. D. Bruyn, Motion and shape of bubbles rising through a yield-stress fluid, Journal of Non-Newtonian Fluid Mechanics. 2009, 159 (13) :10-16.
- C.H. Marks, Measurements of the terminal velocity of bubbles rising in a chain, ASME J. Fluids Eng. 1973, 95:17-22.
- N.M. Omran, P.J. Foster, The terminal velocity of a chain of drops or bubbles in a liquid, Trans. Inst. Chem. Eng. 1977, 55:171-177.
- Zhang, J., Fan, L.S., 2003. On the rise velocity of an interactive bubble in liquids. Chem. Eng. J. 92 (1), 169-176.
- Shi B H, Gong J, Sun C Y, et al. An Inward and Outward Natural Gas Hydrates Growth Shell Model Considering Intrinsic Kinetics, Mass and Heat Transfer. Chem. Eng. J.2011, 171, 1308-1316.
- Li S, Sun C, Liu B, et al. Initial thickness measurements and insights into crystal growth of methane hydrate film[J]. AIChE Journal, 2013, 59(6): 2145-2154.
- Lee S Y, Kim H C, Lee J D. Morphology Study of Methane−Propane Clathrate Hydrates on the Bubble Surface in the Presence of SDS or PVCap. J. Cryst. Growth 2014, 402, 249-259.
- Li S L, Sun C Y, Chen G J, et al. Measurements of hydrate film fracture under conditions simulating the rise of hydrated gas bubbles in deep water. Chem. Eng. Sci. 2014, 116, 109-117.
- Salamatin A N, Hondoh T, Uchida T, et al. Post-nucleation conversion of an air bubble to clathrate air–hydrate crystal in ice. J. Cryst. Growth. 1998, 193(1-2), 197-218.
- Y.H. Mori, T. Mochizuki, Mass transport across clathrate hydrate films—a capillary permeation model, Chem. Eng. Sci. 1997, 52 (20) :3613-3616.
- K. Ogasawara, A. Yamasaki, H. Teng. Mass transfer from CO2 drops traveling in high-pressure and low-temperature water, Energy Fuels. 2001, 15(1):147-150.
- Ogasawara K, Yamasaki A, Teng H. 2001. Mass transfer from CO2 drops traveling in high-pressure and low-temperature water. Energy Fuels 15 (1), 147-150.
- D. Daniel-David, F. Guerton, C. Dicharry, J. Torré, D. Broseta, Hydrate growth at the interface between water and pure or mixed CO2/CH4 gases: Influence of pressure, temperature, gas composition and water-soluble surfactants, Chem. Eng. Sci. 2015,132: 118-127.
- Rehder G, Leifer I, Brewer P G, et al. Controls on Methane Bubble Dissolution Inside and Outside the Hydrate Stability Field from Open Ocean Field Experiments and Numerical Modeling. Mar. Chem. 2009, 114, 19-30.
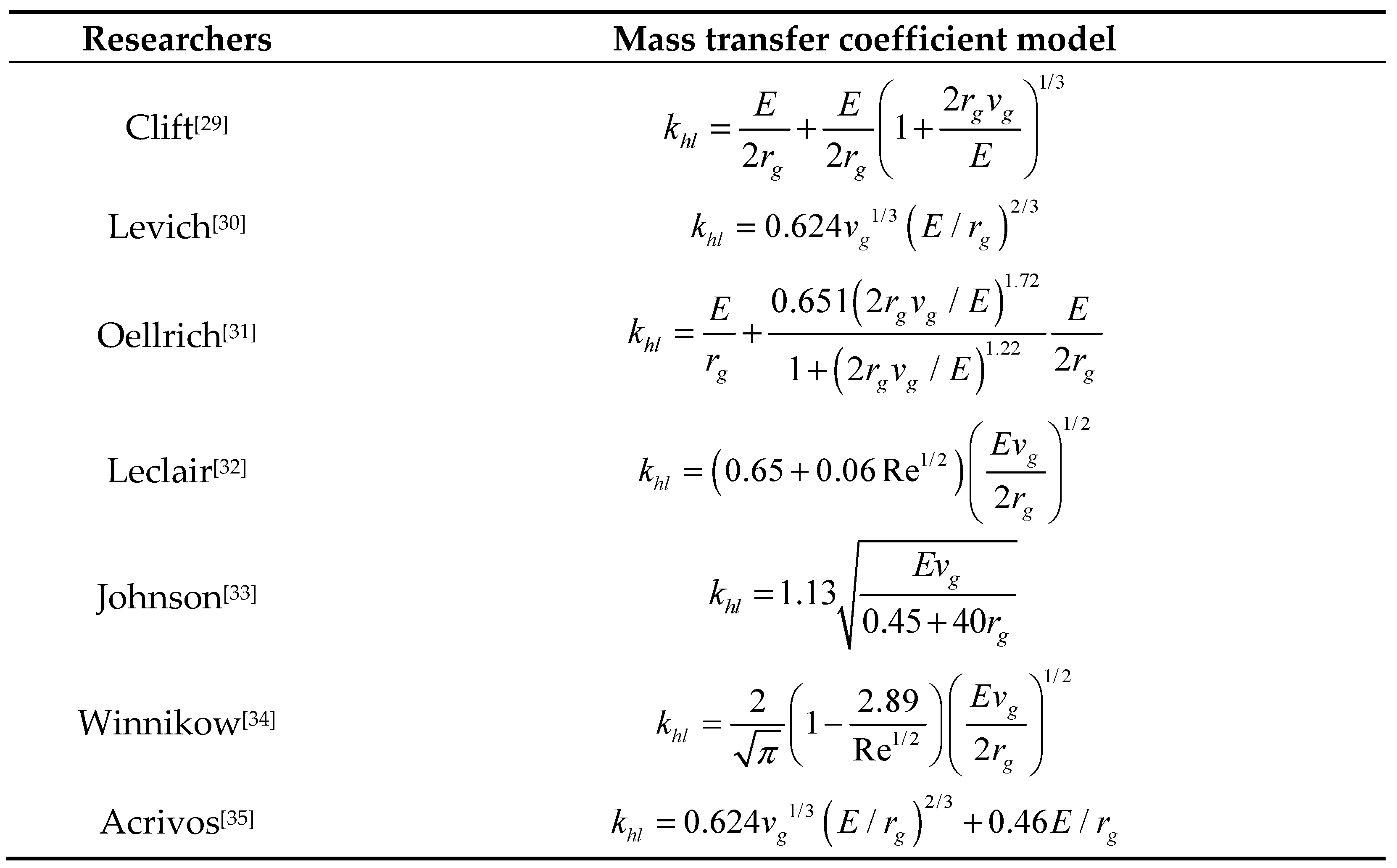
- Clift R C, Grace J R. Bubbles, Drops and Particles[M]. 1978: 58-67.
- V.G. Levich, Physicochemical Hydrodynamics, Prentice Hall, New York, 1962.
- Oellrich L, Schmidt-Traub H. Theoretische berechnung des stofftransports in der umgebung einer einzelblase. Chem. Eng. Sci. 28 (3), 711-721.
- Leclair B P, Hamielec A E. 1971. Viscous flow through particle assemblages at intermediate Reynolds numbers-a cell model for transport in bubble swarms. Can. J. Chem. Eng. 49 (6), 713-720.
- A. Johnson, I. Rezmer-Cooper, T. Bailey, D. Mccann, Gas migration: fast, slow or stopped, Oil Industry. 1995.
- Winnikow S. 1967. Letters to the editor. Chem. Eng. Sci. 22 (3), 477.
- Acrivos A, Goddard J D. 1965. Asymptotic expansions for laminar forced convection heat and mass transfer. J. Fluid Mech. 23 (2), 273-291.
- Rodrigue D, De Kee D, Fong M, et al. A note on the drag coefficient of a single gas bubble in a power-law fluid[J]. The Canadian Journal of Chemical Engineering, 1999, 77(4): 766-768.
- Hao L, Sun B, Gao Y, et al. Study on Rising Velocity of Methane Bubble in Deepwater Wellbore During the Shut-In Period[C]. Abu Dhabi International Petroleum Exhibition & Conference. 2017.
- Mei R, Klausner J F. Unsteady force on a spherical bubble at finite Reynolds number with small fluctuations in the free-stream velocity[J]. Physics of Fluids A: Fluid Dynamics, 1992, 4: 63-70.
- Turton R, Levenspiel O. A short note on the drag correlation for spheres[J]. Powder Technology, 1986, 47(1): 83-86.
- Tomiyama A, Kataoka I, Zun I, et al. Drag coefficients of single bubbles under normal and micro gravity conditions[J]. Jsme International Journal Ser B Fluids & Thermal Engineering, 2008, 41(2): 472-479.
- Wallis G B. The terminal speed of single drops or bubbles in an infinite medium[J]. International Journal of Multiphase Flow, 1974,1(4): 491-511.
- Bozzano G, Dente M. Shape and terminal velocity of single bubble motion: a novel approach[J]. Computers & Chemical Engineering, 2001, 25(4): 571-576.
- Ishii M, Chawla T C. Local drag laws in dispersed two-phase flow[J]. Nasa Sti/recon Technical Report N, 1979.


 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




