1. Introduction
In the last years, Actinidia arguta has attracted the interest of consumers and researchers due to its sensorial properties, nutritional composition, and health benefits [1]. Actinidia arguta, also known as the "mini kiwi," "kiwiberry," "baby kiwi," or "hardy kiwifruit," is a small grape-sized fruit with thin, edible, and hairless skin and an aromatic, sweet flavour. It is one of the most nutrient-dense fruits and an exceptional source of antioxidants (primarily polyphenols), vitamins (especially vitamin C), carotenoids, chlorophylls, sugars, dietary fiber, organic acids and minerals [2–5]. The harvesting and storage conditions of Actinidia arguta highly influence its aroma, taste and physicochemical features [3,6–8]. The most important barriers for the commercialization of these fruits are intensive labour during harvest and their short shelf life (1–2 months) when kept at 0°C. The short shelf-life is due to fruit softening, skin wrinkling due to water loss (dryness), and fruit decay [1,6–8]. For these reasons, Actinidia arguta are usually picked up before fully ripe, when the sugar levels correspond to 6.5 - 8 °Brix [6,7,9,10].
Fruits are known as nutritionally important products, however, these products are considered perishable and cannot be accepted for consumption if they are not managed properly after harvesting [11]. Postharvest diseases of fruits and vegetables are a major problem in fresh horticultural produce storage and significantly affect the cost of food production and produce trade [12]. Efforts to reduce food losses and preserve the quality of fresh food over a longer period has been a priority for the food industry, and the promising use of edible coatings for food applications has attracted great interest as sustainable alternative packaging materials and as a potential source of improved food safety and quality [13,14].
More recently, there have been reports of the application of fabricated coarse emulsion and nanoemulsion coatings with varied compositions and formulations, which have been used to extend the shelf-life of fruits [15–17]. These coatings offer a protective barrier that prevents microbial growth and promotes a modified atmosphere, which reduces the rate of respiration and transpiration, thereby slowing down senescence and prolonging the storage life of fresh produce. [16,18–22]. Formulations of edible coatings utilizing polysaccharides and essential oil components have been extensively utilized in whole fruits, with the primary objective of mitigating water loss and preserving quality. Furthermore, there has been an increasing inclination towards utilizing natural products to manage decay and prolong the shelf-life of perishable goods. [23]. Alginate is a biopolymer consisting of D-mannuronic acid and L-guluronic acid monomers, which is naturally derived from brown seaweed. As a salt of alginic acid, alginate possesses colloidal properties and can form robust gels or insoluble polymers through interaction with multivalent metal cations, such as calcium. The excellent film-forming ability of alginate make it suitable as a potential edible coating material [24]. Essential oils or their compounds, when incorporated into edible coating formulations as an antimicrobial agent, can enhance the effectiveness of coatings in safeguarding foods against microbial spoilage, ultimately extending their postharvest shelf life and preserving their quality [25]. The chemical composition of plant-derived products, such as essential oils, are highly variable with seasonal and cultural practices. The use of single compounds instead of naturally produced essential oil mixtures offers a better approach to obtain edible coatings with constant characteristics [26,27].
The possibility of applications of edible coatings to preserve food quality is not new but this research topic has recently been gaining traction driven by an increasing consumer demand for safer, high quality, and minimally processed foods [19,28].
The type of food product dictates the most appropriate method for coating application, and edible coatings can be applied by different methods such as spraying, dipping, brushing, wrapping, casting, panning, or rolling [29]. Dipping is advantageous when a product requires several applications of a coating to obtain uniformity on an irregular surface; however, spraying provides a more uniform coating [29].
The study aims to achieve three primary objectives. Firstly, to determine the impact of edible coatings use on the quality and storage capacity of fresh Actinidia arguta fruit. Secondly, to evaluate and compare the impacts of using edible alginate coatings in coarse emulsion (C) and nanoemulsion (N) forms, and additionally, assess the effectiveness of two different coating application techniques, namely spraying (S) and dipping (D). Lastly, to evaluate the utilization of Vis-NIR for data analysis and interpretation of coatings related information.
2. Materials and Methods
2.1. Fruit Material
For all trials, kiwiberry (Actinidia arguta cv. ‘Ken´s Red’) were harvested from 5-year-old vines growing in several blocks on a commercially managed orchard located in north Portugal (Baião). In this area, the conventional harvest time occurs during September. Fruits were collected and were transported to the post-harvest laboratory at the University of Algarve at ambient temperature and arriving within 24 h of harvest. Immediately on arrival at the laboratory, 30 fruit were randomly selected for fruit maturity characterization (quality measurements). The remaining fruit were packed into single layer trays and treatments below were performed.
2.2. Chemicals
Food grade sodium alginate (AL) and nonionic surfactant Tween80 (Polyoxyethylene sorbitan MonoOleate) were purchased from Sigma–Aldrich Chemic (Steinhein, Germany). To obtain ultrapure water, a Milli-Q filtration system was used. Sigma-Aldrich Chemic (Steinhein, Germany) provided the calcium chloride, and Scharlau (Barcelona, Spain) provided the ascorbic acid. Citral (Cit) and eugenol (Eug), essential oil components, were bought from Sigma-Aldrich Chemic (Steinhein, Germany). Scharlau (Barcelona, Spain) supplied the ascorbic acid.
2.3. Preparation of Emulsions
Sodium alginate (2%, w/w) was dissolved in MilliQ water at 70 °C, with continuous stirring until complete dissolution, then the solution was cooled down to 25 °C. This solution was the basis for the preparation of the course and nano emulsions [16]. Essential oils compounds (EO´s) concentrations, citral (Cit) and eugenol (Eug), used in the emulsions were based on their minimum inhibitory concentrations (MIC) and the double MIC, 0.15 and 0.3% (w/w) for citral and 0.10 and 0.20% (w/w) for eugenol [33].
The coarse emulsions (C) based on sodium alginate (2%, w/w) and EO´s were formulated as described by Rojas-Graü et al. [34] and Guerreiro et al. [35], by dissolving powders of sodium alginate (AL) in distilled water while stirring until the solution became clear. Coating forming solutions were homogenized with an Ultra Turrax T25(IKA WERKE, Germany).
To prepare the nanoemulsion (N) the EO’s were incorporated into the AL solution (2%, w/w) using a thermomix (Vorwerk & Co.KG, Wuppertal, Germany) in 6 series of 1 min at speed 9 (3028 g), avoiding exceeding 37 °C. Thereafter, the emulsion was mixed with a T-18 Ultraturrax (IKA, Staufen, Germany) for 1 min at 1762 g. Tween 80 concentrations were bound at an oil/surfactant ratio of 1:3 [16].
2.4. Coating’s Characterization
Particle size, polydispersity and z-potential of coarse emulsions and nanoemulsions were determined by dynamic-light-scattering (DLS) and phase-analysis light scattering (PALS) [16]. To sidestep multiple scattering effects, samples were diluted prior to analysis with Milli-Q water (1:10). The emulsions were observed by negative-staining electron microscopy as a direct measurement of their droplet size and shape as reported by Artiga-Artigas et al. [36]. The grids were observed in a transmission electron microscope Morgagni 268D TEM (FEI Company, Netherlands) with a CCD Mega-View camera (Olympus, Tokyo, Japan).
2.5. Spraying Application Method
To apply coarse emulsion and nanoemulsion preparations, the spraying method (S) was performed by spraying uniformly on the whole fruit surface with the use of a paint sprayer (Dexter nozzle 1.5mm, ADEO services, Ronchini - France), at a pressure of 8 bar; the flow rate was 150 L min-1 and spraying distance 20 cm. After coating applications, fruit were fan dried at room temperature for 2–3 min, then a solution of calcium chloride 1% was applied, to form a uniform, transparent, water-insoluble and thermo-irreversible gel at room temperature, by cross-linking with di- or tri-valent ions, promoted by the characteristics of alginates [37]. Then, coatings formed on kiwiberries were allowed to dry during 1 h at room temperature (~20 °C), labelled, weighed, and then randomly packed into polypropylene plastic trays (8 cm × 10 cm × 4 cm), perforated in the cover, and stored at 0.5◦C with relative humidity (RH) 95%. After 2, and 4 weeks, 30 fruits per treatment, were sampled for 5 d shelf-life at ~5 ◦C and 60% RH. After that, shelf-life quality parameters were performed.
2.6. Dipping Application Method (D)
Each treatment, from coarse emulsions and nanoemulsions preparations, was performed in two steps: first, kiwiberries were dipped into the edible coating solution for 2 min; the excess of coating material was allowed to drip off for 30 s before the second dip in the calcium chloride solution for 1 min, to induce a cross linking reaction. Then, fruits were labelled, weighed, and then randomly packed into polypropylene plastic trays (8 cm × 10 cm × 4 cm), perforated in the cover, and stored and sampled as the ones with spraying treatment.
2.7. Quality Measurements
The quality analyses were performed on the control and ten treatments sets resulting from the combination of the multiple emulsion formulations and application methodologies (Table 1).
Colour measurements were based on CIE (Commission International de I’Eclairage). For that, a Minolta Chroma meter CR-300 (EC Minolta, Japan) was used using the CIELab scale (L*, a* and b*). The L* represents colour lightness (0 = black and 100 = white). Hue was calculated as h° = arctan (b*/a*) and colour saturation (chroma) as C* = (a*2 + b*2)0.5 (McGuire, 1992). The firmness (Fm) of kiwiberries was measured at the fruit equator, by compression with a Chatillon TCD200 and Digital Force Gauge DFIS 50 (Jonh Chatillon & Sons, Inc., USA) using a 45 mm diameter piston at a speed of 1 mm s-1 compressing the fruit 2 mm, with the result expressed in Newton (N). Each fruit was then squeezed individually in an automatic squeezer. A fraction of the juice was then used to measure the soluble solids content (SSC, in ºBrix) with a digital refractometer (Atago Co. Ltd., Tokyo, Japan). Determination of dry matter (DMC) was determined using a forced-air oven at 105ºC for 48 h and was expressed as percentage of initial weight. Weight loss (WL) was expressed as percentage of initial weight.
Table 1.
Table of treatments, resulting from the emulsion formulation vs. application methodologies.
Table 1.
Table of treatments, resulting from the emulsion formulation vs. application methodologies.
| |
List of Treatments |
| 1 |
Control |
| 2 |
AL-S |
| 3 |
AL-D |
| 4 |
AL-Cit 0.15+Eug 0.1-N-S |
| 5 |
AL-Cit 0.15+Eug 0.1-N-D |
| 6 |
AL-Cit 0.15+Eug 0.1-C-S |
| 7 |
AL-Cit 0.15+Eug 0.1-C-D |
| 8 |
AL-Cit 0.3+Eug 0.2-N-S |
| 9 |
AL-Cit 0.3+Eug 0.2-N-D |
| 10 |
AL-Cit 0.3+Eug 0.2-C-S |
| 11 |
AL-Cit 0.3+Eug 0.2-C-D |
2.8. Experimental Setup of VIS/NIR Measurements
Non-destructive technology was used as a complementary method of analysis in this experiment. For the acquisition of the kiwiberries diffuse reflectance spectra, we have used an optical setup consisting of a spectrometer (Hamamatsu C9405 CA, Hamamatsu, Japan) working in the range of 432–1147 nm, a tungsten light source (Ocean Optics HL 2000, Ocean Optics, USA) and a bifurcated optical fiber with a customized interactance probe described at Guerreiro et al. [
38]. As usual, the protocol includes the measurement of reference (Ref), dark (D) and sample spectra (S). The reflectance, R, is then calculated as R=(S-D)/(Ref-D). The reference measurements were performed with the interactance probe kept 1 cm above a Spectralon™ disk. The kiwiberries were always measured in the equatorial zone. To achieve best results, the average of 100 scans of 100 ms was taken for each fruit. The reflectance spectra were then grouped in a matrix of n samples by 1024 spectral features (wavelengths) and analyzed to investigate possible interactions between coatings and fruit. The calculations were performed using MATLAB® (Mathworks, USA) and Python.
2.9. Statistical Methods
To further investigate statistical patterns in the acquired data, data analysis was performed using IBMSPSS Statistic 27.0 software (IBM SPSS Inc., NY, USA) on an PC workstation (Intel(R) Core (TM) i7. The study employed an independent and dependent t-test to examine the mean difference in attributes between treatments and weeks at the 0.05 alpha level. The effect of time was determined by ANOVA Repeated Measures (Type III) and Bonferroni post hoc test for a significance level of p < 0.05. We also applied a multiple correspondence analysis (MCA) technique with standardized variables and two categorical independent variables to detect and represent underlying structures in a low-dimensional Euclidean space using a criterion of convergence of .00001 and a maximum iteration of 100 and a squared Euclidean distance [
39]. Since the representation alone is insufficient for creating a complete interpretation of the results, we applied an optimal scaling technique to analyze the relations between cases or among variables.
The organization of information in a hierarchical mode is done through factor axes or vectors specific to which eigenvalues are associated to the contribution of each axis to the observed data. The objective of Multidimensional scaling (MDS) method is to visualize the underlying, hidden structure in a data set. An association is calculated and provides an indication of how similar or dissimilar objects are [
40]. ALSCAL algorithm was used as the basis for obtaining the MDS. To guarantee the viability of the method it is necessary that this algorithm obtains values close to 1 for the R-squared (R
2), the value that represents the proportion of the variance for a dependent variable, while correlation explains the strength of the relationship between an independent and dependent variable. Stress values obtained at quality measures used in the evaluation of ALSCAL algorithm, suggests the following benchmarks for stress values: 0.20 = poor; 0.10 = fair, 0.05= good; 0.025=excellent, and 0.00 = perfect [
41]. The organization of information in a hierarchical mode is done through factor axes or vectors specific to which eigenvalues are associated to the contribution of each axis to the observed data.
3. Results and Discussion
3.1. Coating’s Characteristics
The preparation method is very important for the structure and stability of emulsions. In
Table 2, the droplet size (nm) showed two very distinct patterns: in the case of course emulsions, AL-Cit 0.15 + Eug 0.1-C obtained 1706.67±236.72nm and AL-Cit 0.3 + Eug 0.2-C had 1470.00±101.36nm, although nanoemulsions showed values between 236.73± 93.34 and 236.73± 93.34, for AL-Cit 0.15 + Eug 0.1-N and AL-Cit 0.3 + Eug 0.2-N, respectively. As expected, nanoemulsions had droplets in the nano range, smaller than 500 nm, this very small droplet size can reduce the occurrence of creaming, sedimentation and flocculation during storage [16]. Recently, Gago et al. [16] and Gago et al. [
42] reported that nanoemulsions based on alginate with lemongrass or citral alone presented lower droplets sizes. However, in the same study, it was found that when eugenol is added to the formulations the droplet size increases to values similar to those obtained in our work, so we can say that the presence of eugenol in the formulations will lead to higher values of droplet size, nevertheless in the nano range. According to Salvia-Trujillo et al. [
43] these differences in droplet size might be attributed to their molecular structure, concentration of volatile compounds, interfacial tension or surfactant affinity to each type of EO or their main compounds.
Two conditions are important in the case of polydispersity index, as higher the polydispersity index, the lower the uniformity of the droplet size in the formulation, and polydispersity values less than 0.3 mean good uniformity of particle size within the formulation [16]. The higher value obtained was in treatment AL-Cit 0.15 + Eug 0.1-C (0.82) and the lower was in AL-Cit 0.3 + Eug 0.2-N (0.62) (
Table 2). In the analyzed emulsions higher values of EO´s indicated lower polydispersity index. All these polydispersity values are similar to Gago et al. [
42] results for nanoemulsions using eugenol, though Yang et al. [
44] and Salvia-Trujillo et al. [
45] obtained almost half of these results. This may be due to the emulsions production process, which contributes to reach more uniform size droplets [16].
ζ-potential measures the amplitude of the electrostatic or charge repulsion/attraction between particles; a ζ-potential formed by surfactants can produce repulsive/attractive electrical forces among approaching oil droplets and thus prevents their coalescence (Gago et al., 2019). The results showed distinct values, contrarily to the expected, course emulsions showed better values, -80.97 ± 4.30 mV and -75.53 ± 0.24 mV, for AL-Cit 0.15 + Eug 0.1-C and AL-Cit 0.3 + Eug 0.2-C, respectively (
Table 2). The nanoemulsions had results between -17.10 ± 2.40 mV and -41.13 ± 0.51 mV. According to Heurtault et al. [
46] particles with ζ-potential more positive than +30 mV or more negative than -30 mV are commonly considered to be stable, since electrical charge of droplets is robust enough to assume that repulsive forces among droplets and are prevalent in the nanoemulsion systems. Our results of ζ-potential measurements present better values for course emulsions, which could be explained since emulsions and nanoemulsions were formulated with different essential oils, leading to differences in the dissociation degree and number of ionizable compounds of oils [
42].
In the results obtained, a significant difference in the emulsions’ characteristics is clearly visible; it is possible to state that the manufacturing methodology is the major responsible for these results.
3.2. General Quality Parameters of Fruits
3.2.1. Colour
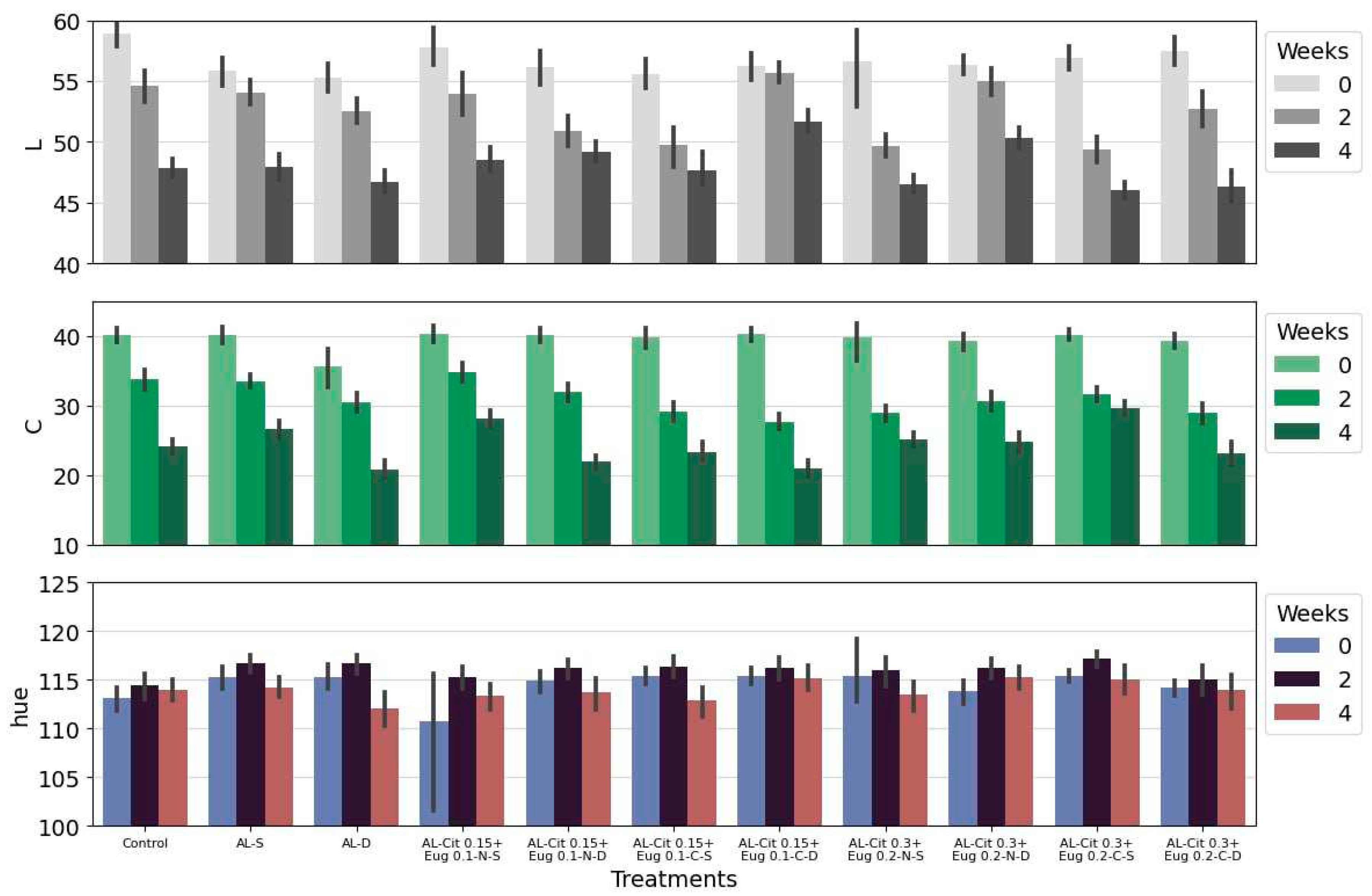
In general, the values obtained from CIE analyses during storage were consistent with visual analyses (
Figure 1). In the case of the lightness (L*) the L* value of the fruit skin suffered a slight decrease over time in all treatments. This decrease in the L* value can be explained by the fact that some compounds present in the fruit, such as pigments, amino acids and phenolic compounds, are related with enzymatic darkening and with the reduction of the luminosity (L* values) of the CIELab colour scale [
47]. However, this decrease was minimal and therefore did not affect the external appearance of the fruit. Statistically, we found some differences, in particular at the end of the four weeks of storage, where the treatments AL-Cit 0.15 + Eug 0.1-C- D and AL-Cit 0.3 + Eug 0.2-N- D recorded the highest values of the parameter L*.
Hue angle (h°) values were from 114 to 118 reflecting greenness, and the colour changes in green kiwifruit are mainly described by quantitative colorimetric parameters rather than qualitative ones (h°) [
48]. In this study, we verified that the values obtained did not change during storage and are within the expected values for kiwifruit harvested at the optimal date of harvest.
Chroma (C*), considered the quantitative attribute of colourfulness, is used to determine the degree of difference of a hue in comparison to a grey colour with the same lightness, whereby, the higher the chroma values, the higher is the colour intensity of samples perceived by humans [
47]. In overall, the chroma values decrease with storage time, initially there are no significant differences between the treatments applied, but after four weeks there is a great decrease in the chroma values obtained. It can be observed that the treatments AL-Cit 0.15+Eug 0.1-N-S and AL-Cit 0.3+Eug 0.2-C-S were the ones that maintained the highest values and with less variation. The values of chroma´s suggest that kiwiberries lose their colour intensity, during storage, independently of the application or non-application of treatments.
Broadly, fruits darkened during storage, losing intensity in colour, and changing from and intense green to dark green at the end. Fisk et al. [20], Stefaniak et al. [4] and Mendes da Silva et al. [
48] obtained similar results in their studies for colour values.
Figure 1.
Colour parameters, L*, hue and Chroma of kiwiberries covered with different alginate (AL) based emulsions during storage at 0.5ºC. Parameters were evaluated at harvest (before treatments) and after 2 and 4 weeks of storage. Values represent the mean±standard deviation of three replicates.
Figure 1.
Colour parameters, L*, hue and Chroma of kiwiberries covered with different alginate (AL) based emulsions during storage at 0.5ºC. Parameters were evaluated at harvest (before treatments) and after 2 and 4 weeks of storage. Values represent the mean±standard deviation of three replicates.
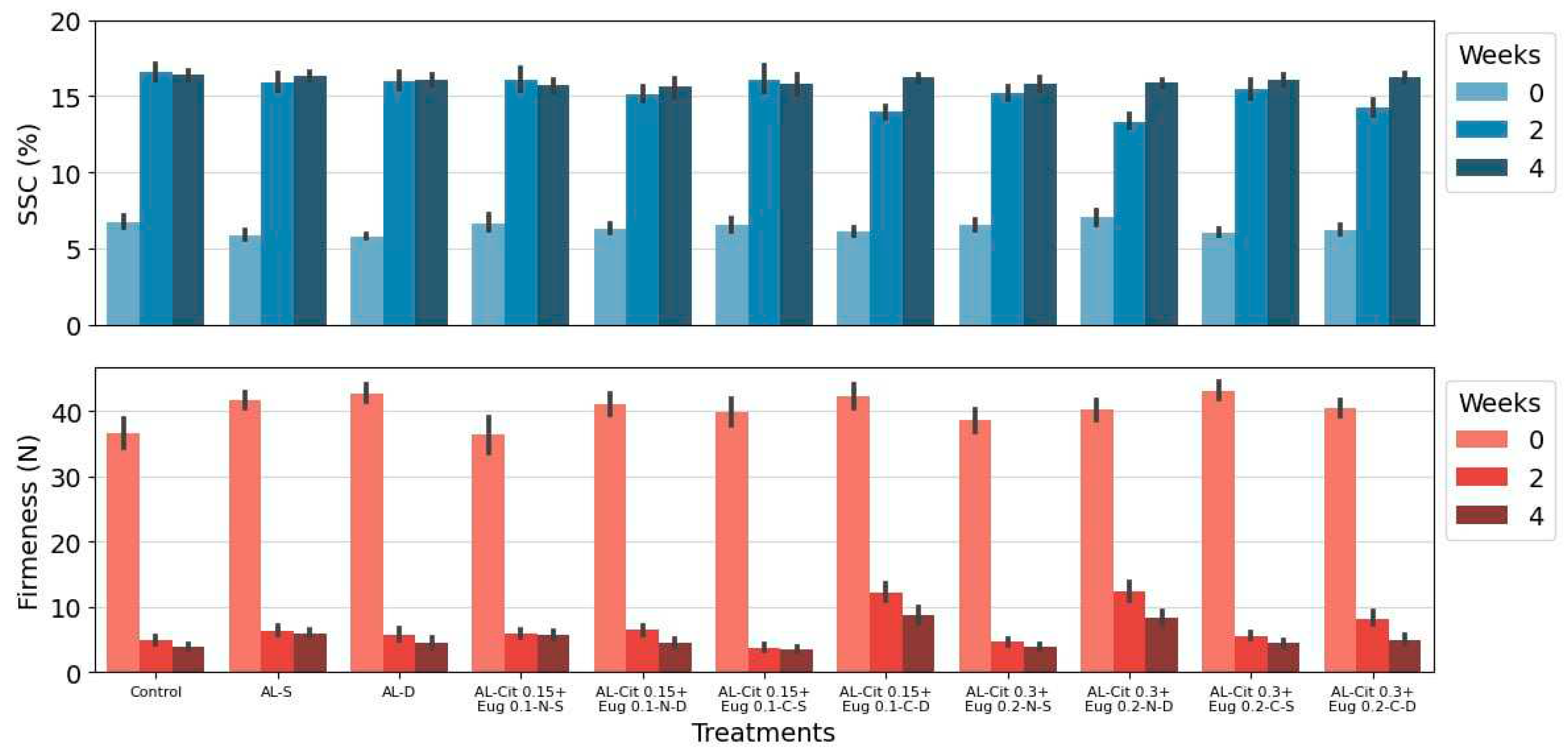
3.2.2. Firmness and SSC
The optimal harvest time for green kiwiberry has been reported as when the SSC of the fruit is reached to 6.5% [9,
49]. The SSC increased over time (
Figure 2), as is expected for a climacteric fruit when harvested at the optimum state of maturity. The fruit at harvest had a brix of 6.5%, after two weeks of storage this had a rapid increase in all treatments and had values of approximately 15%, at the end of four weeks of storage the differences were minimal with values close to 16%. At the end of the storage period there were no differences in the treatments applied or in the method of emulsions application. The observed changes are similar to those obtained in the studies by Krupa et al. [
50], Stefaniak et al. [4] and Han et al. [
49].
Firmness along with SSC are probably the best predictors of kiwifruit ripening and shelf-life [
51,
52]. According to the results obtained for firmness (
Figure 2), it was observed that the values decreased over time in all analysed samples. There was a marked decrease at two weeks of storage, but a slight decrease at the end of the fourth week of storage. The treatments AL-Cit 0.15+Eug 0.1-C-D and AL-Cit 0.3+Eug 0.2-N-D present slightly higher values at the end of the storage period, but these are not statistically significant. The loss of firmness is a physiological process that occurs during ripening and directly affects post-harvest. Short-term storage is a major problem for the kiwiberries, as firmness decreases significantly during storage [
50]. Fisk et al. [9], Stefaniak et al. [4], Burdon et al. [
53] and Oh et al. [7] obtained similar results over the same storage times, even though different cultivars were used.
Interesting to notice that, as for kiwifruit as ‘Hayward’, the higher increase in SSC and the decrease in firmness occurred in the first half storage time, being slight thereafter [
54].
Figure 2.
Firmness and solid soluble content (SSC) of kiwiberries covered with different alginate (AL) based emulsions during storage at 0.5ºC. Parameters were evaluated at harvest (before treatments) and after 2 and 4 weeks of storage. Values represent the mean±standard deviation of three replicates.
Figure 2.
Firmness and solid soluble content (SSC) of kiwiberries covered with different alginate (AL) based emulsions during storage at 0.5ºC. Parameters were evaluated at harvest (before treatments) and after 2 and 4 weeks of storage. Values represent the mean±standard deviation of three replicates.
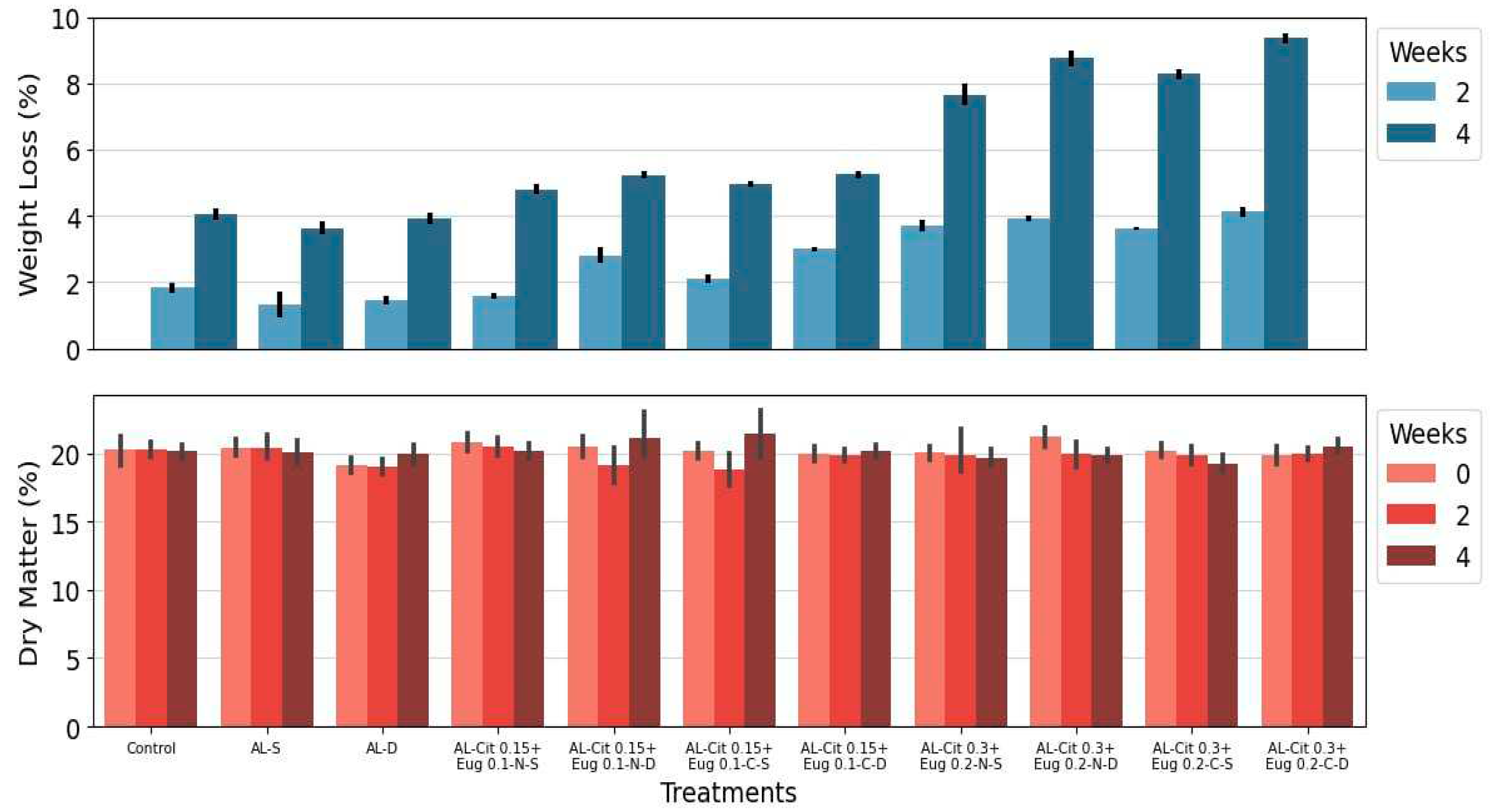
3.2.3. Weight Loss and Dry Matter Content
According to the OECD [
55], the average dry matter should be around 15% at harvest for good-quality fruit. Our values ranged from 15 to 20% (
Figure 3). The dry matter content (DMC) maintained its values stable throughout the storage period, and in the evaluation of this quality parameter we found that there was no effect caused by the type of emulsion or the type of application methodology. The weight loss is indicative of water loss and results in fruit shrivelling. Consumer acceptance and fruit freshness are affected when weight loss is higher than 4-5% [
56]. This is according to our results, that until two weeks of storage, while there are some significant differences between the treatments, the fruits still show a low weight loss. However, at the end of the four weeks of storage, there was a very distinct pattern among the treatments. Treatments with higher amount of EO´s in their formulation showed higher weight losses, while fruits in which only alginate without EO´s was applied obtained lower weight losses. The application methodology did not affect the results.
One of the major causes of fruit weight loss during storage is the migration of water from fruit to the environment [
57]. Edible coatings act as an extra layer which also coats the stomata leading to a decrease in transpiration and in turn, to a reduction in weight loss, as it has been demonstrated in a wide range of fruit including arbutus berries, apples, apricot, pepper, peach, sweet cherry and litchi [33,
58,
59,
60]. Additionally, variations in the ability to reduce weight loss are recognized to the different water vapor permeability of the polysaccharides used in the formulation of the edible coatings, and it is possible to verify results similar to ours, on strawberries and raspberries [35,
61,
62]. For kiwiberries some authors have observed slight increase in weight loss values on non-treat fruits [7,20].
Figure 3.
Weight Loss and Dry Matter Content of kiwiberries covered with different alginate (AL) based emulsions during storage at 0.5ºC. Parameters were evaluated at harvest (before treatments) and after 2 and 4 weeks of storage. Values represent the mean±standard deviation of three replicates.
Figure 3.
Weight Loss and Dry Matter Content of kiwiberries covered with different alginate (AL) based emulsions during storage at 0.5ºC. Parameters were evaluated at harvest (before treatments) and after 2 and 4 weeks of storage. Values represent the mean±standard deviation of three replicates.
3.3. Spectral Analysis
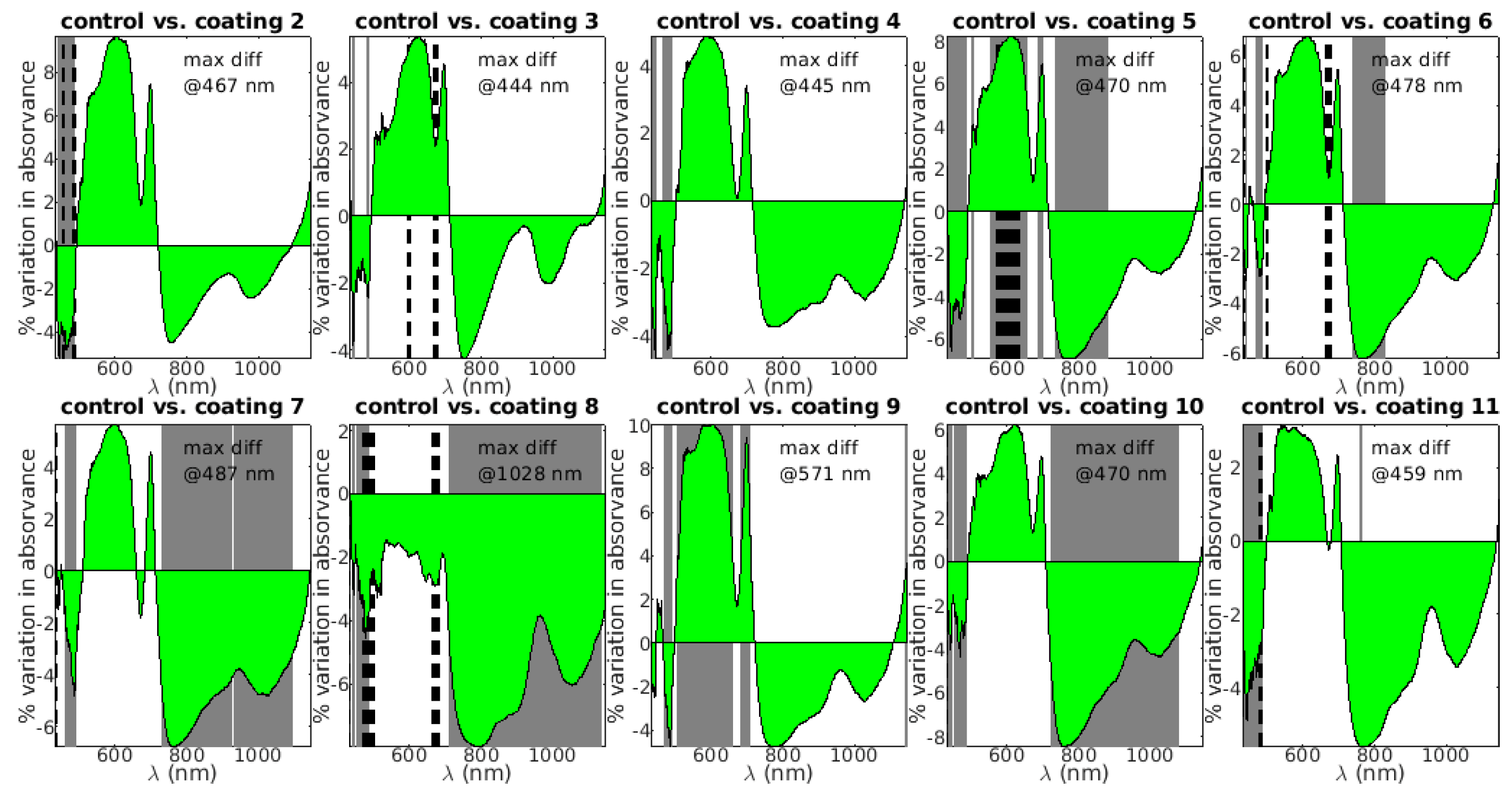
To investigate if the different types of coatings were distinguishable from a spectral point of view, the spectra obtained in fruit at the beginning of the experiment (week 0) were analyzed. Restraining this analysis to the 330 fruit (30 fruit per class) sampled in week 0 allows to separate ripening effects, and effectively focus the onus of the analysis on the coating treatments.
Univariate and multivariate approaches were used. In the univariate approach a t-test in the absorbance spectra (A=log(1/R), where R is the reflectance) was conducted between the control group and all the other groups. The test was performed for each wavelength. The results are depicted in figure 3. The area plot shows the average variation in absorbance between coating and control (the coating group absorbs more than the control in the wavelengths where the area plot is positive). The grey bands mark the regions where the average spectra are different with 95% confidence level and the dashed bands mark the regions where the normality assumption does not hold, as evaluated by the Jacques-Bera test (Jacques and Bera 1987). In these regions the results of the t-test may not hold.
Except for coating 9, the general pattern is the same for all the other coatings: i) a decrease in absorption below approximately 500 nm; ii) an increase in absorption between approx. 500 nm and 720 nm; iii) a decrease in absorption from 720 nm onwards. Since the carotenoids absorb mainly up to 500 nm, observation i) suggests that carotenoid content is higher in the control, meaning that the coating promotes its degradation, while observation ii) suggests, on the contrary, that the coating extends the lifetime of chlorophyll. Finally, observation iii) shows that in all, the NIR plateau is affected, pointing to a global change in tissue structure, possibly increasing scattering and reflection. This would translate in a decrease of absorption since absorption and reflectance are inversely related. It is interesting to note that the baseline of the NIR plateau is indented at the water peak around 960 nm. Because of the dominant NIR plateau shift, it is not clear if water loss is reduced by the coating. The NIR plateau shift is so important is coating 8 that it brings all the spectral amplitudes down. This points to drastic tissue structural changes. Finally, it is also interesting to note that for almost all the coatings (except 8 and 9), the largest difference between the groups is below 500 nm. This suggests that the more important changes occur in the pigments, especially in the carotenoids.
Figure 4.
T-tests in the absorvance spectra between the control group and all the other groups. The test was performed for each wavelength. The area plot shows the average variation in absorvance between coating and control (coating - control). The grey bands mark the regions where the average spectra are different with 95% confidence level and the dashed bands mark the regions where the normality assumption does not hold. The wavelength with maximal difference between the groups in indicated by “max diff”.
Figure 4.
T-tests in the absorvance spectra between the control group and all the other groups. The test was performed for each wavelength. The area plot shows the average variation in absorvance between coating and control (coating - control). The grey bands mark the regions where the average spectra are different with 95% confidence level and the dashed bands mark the regions where the normality assumption does not hold. The wavelength with maximal difference between the groups in indicated by “max diff”.
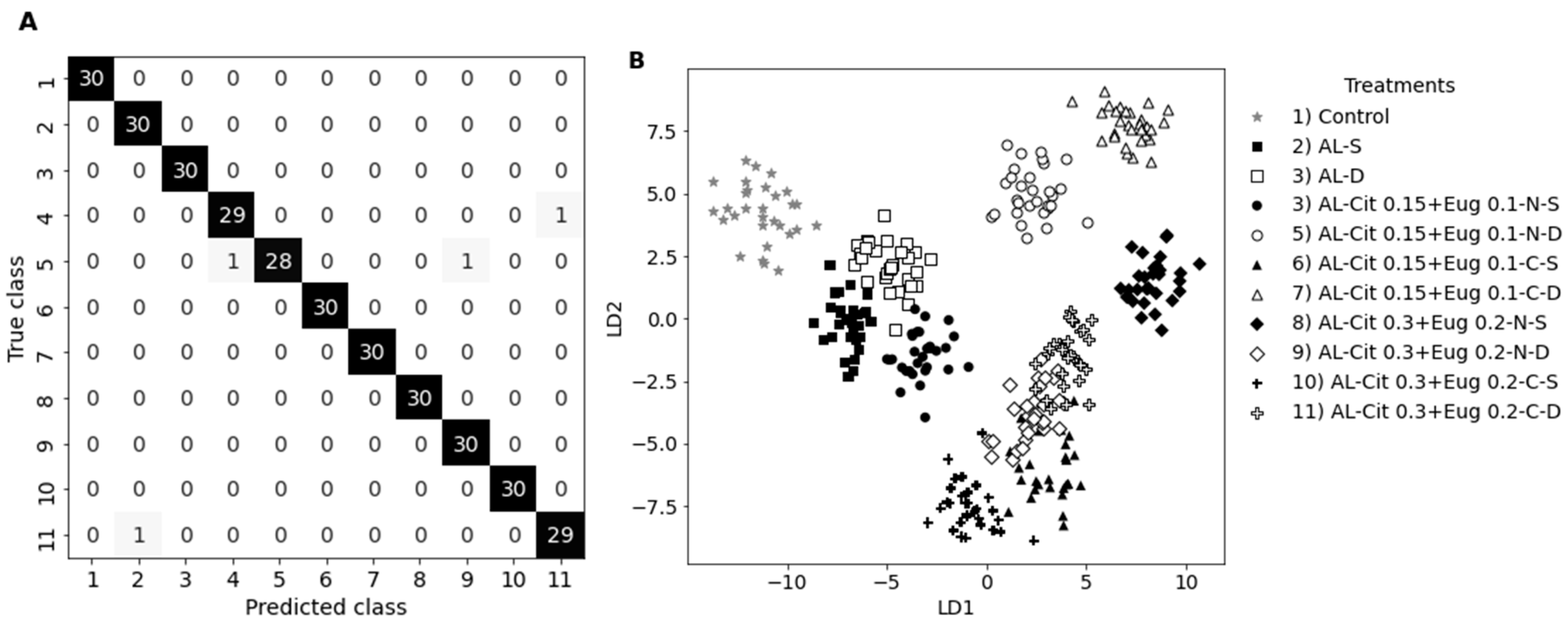
For the multivariate analysis, the different treatments were used as labels for a classification problem, where the independent variables are the 1024 spectral features/wavelengths in the range 432 nm to 1146 nm. A linear discriminant analysis (LDA) with 10 components was used as classifier. The LDA algorithm focuses on maximizing the separability between the classes by fitting class conditional densities to the data and using Bayes’s rule to establish decision boundaries [
63]. Using the reflectance spectra (with no preprocessing applied) as input and a 5-fold cross validation approach, the LDA classifier attains a 99 ± 0.01 % accuracy.
Figure 5A shows the confusion matrix for the several treatments/classes, while
Figure 5B portraits the first two LDA components where most classes are evenly separated (even though this is a low dimensional representation of the clusters).
Figure 5.
A) confusion matrix of the LDA classifier for fruit in week 0 (off diagonal number represent wrong predictions). B) LDA dimensional reduction using the first two components. Gray stars correspond to the control group while black and white symbol help distinguish between sprayed and dipped treatments variants.
Figure 5.
A) confusion matrix of the LDA classifier for fruit in week 0 (off diagonal number represent wrong predictions). B) LDA dimensional reduction using the first two components. Gray stars correspond to the control group while black and white symbol help distinguish between sprayed and dipped treatments variants.
This analysis hints that the reflectance spectra acquired contains enough information to perform a good classification of the different treatments. The same type of analysis performed at weeks 2 and 4 show a slight decrease in accuracy to 97 ± 0.04 % and a very different low dimensional LDA representation.
3.4. Statistical Analysis
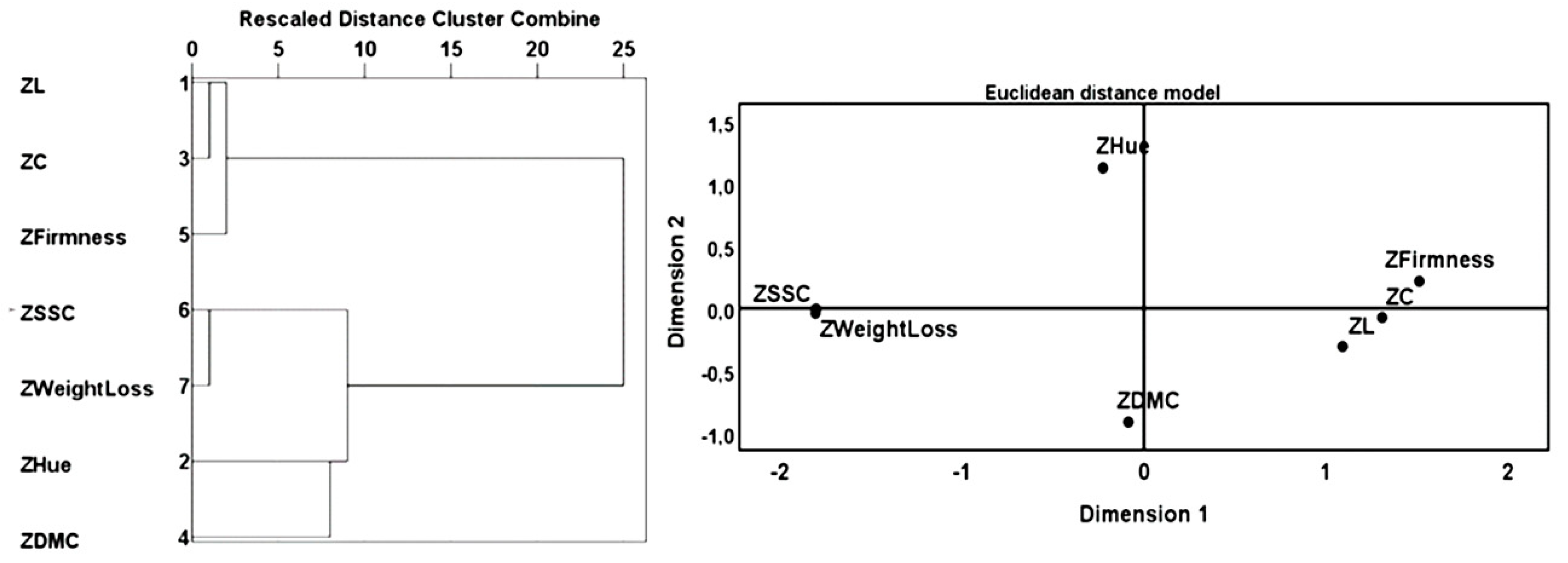
Considering the results obtained, the statistical analysis was done in two phases, initially we verified the effect of the treatments on the variables analysed and then we proceeded to verify the effect of the treatments throughout the time of storage. According to the eigenvalue rule greater than 1, it is possible to summarize the relational information between the variables into two orthogonal components, which explain 91.12% (the total of the Percent of Variance of the two dimensions represented) of the total variance of the original variables. Table 3 reproduces the weights of each variable in each component, the percentage explains the internal consistency (Alpha de Cronbach value). The first component has a high internal consistency (α= 0.898) and the second component has a moderate internal consistency (α= 0.593). The first component is represented by L*; C*; firmness, weight loss and SSC. The second component is represented by Hue and DMC with low contribution values which reveals they are not essential in this type of relationships.
Table 3.
Principal components extracted from the Categorical Principal Components Analysis (CATPCA), with their eigenvalues, Cronbach’s alpha, percentage of variance and weights of each variable.
Table 3.
Principal components extracted from the Categorical Principal Components Analysis (CATPCA), with their eigenvalues, Cronbach’s alpha, percentage of variance and weights of each variable.
| Discrimination Measures |
|---|
| |
Dimension |
|
| 1 |
2 |
Mean |
| L* |
.595 |
.404 |
.499 |
| Hue |
.055 |
.252 |
.154 |
| C* |
.857 |
.497 |
.677 |
| DMC |
.046 |
.158 |
.102 |
| Firmness |
.927 |
.195 |
.561 |
| SSC |
.923 |
.207 |
.565 |
| Weight Loss |
.942 |
.320 |
.631 |
| Eigenvalue |
4.345 |
2.033 |
3.189 |
| Alpha de Cronbach |
0.898 |
0.593 |
0.801a
|
| Percent of Variance |
62.068 |
29.050 |
45,559 |
Using a Cluster method and an MDS (multiple dimensional scaling) -Ascal algorithm for standardized variables (Figure 6), the method has retained two dimensions that reproduce appropriately the relations between them. MDS -Ascal algorithm showed a stress value of 0.02637 and R2 equal to 0.996, these values obtained with this algorithm show that this methodology is highly feasible.
Figure 6.
Dendrogram using a hierarchical cluster using the square Euclidean distance. MDS Alscal using Euclidean distance model.
Figure 6.
Dendrogram using a hierarchical cluster using the square Euclidean distance. MDS Alscal using Euclidean distance model.
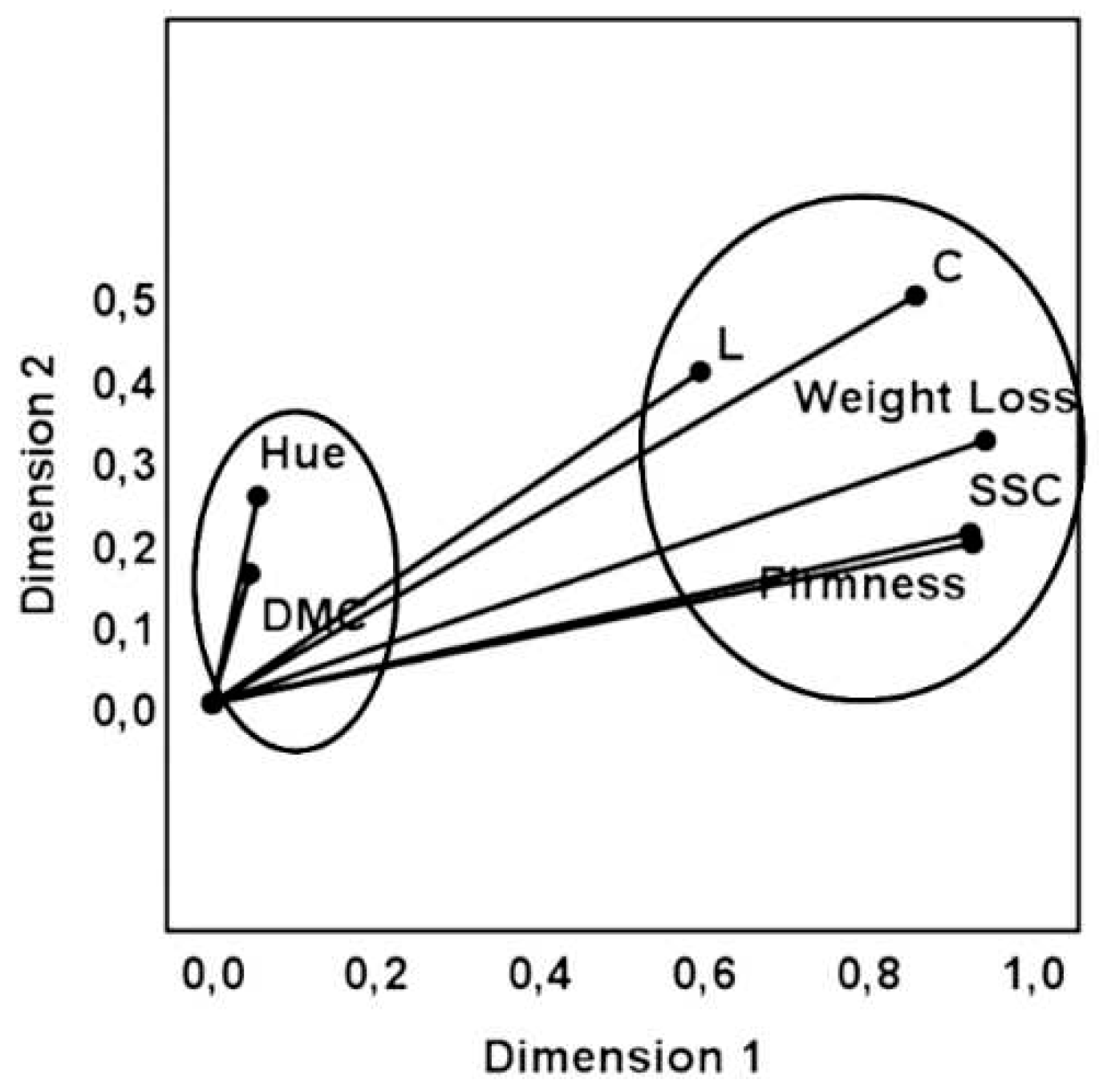
To ensure the reliability of statistical analysis, 3 methodologies were used (MCA, MDS and hierarchical cluster), and we observed that these applied multivariate analysis techniques correspond with each other.
Figure 7.
Quality fruit attributes obtained by Multiple Correspondence Analysis (MCA).
Figure 7.
Quality fruit attributes obtained by Multiple Correspondence Analysis (MCA).
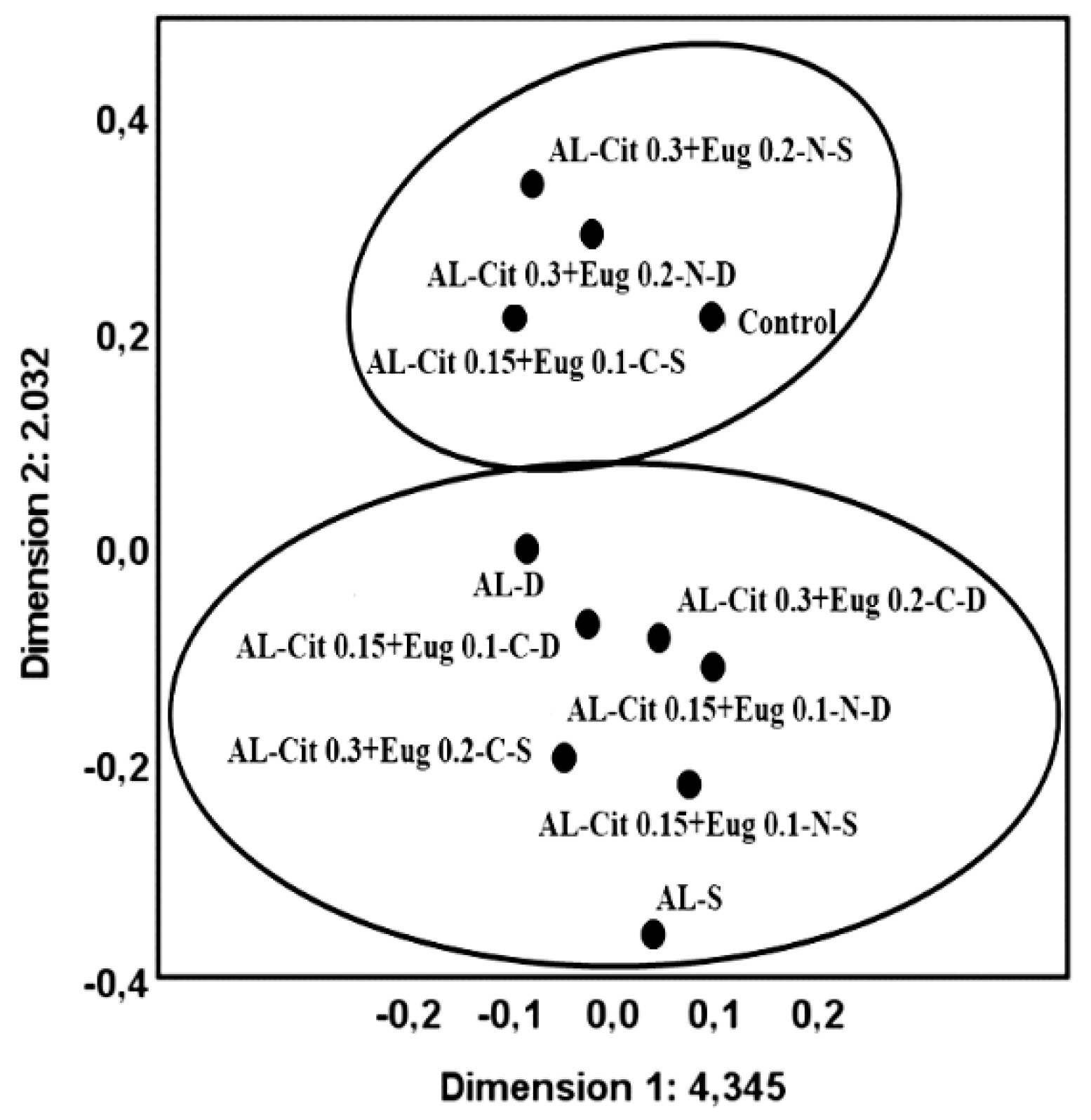
To complete the analysis a MCA for treatments was preformed and in Figure 8 we can see that we have two distinct groups: the first group was formed by Control, AL-Cit 0.15+Eug 0.1-C-S, AL-Cit 0.3+Eug 0.2-N-S and AL-Cit 0.3+Eug 0.2-N-D; and the second group was formed by AL-S, AL-D, AL-Cit 0.15+Eug 0.1-N-S, AL-Cit 0.15+Eug 0.1-N-D, AL-Cit 0.15+Eug 0.1-C-D, AL-Cit 0.3+Eug 0.2-C-S and AL-Cit 0.3+Eug 0.2-C-D. The treatments within each group reveal themselves to be similar to each other.
Figure 8.
Objects points labelled by treatment and the variables in the dimensional map defined by the two main components retained by MCA method and their position relative to the original variables.
Figure 8.
Objects points labelled by treatment and the variables in the dimensional map defined by the two main components retained by MCA method and their position relative to the original variables.
We can conclude from this analysis that the variables that suffer significant changes during storage are the L*, C*, firmness, SSC and weight loss. These variables are the more adequate to be used in the selection of the best treatments.
4. Conclusions
In the present study, we can conclude that we can preserve fresh kiwiberry cv. Ken’s Red up to four weeks at 0 ºC plus 5 d shelf-life at 5ºC. This was confirmed by the qualitative analysis of the fruit throughout the storage period. The use of course emulsions or nanoemulsions did not affect the parameters analysed, neither were found differences between the application method. However, we verified that more important than the method of formulation of the emulsions or their application method, is the quantity of EO’s to be used in their constitution. In the case of kiwiberries we can easily verify that the greater the quantity of EO’s present in the emulsions the worse fruit quality is, the shorter their shelf life and the worse their appearance, based mainly in an increase in weight loss.
In that way we can state that these emulsions can be used as natural postharvest treatments in kiwiberries with the aim to delay senescence and maintain fruit quality up to four weeks at 0°C. Based on the major effect of reducing the weight loss and the scores of the statistical analysis we select as the best treatments for increasing kiwiberry storage the AL-S, AL-D, AL-Cit 0.15+Eug 0.1-N-S, AL-Cit 0.15+Eug 0.1-N-D and AL-Cit 0.15+Eug 0.1-C-D, which performed better than control.
Overall, the spectral analysis shows that: i) significant differences in the spectra are found for all coatings, pointing to some chemical and structural changes either in the skin or pulp; ii) the coatings with better performance (AL-S, AL-D and AL-Cit 0.15+Eug 0.1-N-S) are also those with better t-test comparations, with limited spectral changes and only below 500 nm; iii) the other coatings tend to show also differences in the NIR plateau, indicating important tissue changes; iv) the Vis-NIR spectral analysis could be used in situations where coatings discrimination needs to be performed, e.g. commercial control of coated / non-coated fruit.
Funding
Research work was financial support from the EU (FEDER funds through COMPETE) and National Funds (FCT/MEC, Fundação para a Ciência e Tecnologia/Ministério da Educação e Ciência) through project UIDB/00631/2020 CEOT BASE, co-financed by the EU (FEDER) under the Partnership Agreement PT2020. D. Passos acknowledges funding by projects UIDP/00631/2020 CEOT PROGRAMÁTICO and FCT/RNCA project CPCA-IAC/AV/477942/2022.
Credit author statement
Adriana C. Guerreiro: Conceptualization; Data curation; Formal analysis; Performed the experiments; Investigation; Methodology; Funding acquisition; Writing - original draft, review and editing. Custódia Gago: Performed the experiments; Investigation. Dário Passos: Formal analysis; Data curation; Software. Jaime Martins: Formal analysis. Sandra Cruz: Formal analysis. Fernão Veloso: Performed the experiments; Resources. Rui Guerra: Validation; Funding acquisition; Supervision. Maria D. Antunes: Writing – review and editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
A. Guerreiro acknowledges a grant for Stimulus of scientific employment from FCT (CEECIND/01009/2017). D. Passos acknowledges funding by projects UIDP/00631/2020 CEOT PROGRAMÁTICO and FCT/RNCA project CPCA-IAC/AV/477942/2022.
References
- Pinto, D.; Delerue-Matos, C.; Rodrigues, F. Bioactivity, Phytochemical Profile and pro-Healthy Properties of Actinidia Arguta: A Review. Food Res. Int. 2020, 136, 109449. [Google Scholar] [CrossRef] [PubMed]
- Latocha, P. The Nutritional and Health Benefits of Kiwiberry (Actinidia Arguta)—a Review. Plant Foods Hum. Nutr. 2017, 72, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Krupa, T.; Latocha, P.; Liwińska, A. Changes of Physicochemical Quality, Phenolics and Vitamin C Content in Hardy Kiwifruit (Actinidia Arguta and Its Hybrid) during Storage. Sci. Hortic. 2011, 130, 410–417. [Google Scholar] [CrossRef]
- Stefaniak, J.; Sawicka, M.; Krupa, T.; Latocha, P.; Łata, B. Effect of Kiwiberry Pre-Storage Treatments on the Fruit Quality during Cold Storage. Zemdirb. -Agric. 2017, 104, 235–242. [Google Scholar] [CrossRef]
- Wojdyło, A.; Figiel, A.; Legua, P.; Lech, K.; Carbonell-Barrachina, Á.A.; Hernández, F. Chemical Composition, Antioxidant Capacity, and Sensory Quality of Dried Jujube Fruits as Affected by Cultivar and Drying Method. Food Chem. 2016, 207, 170–179. [Google Scholar] [CrossRef]
- Lim, S.; Han, S.H.; Kim, J.; Lee, H.J.; Lee, J.G.; Lee, E.J. Inhibition of Hardy Kiwifruit (Actinidia Aruguta) Ripening by 1-Methylcyclopropene during Cold Storage and Anticancer Properties of the Fruit Extract. Food Chem. 2016, 190, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.B.; Muneer, S.; Kwack, Y.B.; Shin, M.H.; Kim, J.G. Characteristic of Fruit Development for Optimal Harvest Date and Postharvest Storability in ‘Skinny Green’ Baby Kiwifruit. Sci. Hortic. 2017, 222, 57–61. [Google Scholar] [CrossRef]
- Kim, A.N.; Kim, H.J.; Chun, J.; Heo, H.J.; Kerr, W.L.; Choi, S.G. Degradation Kinetics of Phenolic Content and Antioxidant Activity of Hardy Kiwifruit (Actinidia Arguta) Puree at Different Storage Temperatures. Lwt 2018, 89, 535–541. [Google Scholar] [CrossRef]
- Fisk, C.L.; McDaniel, M.R.; Strik, B.C.; Zhao, Y. Physicochemical, Sensory, and Nutritive Qualities of Hardy Kiwifruit (Actinidia Arguta ’Ananasnaya’) as Affected by Harvest Maturity and Storage. J. Food Sci. 2006, 71. [Google Scholar] [CrossRef]
- Latocha, P.; Krupa, T.; Jankowski, P.; Radzanowska, J. Changes in Postharvest Physicochemical and Sensory Characteristics of Hardy Kiwifruit (Actinidia Arguta and Its Hybrid) after Cold Storage under Normal versus Controlled Atmosphere. Postharvest Biol. Technol. 2014, 88, 21–33. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Mohammadi Nafchi, A.; Salehabadi, A.; Oladzad-abbasabadi, N.; Jafari, S.M. Application of Bio-Nanocomposite Films and Edible Coatings for Extending the Shelf Life of Fresh Fruits and Vegetables. Adv. Colloid Interface Sci. 2021, 291, 102405. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S.; Moalemiyan, M.; Kushalappa, A.C. Effect of Pectin-Based Edible Emulsion Coating on Changes in Quality of Avocado Exposed to Lasiodiplodia Theobromae Infection. Carbohydr. Polym. 2007, 68, 341–349. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of Chitosan-Beeswax Edible Coatings on the Quality of Fresh Strawberries (Fragaria Ananassa Cv Camarosa) under Commercial Storage Conditions. LWT - Food Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Leceta, I.; Molinaro, S.; Guerrero, P.; Kerry, J.P.; Caba, K. De Postharvest Biology and Technology Quality Attributes of Map Packaged Ready-to-Eat Baby Carrots by Using Chitosan-Based Coatings. Postharvest Biol. Technol. 2015, 100, 142–150. [Google Scholar] [CrossRef]
- Guerreiro, A.; Gago, C.; Miguel, M.G.; Faleiro, M.L.; Antunes, M.D. Improving the Shelf-Life of Strawberry Fruit with Edible Coatings Enriched with Essential Oils. Acta Hortic. 2021, 1327, 597–606. [Google Scholar] [CrossRef]
- Gago, C.; Antão, R.; Dores, C.; Guerreiro, A.; Miguel, M.G.; Faleiro, M.L.; Figueiredo, A.C.; Antunes, M.D. The Effect of Nanocoatings Enriched with Essential Oils on “Rocha” Pear Long Storage. Foods 2020, 9, 240. [Google Scholar] [CrossRef]
- Rusková, M.; Opálková Šišková, A.; Mosnáčková, K.; Gago, C.; Guerreiro, A.; Bučková, M.; Puškárová, A.; Pangallo, D.; Antunes, M.D. Biodegradable Active Packaging Enriched with Essential Oils for Enhancing the Shelf Life of Strawberries. Antioxidants 2023, 12, 755. [Google Scholar] [CrossRef]
- Antunes, M.D.C.M.; Gago, C.; Cavaco, A.; Miguel, M.G. Edible Coatings Enriched with Essential Oils and Their Compounds for Fresh and Fresh-Cut Fruit. Recent Pat. Food. Nutr. Agric. 2012, 4, 114–122. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y. Innovations in the Development and Application of Edible Coatings for Fresh and Minimally Processed Fruits and Vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Fisk, C.L.; Silver, A.M.; Strik, B.C.; Zhao, Y. Postharvest Quality of Hardy Kiwifruit (Actinidia Arguta ‘Ananasnaya’) Associated with Packaging and Storage Conditions. Postharvest Biol. Technol. 2008, 47, 338–345. [Google Scholar] [CrossRef]
- Gonzalez-Aguilar, G. a.; Celis, J.; Sotelo-Mundo, R.R.; de la Rosa, L. a.; Rodrigo-Garcia, J.; Alvarez-Parrilla, E. Physiological and Biochemical Changes of Different Fresh-Cut Mango Cultivars Stored at 5 °C. Int. J. Food Sci. Technol. 2008, 43, 91–101. [Google Scholar] [CrossRef]
- Salinas-Roca, B.; Guerreiro, A.; Welti-Chanes, J.; Antunes, M.D.C.; Martín-Belloso, O. Improving Quality of Fresh-Cut Mango Using Polysaccharide-Based Edible Coatings. Int. J. Food Sci. Technol. 2018, 53, 938–945. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Cánovas, G. V Edible Coatings for Fresh-Cut Fruits. Crit. Rev. Food Sci. Nutr. 2005, 45, 657–670. [Google Scholar] [CrossRef]
- Jiang, T. Effect of Alginate Coating on Physicochemical and Sensory Qualities of Button Mushrooms (Agaricus Bisporus) under a High Oxygen Modified Atmosphere. Postharvest Biol. Technol. 2013, 76, 91–97. [Google Scholar] [CrossRef]
- Antunes, M.M.D.C.; Cavaco, A.M.A. The Use of Essential Oils for Postharvest Decay Control. A Review. Flavour Fragr. J. 2010, 25, 351–366. [Google Scholar] [CrossRef]
- Azevedo, A.N.; Buarque, P.R.; Cruz, E.M.O.; Blank, A.F.; Alves, P.B.; Nunes, M.L.; Santana, L.C.L.D.A. Response Surface Methodology for Optimisation of Edible Chitosan Coating Formulations Incorporating Essential Oil against Several Foodborne Pathogenic Bacteria. Food Control 2014, 43, 1–9. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant Activity of Medicinal and Aromatic Plants. A Review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Andrade, R.D.; Skurtys, O.; Osorio, F.A. Atomizing Spray Systems for Application of Edible Coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337. [Google Scholar] [CrossRef]
- Martín-Belloso, O.; Rojas-Graü, M.A.; Soliva-Fortuny, R. Delivery of Flavor and Active Ingredients Using Edible Films and Coatings. In Edible Films and Coatings for Food Applications; Huber, K.C., Embuscado, M.E., Eds.; Springer New York: New York, NY, 2009; pp. 295–313. ISBN 978-0-387-92824-1. [Google Scholar]
- Pires, R.; Guerra, R.; Cruz, S.P.; Antunes, M.D.; Brázio, A.; Afonso, A.M.; Daniel, M.; Panagopoulos, T.; Gonçalves, I.; Cavaco, A.M. Ripening Assessment of ‘Ortanique’ (Citrus Reticulata Blanco x Citrus Sinensis (L) Osbeck) on Tree by SW-NIR Reflectance Spectroscopy-Based Calibration Models. Postharvest Biol. Technol. 2022, 183. [Google Scholar] [CrossRef]
- Cruz, S.; Guerra, R.; Brazio, A.; Cavaco, A.M.; Antunes, D.; Passos, D. Nondestructive Simultaneous Prediction of Internal Browning Disorder and Quality Attributes in ‘Rocha’ Pear (Pyrus Communis L.) Using VIS-NIR Spectroscopy. Postharvest Biol. Technol. 2021, 179. [Google Scholar] [CrossRef]
- Jamshidi, B.; Mohajerani, E.; Jamshidi, J. Developing a Vis/NIR Spectroscopic System for Fast and Non-Destructive Pesticide Residue Monitoring in Agricultural Product. Measurement 2016, 89, 1–6. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The Effect of Alginate-Based Edible Coatings Enriched with Essential Oils Constituents on Arbutus Unedo L. Fresh Fruit Storage. Postharvest Biol. Technol. 2015, 100, 226–233. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; McHugh, T.H.; Martín-Belloso, O. Apple Puree-Alginate Edible Coating as Carrier of Antimicrobial Agents to Prolong Shelf-Life of Fresh-Cut Apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. Raspberry Fresh Fruit Quality as Affected by Pectin- and Alginate-Based Edible Coatings Enriched with Essential Oils. Sci. Hortic. (Amsterdam). 2015, 194, 138–146. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Acevedo-Fani, A.; Martín-Belloso, O. Effect of Sodium Alginate Incorporation Procedure on the Physicochemical Properties of Nanoemulsions. Food Hydrocoll. 2017, 70, 191–200. [Google Scholar] [CrossRef]
- Comaposada, J.; Gou, P.; Marcos, B.; Arnau, J. Physical Properties of Sodium Alginate Solutions and Edible Wet Calcium Alginate Coatings. LWT - Food Sci. Technol. 2015, 64, 212–219. [Google Scholar] [CrossRef]
- Dores, C.S.; Vieira, A.I.; Guerreiro, A.C.; Gago, C.L.; Miguel, M.G.; Faleiro, M.L.; Dantuma, A.; Antunes, M.D. The Effect of Active Packaging on the Storage of Kiwifruit Snacks. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science (ISHS), Leuven, Belgium, 31 October 2018; pp. 541–548. [Google Scholar]
- Sokal, R.R. Clustering and Classification: Background and Current Directions. In Classification and Clustering; Van Ryzin, J., Ed.; Academic Press, 1977; pp. 1–15. ISBN 978-0-12-714250-0. [Google Scholar]
- De Pelsmacker, P.; Van Kenhove, P.; Janssens, W.; Wijnen, K. Marketing Research with SPSS EBook; Pearson Education; ISBN 9780273732280.
- Borg, I.; Groenen, P.J.F. Modern Multidimensional Scaling; Springer Series in Statistics; Springer New York: New York, NY, 2005; ISBN 978-0-387-25150-9. [Google Scholar]
- Gago, C.M.L.; Artiga-Artigas, M.; Antunes, M.D.C.; Faleiro, M.L.; Miguel, M.G.; Martín-Belloso, O. Effectiveness of Nanoemulsions of Clove and Lemongrass Essential Oils and Their Major Components against Escherichia Coli and Botrytis Cinerea. J. Food Sci. Technol. 2019, 56, 2721–2736. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical Characterization and Antimicrobial Activity of Food-Grade Emulsions and Nanoemulsions Incorporating Essential Oils. Food Hydrocoll. 2015, 43, 547–556. [Google Scholar] [CrossRef]
- Yang, R.; Miao, J.; Shen, Y.; Cai, N.; Wan, C.; Zou, L.; Chen, C.; Chen, J. Antifungal Effect of Cinnamaldehyde, Eugenol and Carvacrol Nanoemulsion against Penicillium Digitatum and Application in Postharvest Preservation of Citrus Fruit. Lwt 2021, 141, 110924. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of Antimicrobial Nanoemulsions as Edible Coatings: Impact on Safety and Quality Attributes of Fresh-Cut Fuji Apples. Postharvest Biol. Technol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- Heurtault, B.; Saulnier, P.; Pech, B.; Proust, J.E.; Benoit, J.P. Physico-Chemical Stability of Colloidal Lipid Particles. Biomaterials 2003, 24, 4283–4300. [Google Scholar] [CrossRef] [PubMed]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Mendes da Silva, T.; Briano, R.; Peano, C.; Giuggioli, N.R. The Use of a New Explanatory Methodology to Assess Maturity and Ripening Indices for Kiwiberry (Actinidia Arguta): Preliminary Results. Postharvest Biol. Technol. 2020, 163, 111122. [Google Scholar] [CrossRef]
- Han, N.; Park, H.; Kim, C.W.; Kim, M.S.; Lee, U. Physicochemical Quality of Hardy Kiwifruit (Actinidia Arguta L. Cv. Cheongsan) during Ripening Is Influenced by Harvest Maturity. Forest Sci. Technol. 2019, 15, 187–191. [Google Scholar] [CrossRef]
- Krupa, T.; Latocha, P.; Liwińska, A. Changes of Physicochemical Quality, Phenolics and Vitamin C Content in Hardy Kiwifruit (Actinidia Arguta and Its Hybrid) during Storage. Sci. Hortic. (Amsterdam). 2011, 130, 410–417. [Google Scholar] [CrossRef]
- Koukounaras, A.; Sfakiotakis, E. Effect of 1-MCP Prestorage Treatment on Ethylene and CO2 Production and Quality of ‘Hayward’ Kiwifruit during Shelf-Life after Short, Medium and Long Term Cold Storage. Postharvest Biol. Technol. 2007, 46, 174–180. [Google Scholar] [CrossRef]
- Hu, W.; Sun, D.-W.; Blasco, J. Rapid Monitoring 1-MCP-Induced Modulation of Sugars Accumulation in Ripening ‘Hayward’ Kiwifruit by Vis/NIR Hyperspectral Imaging. Postharvest Biol. Technol. 2017, 125, 168–180. [Google Scholar] [CrossRef]
- Burdon, J.; Pidakala, P.; Martin, P.; Billing, D. Softening of ‘Hayward’ Kiwifruit on the Vine and in Storage: The Effects of Temperature. Sci. Hortic. (Amsterdam). 2017, 220, 176–182. [Google Scholar] [CrossRef]
- Antunes, M.D.C.; Sfakiotakis, E. Ethylene Biosynthesis and Ripening Behaviour of ‘Hayward’ Kiwifruit Subjected to Some Controlled Atmospheres. Postharvest Biol. Technol. 2002, 26, 167–179. [Google Scholar] [CrossRef]
- Organization For Economic Co-operation and development. International Standardisation of Fruit and Vegetables (Kiwifruit); 2008; ISBN 92-64-009712-0. [Google Scholar]
- Nunes, M.C. do N. Correlations between Subjective Quality and Physicochemical Attributes of Fresh Fruits and Vegetables. Postharvest Biol. Technol. 2015, 107, 43–54. [Google Scholar] [CrossRef]
- Duan, J.; Wu, R.; Strik, B.C.; Zhao, Y. Effect of Edible Coatings on the Quality of Fresh Blueberries (Duke and Elliott) under Commercial Storage Conditions. Postharvest Biol. Technol. 2011, 59, 71–79. [Google Scholar] [CrossRef]
- Ayranci, E.; Tunc, S. A Method for the Measurement of the Oxygen Permeability and the Development of Edible Films to Reduce the Rate of Oxidative Reactions in Fresh Foods. Food Chem. 2003, 80, 423–431. [Google Scholar] [CrossRef]
- Hassimotto, N.M.A.; Pinto, M.D.S.; Lajolo, F.M. Antioxidant Status in Humans after Consumption of Blackberry (Rubus Fruticosus L.) Juices with and without Defatted Milk. J. Agric. Food Chem. 2008, 56, 11727–11733. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Mula, H.M.; Serrano, M.; Valero, D. Alginate Coatings Preserve Fruit Quality and Bioactive Compounds during Storage of Sweet Cherry Fruit. Food Bioprocess Technol. 2012, 5, 2990–2997. [Google Scholar] [CrossRef]
- Vargas, M.; Pastor, C.; Chiralt, A.; McClements, D.J.; González-Martínez, C. Recent Advances in Edible Coatings for Fresh and Minimally Processed Fruits. Crit. Rev. Food Sci. Nutr. 2008, 48, 496–511. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Miguel, M.G.C.; Faleiro, M.L.; Antunes, M.D.C. The Influence of Edible Coatings Enriched with Citral and Eugenol on the Raspberry Storage Ability, Nutritional and Sensory Quality. Food Packag. Shelf Life 2016, 9, 20–28. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning; Springer Series in Statistics; Springer New York: New York, NY, 2009; ISBN 978-0-387-84857-0. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).