1. Introduction
The phototransduction cascade in vertebrate photoreceptors allows the visual system to operate over a wide range of light levels, from moonless nights to bright midday. This wide range of sensitivity is provided by a multi-stage biochemical amplification system, with powerful feedback loops at each stage. Like other tissues, the retina relies on second messengers to carry out various functions, including phototransduction. Two major cyclic nucleotide monophosphates, cGMP and cAMP, have distinct roles in the vertebrate phototransduction cascade. cGMP regulates the permeability of plasma membrane channels in photoreceptors and its role has been extensively studied (for review see [
1,
2]). After absorbing a quantum of light, a molecule of the visual pigment rhodopsin becomes active and activates several hundred molecules of the G-protein transducin. Each transducin molecule activates a cGMP-specific phosphodiesterase type 6 (PDE6), which reduces the intracellular concentration of cGMP. As a result, cGMP-dependent cation channels in the outer segment of the photoreceptor are closed, leading to hyperpolarisation of the cell and generation of a presynaptic signal at the synaptic end of the photoreceptor.
It is thought that the feedbacks that regulate all loops of the phototransduction cascade are controlled by calcium concentration via a set of calcium-sensitive proteins associated with each stage of phototransduction. Turning on or changing the intensity of the background illumination causes a change in the level of photoreceptor polarisation, which is initially transient and then settles to a new constant level. It is assumed that at each steady state level of the intracellular potential, the calcium concentration is unchanged, albeit at different levels, which in turn should mean that the parameters of the phototransduction cascade are unchanged. However, real physiological data indicate that when the photoreceptor is tested in steady-state at background illumination, slow processes with characteristic times of seconds and tens of seconds are observed [
3]. To explain the nature of the relatively slow changes in the parameters of the phototransduction cascade under steady-state stimulation, either an unknown mechanism of slow and delayed regulation of the intracellular calcium concentration in the photoreceptor or another mechanism of cascade regulation in addition to the one already described should be assumed. The regulatory effects of cAMP have been demonstrated in several cellular components of the retina. Although not as extensively studied as cGMP in the phototransduction cascade, cAMP has been implicated in processes such as circadian rhythms, intercellular contacts and retinomotor effects [
4,
5,
6,
7], for review see [
8,
9]
The effect of cAMP on the phototransduction cascade may be one such regulatory effect. Almost all key proteins of the phototransduction cascade have PKA phosphorylation sites, and for rhodopsin kinase the regulatory effects of cAMP and PKA have been shown
in vivo [
10,
11]. It follows that changes in cAMP levels could have a regulatory effect on proteins of the phototransduction cascade through their phosphorylation by PKA. It is also known that the level of cAMP in the rod cytoplasm changes cyclically throughout the day, and if these changes are artificially reproduced, significant functional changes in the operation of the phototransduction cascade can be recorded [
12] . However, for a regulatory effect on the time scale of seconds and tens of seconds, it is necessary that cAMP changes significantly in this time range. Currently, there are no data on the dynamics of changes in intracellular cAMP levels on the time scale of seconds and minutes. This is largely due to the specificity of the sensory modality of photoreceptors, where it is practically impossible to use conventional experimental approaches based on fluorescence methods. In the present work, we used the method of rapid cryofixation of retinal samples after light stimulation and subsequent isolation of outer segment preparations. In these preparations, the levels of cAMP was measured using highly sensitive metabolomics approaches. In addition, PKA activity was measured in these samples. We show that when illuminated with near-saturating but still moderate light, cAMP levels rise transiently within approximately the first second and then return to pre-stimulus levels. The light-dependent increase in cAMP is intensity-dependent and is not seen after continuous light of lower intensity or after short flashes. Increase of cAMP activate PKA which leads to phosphorylation of PKA specific substrates in frog retina outer segments.
2. Results
2.1. Dark level of cAMP and correlation of cAMP levels in two frog eyes
To estimate the dark level of cAMP in rod outer segments (ROS) of frog retina, we analyzed its level in all samples cryofixed in the dark. Based on 72 samples, we found the dark cAMP level to be 11.4±0.5 pmol/mg of protein (mean±SEM). Experimentally, we found that the minimum sample size to be measured by the method described in the Materials and Methods section, is ¼ of the retina from one eye. The retinal specimen cryofixation unit allows for simultaneous fixation of up to six retinal specimens according to a preset programme. Thus, we used the division of the retina of one eye into two or three segments. Using six retinal samples from two eyes to simultaneously measure the dynamics of cAMP levels for five values of time delay after stimulus onset (plus one dark value) implies that the cAMP levels in both eyes of the animal are approximately the same. To test this assumption, we performed an experiment in which we compared the dark levels of cAMP in the halves of each eye. The ratio of cAMP content (1 eye)/(2 eyes) (according to the order in which they were dissected) was 1.04 ± 0.07 (mean±SEM, n=24). Thus the difference in intracellular cAMP content in two eyes of the same animal is statistically insignificant (p=0.55), and we used retinal preparations to measure four to six different values of the post-stimulus in one animal.
2.2. Application of IBMX and Forskolin leads to an increase in cAMP levels.
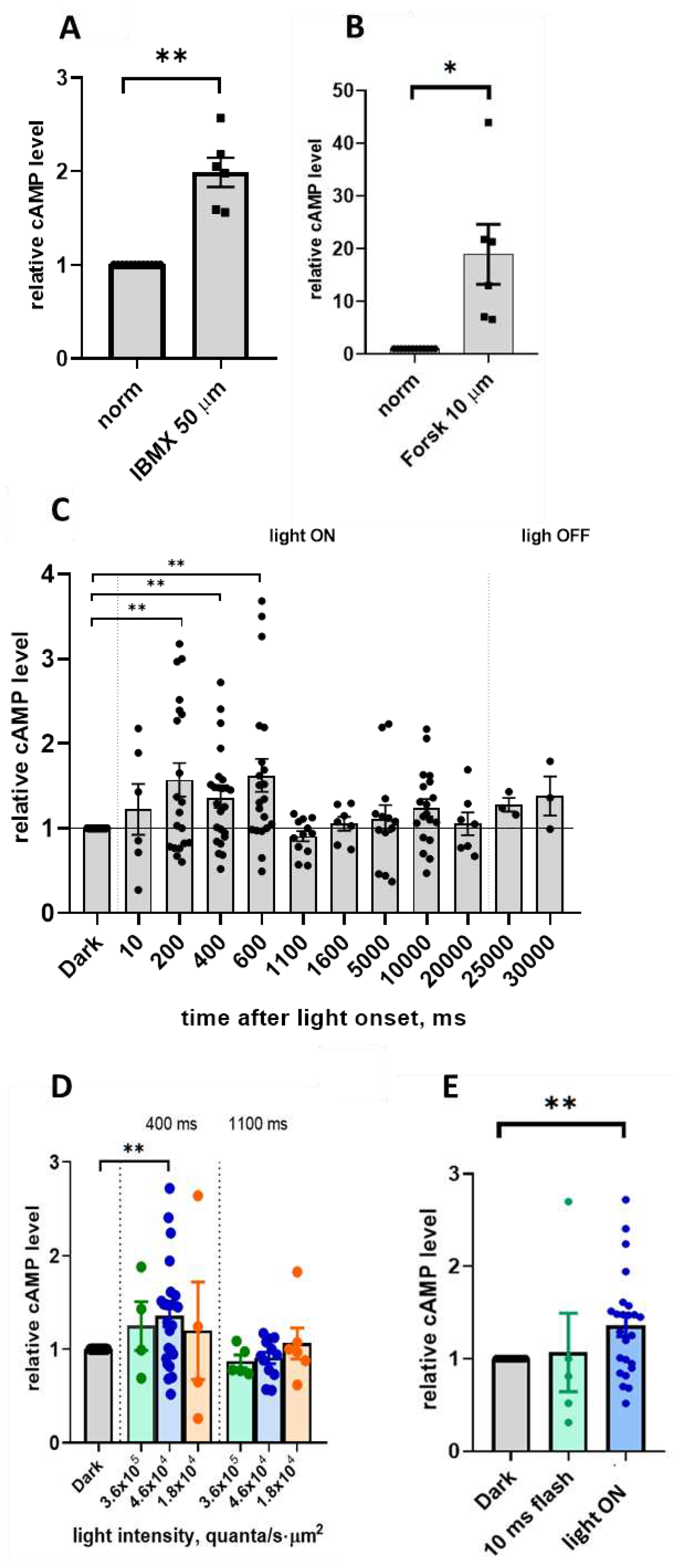
As a positive control, we used the application of a nonspecific phosphodiesterase inhibitor IBMX and a direct activator of adenylate cyclases, forskolin. In both cases, the retina of one eye was cut into two halves, of which one half was incubated in normal Ringer's solution and used as a control, and the other half was placed for 5 minutes in a solution containing 50 μM IBMX or for 20 minutes in a solution containing 10 μM forskolin. All measurements were taken in the dark. As a result, cAMP level statistically significant rised in IBMX 1.98 ± 0.16 times (mean±SEM, p=0.0014; n=6) and in Forskolin 18.9 ± 5.7 times (p=0.025; n=6) (
Figure 1 A,B and
Supplementary Table S2).
2.3. The dynamics of cAMP levels in the rod outer segments after onset of light stimulation
The result is shown in
Figure 1С and in
Supplementary Table S1. For samples with a time delay after onset of light stimulation of up to 2 seconds, a 2-seconds light stimulus was used; for samples with a time delays of 5, 10 and 20 seconds, a 20-second light stimulus was used. As slightly different time delay protocols were used for the early and late measurements, we combined the results of measurements with time delays of 0.2 s (n=15) and 0.16 s (n=5) into a "0.2 s" group, and the results of measurements with time delays of 0.4 s (n=20) and 0.31 s (n=5) into a "0.4 s" group. The result shows that for time delays of 0.2 - 0.6 s after stimulus onset, the level of cAMP is significantly (p≤0.05) increased relative to the dark level. For time delays of 5, 10 and 20 seconds after the onset of the stimulus, the level of cAMP is not significantly different from the level in the dark. Thus, we have shown that as early as 200 ms after onset of a saturating step of light with an intensity of 4.6x10
4 quanta/(s·µm
2), the level of cAMP in photoreceptor outer segments increases reliably, remains elevated for about 1 second, and then decreases again to approximately dark levels.
Figure 1.
cAMP content in frog retina rod outer segments. A, B: cAMP level in ROS after incubation for 5 min in IBMX 50 µM Ringer solution (A) or incubation for 20 min in Forskolin 10 µM Ringer solution (B). Data are relative to cAMP content in normal Ringer's solution in the dark. (C) cAMP concentration in ROS after various time after onset of light step of intensity 4.6x104 quanta/(s·µm2) lasting 20 seconds. Vertical dashed lines on the graph mark the beginning and end of light stimulation. All values shown as relative to measurement of the dark-adapted sample on the same eye or on the same frog. Time point 200 ms includes also data measured at 160 ms, point 400 ms includes data taken at 310 ms (see Materials and Methods). D. cAMP level in ROS measured 400 and 1100 ms after light step onset, with three different light intensities of the light - 1.8x104, 4.6x104 and 3.6x105 quanta/(s·µm2). E. cAMP level in ROS measured after 400 ms after short 10 ms flash of light light or light step onset of the same intesity 4.6x104 quanta/(s·µm2). Error bars indicates SEM, stars indicate significant difference (one-sample t-test). .
Figure 1.
cAMP content in frog retina rod outer segments. A, B: cAMP level in ROS after incubation for 5 min in IBMX 50 µM Ringer solution (A) or incubation for 20 min in Forskolin 10 µM Ringer solution (B). Data are relative to cAMP content in normal Ringer's solution in the dark. (C) cAMP concentration in ROS after various time after onset of light step of intensity 4.6x104 quanta/(s·µm2) lasting 20 seconds. Vertical dashed lines on the graph mark the beginning and end of light stimulation. All values shown as relative to measurement of the dark-adapted sample on the same eye or on the same frog. Time point 200 ms includes also data measured at 160 ms, point 400 ms includes data taken at 310 ms (see Materials and Methods). D. cAMP level in ROS measured 400 and 1100 ms after light step onset, with three different light intensities of the light - 1.8x104, 4.6x104 and 3.6x105 quanta/(s·µm2). E. cAMP level in ROS measured after 400 ms after short 10 ms flash of light light or light step onset of the same intesity 4.6x104 quanta/(s·µm2). Error bars indicates SEM, stars indicate significant difference (one-sample t-test). .
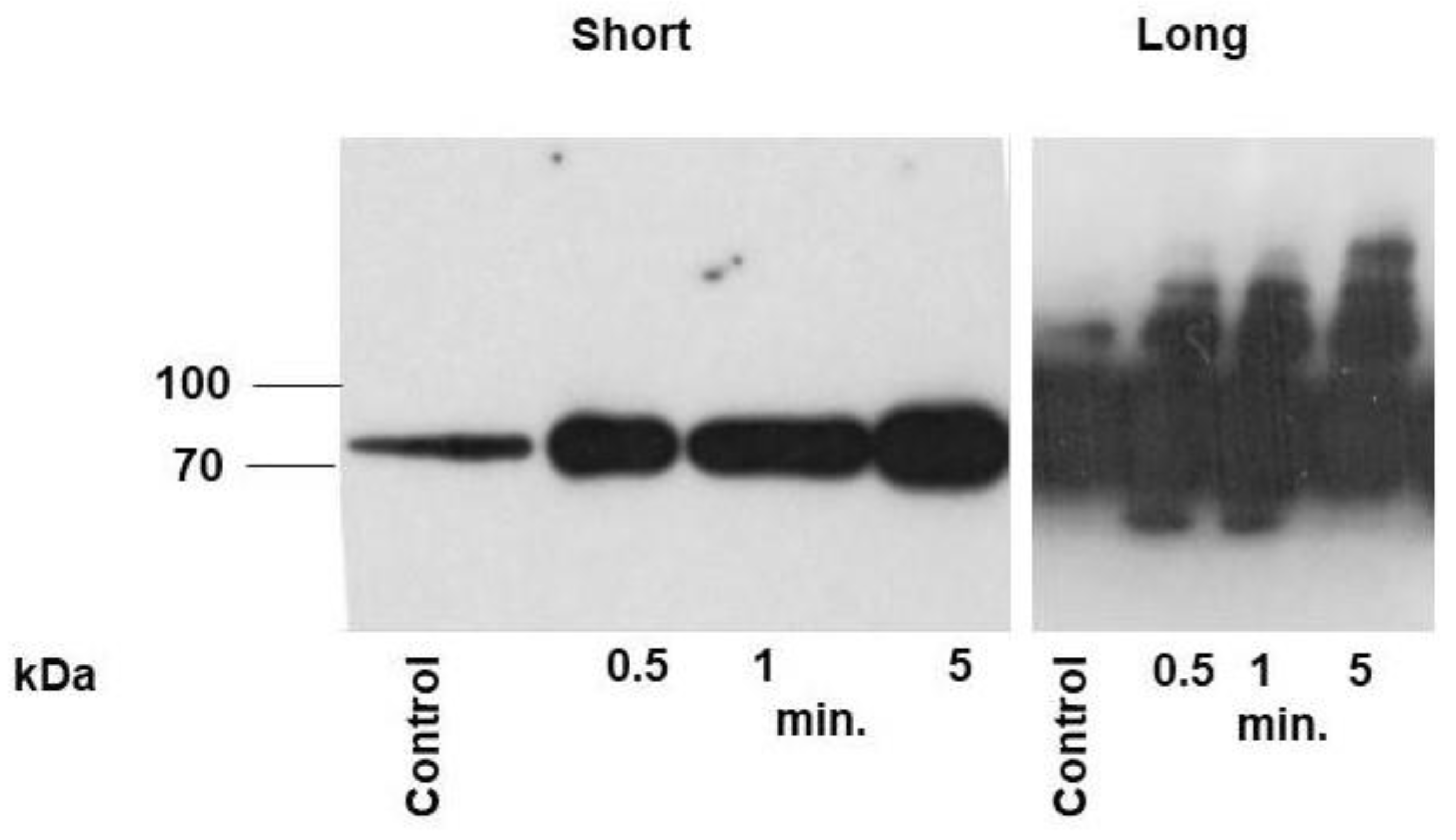
2.4. Light stimulation activates PKA in frog outer segments.
To evaluate whether relatively small increase of cAMP (
Figure 1) can activate PKA in frog outer segments we used Western blot analysis. Because there are no antibodies that can recognize some specific PKA substrates in frog retina we used antibodies that recognized phosphorylated PKA substrate (P-S/T). Equal loading of proteins were controlled by Ponceau S staining (
Supplementary Figure S1). The samples were collected in the dark (control) and after onset of light stimulation during 0.5, 1, and 5 min (
Figure 2). Strong increase of the signal was detected already after 0.5 min after light stimulation which did not decreased during five min after stimulation. Interestingly, the strong signal was detected only on the proteins around 70 kDa (see
Figure 2 left panel, short exposure). To test whether such unexpected recognition of a single substrate is not connected with some defect of antibodies, we used this antibodies in our well-established system, human platelets [
13] and showed that in human platelets stimulated with forskolin numerous bands of different molecular weight are detected (
Supplementary Figure S2), indicating that in frog outer segment the most prominent PKA substrate are some proteins with the molecular weight round 70 kDa.
3. Discussion
The change in PKA activity in response to light stimulation in the mouse photoreceptor layer was previously shown in the work of Sato et al [
14]. They showed that stimulation with light of saturating intensity initially leads to a short-term decrease in PKA activity, which is then followed by a reverse increase in PKA activity that significantly exceeds the initial dark level. They also showed that the increase in PKA activity does not begin until the light stimulus is turned off, and is therefore a response to turning off the stimulus. Sato and colleagues suggested that switching the light stimulus on and off triggers two multidirectional processes of PKA inactivation and activation, with the PKA activation process increasing in intensity as the stimulus light intensity increases, including in an intensity range four orders of magnitude greater than the working intensity range of the rods. The spectrum of the process responsible for PKA activation shifts into the red region with increasing light intensity, and Sato suggests that this process is provided by melanopsin in dopaminergic retinal ganglion cells.
In our work, we have applied a new, original approach that allows us to measure the dynamics of changes in cAMP content in the outer segments of frog photoreceptors for a period of less than one second after switching light stimulation on or off. The main advantage of the presented method is that the experimental protocol does not use light sources as required in optical fluorescence approaches. We have shown that almost immediately after switching on a light stimulus with an intensity close to the saturating intensity for rods (4.6x10
4 quanta/s·µm
2), the level of cAMP increases by about 50-70% relative to the dark level and then returns almost to the dark level after about 1 second and remains there during the continuation of the light step and during the first 10 s after switching the light off (Figure1C). The intensity of the light stimulus was three orders of magnitude lower than in Sato's experiments, but our experience shows that such an intensity is saturating or close to saturation for frog rods [
3].
The small but statistically significant increase in the concentration of cAMP immediately after the onset of moderate-intensity light stimulation is puzzling. In a standard scheme of dynamic maintenance of the level of a signalling molecule at a stable level, there should be synthesising and degrading elements working in synchrony. In the case of cyclic mononucleosides, and in particular cyclic adenosine monophosphate, synthesis is carried out by one or more adenylate cyclases and hydrolysis by phosphodiesterase. The exact composition of these enzymes in the outer segments of frog
P. ridibundus photoreceptors is unknown. Adenylate cyclase isoforms are divided into four functional groups, which differ in their mechanism of activation by G-proteins and calcium [
15]. The first group includes the calcium-activated adenylate cyclases (types 1, 3 and 8). Of these, the presence of AC1 in significant amounts has been shown in chick [
6] and rat [
16] photoreceptors, and the presence of AC8 has also been shown in chick photoreceptors [
6] (for a review see [
9]. In addition, group 2 adenylate cyclases characterised by calcium inhibition have also been identified, including AC2, which has been found in the retinas of rodless knockout mice [
17] and in the inner segments of fish photoreceptors [
18].
The composition of the enzymes that utilise cAMP in vertebrate photoreceptors is also not well understood. The outer segments of photoreceptors contain a large amount of phosphodiesterase 6 which is specific for cGMP but can also hydrolyse cAMP with low efficiency [
19]. In addition, the presence of PDE1A in photoreceptore outer segment [
20] and PDE4B in outer and inner segments [
21] has been demonstrated in the rat. PDE1A hydrolyses both cGMP and cAMP and its level of activity is positively dependent on calcium, whereas PDE4B is a cAMP-specific phosphodiesterase and its activity is thought to be independent of calcium levels [
22].
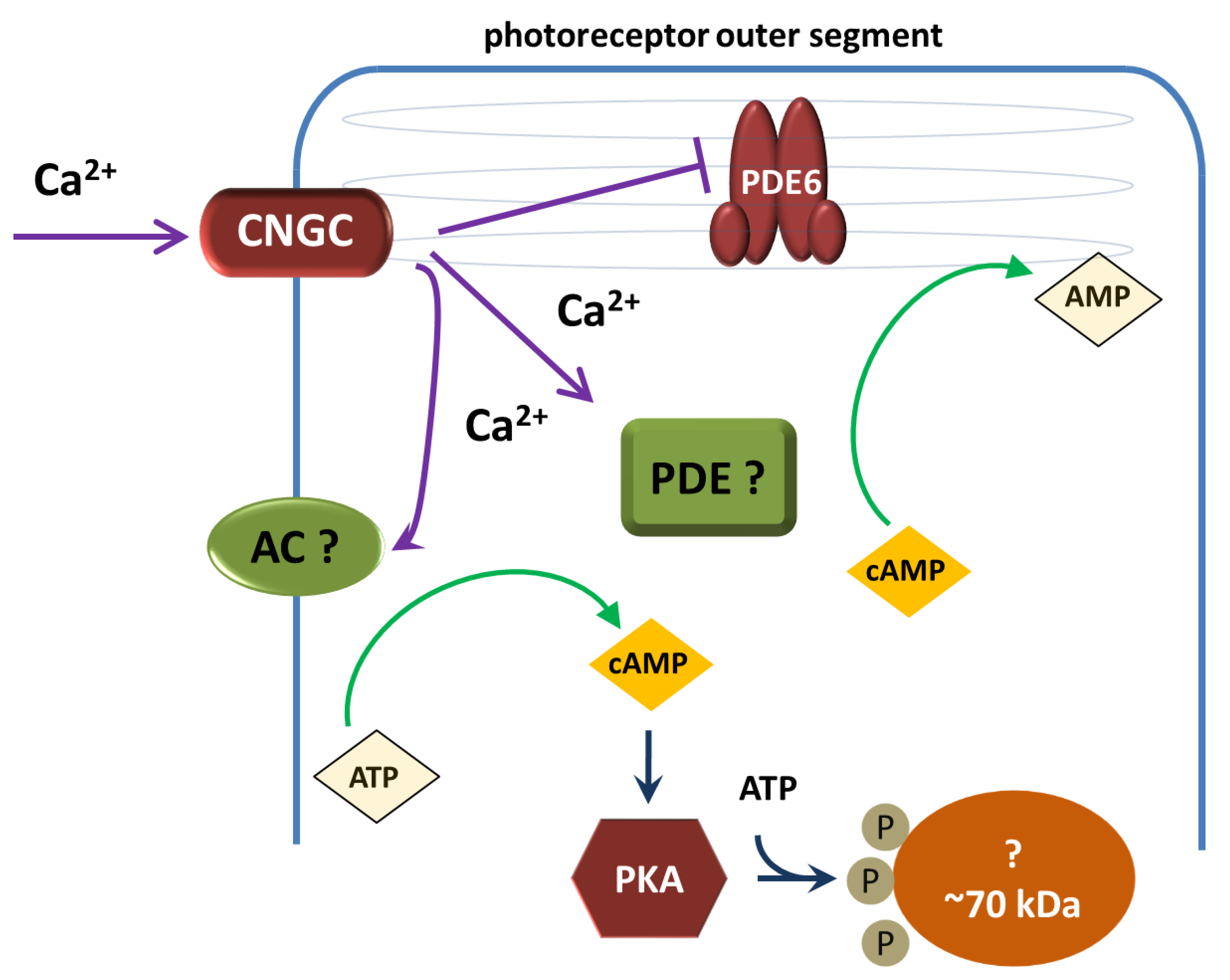
Dark level of free cytoplasmic calcium in amphibian rod photoreceptors is about 600 nM and it drops up to 30 nM when rod is illuminated by saturating light [
23]. A hypothetical scheme to explain the turnover of cAMP in the photoreceptor outer segment of the frog retina should include specific types of adenylate cyclase and phosphodiesterases to determine the sign of the response of these enzymes to a decrease in calcium concentration (
Figure 3). Unfortunately, we do not yet have information on the repertoire of these enzymes in frog photoreceptors, so we cannot directly correlate the results of our experiments with this scheme.
An increase in cAMP levels leads to an increase in PKA activity, which is also measured in photoreceptor outer segments. We have shown that PKA activity increases from 30 seconds after the light stimulus is turned on and continues to increase for up to 5 minutes after the stimulus is turned on. The light intensities we used approximate the light intensities used in physiological experiments where the existence of slow processes at the level of individual photoreceptors and isolated retina has been demonstrated [
24]. At the same time, these intensities are three orders of magnitude lower than those at which Sato et al. demonstrated the effect of PKA on light stimulus activation, and four orders of magnitude lower than the intensities that elicited a significant OFF-response. Оur data indicate that the main target of phosphorylation by protein kinase A is a protein or a small group of proteins with a molecular mass of about 70 kDa. Some key proteins of the phototransduction cascade (such as rhodopsin kinase or the alpha and beta subunits of guanylate cyclase) have molecular masses close to this value, but additional experiments are needed for reliable identification.
4. Materials and Methods
4.1. Drugs and chemicals.
Cyclic adenosine monophosphate (cAMP) was obtained from Merck (USA, purity not less than 98%), аcetonitrile (HPLC grade) from ITW Group (USA), formic acid from Sigma-Aldrich (USA), isopropylic alcohol (purity not less than 99.8%) from Lenreactiv (Russia), isotope-labeled cyclic adenosine monophosphate (cAMP-13C5, purity not less than 99%) prodused by “TRC” (Canada) was used as an internal standard. IBMX, forskolin and all the chemicals used in the preparation of the Ringer's solution were purchased from Sigma-Aldrich..
4.2. Animals and preparation of retinal samples.
Adult marsh frogs (P. ridibundus) were collected from the wild in southern Russia. The frogs were housed in water tanks at temperatures of 6-8°C for a maximum of 8 months. Animals were handled in accordance with the Council Directive of the European Communities (24 November 1986; 86/609/EEC) and the experimental protocol was approved by the local Institutional Animal Care and Use Committee (protocol # 4/22 at 28.01.2022). Prior to the experiment, animals were transferred to room temperature and a 12/12 light cycle for several days. Animals were dark adapted overnight and then decapitated under dark red light. Both eyes were isolated and enucleated, the hemisphere of each eye was cut into 2, 3 or 4 equal segments. The retinas were then separated and layered on filter paper. Before use in the cryofixation setup, retinal sections layered on filter paper were stored on a moist substrate at room temperature in the dark for no more than a few minutes.The normal Ringer solution used for the frog preparations and perfusion was (in mM): NaCl 90, KCl 2.5, MgCl2 1.4, CaCl2 1.05, NaHCO3 5, HEPES 5, glucose 10, EDTA 0.05, pH adjusted to 7.6 with sodium hydroxide. In experiments with IBMX-induced and forskolin-induced changes in cAMP concentration, Ringer's solution was prepared by adding either 225 mM IBMX stock solution in dimethylsulfoxide (DMSO) or 10 mM forskolin stock solution in DMSO. The final concentration of IBMX in the Ringer solution was 50 μM and forskolin was 10 μM. The incubation time prior to cryofixation was 5 min for IBMX Ringer solution and 20 min for Forskolin Ringer solution.
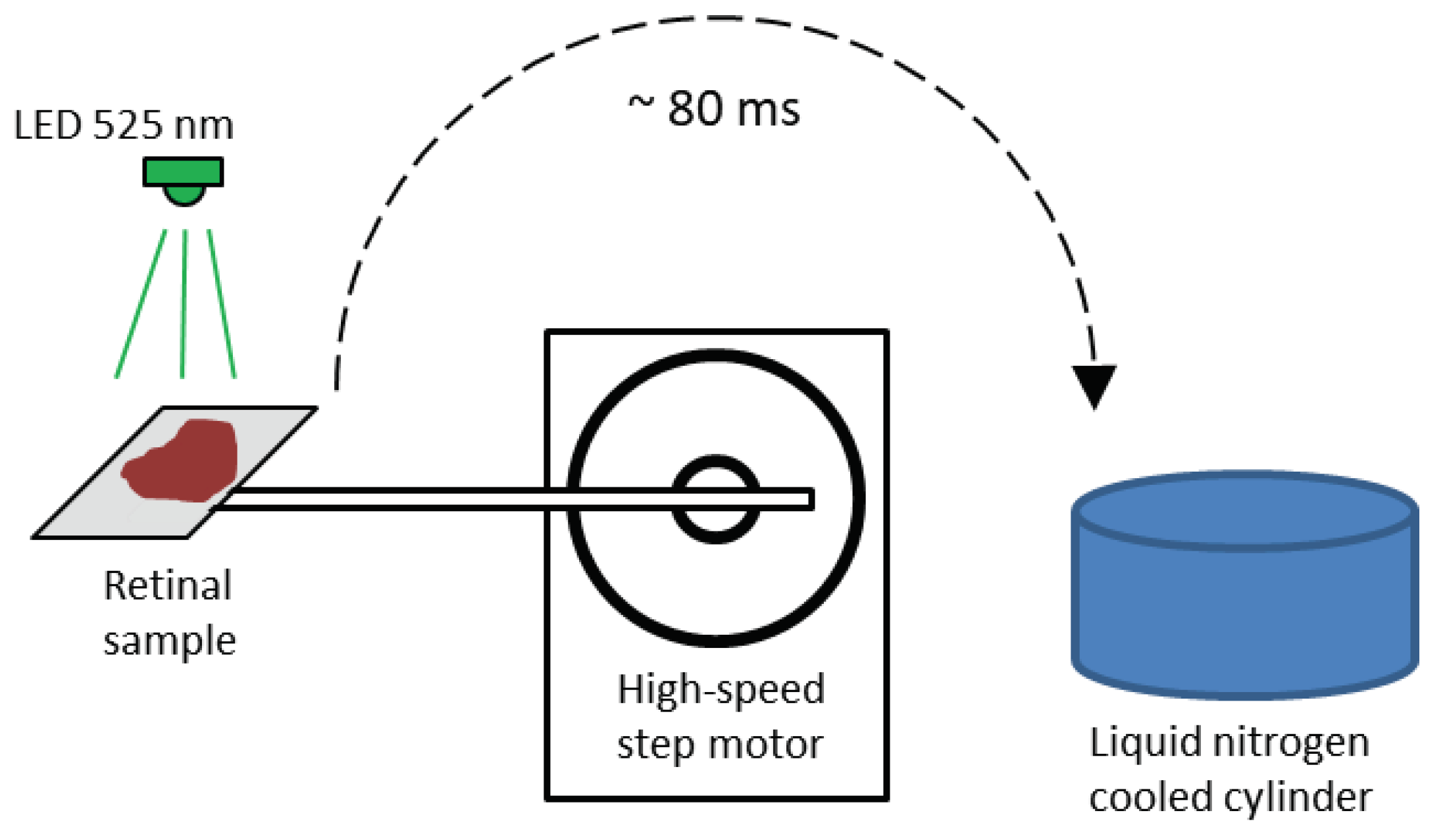
4.3. Rapid retinal sample cryofixation.
The setup for rapid cryofixation of retinal samples is a system of six identical sections with a fast stepper motor (max speed 150,000 rpm) driving a lever with an attached retinal sample pad (
Figure 4). The sample is placed horizontally on the pad and is illuminated with LED light (lambda max 525 nm) by computer command. The light intensities used to stimulate the retina were 1.8x10
4, 4.6x10
4 and 3.6x10
5 quanta/(s·µm
2). After a programmable delay, the lever with the specimen makes an arc of 180
0 in appr. 80 ms and presses the retinal specimen against the polished surface of a copper cylinder cooled to the temperature of liquid nitrogen. For cryofixation of dark-frozen or light-stimulated retinal samples, the sample was placed in a light-isolated cryo-container and stored for no more than two hours before use on the cryotome. The samples were then placed on the Leica RM2265 rotary freezing cryotome, then retinas were serially sectioned at 10-μm thickness until the orange-pink layer containing the photoreceptors was completely excised. The resulting cells shavings were collected with a spatula into a pre-cooled Eppendorf tubes containing 500 µL of 0.1 M HCl. The harvested cells shavings were lysed and dissolved by sonication with a liquid nitrogen pre-cooled ultrasonic tip (Vibra-Cell VCX 130, Sonics, Newtown, CT). The resulting suspension was stored at +0.1°C until aliquoted for protein assay and then at -80°C until further measurements.
4.4. Protein assay.
A 50-μL aliquot of each sample was collected for protein concentration measurement using the Bradford Reagent. All assays were performed in triplicate using BSA as the standard.Absorbance at 595 nm was measured using flat-bottomed 96-well plates in the CLARIOstar Plus Microplate Reader (Labtech International, UK). Protein concentrations were determined by plotting standard curves using Microsoft Excel (Microsoft, Redmond, WA, USA).
4.5. Western blot analysis for detection of PKA activity
PKA activity was determined by antibodies that recognized phosphorylated PKA substrate (P-S/T PKA substrate antibodies, Cell Signaling, cat. #9621S). Frozen sections were homogenize in 100 µl of the buffer (10 mM HEPES, 150 mM NaCl, 0.1% Triton X-100, with protease and phosphatase inhibitors), then equal amount of Laemmli solution was added and samples were boiled at 95oC for 5 min. For Western blot analysis proteins (10 µg of protein/lane) were separated by SDS-PAGE (10% gel) and transferred to nitrocellulose membrane and the membranes were incubated with PKA substrate antibodies (dilution 1 : 1000) overnight at 4oC in 3% nonfat milk in Tris buffer with 0.1% Tween (TBST). After washing in TBST, membranes were incubated with goat anti-rabbit IGG-conjugated with horseradish peroxidase and the signal was visualized by ECL detection (Amersham Pharmacia Biotech).
4.6. cAMP measurement.
4.6.1. Liquid chromatography- tandem mass spectrometry (LC–MS/MS)
Sample extracts were analyzed using a high resolution HPLC–MS/MS system consisting of a Dionex UltiMate 3000 HPLC (Thermo Scientific) with Q Exactive detector (Thermo Scientific) with electrospray ionization (ESI). After various tests to obtain the best signal response for each compound, chromatographic separation was achieved by injecting 20 µl of the sample extract into a Zorbax SB-C8 150mm × 4.6mm × 1.8 µm column. The mobile phase was a gradient mixture of two components: solvent A - 0.1M ammonium formiate in water, solvent B - acetonitrile. The flow rate was set to 0.400 ml/min with the following gradient programme: 0.0-2.0 min 2% solvent B, then the B content increased to 30% at 8.0 min, and remained so until 9 min, then decreased to 2% at 9.1 min and remained so until the end of the programme. Mass spectrometric detection was performed using negative electrospray ESI(-). The analytes were identified by selecting characteristic target reactions (MRM transitions) and the retention time of the analytes. An example of a chromatogram and mass spectrа of the analytes is given in
Supplementary Figure S3. The following MS parameters were kept constant during the analysis: nebulisation voltage 4800 V for positive ionization. The temperature of the cone was set at 300°C, the temperature of the heated probe at 400°C, the gas flow through the nebulizer at 3 l/min, the flow rate of the drying gas 10 l/min. Product ions and precursor ions were selected for analyte identification (
Table 1).
4.6.2. Preparation of Standard Solutions.
To prepare the stock internal standard solution, 10 mg of cAMP-13C5 was accurately weighed (±0.1 mg) using an AUW-220D analytic balance (Shimadzu, Japan), transferred to a 1000 mL volumetric flask and dissolved in 0.1M HCl in water. Working internal standard solutions (10 ng/ml) were prepared by diluting the stock solutions with 0.1M HCl in water. Stock solutions were stored at +4°C for no longer than one week.
To prepare the standard solutions, 10 mg of each substance was accurately weighed (±0.1 mg) using an AUW-220D analytic balance (Shimadzu, Japan), transferred to a 25 mL volumetric flask and dissolved in the working internal standard solution. Calibration solutions were prepared from the stock solution by dilution with working internal standard solution. All stock solutions were stored at +4°C for no longer than one week.
4.6.3. Sample preparation.
50 μl of the working internal standard solution was added to Eppendorf tubes with samples and thoroughly mixed using a rotary shaker (15 min) and then ultrasonic unit. After ultrasonic stirring for 15 minutes, the tubes were centrifuged at 14,000 rpm for 5 minutes. Approximately 40 μl of the supernatant was decanted, transferred into glass vials for HPLC analysis.
4.7. Statistical Analysis.
All values are expressed as the mean ± SEM. All statistical analyses were performed using GraphPad Prism 8 software (GraphPad, La Jolla, CA, USA). For all data analyzed one-sample t-test was used . The data were checked for normality by Shapiro-Wilk test, outliners were removed. A value of p < 0.05 was considered statistically significant (* p < 0.05, ** p < 0.01).
5. Conclusions
In the present study, we have used an original cryofixation method to investigate the dynamics of changes in the concentration of cAMP in toad photoreceptor outer segments in response to light stimulation. We have shown that in response to a light stimulus, the concentration of cAMP increases by 50-70% during the first second and then returns to the dark level. An increase in cAMP concentration for no more than 30 seconds leads to the activation of PKA. The target of PKA phosphorylation is a protein with a molecular mass of about 70 kDa.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
Conceptualization, M.F. and S.G.; methodology, M.F.,M.B., D.M. and S.G.; formal analysis, M.F. and O.C..; investigation, O.C., M.B. and N.E..; writing, M.F.,M.B. and S.G.; supervision and funding acquisition, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by RSF grant # 22-25-00656 to MF.
Institutional Review Board Statement
The study was conducted in accordance with the Council Directive of the European Communities (24 November 1986; 86/609/EEC) and the experimental protocol # 4/22 was approved by the local Animal Care and Use Committee of Sechenov Institute at 28.01.2022.
Conflicts of Interest
The authors declare no conflict of interest
References
- Lamb, T.D. Photoreceptor physiology and evolution: Cellular and molecular basis of rod and cone phototransduction. The Journal of Physiology 2022, 600, 4585–4601. [Google Scholar] [CrossRef]
- Dell’Orco, D.; Koch, K.-W.; Rispoli, G. Where vision begins. Springer: 2021; Vol. 473, pp 1333-1337.
- Nikolaeva, D.A.; Nekrasova, M.A.; Rotov, A.Y.; Astakhova, L.A. Adaptation memory in photoreceptors: Different mechanisms in rods and cones. Frontiers in Molecular Neuroscience 2023, 16, 1135088. [Google Scholar] [CrossRef]
- Burnside, B.; Ackland, N. Effects of circadian rhythm and camp on retinomotor movements in the green sunfish, lepomis cyanellus. Invest Ophthalmol.Vis.Sci. 1984, 25. [Google Scholar]
- Hasegawa, M.; Cahill, G.M. Cyclic amp resets the circadian clock in cultured xenopus retinal photoreceptor layers. J.Neurochem. 1998, 70, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, S.S.; Haque, R.; Pozdeyev, N.; Jackson, C.R.; Iuvone, P.M. Temporal coupling of cyclic amp and ca/calmodulin-stimulated adenylyl cyclase to the circadian clock in chick retinal photoreceptor cells. J.Neurochem. 2006, 99, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Cahill, G.M. A role for cyclic amp in entrainment of the circadian oscillator in xenopus retinal photoreceptors by dopamine but not by light. J.Neurochem. 1999, 72, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Steinle, J.J. Review: Role of camp signaling in diabetic retinopathy. Molecular vision 2020, 26, 355–358. [Google Scholar] [PubMed]
- Erofeeva, N.; Meshalkina, D.; Firsov, M. Multiple roles of camp in vertebrate retina. Cells 2023, 12, 1157. [Google Scholar] [CrossRef] [PubMed]
- Osawa, S.; Jo, R.; Weiss, E.R. Phosphorylation of grk7 by pka in cone photoreceptor cells is regulated by light. J.Neurochem. 2008, 107, 1314–1324. [Google Scholar] [CrossRef]
- Osawa, S.; Jo, R.; Xiong, Y.; Reidel, B.; Tserentsoodol, N.; Arshavsky, V.Y.; Iuvone, P.M.; Weiss, E.R. Phosphorylation of g protein-coupled receptor kinase 1 (grk1) is regulated by light but independent of phototransduction in rod photoreceptors. J.Biol.Chem. 2011, 286, 20923–20929. [Google Scholar] [CrossRef]
- Astakhova, L.A.; Samoiliuk, E.V.; Govardovskii, V.I.; Firsov, M.L. Camp controls rod photoreceptor sensitivity via multiple targets in the phototransduction cascade. J.Gen.Physiol 2012, 140, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Gambaryan, S.; Kobsar, A.; Rukoyatkina, N.; Herterich, S.; Geiger, J.; Smolenski, A.; Lohmann, S.M.; Walter, U. Thrombin and collagen induce a feedback inhibitory signaling pathway in platelets involving dissociation of the catalytic subunit of protein kinase a from an nfκb-iκb complex. Journal of Biological Chemistry 2010, 285, 18352–18363. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Yamashita, T.; Matsuda, M. Rhodopsin-mediated light-off-induced protein kinase a activation in mouse rod photoreceptor cells. Proc.Natl.Acad.Sci U.S.A 2020, 117, 26996–27003. [Google Scholar] [CrossRef]
- Halls, M.L.; Cooper, D.M. Regulation by ca2+-signaling pathways of adenylyl cyclases. Cold Spring Harb Perspect Biol 2011, 3, a004143. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, C.; Liu, C.; Ivanova, T.N.; Chan, G.C.; Storm, D.R.; Iuvone, P.M.; Tosini, G. Gating of the camp signaling cascade and melatonin synthesis by the circadian clock in mammalian retina. J.Neurosci. 2004, 24, 1803–1811. [Google Scholar] [CrossRef]
- Yoshida, S.; Mears, A.J.; Friedman, J.S.; Carter, T.; He, S.; Oh, E.; Jing, Y.; Farjo, R.; Fleury, G.; Barlow, C. Expression profiling of the developing and mature nrl−/− mouse retina: Identification of retinal disease candidates and transcriptional regulatory targets of nrl. Human molecular genetics 2004, 13, 1487–1503. [Google Scholar] [CrossRef]
- Nakao, T.; Tsujikawa, M.; Notomi, S.; Ikeda, Y.; Nishida, K. The role of mislocalized phototransduction in photoreceptor cell death of retinitis pigmentosa. PloS one 2012, 7, e32472. [Google Scholar] [CrossRef]
- Cote, R.H.; Gupta, R.; Irwin, M.J.; Wang, X. Photoreceptor phosphodiesterase (pde6): Structure, regulatory mechanisms, and implications for treatment of retinal diseases. Advances in experimental medicine and biology 2022, 1371, 33–59. [Google Scholar] [PubMed]
- Santone, R.; Giorgi, M.; Maccarone, R.; Basso, M.; Deplano, S.; Bisti, S. Gene expression and protein localization of calmodulin-dependent phosphodiesterase in adult rat retina. Journal of neuroscience research 2006, 84, 1020–1026. [Google Scholar] [CrossRef]
- Whitaker, C.M.; Cooper, N.G. The novel distribution of phosphodiesterase-4 subtypes within the rat retina. Neuroscience 2009, 163, 1277–1291. [Google Scholar] [CrossRef]
- Omori, K.; Kotera, J. Overview of pdes and their regulation. Circ.Res. 2007, 100, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Pugh, E.N., Jr.; Nikonov, S.; Lamb, T.D. Molecular mechanisms of vertebrate photoreceptor light adaptation. Current opinion in neurobiology 1999, 9, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Rotov, A.Y.; Astakhova, L.; Firsov, M.; Govardovskii, V. Light adaptation of retinal rods, adaptation memory, and afterimages. Neuroscience and Behavioral Physiology 2021, 51, 116–122. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).