Submitted:
30 October 2023
Posted:
31 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of nanocomposites
2.2.1. Co nanoparticles synthesis
2.2.2. Co3O4 nanoparticles synthesis
2.2.3. MnO2 nanoparticles synthesis
2.2.4. Co3O4/Zeolite nanocomposite synthesis
2.2.5. CeO2 nanoparticle synthesis
2.2.6. Co3O4 /CeO2 nanocomposite synthesis
2.2.7. Co3O4 /MnO2 nanocomposite synthesis
2.3. Characterization of catalysts
2.4. Catalytic activity test
3. Results and Discussion
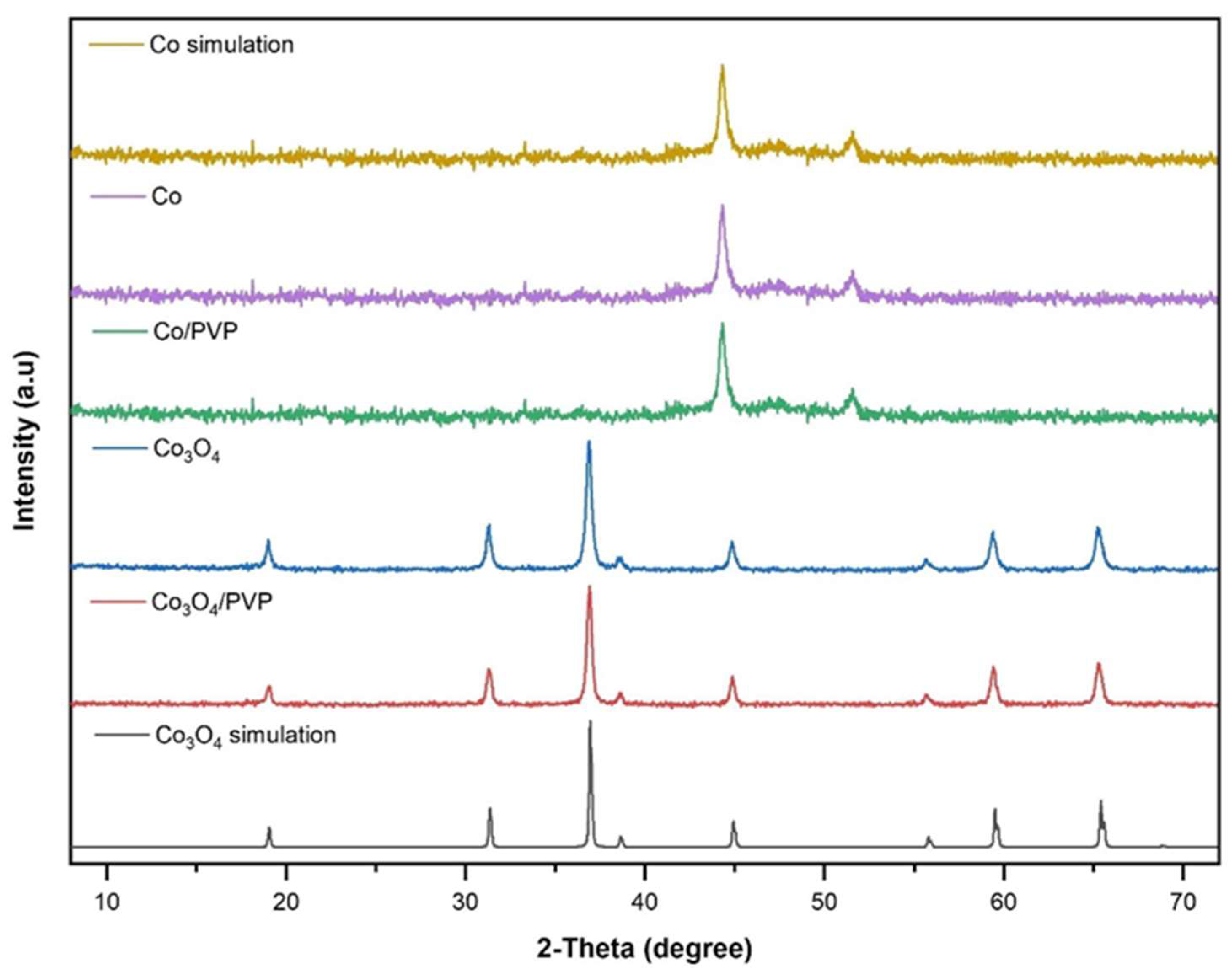
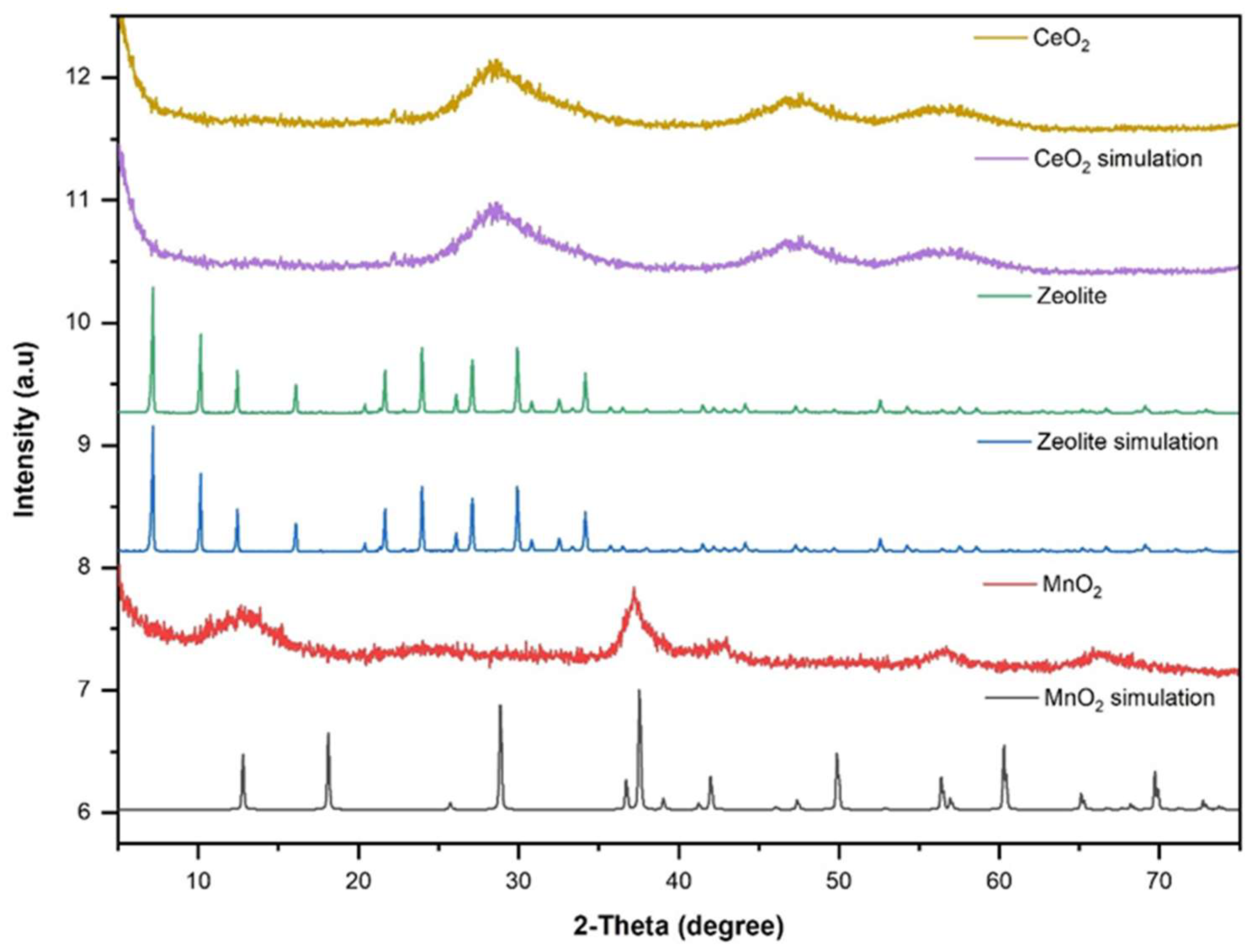
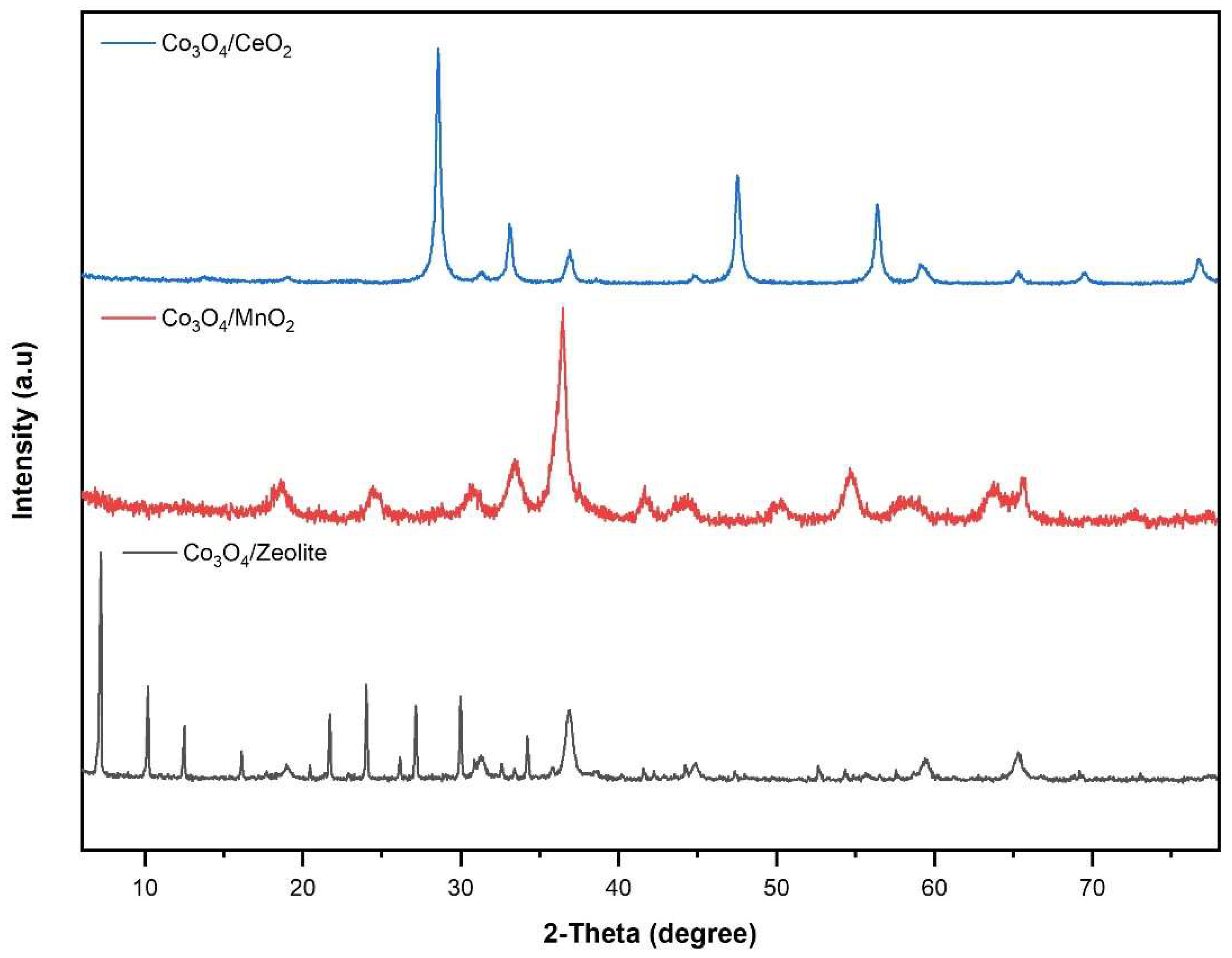
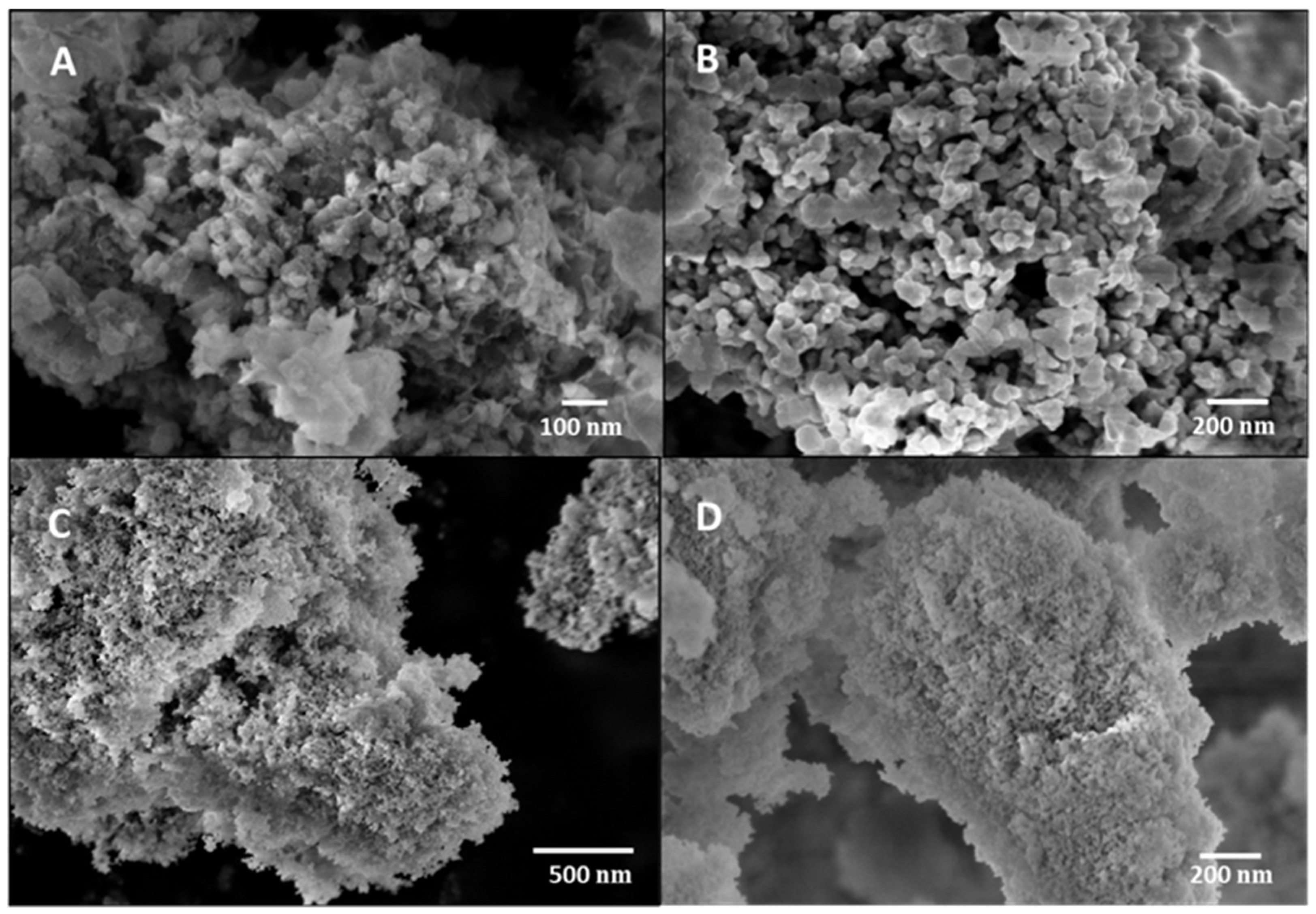
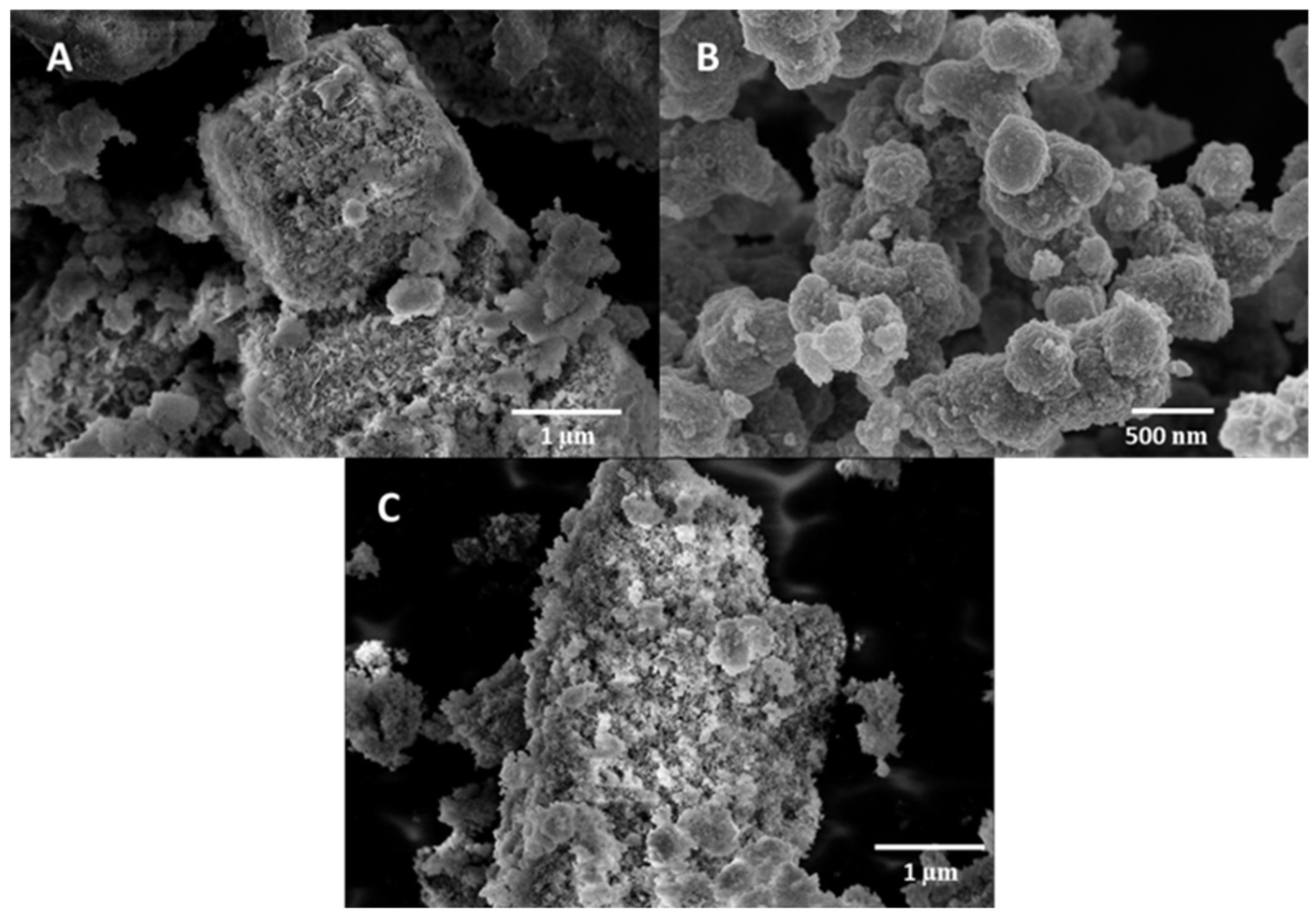
3.1. Structural and morphological characterization of nanomaterials
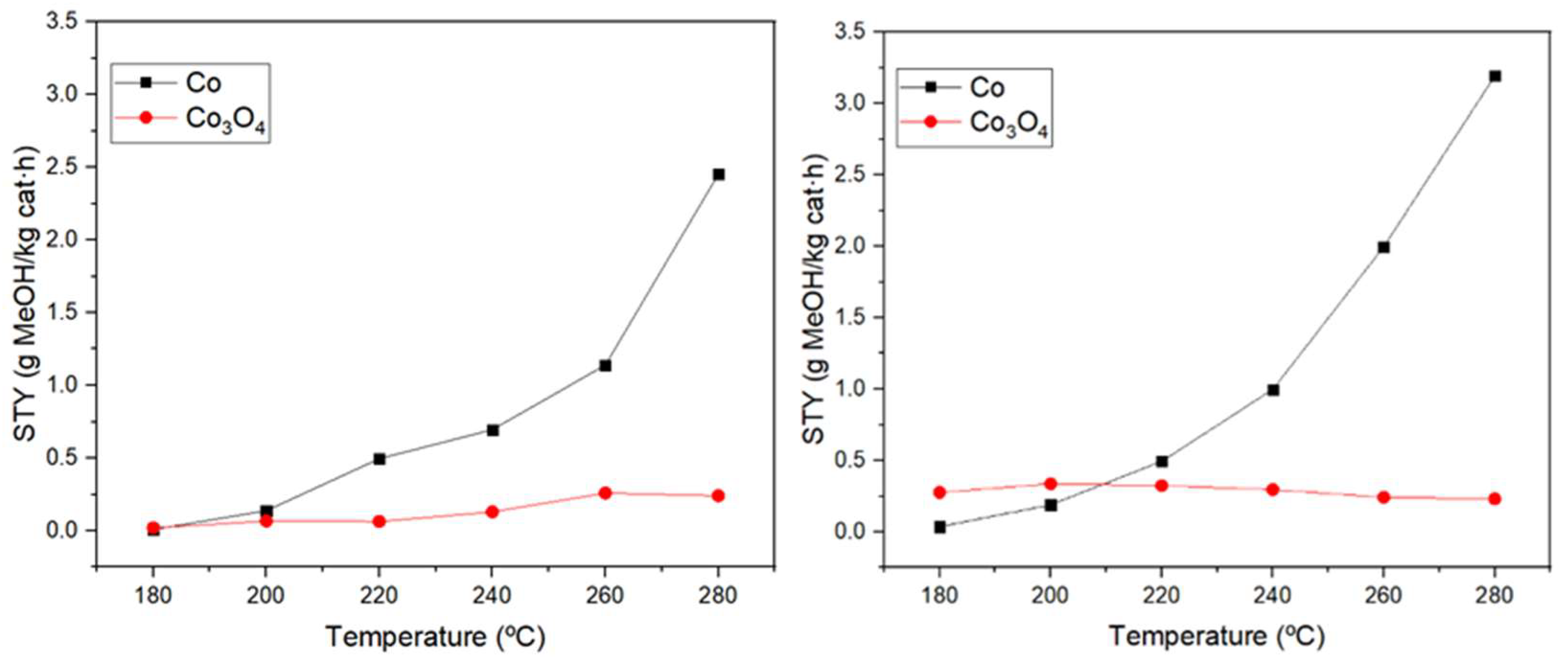
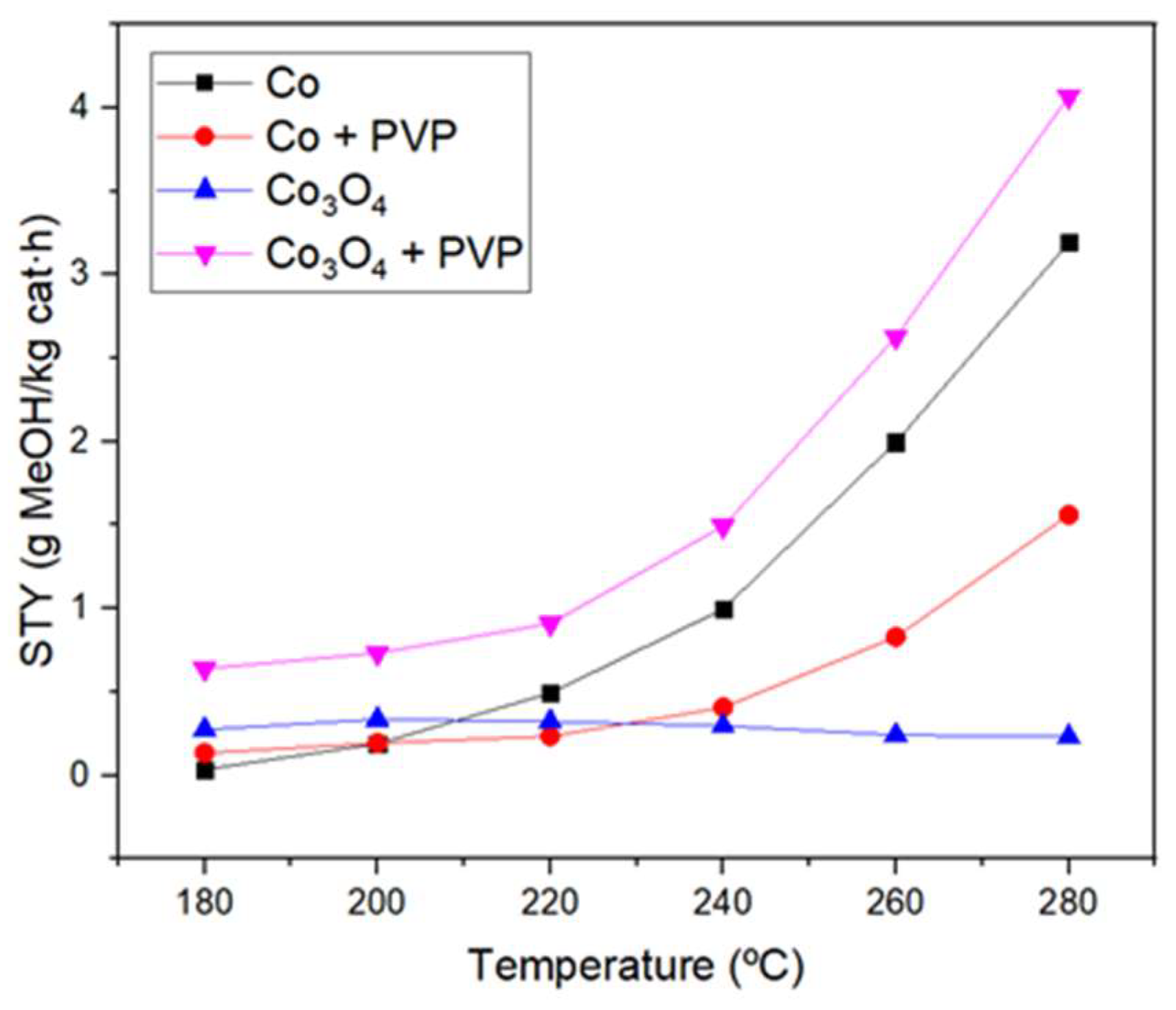
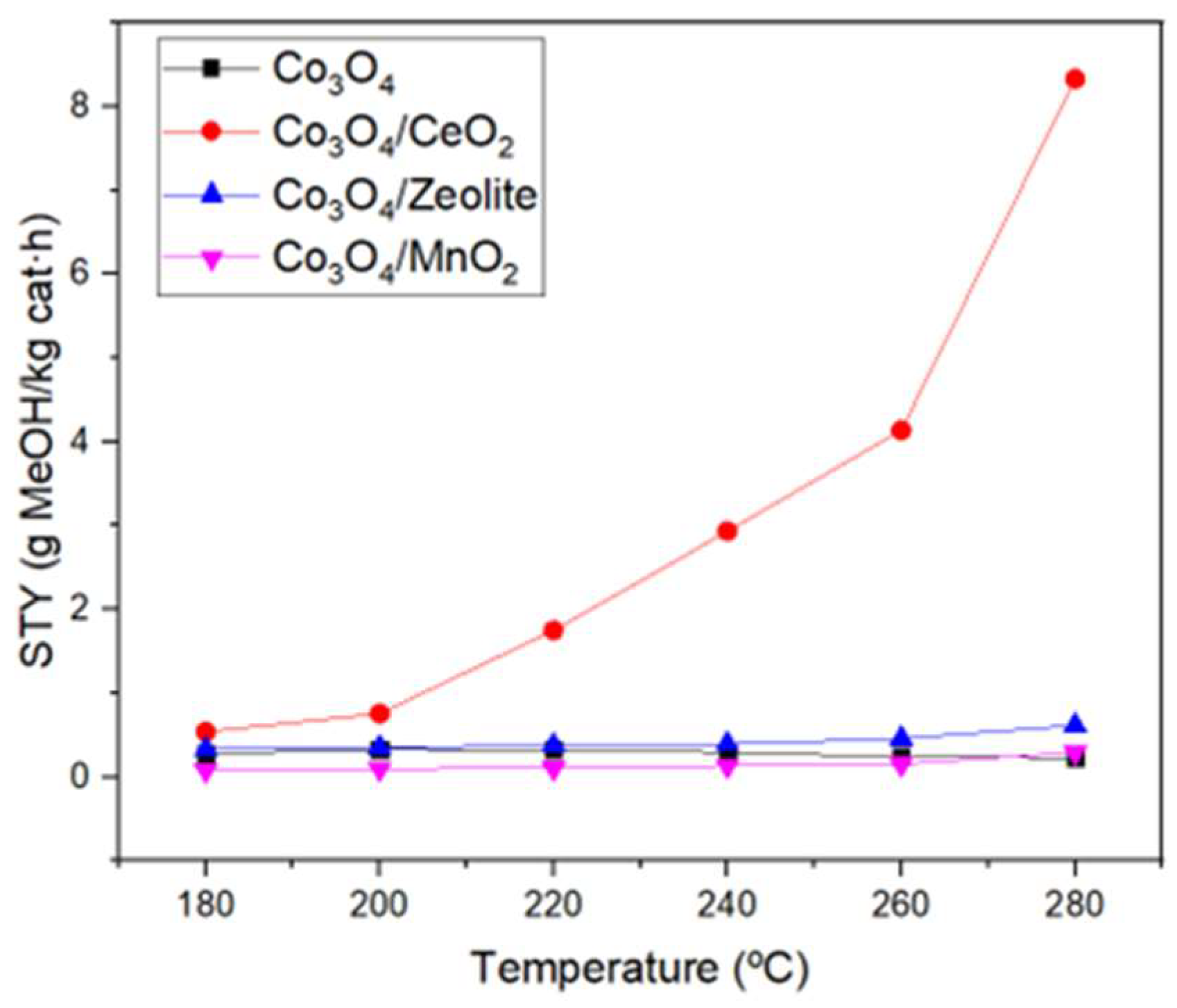
3.2. Catalytic activity of the catalysts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masson-Delmotte, V.; Zhai P.; Pirani A.; Connors S.L.; Péan C.; Berger S., Caud N., Chen Y. IPCC, 2021: Summary of Policymakers. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. En Climate Change 2021: The Physical Science Basis. Cambridge University Press, United Kingdom and New York, NY, USA 2021, No. In Press, 3949.
- Yadav, S.; Mondal, S.S. A review on the progress and prospects of oxy-fuel carbon capture and sequestration (CCS) Technology. Fuel 2022, 308, 122057. [Google Scholar] [CrossRef]
- Sabri, M.A.; Al Jitan, S.; Bahamon, D.; Vega, L.F.; Palmisano, G. Current and future perspectives on catalytic-based integrated carbon capture and utilization. Sci. Total Environ. 2021, 790, 148081. [Google Scholar] [CrossRef] [PubMed]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Olah, G.A. Beyond Oil and Gas: The Methanol Economy. Angew. Chemie Int. Ed. 2005, 44, 2636–2639. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.G.; Vaidya, P.D.; Bhanage, B.M.; Joshi, J.B. Catalytic carbon dioxide hydrogenation to methanol: A review of recent studies. Chem. Eng. Res. Des. 2014, 92, 2557–2567. [Google Scholar] [CrossRef]
- Li, M.M.J.; Tsang, S.C.E. Bimetallic catalysts for green methanol production via CO2 and renewable hydrogen: A mini-review and prospects. Catal. Sci. Technol. 2018, 8, 3450–3464. [Google Scholar] [CrossRef]
- Sha, F.; Han, Z.; Tang, S.; Wang, J.; Li, C. Hydrogenation of Carbon Dioxide to Methanol over Non−Cu-based Heterogeneous Catalysts. ChemSusChem 2020, 13, 6160–6181. [Google Scholar] [CrossRef] [PubMed]
- Have, I.C.; Kromwijk, J.J.G.; Monai, M.; Ferri, D.; Sterk, E.B.; Meirer, F.; Weckhuysen, B.M. Uncovering the reaction mechanism behind CoO as active phase for CO2 hydrogenation. Nat. Commun. 2022, 13, 324. [Google Scholar] [CrossRef]
- Tan, Q.; Shi, Z.; Wu, D. CO2 Hydrogenation to Methanol over a Highly Active CuNi/CeO2-Nanotube Catalyst. Ind. Eng. Chem. Res. 2018, 57, 10148–10158. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.Y.; Ding, Y.; Yu, Z. Mesoporous manganese-cobalt oxide spinel catalysts for CO2 hydrogenation to methanol. J. CO2 Util. 2019, 32, 146–154. [Google Scholar] [CrossRef]
- Wang, L.; Guan, E.; Wang, Y.; Wang, L.; Gong, Z.; Cui, Y.; Meng, X.; Gates, B.C.; Xiao, F.S. Silica accelerates the selective hydrogenation of CO2 to methanol on cobalt catalysts. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, Y.; Qin, Z.; Xie, Q.; Ji, H. Influence of Zr, Ce, and La on Co3O4 catalyst for CO2 methanation at low temperature. Chinese J. Chem. Eng. 2018, 26, 768–774. [Google Scholar] [CrossRef]
- Suslova, E.V.; Chernyak, S.A.; Egorov, A.V.; Savilov, S.V.; Lunin, V.V. CO2 hydrogenation over cobalt-containing catalysts. Kinet. Catal. 2015, 56, 646–654. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Zhang, J.; Liu, X.; Wang, H.; Zhng, W.; Yang, Q.; Ma, J.; Dong, X.; Jo Yoo, S.; Kim, J.G.; Meng, X.; Xiao, F.S. Selective Hydrogenation of CO2 to Ethanol over Cobalt Catalysts. Angew. Chemie Int. Ed. 2018, 57, 6104–6108. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.; Xiong, S.; Zhang, Y.; Liu, B.; Li, J. The study of morphology effect of Pt/Co3O4 catalysts for higher alcohol synthesis from CO2 hydrogenation. Appl. Catal. A Gen. 2017, 543, 189–195. [Google Scholar] [CrossRef]
- Tursunov, O.; Tilyabaev, Z. Hydrogenation of CO2 over Co supported on carbon nanotube, carbon nanotube-Nb2O5, carbon nanofiber, low-layered graphite fragments and Nb2O5. J. Energy Inst. 2019, 92, 18–26. [Google Scholar] [CrossRef]
- Fan, T.; Liu, H.; Shao, S.; Gong, Y.; Li, G.; Tang, Z. Cobalt Catalysts Enable Selective Hydrogenation of CO2 toward Diverse Products: Recent Progress and Perspective. J. Phys. Chem. Lett. 2021, 12, 10486–10496. [Google Scholar] [CrossRef] [PubMed]
- Carrasco Garcia, A.; Moral-Vico, J.; Markeb, A.A.; Sanchez, A. Conversion of Carbon Dioxide into Methanol Using Cu–Zn Nanostructured Materials as Catalysts. Nanomaterials 2022, 12, 999. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Tang, Y.; Nie, L.; Andolina, C.M.; Zhang, X.; House, S.; Li, Y.; Yang, J.; Tao, F. Complete Oxidation of Methane on Co3O4/CeO2 Nanocomposite: A Synergic Effect. Catal. Today 2018, 311, 48–55. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, K.; Wang, L.; Wang, B.; Li, Y. Oxygen vacancy clusters promoting reducibility and activity of ceria nanorods. J. Am. Chem. Soc. 2009, 131, 3140–3141. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; ul Ain, N.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: recent trends and future prospects. J. Nanobiotechnology 2020, 18, 1–15. [Google Scholar] [CrossRef]
- Zhao, Y.W.; Zheng, R.K.; Zhang, X.X.; Xiao, J.Q. A simple method to prepare uniform Co nanoparticles. IEEE Trans. Magn. 2003, 39, 2764–2766. [Google Scholar] [CrossRef]
- Worku, A.K.; Ayele, D.W.; Habtu, N.G.; Yemata, T.A. Engineering Co3O4/MnO2 nanocomposite materials for oxygen reduction electrocatalysis. Heliyon 2021, 7, e08076. [Google Scholar] [CrossRef] [PubMed]
- Natile, M.M.; Glisenti, A. CoO x / CeO2 Nanocomposite Powders : Synthesis, Characterization, and Reactivity,”. Chem. Mater. 2005, 17, 3403–3414. [Google Scholar] [CrossRef]
- Jincy, C.S.; Meena, P. Synthesis of Co3O4 nanoparticles for sensing toxic gas at room temperature. Mater. Today Proc. 2020, 33, 2362–2365. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Hua, W.C.; Guo, Y.; Lu, G.Z.; Gil, S.; Giroir-Fendler, A. Catalytic oxidation of vinyl chloride emissions over Co-Ce composite oxide catalysts. Chem. Eng. J. 2017, 315, 392–402. [Google Scholar] [CrossRef]
- Palacio, R.; Torres, S.; Lopez, D.; Hernandez, D. Selective glycerol conversion to lactic acid on Co3O4/CeO2 catalysts. Catal. Today 2018, 302, 196–202. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, G.; Du, Q.; Su, L.; Ma, Z.; Qin, X.; Shao, G. Co3O4@MnO2 core shell arrays on nickel foam with excellent electrochemical performance for aqueous asymmetric supercapacitor. Ionics (Kiel) 2017, 23, 1637–1643. [Google Scholar] [CrossRef]
- Davar, F.; Fereshteh, Z.; Shoja Razavi, H.; Razavi, R.S.; Loghman-Estarki, M.R. Synthesis and characterization of cobalt oxide nanocomposite based on the Co3O4–zeolite Y. Superlattices Microstruct. 2014, 66, 85–95. [Google Scholar] [CrossRef]
- Gupta, S.; Ciotonea, C.; Royer, S.; Dacquin, J.; Vinod, C.P. Engineering pore morphology using silica template route over mesoporous cobalt oxide and its implications in atmospheric pressure carbon dioxide hydrogenation to olefins. Appl. Mater. Today 2020, 19, 100586. [Google Scholar] [CrossRef]
- Jampaiah, D.; Damma, D.; Chalkidis, A.; Venkataswamy, P.; Bhargava, S.K.; Reddy, B.M. MOF-derived ceria-zirconia supported Co3O4 catalysts with enhanced activity in CO2 methanation. Catal. Today 2020, 356, 519–526. [Google Scholar] [CrossRef]
- Díez-Ramírez, J.; Sanchez, P.; Kyriakou, V.; Zafeiratos, S.; Marnellos, G.E.; Konsolakis, M.; Dorado, F. Effect of support nature on the cobalt-catalyzed CO2 hydrogenation. J. CO2 Util. 2017, 21, 562–571. [Google Scholar] [CrossRef]
- Gabe, A.; García-Aguilar, J.; Berenguer-Murcia, A.; Morallón, E.; Cazorla-Amorós, D. Key factors improving oxygen reduction reaction activity in cobalt nanoparticles modified carbon nanotubes. Appl. Catal. B: Environ. 2017, 217, 303–312. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, S.; Liu, B.; Liu, J.; Wang, L.; Xiao, Y.; Xu, Y.; Liu, X. Insights into the Influence of CeO2 Crystal Facet on CO2 Hydrogenation to Methanol over Pd/CeO2 Catalysts. ACS Catal. 2020, 10, 11493–11509. [Google Scholar] [CrossRef]
- Sha, F.; Han, Z.; Tang, S.; Wang, J.; Li, C. Hydrogenation of Carbon Dioxide to Methanol over Non−Cu-based Heterogeneous Catalysts. ChemSusChem 2020, 13, 6160–6181. [Google Scholar]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef] [PubMed]
- Ronda-Lloret, M.; Wang, Y.; Oulego, P.; Rothenberg, G.; Tu, X.; Shiju, N.R. CO2 Hydrogenation at Atmospheric Pressure and Low Temperature Using Plasma Enhanced Catalysis over Supported Cobalt Oxide Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 17397–17407. [Google Scholar] [CrossRef]
- Li, C.S.; Melaet, G.; Ralston, W.T.; An, K.; Brooks, C.; Ye, Y.; Liu, Y.S.; Zhu, J.; Guo, J.; Alayoglu, S.; Somorjai, G.A. High-performance hybrid oxide catalyst of manganese and cobalt for low-pressure methanol synthesis. Nat. Commun. 2015, 6, 1–5. [Google Scholar] [CrossRef]

| Element | Weight (%) | Atomic (%) |
|---|---|---|
| C | 28.60 | 43.21 |
| O | 38.52 | 43.68 |
| Mn | 14.38 | 4.75 |
| Co | 14.15 | 4.36 |
| Na | 0.54 | 0.42 |
| Element | Weight (%) | Atomic (%) |
|---|---|---|
| C | 5.44 | 16.78 |
| O | 24.06 | 55.74 |
| Co | 21.43 | 13.48 |
| Ce | 48.46 | 12.82 |
| Element | Weight (%) | Atomic (%) |
|---|---|---|
| C | 46.01 | 58.85 |
| O | 35.51 | 34.10 |
| Si | 2.12 | 1.16 |
| Al | 2.25 | 1.28 |
| Co | 11.83 | 3.08 |
| Na | 2.29 | 1.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





