Submitted:
31 October 2023
Posted:
31 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Field experimental site and soils
2.2. Fertilizers and rice materials
2.3. Experimental design
2.3.1. Experiment 1
2.3.2. Experiment 2
2.4. Rice and soil sampling
2.5. Chemical analysis of samples
2.6. Data and statistical analyses
3. Results
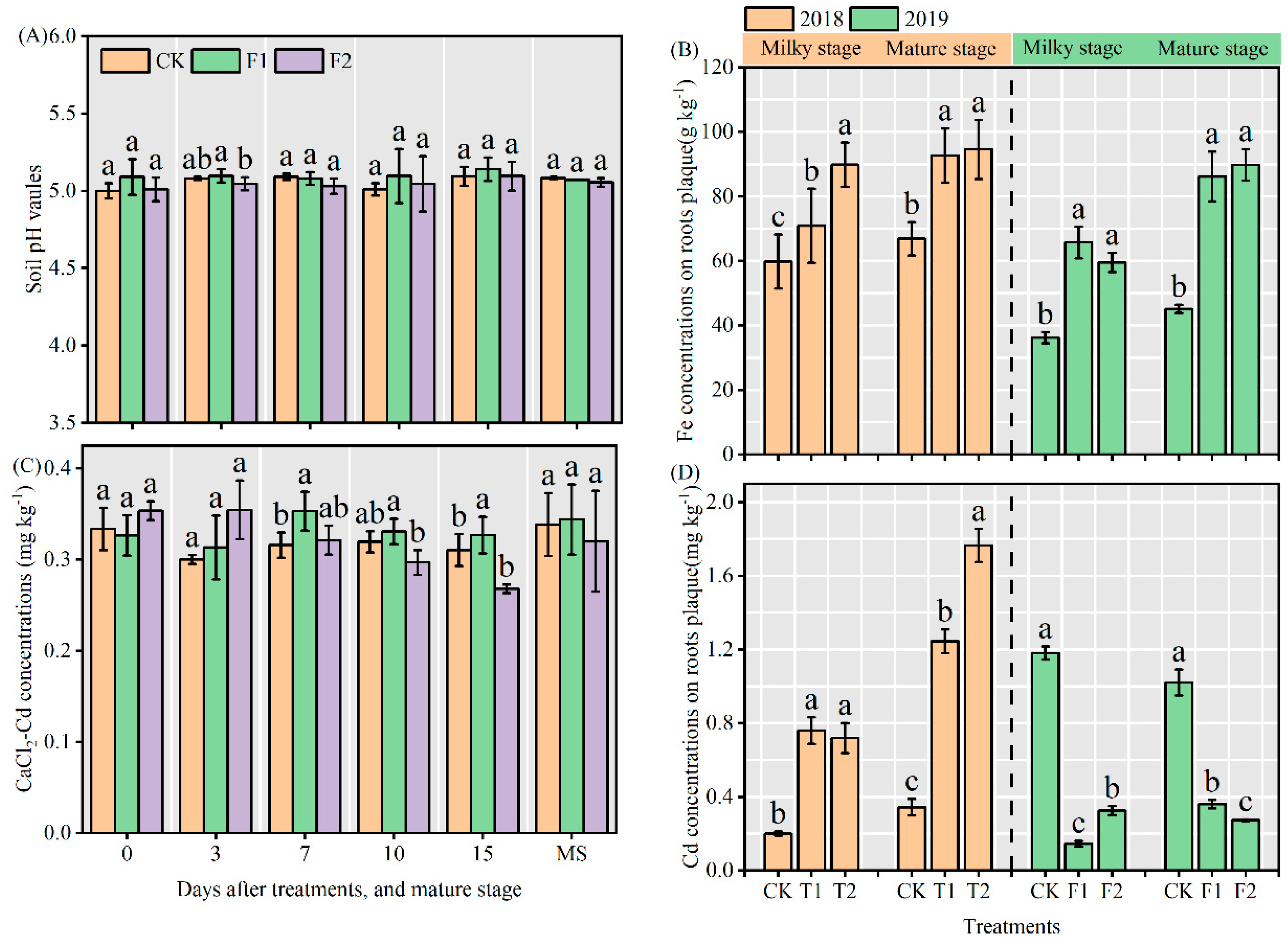
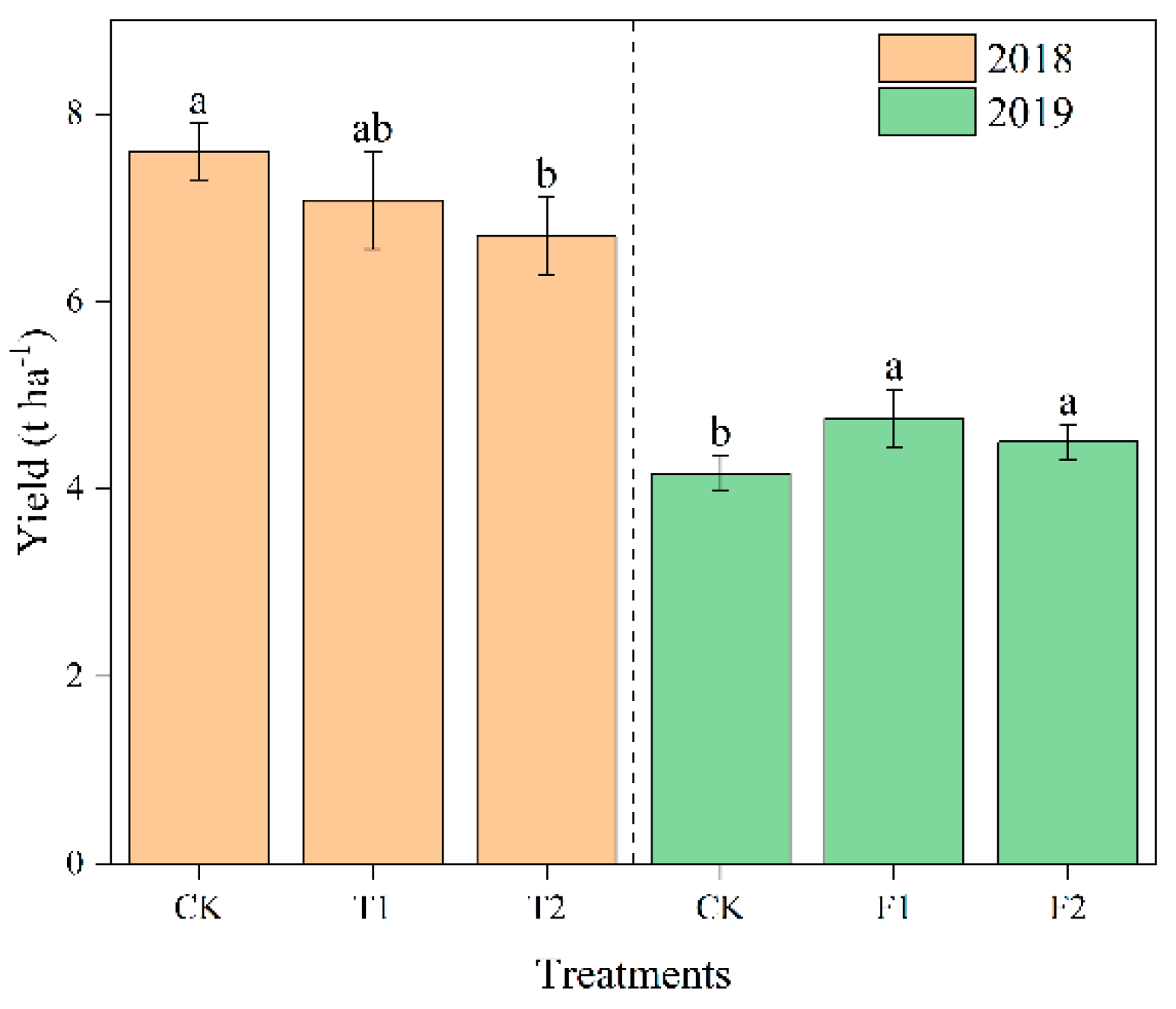
3.1. Rice yields
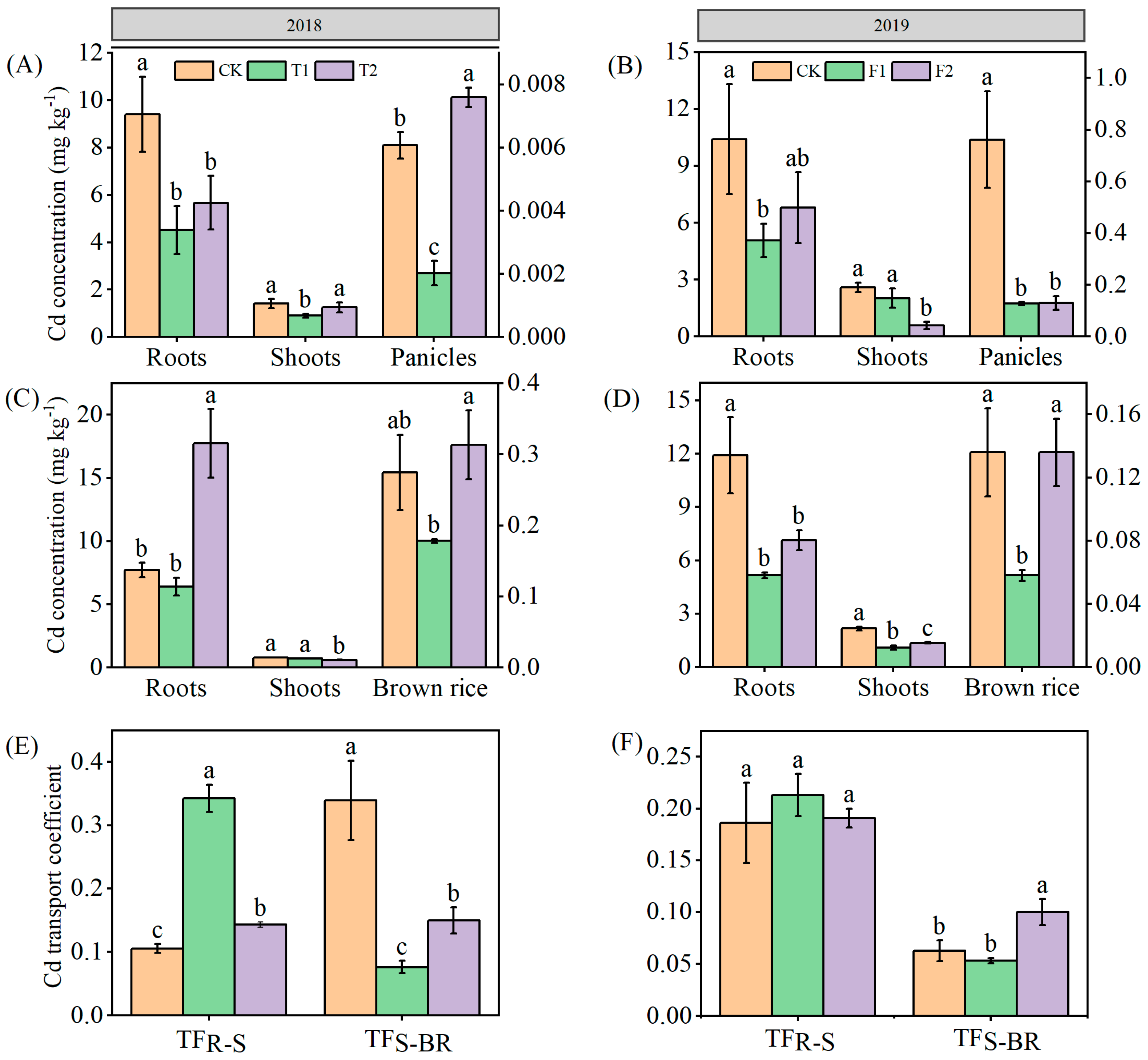
3.2. Cd concentrations in rice different parts
3.3. Soil pH values and CaCl2-Cd concentrations in soil
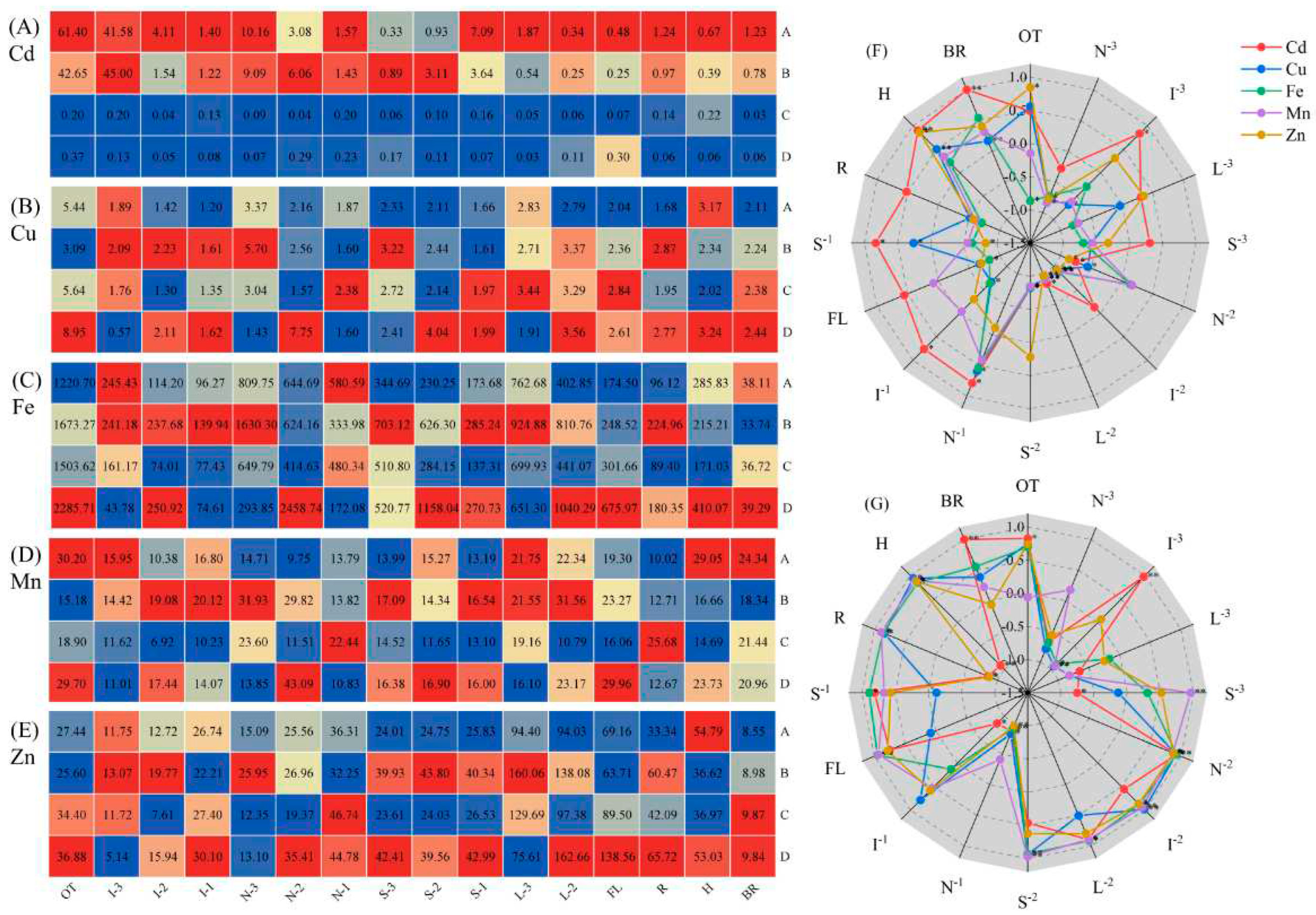
3.4. Concentrations of Fe and Cd on roots plaque
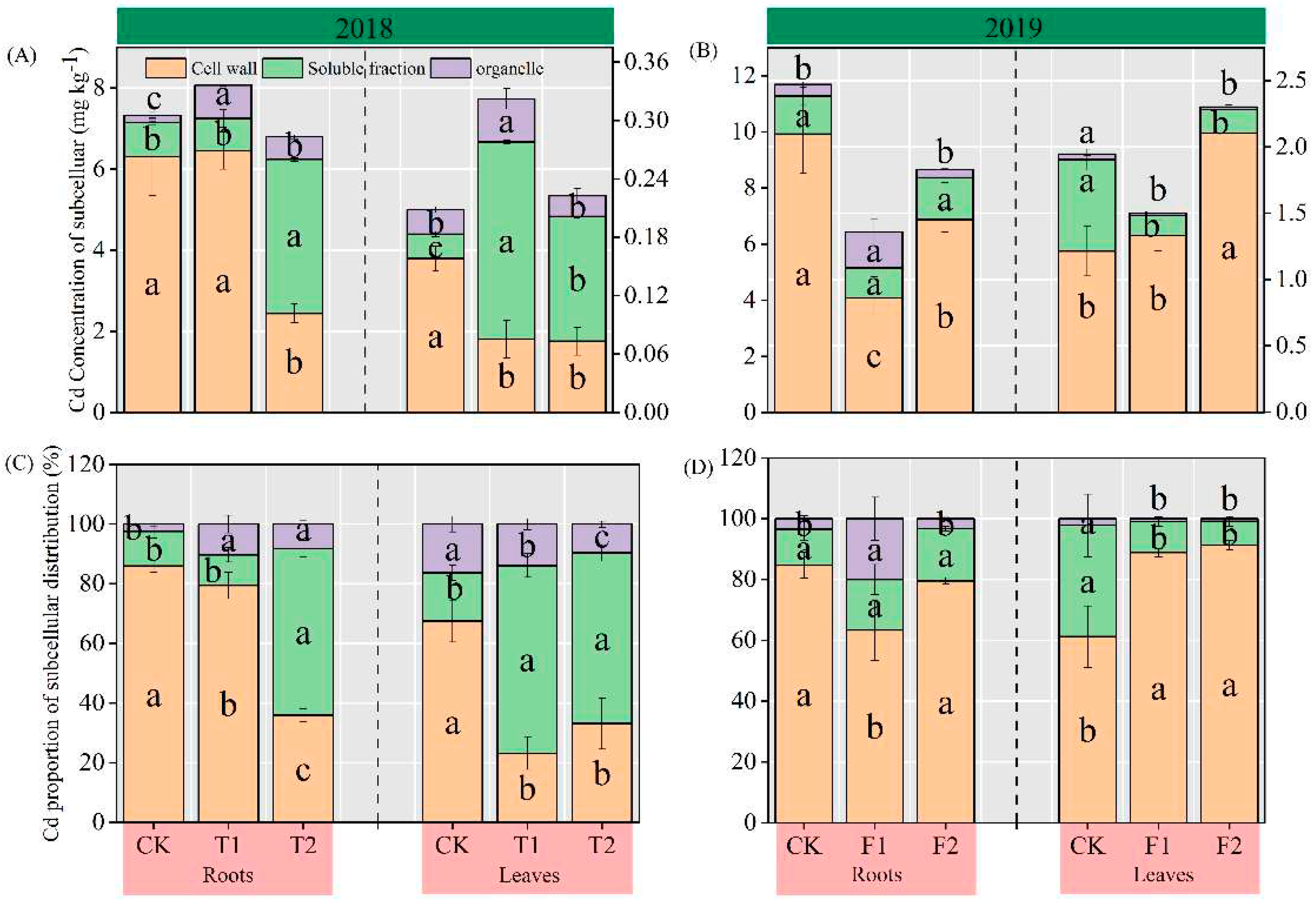
3.5. Cd concentration of subcellular in roots and leaves
3.6. Concentrations of Cd in the aerial parts and brown rice in the Hydroponic culture
4. Discussion
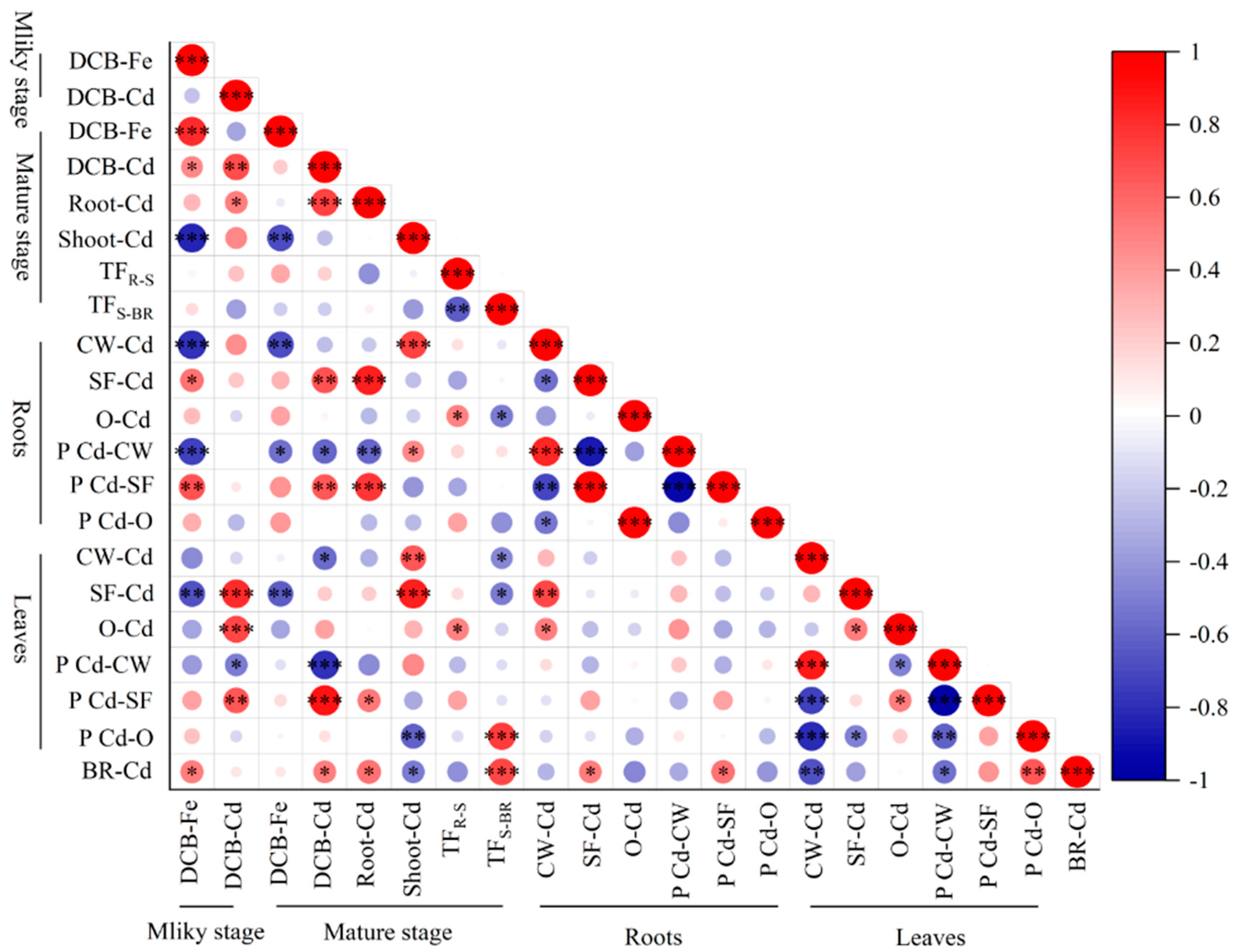
4.1. The effect of late nitrogen application on Cd of brown rice was not caused by soil
4.2. Fe and Mn accumulation in rice significantly affected Cd accumulation in brown rice.
5. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, P.; Chen, H.; Kopittke, P.M.; Zhao, F.-J. Cadmium contamination in agricultural soils of China and the impact on food safety. Environ. Pollut. 2019, 249, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Uraguchi, S.; Fujiwara, T. Cadmium transport and tolerance in rice: perspectives for reducing grain cadmium accumulation. Rice. 2012, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, E.; Agapiou, A.; Anastopoulos, I.; Omirou, M.; Ioannides, I.M. The effects of different soil nutrient management schemes in nitrogen cycling. J. Environ. Manag. 2019, 243, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, H.; Huang, D.; Xu, C.; Zhu, H.; Zhu, Q. Water managements limit heavy metal accumulation in rice: Dual effects of iron-plaque formation and microbial communities. Sci. Total Environ. 2019, 687, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Zhuang, P.; Li, F.; Mcbride, M.B.; Ren, W.D.; Li, Y.Y.; Li, Y.W.; Mo, H.; Fu, H.Y.; Li, Z.A. Phosphate addition diminishes the efficacy of wollastonite in decreasing Cd uptake by rice (Oryza sativa L.) in paddy soil. Sci. Total Environ. 2019, 687, 441–450. [Google Scholar] [CrossRef]
- Kong, F.; Lu, S. Effects of microbial organic fertilizer (MOF) application on cadmium uptake of rice in acidic paddy soil: Regulation of the iron oxides driven by the soil microorganisms. Environ. Pollut. 2022, 307, 119447. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, C.; Wang, C.; Huang, Y.; Liu, Z. Increasing phosphate inhibits cadmium uptake in plants and promotes synthesis of amino acids in grains of rice. Environ. Pollut. 2019, 257, 113496. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhu, T.; Gao, Y.; Wang, Y.; Ning, C.; Björn, L.O.; Chen, D.; Li, S. Effects of Ca addition on the uptake, translocation, and distribution of Cd in Arabidopsis thaliana. Ecotoxicol. Environ Saf. 2017, 139, 228–237. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, J.; Tao, L.; Cao, Z.; Tang, W.; Zhang, J.; Yu, X.; Fu, G.; Zhang, X.; Lu, Y. Regulatory Mechanisms of Nitrogen (N) on Cadmium (Cd) Uptake and Accumulation in Plants: A Review. Sci. Total Environ. 2019, 708, 135186. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Liu, T.; Fu, H.; Yuan, X.; Cheng, Q.; Liao, Q.; Zhang, Y.; Li, W.; Sun, Y.; Yang, Z.; Ma, J.; Li, X. Strategies for fertilizer management to achieve higher yields and environmental and fertilizer benefits of rice production in China. Sci. Total Environ. 2023, 904, 166325. [Google Scholar] [CrossRef]
- Akahane, I.; Makino, T.; Maejima, Y. Effects of Nitrogen Fertilizer, pH, and Electrical Conductivity on the Solubility of Cadmium in Soil Solution. Pedologist. 2010, 53, 101–107. [Google Scholar] [CrossRef]
- Jalloh, M.A.; Chen, J.; Zhen, F.; Zhang, G. Effect of different N fertilizer forms on antioxidant capacity and grain yield of rice growing under Cd stress. J. Hazard. Mater. 2009, 162, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.J.; Wang, F.; Ali, S.; Zhang, G. Toxic Effect of Cadmium on Rice as Affected by Nitrogen Fertilizer Form. Plant Soil. 2005, 277, 359–365. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, W.; Xu, S.; Shi, S.; Wen, D.; Huang, Y.; Peng, L.; Deng, T.; Du, R.; Li, F.; Wang, X.; Wang, F. Increasing ammonium nutrition as a strategy for inhibition of cadmium uptake and xylem transport in rice (Oryza sativa L.) exposed to cadmium stress. Environ. Exp. Bot. 2018, 155, 734–741. [Google Scholar] [CrossRef]
- Xu, C.; Wu, Z.; Zhu, Q.; Zhu, H.; Zhang, Y.; Huang, D. Effect of coated urea on cadmium accumulation in Oryza sativa L. grown in contaminated soil. Environ. Monit. Assess. 2015, 187, 716. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Chen, Y.; Yang, Y.; Lu, L.; Yuan, X.; Zeng, H.; Zeng, Q. Cadmium accumulation in rice (Oryza sativa L.) alleviated by basal alkaline fertilizers followed by topdressing of manganese fertilizer. Environ. Pollut. 2020, 262, 114289. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiong, J.; Chen, R.; Fu, G.; Chen, T.; Tao, L. Excessive nitrate enhances cadmium (Cd) uptake by up-regulating the expression of OsIRT1 in rice (Oryza sativa). Environ. Exp. Bot. 2016, 122, 141–149. [Google Scholar] [CrossRef]
- Liu, J.; Qu, P.; Zhang, W.; Dong, Y.; Li, L.; Wang, M. Variations among rice cultivars in subcellular distribution of Cd: The relationship between translocation and grain accumulation. Environ. Exp. Bot. 2014, 107, 5–31. [Google Scholar] [CrossRef]
- Chai, M.; Li, R.; Shen, X.; Tam, N.F.Y.; Zan, Q.; Li, R. Does ammonium nitrogen affect accumulation, subcellular distribution and chemical forms of cadmium in Kandelia obovata? Ecotoxicol. Environ Saf. 2018, 162, 430–437. [Google Scholar] [CrossRef]
- Tian, T.; Zhou, H.; Gu, J.; Jia, R.; Li, H.; Wang, Q.; Zeng, M.; Liao, B. Cadmium accumulation and bioavailability in paddy soil under different water regimes for different growth stages of rice (Oryza sativa L.). Plant Soil. [CrossRef]
- Rodda, M.S.; Li, G.; Reid, R.J. The timing of grain Cd accumulation in rice plants: the relative importance of remobilisation within the plant and root Cd uptake post-flowering. Plant Soil. 2011, 347, 105–114. [Google Scholar] [CrossRef]
- Fujimaki, S.; Suzui, N.; Ishioka, N.S.; Kawachi, N.; Ito, S.; Chino, M.; Nakamura, S.I. Tracing Cadmium from Culture to Spikelet: Noninvasive Imaging and Quantitative Characterization of Absorption, Transport, and Accumulation of Cadmium in an Intact Rice Plant. Plant Physiol. 2010, 152, 1796–1806. [Google Scholar] [CrossRef]
- GARNETT, T.P. Distribution and Remobilization of Iron and Copper in Wheat. Ann. Bot. 2005, 95, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhu, W.; Yang, W.; Gu, J.; Gao, Z.; Chen, L.; Du, W.; Zhang, P.; Peng, P.; Liao, B. Cadmium uptake, accumulation, and remobilization in iron plaque and rice tissues at different growth stages. Ecotoxicol. Environ Saf. 2018, 152, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, J.; Huang, Q.; Tang, S.; Wang, J.; Hu, P.; Shao, G. Can liming reduce cadmium (Cd) accumulation in rice (Oryza sativa) in slightly acidic soils? A contradictory dynamic equilibrium between Cd uptake capacity of roots and Cd immobilisation in soils. Chemosphere. 2018, 193, 547–556. [Google Scholar] [CrossRef]
- Deng, X.; Chen, B.; Chen, Y.; Jiang, L.; Hu, Y.; Yang, Y.; Rong, X.; Peng, L.; Zeng, Q. Flag leaf cell wall functional groups and components play a crucial role in the accumulation and translocation of Cd in rice grain via foliage application of humic acid. Ecotoxicol. Environ Saf. 2022, 239, 113658. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Deng, X.; Ma, Q.; Zhao, Y.; Wang, A.; Zhang, X.; Zeng, Q. Cadmium accumulation in brown rice (Oryza sativa L.) depends on environmental factors and nutrient transport: A three-year field study. Sci. Total Environ. 2023, 903, 166942. [Google Scholar] [CrossRef]
- Hinsinger, P.; Plassard, C.; Tang, C.; Jaillard, B. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil. 2003, 248, 43–59. [Google Scholar] [CrossRef]
- Hong-Duck, R.; Min-Seob, K.; Chung, E.; Baek, U.; Kim, S.; Kim, D.; Kim, Y.; Lee, J. Assessment and identification of nitrogen pollution sources in the Cheongmi River with intensive livestock farming areas, Korea. Environ. Sci. Pollut. Res. 2018, 25, 13499–13510. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, Z. Mechanisms of cadmium phytoremediation and detoxification in plants. Crop J. 2021, 9, 521–529. [Google Scholar] [CrossRef]
- Li, A.; Deng, H.; Jiang, Y.; Ye, C.; Yu, B.; Zhou, X.; Ma, A. Superefficient removal of heavy metals from wastewater by Mg-loaded biochars: adsorption characteristics and removal mechanisms. Langmuir. 2020, 36, 9160–9174. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, X.; Chen, F.; Dong, X.; Lan, P.; Ma, J.; Shen, R. Altered cell wall properties are responsible for ammonium-reduced aluminium accumulation in rice roots. Plant Cell Environ. 2015, 38, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Nkoh, J.N.; Abdulaha-Al Baquy, M.; Dong, G.; Li, J.; Xu, R. Plants alter surface charge and functional groups of their roots to adapt to acidic soil conditions. Environ. Pollut. 2020, 267, 115590. [Google Scholar] [CrossRef] [PubMed]
- Okamura, M.; Arai-Sanoh, Y.; Yoshida, H.; Mukouyama, T.; Adachi, S.; Yabe, S.; Nakagawa, H.; Tsutsumi, K.; Taniguchi, Y.; Kobayashi, N.; Kondo, M. Characterization of high-yielding rice cultivars with different grain-filling properties to clarify limiting factors for improving grain yield. Field Crop Res. 2018, 219, 139–147. [Google Scholar] [CrossRef]
- Li, G.; Pan, J.; Cui, K.; Yuan, M.; Hu, Q.; Wang, W.; Mohapatra, P.; Nie, L.; Huang, J.; Peng, S. Limitation of Unloading in the Developing Grains Is a Possible Cause Responsible for Low Stem Non-structural Carbohydrate Translocation and Poor Grain Yield Formation in Rice through Verification of Recombinant Inbred Lines. FRONT PLANT SCI. 2017, 8, 1369. [Google Scholar] [CrossRef] [PubMed]
- Hiromi, N.; Ippei, O.; Yasuhiro, I.; Satoshi, M.; Naoko, K.N. Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci. Plant Nutr. 2006, 52, 464–469. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, D.; Xu, C.; Zhu, H.; Feng, R.; Zhu, Q. Fe fortification limits rice Cd accumulation by promoting root cell wall chelation and reducing the mobility of Cd in xylem. Ecotoxicol. Environ Saf. 2022, 240, 113700. [Google Scholar] [CrossRef]
- Huang, G.; Ding, C.; Hu, Z.; Cui, C.; Zhang, T.; Wang, X. Topdressing iron fertilizer coupled with pre-immobilization in acidic paddy fields reduced cadmium uptake by rice (Oryza sativa L.). Sci. Total Environ. 2018, 636, 1040–1047. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Zhang, F. Role of iron plaque in Cd uptake by and translocation within rice (Oryza sativa L.) seedlings grown in solution culture. Environ. Exp. Bot. 2007, 59, 314–320. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



