Submitted:
31 October 2023
Posted:
31 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Insect collection
2.2. Dissection and extraction of eggs
2.3. Egg activation
2.4. Egg activation and subsequent development with treatments
2.5. Rearing of Sirex noctilio larvae, emerging from activated eggs, on a modified artificial diet
2.6. Statistical analysis
3. Results
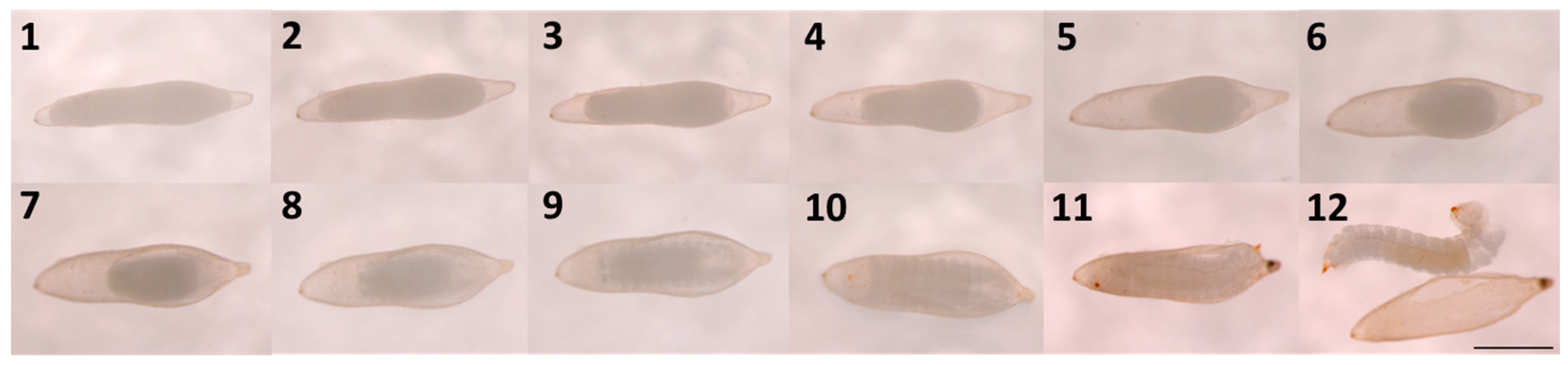
3.1. Egg activation
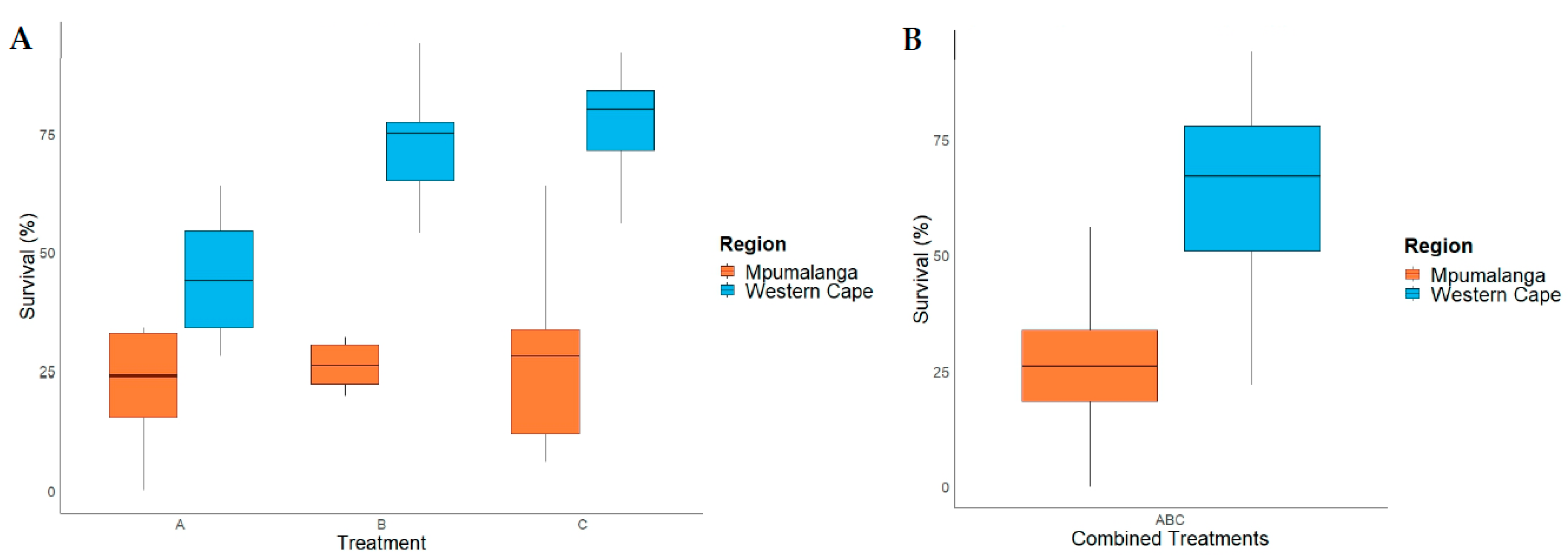
3.2. Egg activation and subsequent development with treatments
3.3. Rearing of Sirex noctilio larvae, emerging from activated eggs, on a modified artificial diet
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sartain CV & Wolfner MF (2013) Calcium and egg activation in Drosophila. Cell Calcium 53: 10-15. [CrossRef]
- Yamamoto DS, Hatakeyama M & Matsuoka H (2013) Artificial activation of mature unfertilized eggs in the malaria vector mosquito, Anopheles stephensi (Diptera, Culicidae). Journal of Experimental Biology 216: 2960-2966. [CrossRef]
- Horner VL & Wolfner MF (2008) Transitioning from egg to embryo: triggers and mechanisms of egg activation. Developmental Dynamics 237: 527-544. [CrossRef]
- Sasaki K, Sobajima H, Satoh T & Obara Y (1997) Activation in vitro of unfertilized egg development in honeybee queens. Naturwissenschaften 84: 74-76. [CrossRef]
- Sperling AL & Glover DM (2023) Parthenogenesis in dipterans: a genetic perspective. Proceedings: Biological Sciences 290: 20230261. [CrossRef]
- Suomalainen E (1962) Significance of parthenogenesis in the evolution of insects. Annual Review of Entomology 7: 349-366. [CrossRef]
- Rabeling C & Kronauer DJ (2013) Thelytokous parthenogenesis in eusocial Hymenoptera. Annual Review of Entomology 58: 273-292. [CrossRef]
- Miura K & Tagami Y (2004) Comparison of life history characters of arrhenotokous and Wolbachia-associated thelytokous Trichogramma kaykai Pinto and Stouthamer (Hymenoptera: Trichogrammatidae). Annals of the Entomological Society of America 97: 765-769. [CrossRef]
- Pearcy M, Hardy O & Aron S (2006) Thelytokous parthenogenesis and its consequences on inbreeding in an ant. Heredity (Edinb) 96: 377-382. [CrossRef]
- Went DF (1982) Egg activation and parthenogenetic reproduction in insects. Biological Reviews 57: 319-344. [CrossRef]
- Pijnacker LP & Ferwerda MA (1976) Experiments on blocking and unblocking of first meiotic metaphase in eggs of the parthenogenetic stick insect Carausius morosus Br. (Phasmida, Insecta). Journal of Embryology Experimental Morphology 36: 383-394.
- Mahowald AP, Goralski TJ & Caulton JH (1983) In vitro activation of Drosophila eggs. Developmental Biology 98: 437-445. [CrossRef]
- Sawa M & Oishi K (1989) Studies on the sawfly, Athalia rosae (Insecta, Hymenoptera, Teethredinidae). II. Experimental activation of mature unfertilized eggs. Zoological Science 6: 549-556.
- York-Andersen AH, Wood BW, Wilby EL, Berry AS & Weil TT (2021) Osmolarity-regulated swelling initiates egg activation in Drosophila. Open Biology 11: 210067. [CrossRef]
- Went DF & Krause G (1974) Alteration of egg architecture and egg activation in an endoparasitic Hymenopteran as a result of natural or imitated oviposition. Wilhelm Roux' Archiv für Entwicklungsmechanik der Organismen 175: 173-184. [CrossRef]
- Went DF & Krause G (1973) Normal development of mechanically activated, unlaid eggs of an endo-parasitic hymenopteran. Nature 244: 454-455. [CrossRef]
- King PE & Rafai J (1973) A possible mechanism for initiating the parthenogenetic development of eggs in a parasitoid Hymenopteran, Nasonia vitripennis (Walker) (Pteromalidae). The Entomologist 106: 118-120.
- Sasaki K & Obara Y (2002) Egg activation and timing of sperm acceptance by an egg in honeybees (Apis mellifera L.). Insectes Sociaux 49: 234-240. [CrossRef]
- Vinson SB & Jang H (1987) Activation of Campoletis sonorensis (Hymenoptera: Ichneumonidae) eggs by artificial means. Annals of the Entomological Society of America 80: 486-489. [CrossRef]
- Sørensen JG, Addison MF & Terblanche JS (2012) Mass-rearing of insects for pest management: challenges, synergies and advances from evolutionary physiology. Crop Protection 38: 87-94. [CrossRef]
- Taning CNT, Van Eynde B, Yu N, Ma S & Smagghe G (2017) CRISPR/Cas9 in insects: Applications, best practices and biosafety concerns. Journal of Insect Physiology 98: 245-257. [CrossRef]
- Pacheco ID, Walling LL & Atkinson PW (2022) Gene editing and genetic control of hemipteran pests: progress, challenges and perspectives. Frontiers in Bioengineering and Biotechnology 10: 900785. [CrossRef]
- Yan Y, Aumann RA, Häcker I & Schetelig MF (2023) CRISPR-based genetic control strategies for insect pests. Journal of Integrative Agriculture 22: 651-668. [CrossRef]
- Espinosa del Alba L & Petschenka G (2023) A simple artificial diet for feeding and sequestration assays for the milkweed bugs Oncopeltus fasciatus and Spilostethus saxatilis. Entomologia Experimentalis et Applicata 171: 658-667. [CrossRef]
- Ajó Fernández AAF, Martínez AS, Villacide JM & Corley JC (2015) Behavioural response of the woodwasp Sirex noctilio to volatile emissions of its fungal symbiont. Journal of Applied Entomology 139: 654–659.
- Ciesla WM (2003) European woodwasp: a potential threat to North America's conifer forests. Journal of Forestry 101: 18-23. [CrossRef]
- Bedding RA (2009) Controlling the pine-killing woodwasp, Sirex noctilio, with nematodes: Use of microbes for control and eradication of invasive arthropods (ed. by AE Hajek, TR Glare & M O’Callaghan) Springer, Dordrecht, pp. 213-235.
- Hoebeke E, Haugen DA & Haack R (2005) Sirex noctilio: discovery of a Palearctic siricid woodwasp in New York. Newsletter of the Michigan Entomological Society 50: 24-25.
- Thompson BM, Bodart J, McEwen C & Gruner DS (2014) Adaptations for symbiont-mediated external digestion in Sirex noctilio (Hymenoptera: Siricidae). Annals of the Entomological Society of America 107: 453-460. [CrossRef]
- Coutts MP (1969) The mechanism of pathogenicity of Sirex noctilio on Pinus radiata. I. Effects of the symbiotic fungus Amylostereum sp. (Thelophoraceae). Australian Journal of Biological Sciences, 22, 915-924.
- Tribe GD (2006) A wasp counterattack to save pine trees. Village Life 18: 38-40.
- Hurley BP, Slippers B & Wingfield MJ (2007) A comparison of control results for the alien invasive woodwasp, Sirex noctilio, in the Southern Hemisphere. Agricultural and Forest Entomology 9: 159-171. [CrossRef]
- Ryan K & Hurley BP (2012) Life history and biology of Sirex noctilio: The Sirex woodwasp and its fungal symbiont (ed. by B Slippers, P De Groot & MJ Wingfield) Springer, Dordrecht, pp. 15-30.
- Coutts MP & Dolezal JE (1969) Emplacement of fungal spores by the woodwasp, Sirex noctilio, during oviposition. Forest Science 15: 412-416. [CrossRef]
- Corley JC, Lantschner MV, Martínez AS, Fischbein D & Villacide JM (2018) Management of Sirex noctilio populations in exotic pine plantations: critical issues explaining invasion success and damage levels in South America. Journal of Pest Science 92: 131-142. [CrossRef]
- Slippers B, Hurley BP & Wingfield MJ (2015) Sirex woodwasp: a model for evolving management paradigms of invasive forest pests. Annual Review of Entomology 60: 601-619. [CrossRef]
- Kraus S, Monchanin C, Gomez-Moracho T & Lihoreau M (2019) Insect Diet: Encyclopedia of animal cognition and behavior (ed. by J Vonk & T Shackelford) Springer International Publishing, Cham, pp. 1-9.
- Hull-Sanders H, Pepper E, Davis K & Trotter RT, 3rd (2017) Description of an establishment event by the invasive Asian longhorned beetle (Anoplophora glabripennis) in a suburban landscape in the northeastern United States. PLoS One 12: e0181655. [CrossRef]
- Hajek AE, Haavik LJ & Stephen FM (2021) Biology and ecology of Sirex noctilio in North America. USDA, Forest Service, U.S. Department of Argiculture, Forest Health Assessment and Applied Sciences Team.
- Zhao J, Ogura N, & Isono M (1998) Artificial rearing of Anoplophora glabripennis. Projects of Forest Protection Institute of Ningxia 5: 1-6.
- Dubois T, Hajek AE & Smith S (2002) Methods for rearing the asian longhorned beetle (Coleoptera: Cerambycidae) on artificial diet. Annals of the Entomological Society of America 95: 223-230. [CrossRef]
- Keena MA (2005) Pourable artificial diet for rearing Anoplophora glabripennis (Coleoptera: Cerambycidae) and methods to optimize larval survival and synchronize development. Annals of the Entomological Society of America 98: 536-547. [CrossRef]
- Mason CJ, Scully ED, Geib SM & Hoover K (2016) Contrasting diets reveal metabolic plasticity in the tree-killing beetle, Anoplophora glabripennis (Cerambycidae: Lamiinae). Scientific Reports 6: 33813. [CrossRef]
- Favaro R, Lupi D, Jucker C, Cappellozza S & Faccoli M (2017) An artificial diet for rearing three exotic longhorn beetles invasive to Europe. Bulletin of Insectology 70: 91-99.
- Madden JL (1981) Egg and larval development in the woodwasp, Sirex noctilio F. Australian Journal of Zoology 29: 493-506.
- Kaneuchi T, Sartain CV, Takeo S, Horner VL, Buehner NA, Aigaki T & Wolfner MF (2015) Calcium waves occur as Drosophila oocytes activate. Proceedings of the National Academy of Sciences of the United States of America 112: 791-796. [CrossRef]
- Boitano S, Sanderson MJ & Dirksen ER (1994) A role for Ca(2+)-conducting ion channels in mechanically-induced signal transduction of airway epithelial cells. Journal of Cell Science 107: 3037-3044. [CrossRef]
- Davis MJ, Meininger GA & Zawieja DC (1992) Stretch-induced increases in intracellular calcium of isolated vascular smooth muscle cells. American Journal of Physiology 263: H1292-1299. [CrossRef]
- Sakaki K, Dechev N, Burke RD & Park EJ (2009) Development of an autonomous biological cell manipulator with single-cell electroporation and visual servoing capabilities. IEEE Transactions on Biomedical Engineering 56: 2064-2074. [CrossRef]
- Landman WA, Malherbe J & Engelbrecht F (2017) South Africa’s present-day climate: South African risk and vulnerability atlas: understanding the social & environmental implications of global change. (ed. by J Mambo & K Faccer) AFRICAN SUN MeDIA, Stellenbosch, pp. 7-12.
- Yousuf F, Carnegie AJ, Bedding RA, Bashford R, Nicol HI & Gurr GM (2014) Effect of temperature on woodwasp (Sirex noctilio F.) development and parasitism by the entomopathogenic nematode, Deladenus siricidicola. Biological Control 79: 67-74. [CrossRef]
- Fischer K, Bot ANM, Zwaan BJ & Brakefield PM (2004) Genetic and environmental sources of egg size variation in the butterfly Bicyclus anynana. Heredity 92: 163-169. [CrossRef]
- Gibbs M, Breuker CJ & Van Dyck H (2010) Flight during oviposition reduces maternal egg provisioning and influences offspring development in Pararge aegeria (L.). Physiological Entomology 35: 29-39. [CrossRef]
- Queffelec J, Wooding AL, Greeff JM, Garnas JR, Hurley BP, Wingfield MJ & Slippers B (2019) Mechanisms that influence sex ratio variation in the invasive hymenopteran Sirex noctilio in South Africa. Ecol Evol 9: 7966-7973. [CrossRef]
- Markert JA, Champlin DM, Gutjahr-Gobell R, Grear JS, Kuhn A, McGreevy TJ, Roth A, Bagley MJ & Nacci DE (2010) Population genetic diversity and fitness in multiple environments. BMC Evolutionary Biology 10: 205. [CrossRef]
- Hongwane P, Mitchell G, Kanzler A, Verryn S, Lopez J & Chirwa P (2018) Alternative pine hybrids and species to Pinus patula and P. radiata in South Africa and Swaziland. Southern Forests: a Journal of Forest Science 80: 301-310. [CrossRef]
- Luo D, Lai M, Xu C, Shi H & Liu X (2018) Life history traits in a capital breeding pine caterpillar: effect of host species and needle age. BMC Ecology 18: 24. [CrossRef]
- Madden JL (1968) Physiological aspects of host tree favourability for the woodwasp, Sirex noctilio F. Proceedings of the Ecological Society of Australia 3: 147-149.
- Madden JL & Coutts MP (1979) The role of fungi in the biology and ecology of woodwasps (Hymenoptera: Siricidae): Insect-fungus symbiosis (ed. by Batra LR) Allanheld, Osmun & Co., Montclair, N.J., pp. 165-174.
- Wang P, Lu P, Zheng X, Chen L, Lei C & Wang X (2013) New artificial diet for continuous rearing of the bean pod borer, Maruca vitrata. Journal of Insect Science 13:121. [CrossRef]
- Snodgrass RE (1954) Insect metamorphosis. Smithsonian Miscellaneous Collections 122: 1-124.
- Arrese EL & Soulages JL (2010) Insect fat body: energy, metabolism, and regulation. Annual Review of Entomology 55: 207-225. [CrossRef]
- Li J, Li C, Wang M, Wang L, Liu X, Gao C, Ren L & Luo Y (2021) Gut structure and microbial communities in Sirex noctilio (Hymenoptera: Siricidae) and their predicted contribution to larval nutrition. Frontiers in Microbiology 12: 641141. [CrossRef]


| Ingredient | Amount |
|---|---|
| Distilled water | 257.81 ml |
| Agar | 8.20 g |
| Group 1 | |
| Raw wheat germ | 19.92 g |
| Torula yeast powder | 10.55 g |
| Group 2 | |
| Wesson salt mixture | 1.05 g |
| Sorbic acid | 0.73 g |
| Methyl paraben | 0.73 g |
| Sucrose | 5.86 g |
| Casein from bovine milk | 3.52 g |
| Sodium propionate | 0.45 g |
| Group 3 | |
| Cholesterol | 0.35 g |
| Autoclaved wheatgerm oil | 1.64 ml |
| Group 4 | |
| Choline chloride | 0.09 g |
| Vanderzant vitamin mixture for insects | 1.55 g |
| Vitamin A beadlets | 0.12 g |
| Group 5 | |
| Alpha cellulose | 45.70 g |
| Group 6 | |
| Streptomycin sulfate salt | 0.3 g |
| SABAX pour water | 2 ml |
| Group 7 | |
| Autoclaved pine sawdust | 50 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





