Submitted:
31 October 2023
Posted:
01 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Classification of Bacteriophages
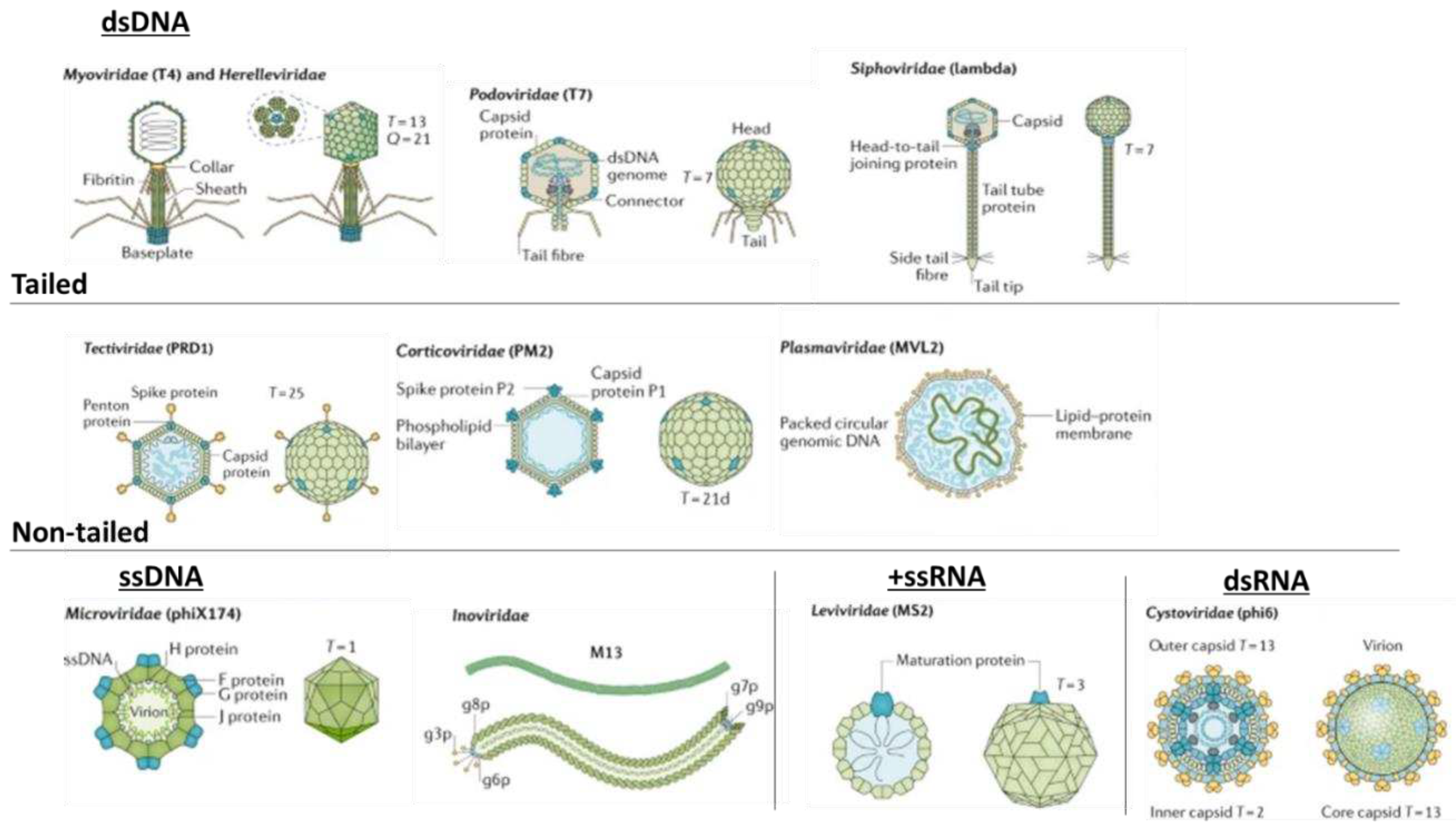
| Family | Genome | Morphology | Host | Phage |
|---|---|---|---|---|
| Myoviridae | dsDNA | Contractile-tailed, non-enveloped | Enterobacteria, Escherichia coli, and Leptospira spp. | T4, LE3, and phi29 |
| Podoviridae | dsDNA | Short-tailed, non-enveloped | Enterobacteria, E. coli, Bacillus spp., and Bordetella spp. | T7, phi29, and BPP-1 |
| Siphoviridae | dsDNA | Long non-contractile-tailed, non-enveloped | Proteobacteria, Lactococcus spp., Streptomyces spp., and Mycobacterium spp. | Lambda, DS6A, PA-2, phiC31, and D29 |
| Corticoviridae | dsDNA | Non-tailed, non-enveloped | Pseudoalteromonas spp. | PM2 |
| Plasmaviridae | dsDNA | Non-tailed, enveloped, pseudo-spherical | Acholeplasma spp. | MVL2 and AVL2 |
| Tectiviridae | dsDNA | Non-tailed, non-enveloped | Microbacterium spp., Streptomyces spp., and Pseudomonas spp. | PRD1, PR4, and Bam35 |
| Inoviridae | ssDNA | Filamentous, helical capsid | Enterobacteria, Vibrio spp., Spiroplasma spp., Salmonella spp., and Rastonia spp. | M13, fs1, 1-C74, Ike, and RSM1 |
| Microviridae | ssDNA | Non-enveloped, non-tailed, icosahedral capsid | Enterobacteria, Bdellovibrio spp., and Chlamydia spp. | phiX174, Chp1, and PhiMH2K |
| Cystoviridae | dsRNA | Enveloped | Pseudomonas spp. | phi6 |
| Leviviridae | ssRNA | Enveloped | Enterobacteriaceae and E. coli | MS2 |
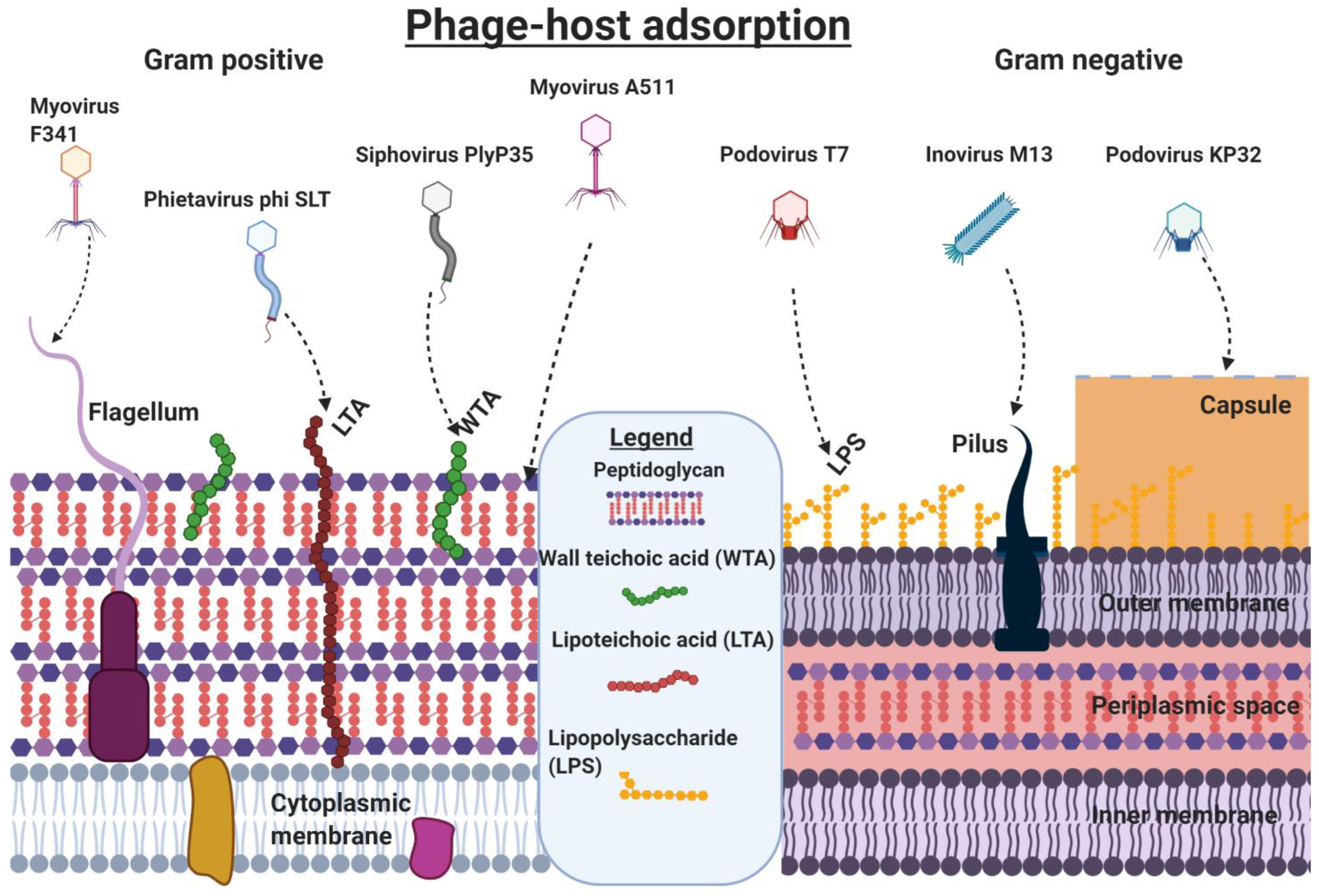
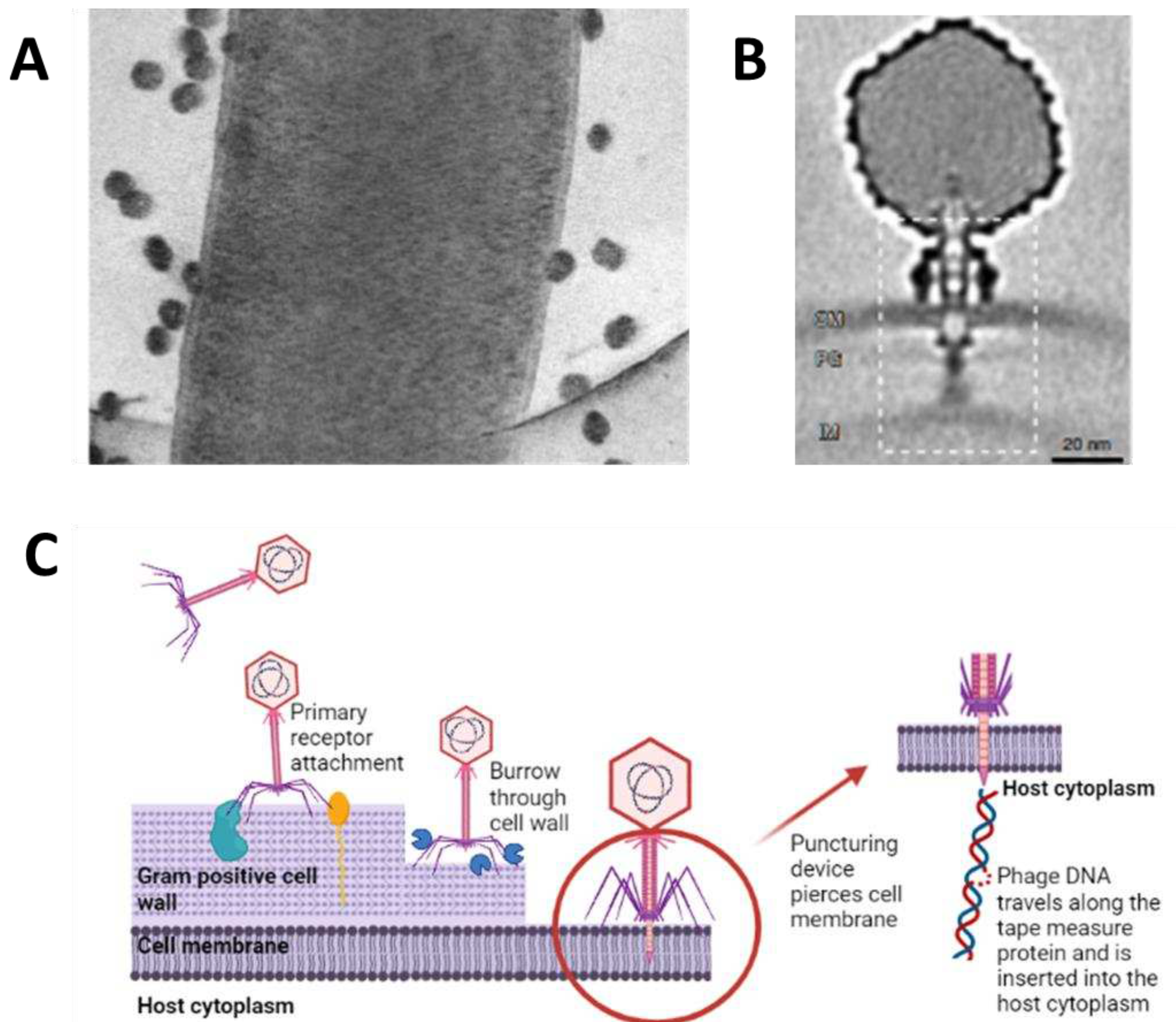
3. Phage˗Host Adsorption and Cell Entry Strategies
4. Phage-Host Interactions
5. Resistance to Phage Attack
6. Bacterial Immunity to Phage Infections
7. Bacteriophage-Based Therapeutics
7.1. Phage Therapy
| Infection(s)/phage trial interest | Causative agent(s)/agents of interest | Outcomes/comments | Reference/clinical trial identifier |
|---|---|---|---|
| Suppurative skin infections* | Pseudomonas, Staphylococcus, Klebsiella, Proteus, and E. coli | Thirty-one patients were treated orally and locally for chronically infected skin ulcers with a 74% success rate | [110] |
| Acute postoperative empyema in chronic suppurative lung diseases* | Staphylococcus, Streptococcus, E. coli, and Proteus | Phage-antibiotic combinations were used in the successful treatment of 45 patients | [111] |
| Complications due to bacterial infections in cancer patients* | Staphylococcus and Pseudomonas | 82% (65) successful treatment with phages compared to patients treated with antibiotics 61% (66) | [112] |
| Recurrent subphrenic abscess* | Antibiotic-resistant E. coli | A single patient was successfully treated with phages after 33 days | [113] |
| Urinary tract infections* | Staphylococcus, E. coli, and Proteus | Forty-six UTI patients were treated with phages with 92% making clinical improvements and 84% achieving bacterial clearance | [114] |
| Rhinitis, pharyngitis, dermatitis, and conjunctivitis* | Staphylococcus, Streptococcus, E. coli, Proteus, enterococci, and P. aeruginosa | Patients were treated with phages (360), antibiotics (404), and phage-antibiotic combinations (576). Clinical improvements of 86%, 48%, and 83% across the treatment regimes, respectively | [115] |
| Cerebrospinal meningitis * | K. pneumoniae | Successful treatment with orally administered phages in a newborn. | [116] |
| Bacterial diarrhea | E. coli | Orally administered coliphages showed no improvement in clinical outcome, some dysbiosis with streptococci was observed | [117] |
| Complicated or recurrent UTI patients with transurethral resection of the prostate | Enterococcus, E. coli, streptococci, P. mirabilis, P. aeruginosa, staphylococci | Patients with intravesical administered pyophage cocktail, orally administered antibiotics, and a placebo bladder irrigation. Success rates of 18%, 28% and 37% were observed, respectively | [118] |
| Burn wounds | P. aeruginosa | Phages PP1131 showed no significant difference to standard of care antibiotics - patients treated with PP1131 were found to have phage-resistant P. aeruginosa | [119] |
| Prosthetic joint infections | S. aureus, S. epidermidis, S. lugdunensis, Streptococcus sp., E. faecium, E. faecalis, E. coli, P. aeruginosa, and/or K. pneumoniae | Phage treatment, with intraoperative and intravenous PhageBank™ bacteriophages, in conjunction with standard-of-care antibiotics/Debridement, Antibiotics, and Implant Retention (DAIR) procedures. Completion is predicted in 2024 | [120] |
| Diabetic foot ulcers (DFU) | Staphylococcus sp., wound microbiome | Use of anti-staphylococcal phage gel (Intralytix Inc, Baltimore, Maryland, USA). Effect on bacterial microbiome of DFU wounds and patient outcomes. Trial was abandoned for funding reasons | [121] |
| Probiotic application for overall gut health | Bifidobacterium animalis subsp. lactis BL04 | The use of bacteriophages (PreforPro) increased the survival and efficacy of probiotic bacteria administered vs probiotics only vs placebo | [122] |
| Phages preventing the acquisition of multi-resistant enterobacteria (PHAGE-BMR) | E. coli or K. pneumoniae containing ESBL or carbapenemases | Collection of multidrug-resistant bacteria from patients in intensive care, subsequent search for presence and absence of phages in carriers/non-carriers. Currently active but of unknown status | [123] |
| Phage dynamics and influences during human gut microbiome establishment (METAKIDS) | A broad range of bacteriophage and bacterial hosts. | Characterize phage and bacterial genomes, abundance, and variations during infant gut development. Terminated | [124] |
| Bacterial infection in cystic fibrosis patients | P. aeruginosa | A cocktail of 10 bacteriophages was used to reduce Pseudomonas presence after 6 and 24 h including sensitivity of isolates. Completed with no recorded outcomes | [125] |
| Prebiotic | Escherichia coli and microbiota | Commercial coliphage cocktail effects on the microbiota and systemic inflammation. No disruption to microbiota and no effect on inflammatory markers | [126] |
| Venous leg ulcers | P. aeruginosa, S. aureus, and E. coli | Polyvalent phage preparation of 8 bacteriophages was assessed for their safety and efficacy. No available outcomes but the trial was completed | [127] |
| Lower urinary tract colonization | E. coli | Assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of phage cocktail LBP-EC01 | [128,129] |
| Safety of topical phage solution intended for wound infections | S. aureus | Evaluating the safety and skin reactions to ascending doses of phages compared to the placebo | [130] |
| Infection(s) | Bacterial specie(s) | Outcome/comment | Reference |
|---|---|---|---|
| Complicated necrotizing pancreatitis | Acinetobacter baumannii | Clearance of A. baumannii and return to health using intravenously (IV) and percutaneously administered (9) phages screened from a phage bank | [131] |
| Bacteremia | P. aeruginosa | An IV-administered bacteriophage cocktail comprised of two phages cleared the bacteremia, but the patient succumbed to other complications | [132] |
| Lung infection and transplant recipient | P. aeruginosa | An IV and nebulizer-administered bacteriophage cocktail, AB-PA01 and Navy, with the patient recovering from pneumonia | [133] |
| Infection of left ventricular assist device | P. aeruginosa | Six-week IV-administered (3) phage cocktail, the patient was clear and then relapsed but a change in antibiotics led to recovery | [132] |
| Osteomyelitis | A. baumannii and K. pneumoniae | The patient developed post-operative infection with multidrug-resistant isolates. IV bacteriophage-antibiotic combination led to the patient’s full recovery without the need for amputation | [134] |
| UTI | ESBL E. coli | Phage treatment with two phages over 23 days in conjunction with antibiotic treatment led to negative urine cultures and full recovery of the patient | [132] |
| CNS infection of a recovering trauma patient | A. baumannii | IV treatment with an A. baumannii phage for 8 days led to CSF cultures coming back negative for A. baumannii but positive for K. pneumoniae and S. aureus. The patient was declared brain dead and later announced deceased | [132] |
| Lung infection of cystic fibrosis patient | Achromobacter xylosoxidans | Cefiderocol and phage treatment were performed for 5 days followed by continuous phage therapy. The patient recovered and was discharged | [135] |
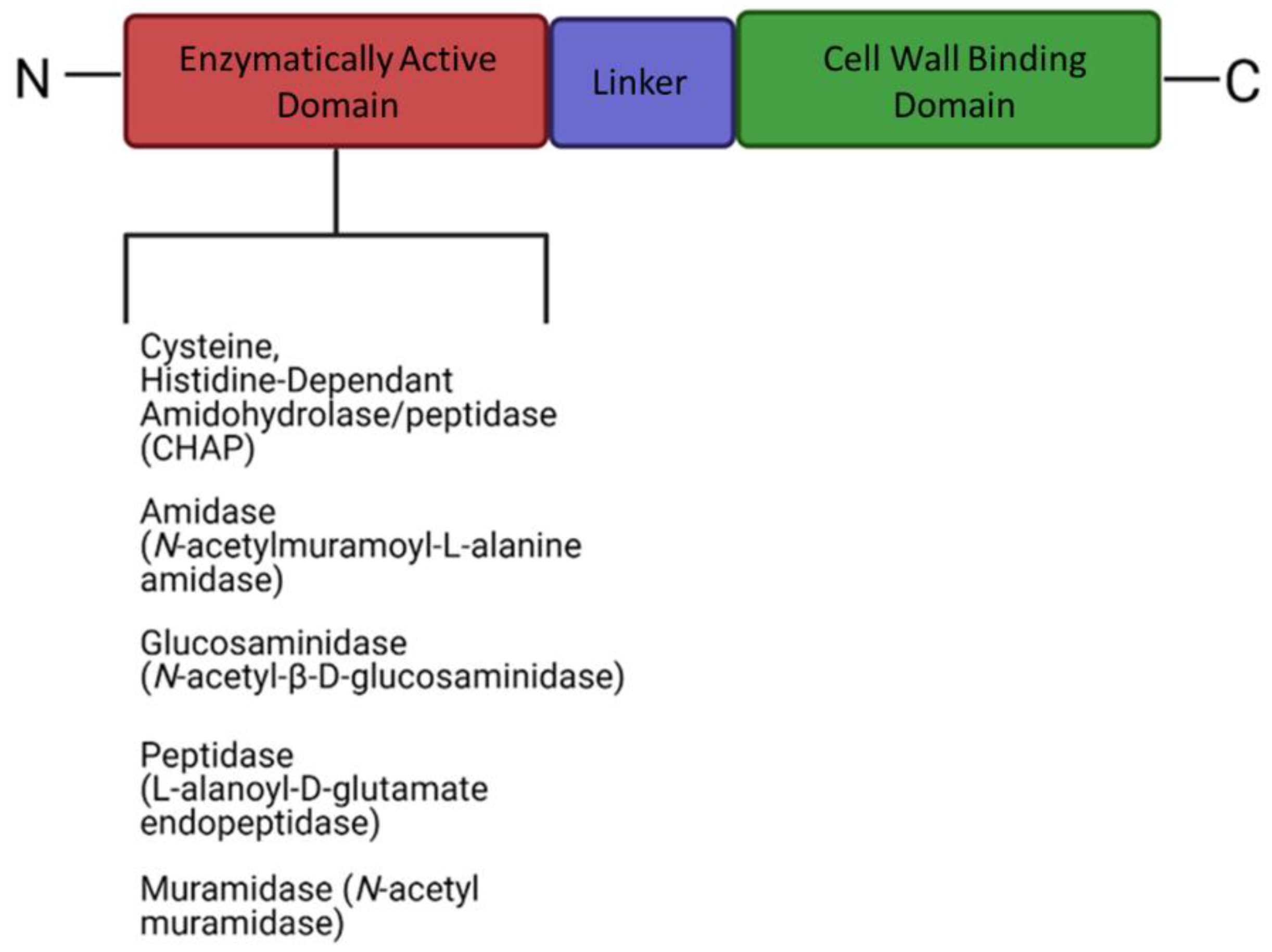
7.2. Therapeutic Potential of Phage-Derived Proteins
| Enzyme class | Phage/enzyme | Polymer substrates | Targeted genera | Reference |
|---|---|---|---|---|
| Hydrolases | ||||
| Sialidases | Phi92 | Polysialic acid | E. coli K1 & K92 | [146] |
| K1E | E. coli K5 | [157] | ||
| K1F | E. coli K1 | [158] | ||
| Levanase | SP10 | Levan | Bacillus species | [150] |
| SPG24 | ||||
| Rhamnosidase | Sf6 | O-antigen LPS | Shigella flexneri | [151,159] |
| P22 | Rhamnogalacturonan | Salmonella (ser.) Typhimurium | ||
| Cellulases | S6 | Cellulose | Erwinia amylovora | [160] |
| Peptidases | CHAPK | Pentaglycine cross-bridge peptidoglycan | Staphylococcus aureus | [161,162] |
| phiNIT1 | Poly-γ-glutamate | Bacillus spp. | ||
| Lyases | ||||
| Hyaluronidases | Prophages | Hyaluronan | Streptococcus equi | [163,164] |
| H4489A | Streptococcus pyogenes | |||
| Alginate lyases | PT 6 | Alginic acid | P. aeruginosa | [12,65] |
| AF | P. putida | |||
| Pectin/pectate lyases | ΦIPLA7 | Pectin* | Staphylococcal spp. | [165] |
| Others | ||||
| Colanidase | Phi92 | Colonic acid | E. coli | [63] |
| Lipases/triacylglycerol hydrolases | Phi3ST:2 | Carboxyl ester bonds* | Cellulophaga spp. | [166] |
| Tf | Pseudomonas spp. | |||
8. Limitations of Phage Therapy
9. Genomic Engineering of Phages
10. Diagnostic Potential of Phages and Phage-Derived Proteins
11. Conclusions
References
- Wall, S. Prevention of Antibiotic Resistance – an Epidemiological Scoping Review to Identify Research Categories and Knowledge Gaps. Glob Health Action 2019, 12, 1756191. [Google Scholar] [CrossRef]
- Forde, A.; Hill, C. Phages of Life – the Path to Pharma. Br J Pharmacol 2018, 175, 412–418. [Google Scholar] [CrossRef]
- Seed, K.D. Battling Phages: How Bacteria Defend against Viral Attack. PLoS Pathog 2015, 11, e1004847. [Google Scholar] [CrossRef]
- Riede, I.; Eschbach, M.-L. Evidence That TraT Interacts with OmpA of Escherichia Coli. FEBS Lett 1986, 205, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Deora, R.; Doulatov, S.R.; Gingery, M.; Eiserling, F.A.; Preston, A.; Maskell, D.J.; Simons, R.W.; Cotter, P.A.; Parkhill, J.; et al. Reverse Transcriptase-Mediated Tropism Switching in Bordetella Bacteriophage. Science (1979) 2002, 295, 2091–2094. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, V.A. Bacteriophage Lysins as Effective Antibacterials. Curr Opin Microbiol 2008, 11, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.-N.; Smith, D.L.; Young, R. Holins: The Protein Clocks of Bacteriophage Infections. Annu Rev Microbiol 2000, 54, 799–825. [Google Scholar] [CrossRef] [PubMed]
- Young, R. Phage Lysis: Three Steps, Three Choices, One Outcome. Journal of Microbiology 2014, 52, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, V.A. Bacteriophage Lysins as Effective Antibacterials. Curr Opin Microbiol 2008, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Akoh, C.C.; Lee, G.-C.; Liaw, Y.-C.; Huang, T.-H.; Shaw, J.-F. GDSL Family of Serine Esterases/Lipases. Prog Lipid Res 2004, 43, 534–552. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gupta, N.; Rathi, P. Bacterial Lipases: An Overview of Production, Purification and Biochemical Properties. Appl Microbiol Biotechnol 2004, 64, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Glonti, T.; Chanishvili, N.; Taylor, P.W. Bacteriophage-Derived Enzyme That Depolymerizes the Alginic Acid Capsule Associated with Cystic Fibrosis Isolates of Pseudomonas Aeruginosa. J Appl Microbiol 2010, 108, 695–702. [Google Scholar] [CrossRef]
- Hatfull, G.F.; Hendrix, R.W. Bacteriophages and Their Genomes. Curr Opin Virol 2011, 1, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Demina, T.A.; Roux, S.; Aiewsakun, P.; Kazlauskas, D.; Simmonds, P.; Prangishvili, D.; Oksanen, H.M.; Krupovic, M. Diversity, Taxonomy, and Evolution of Archaeal Viruses of the Class Caudoviricetes. PLoS Biol 2021, 19, e3001442. [Google Scholar] [CrossRef]
- Brüssow, H.; Hendrix, R.W. Phage Genomics. Cell 2002, 108, 13–16. [Google Scholar] [CrossRef]
- Adriaenssens, E.M.; Sullivan, M.B.; Knezevic, P.; van Zyl, L.J.; Sarkar, B.L.; Dutilh, B.E.; Alfenas-Zerbini, P.; Łobocka, M.; Tong, Y.; Brister, J.R.; et al. Taxonomy of Prokaryotic Viruses: 2018-2019 Update from the ICTV Bacterial and Archaeal Viruses Subcommittee. Archives of Virology 2020 165:5 2020, 165, 1253–1260. [Google Scholar] [CrossRef]
- Santamaria, R.M.; Innes, N.P.T.; Machiulskiene, V.; Evans, D.J.P.; Splieth, C.H. Caries Management Strategies for Primary Molars: 1-Yr Randomized Control Trial Results. J Dent Res 2014, 93, 1062–1069. [Google Scholar] [CrossRef]
- Pantůcek, R.; Rosypalová, A.; Doskar, J.; Kailerová, J.; Růzicková, V.; Borecká, P.; Snopková, S.; Horváth, R.; Götz, F.; Rosypal, S. The Polyvalent Staphylococcal Phage Phi 812: Its Host-Range Mutants and Related Phages. Virology 1998, 246, 241–252. [Google Scholar] [CrossRef]
- Cazares, D.; Cazares, A.; Figueroa, W.; Guarneros, G.; Edwards, R.A.; Vinuesa, P. A Novel Group of Promiscuous Podophages Infecting Diverse Gammaproteobacteria from River Communities Exhibits Dynamic Intergenus Host Adaptation. mSystems 2021, 6. [Google Scholar] [CrossRef]
- Dion, M.B.; Oechslin, F.; Moineau, S. Phage Diversity, Genomics and Phylogeny. Nat Rev Microbiol 2020, 18, 125–138. [Google Scholar] [CrossRef]
- Hulo, C.; de Castro, E.; Masson, P.; Bougueleret, L.; Bairoch, A.; Xenarios, I.; Le Mercier, P. ViralZone: A Knowledge Resource to Understand Virus Diversity. Nucleic Acids Res 2011, 39, D576–D582. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi Silva, J.; Storms, Z.; Sauvageau, D. Host Receptors for Bacteriophage Adsorption. FEMS Microbiol Lett 2016, 363, fnw002. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, S.; Tsurumi, S.; Imai, N. Crossover Behavior for Brownian Motion. J Chem Phys 1986, 84, 539–540. [Google Scholar] [CrossRef]
- Heller, K.J.; Schwarz, H.; Tubingen, U.; Mikrobiologie, L.I. Irreversible Binding to the Receptor of Bacteriophages T5 and BF23 Does Not Occur with the Tip of the Tail. J Bacteriol 1985, 162, 621–625. [Google Scholar] [CrossRef]
- Wolf, S.G.; Shimoni, E.; Elbaum, M.; Houben, L. STEM Tomography in Biology. 2018, 33–60. [CrossRef]
- Wang, C.; Tu, J.; Liu, J.; Molineux, I.J. Structural Dynamics of Bacteriophage P22 Infection Initiation Revealed by Cryo-Electron Tomography. Nature Microbiology 2019 4:6 2019, 4, 1049–1056. [Google Scholar] [CrossRef]
- Dunne, M.; Hupfeld, M.; Klumpp, J.; Loessner, M.J. Molecular Basis of Bacterial Host Interactions by Gram-Positive Targeting Bacteriophages. Viruses 2018, 10. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.; Park, B.; Ryu, S. Core Lipopolysaccharide-Specific Phage SSU5 as an Auxiliary Component of a Phage Cocktail for Salmonella Biocontrol. Appl Environ Microbiol 2014, 80, 1026. [Google Scholar] [CrossRef] [PubMed]
- Sandulache, R.; Prehm, P.; Kamp, D. Cell Wall Receptor for Bacteriophage Mu G(+). J Bacteriol 1984, 160, 299–303. [Google Scholar] [CrossRef]
- Munsch-Alatossava, P.; Alatossava, T. The Extracellular Phage-Host Interactions Involved in the Bacteriophage LL-H Infection of Lactobacillus Delbrueckii Ssp. Lactis ATCC 15808. Front Microbiol 2013, 4. [Google Scholar] [CrossRef]
- Wolin, M.J.; Douglas, L.J. Cell Wall Polymers and Phage Lysis of Lactobacillus Plantarum. Biochemistry 1971, 10, 1551–1555. [Google Scholar] [CrossRef]
- Wendlinger, G.; Loessner, M.J.; Scherer, S. Bacteriophage Receptors on Listeria Monocytogenes Cells Are the N-Acetylglucosamine and Rhamnose Substituents of Teichoic Acids or the Peptidoglycan Itself. Microbiology (N Y) 1996, 142, 985–992. [Google Scholar] [CrossRef]
- Moak, M.; Molineux, I.J. Peptidoglycan Hydrolytic Activities Associated with Bacteriophage Virions. Mol Microbiol 2004, 51, 1169–1183. [Google Scholar] [CrossRef]
- Barbirz, S.; Müller, J.J.; Uetrecht, C.; Clark, A.J.; Heinemann, U.; Seckler, R. Crystal Structure of Escherichia Coli Phage HK620 Tailspike: Podoviral Tailspike Endoglycosidase Modules Are Evolutionarily Related. Mol Microbiol 2008, 69, 303–316. [Google Scholar] [CrossRef]
- Baptista, C.; Santos, M.A.; São-José, C. Phage SPP1 Reversible Adsorption to Bacillus Subtilis Cell Wall Teichoic Acids Accelerates Virus Recognition of Membrane Receptor YueB. J Bacteriol 2008, 190, 4989–4996. [Google Scholar] [CrossRef]
- Xu, J.; Xiang, Y. Membrane Penetration by Bacterial Viruses. J Virol 2017, 91. [Google Scholar] [CrossRef]
- Olsen, R.H.; Siak, J.-S.; Gray, R.H. Characteristics of PRD1, a Plasmid-Dependent Broad Host Range DNA Bacteriophage. J Virol 1974, 14, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Ojala, V.; Laitalainen, J.; Jalasvuori, M. Fight Evolution with Evolution: Plasmid-dependent Phages with a Wide Host Range Prevent the Spread of Antibiotic Resistance. Evol Appl 2013, 6, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, R.A.; Pickard, D.; Dougan, S.; Goulding, D.; Cormie, C.; Hardy, J.; Li, F.; Grinter, R.; Harcourt, K.; Yu, L.; et al. The Flagellotropic Bacteriophage YSD1 Targets Salmonella Typhi with a Chi-like Protein Tail Fibre. Mol Microbiol 2019, 112, 1831–1846. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.M.; Dunstan, R.A.; Grinter, R.; Belousoff, M.J.; Wang, J.; Pickard, D.; Venugopal, H.; Dougan, G.; Lithgow, T.; Coulibaly, F. The Architecture and Stabilisation of Flagellotropic Tailed Bacteriophages. Nature Communications 2020 11:1 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Majkowska-Skrobek, G.; Łątka, A.; Berisio, R.; Maciejewska, B.; Squeglia, F.; Romano, M.; Lavigne, R.; Struve, C.; Drulis-Kawa, Z. Capsule-Targeting Depolymerase, Derived from Klebsiella KP36 Phage, as a Tool for the Development of Anti-Virulent Strategy. Viruses 2016, 8. [Google Scholar] [CrossRef]
- Song, L.; Yang, X.; Huang, J.; Zhu, X.; Han, G.; Wan, Y.; Xu, Y.; Luan, G.; Jia, X. Phage Selective Pressure Reduces Virulence of Hypervirulent Klebsiella Pneumoniae Through Mutation of the Wzc Gene. Front Microbiol 2021, 12, 2904. [Google Scholar] [CrossRef]
- Song, L.; Yang, X.; Huang, J.; Zhu, X.; Han, G.; Wan, Y.; Xu, Y.; Luan, G.; Jia, X. Phage Selective Pressure Reduces Virulence of Hypervirulent Klebsiella Pneumoniae Through Mutation of the Wzc Gene. Front Microbiol 2021, 12. [Google Scholar] [CrossRef]
- Tzagoloff, H.; Pratt, D. The Initial Steps in Infection with Coliphage M13. Virology 1964, 24, 372–380. [Google Scholar] [CrossRef]
- Roncero, C.; Darzins, A.; Casadaban, M.J. Pseudomonas Aeruginosa Transposable Bacteriophages D3112 and B3 Require Pili and Surface Growth for Adsorption. J Bacteriol 1990, 172, 1899–1904. [Google Scholar] [CrossRef]
- Manchak, J.; Anthony. G.; Frost, L.S. Mutational Analysis of F-pilin Reveals Domains for Pilus Assembly, Phage Infection and DNA Transfer. Mol Microbiol 2002, 43, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Jalasvuori, M.; Friman, V.-P.; Nieminen, A.; Bamford, J.K.H.; Buckling, A. Bacteriophage Selection against a Plasmid-Encoded Sex Apparatus Leads to the Loss of Antibiotic-Resistance Plasmids. Biol Lett 2011, 7, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.; Hupfeld, M.; Klumpp, J.; Loessner, M. Molecular Basis of Bacterial Host Interactions by Gram-Positive Targeting Bacteriophages. Viruses 2018, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Kanamaru, S.; Leiman, P.G.; Kostyuchenko, V.A.; Chipman, P.R.; Mesyanzhinov, V. v.; Arisaka, F.; Rossmann, M.G. Structure of the Cell-Puncturing Device of Bacteriophage T4. Nature 2002 415:6871 2002, 415, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Mahony, J.; Alqarni, M.; Stockdale, S.; Spinelli, S.; Feyereisen, M.; Cambillau, C.; van Sinderen, D. Functional and Structural Dissection of the Tape Measure Protein of Lactococcal Phage TP901-1. Scientific Reports 2016 6:1 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, P.; Jacquot, P.; Plançon, L.; Chami, M.; Engel, A.; Parquet, C.; Herbeuval, C.; Letellier, L. Phage T5 Straight Tail Fiber Is a Multifunctional Protein Acting as a Tape Measure and Carrying Fusogenic and Muralytic Activities. Journal of Biological Chemistry 2008, 283, 13556–13564. [Google Scholar] [CrossRef]
- Fokine, A.; Rossmann, M.G. Molecular Architecture of Tailed Double-Stranded DNA Phages. Bacteriophage 2014, 4, e28281. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, R.; Bradley, R.; Paranchych, W. The Effect of M13 Phage Infection upon the F Pili of E. Coli. Virology 1973, 54, 220–229. [Google Scholar] [CrossRef]
- Grahn, A.M.; Daugelavičius, R.; Bamford, D.H. Sequential Model of Phage PRD1 DNA Delivery: Active Involvement of the Viral Membrane. Mol Microbiol 2002, 46, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Daugelavičius, R.; Cvirkaitė, V.; Gaidelytė, A.; Bakienė, E.; Gabrėnaitė-Verkhovskaya, R.; Bamford, D.H. Penetration of Enveloped Double-Stranded RNA Bacteriophages Φ13 and Φ6 into Pseudomonas Syringae Cells. J Virol 2005, 79, 5017–5026. [Google Scholar] [CrossRef] [PubMed]
- Cvirkaitė-Krupovič, V.; Poranen, M.M.; Bamford, D.H. Phospholipids Act as Secondary Receptor during the Entry of the Enveloped, Double-Stranded RNA Bacteriophage Φ6. Journal of General Virology 2010, 91, 2116–2120. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Bossi, N.; Uzzau, S.; Maloriol, D.; Bossi, L. Variable Assortment of Prophages Provides a Transferable Repertoire of Pathogenic Determinants in Salmonella. Mol Microbiol 2001, 39, 260–272. [Google Scholar] [CrossRef]
- McAllister, W.T.; Barrett, C.L. Roles of the Early Genes of Bacteriophage T7 in Shutoff of Host Macromolecular Synthesis. J Virol 1977, 23, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage Resistance Mechanisms. Nat Rev Microbiol 2010, 8, 317–327. [Google Scholar] [CrossRef]
- Scanlan, P.D.; Buckling, A.; Hall, A.R. Experimental Evolution and Bacterial Resistance: (Co)Evolutionary Costs and Trade-Offs as Opportunities in Phage Therapy Research. Bacteriophage 2015, 5, e1050153. [Google Scholar] [CrossRef]
- Meyer, J.R.; Dobias, D.T.; Weitz, J.S.; Barrick, J.E.; Quick, R.T.; Lenski, R.E. Repeatability and Contingency in the Evolution of a Key Innovation in Phage Lambda. Science 2012, 335, 428–432. [Google Scholar] [CrossRef]
- Scholl, D.; Adhya, S.; Merril, C. Escherichia Coli K1’s Capsule Is a Barrier to Bacteriophage T7. Appl Environ Microbiol 2005, 71, 4872–4874. [Google Scholar] [CrossRef]
- Schwarzer, D.; Buettner, F.F.R.; Browning, C.; Nazarov, S.; Rabsch, W.; Bethe, A.; Oberbeck, A.; Bowman, V.D.; Stummeyer, K.; Mühlenhoff, M.; et al. A Multivalent Adsorption Apparatus Explains the Broad Host Range of Phage Phi92: A Comprehensive Genomic and Structural Analysis. J Virol 2012, 86, 10384. [Google Scholar] [CrossRef] [PubMed]
- Patro, L.P.P.; Rathinavelan, T. Targeting the Sugary Armor of Klebsiella Species. Front Cell Infect Microbiol 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, A.; Ceyssens, P.-J.; Krylov, V.N.; Noben, J.-P.; Volckaert, G.; Lavigne, R. Identification of EPS-Degrading Activity within the Tail Spikes of the Novel Pseudomonas Putida Phage AF. Virology 2012, 434, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Yang, R.; Fan, K.; Dong, H.; Gao, C.; Wang, S.; Yu, L.; Cheng, Z.; Lei, L. External Lysis of Escherichia Coli by a Bacteriophage Endolysin Modified with Hydrophobic Amino Acids. AMB Express 2019, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Samson, J.E.; Magadán, A.H.; Sabri, M.; Moineau, S. Revenge of the Phages: Defeating Bacterial Defences. Nat Rev Microbiol 2013, 11, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Holst Sørensen, M.C.; van Alphen, L.B.; Fodor, C.; Crowley, S.M.; Christensen, B.B.; Szymanski, C.M.; Brøndsted, L. Phase Variable Expression of Capsular Polysaccharide Modifications Allows Campylobacter Jejuni to Avoid Bacteriophage Infection in Chickens. Front Cell Infect Microbiol 2012, 2, 11. [Google Scholar] [CrossRef]
- Sørensen, M.C.H.; van Alphen, L.B.; Fodor, C.; Crowley, S.M.; Christensen, B.B.; Szymanski, C.M.; Brøndsted, L. Phase Variable Expression of Capsular Polysaccharide Modifications Allows Campylobacter Jejuni to Avoid Bacteriophage Infection in Chickens. Front Cell Infect Microbiol 2012, 2. [Google Scholar] [CrossRef]
- Manning, A.J.; Kuehn, M.J. Contribution of Bacterial Outer Membrane Vesicles to Innate Bacterial Defense. BMC Microbiol 2011, 11, 258. [Google Scholar] [CrossRef]
- Bernheim, A.; Sorek, R. The Pan-Immune System of Bacteria: Antiviral Defence as a Community Resource. Nat Rev Microbiol 2020, 18, 113–119. [Google Scholar] [CrossRef]
- Murray, N.E. Immigration Control of DNA in Bacteria: Self versus Non-Self. Microbiology (N Y) 2002, 148, 3–20. [Google Scholar] [CrossRef]
- Auer, B.; Schweiger, M. Evidence That Escherichia Coli Virus T1 Induces a DNA Methyltransferase. J Virol 1984, 49, 588–590. [Google Scholar] [CrossRef]
- Walkinshaw, M.D.; Taylor, P.; Sturrock, S.S.; Atanasiu, C.; Berge, T.; Henderson, R.M.; Edwardson, J.M.; Dryden, D.T.F. Structure of Ocr from Bacteriophage T7, a Protein That Mimics B-Form DNA. Mol Cell 2002, 9, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Dillingham, M.S.; Kowalczykowski, S.C. RecBCD Enzyme and the Repair of Double-Stranded DNA Breaks. Microbiology and Molecular Biology Reviews 2008, 72, 642–671. [Google Scholar] [CrossRef]
- Murphy, K.C. The λ Gam Protein Inhibits RecBCD Binding to DsDNA Ends. J Mol Biol 2007, 371, 19–24. [Google Scholar] [CrossRef]
- d’Adda di Fagagna, F.; Weller, G.R.; Doherty, A.J.; Jackson, S.P. The Gam Protein of Bacteriophage Mu Is an Orthologue of Eukaryotic Ku. EMBO Rep 2003, 4, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Marraffini, L.A. CRISPR-Cas Systems: Prokaryotes Upgrade to Adaptive Immunity. Mol Cell 2014, 54, 234–244. [Google Scholar] [CrossRef]
- Deveau, H.; Barrangou, R.; Garneau, J.E.; Labonté, J.; Fremaux, C.; Boyaval, P.; Romero, D.A.; Horvath, P.; Moineau, S. Phage Response to CRISPR-Encoded Resistance in Streptococcus Thermophilus. J Bacteriol 2008, 190, 1390–1400. [Google Scholar] [CrossRef]
- Seed, K.D.; Lazinski, D.W.; Calderwood, S.B.; Camilli, A. A Bacteriophage Encodes Its Own CRISPR/Cas Adaptive Response to Evade Host Innate Immunity. Nature 2013, 494, 489–491. [Google Scholar] [CrossRef]
- Bondy-Denomy, J.; Pawluk, A.; Maxwell, K.L.; Davidson, A.R. Bacteriophage Genes That Inactivate the CRISPR/Cas Bacterial Immune System. Nature 2013, 493, 429–432. [Google Scholar] [CrossRef]
- Pawluk, A.; Bondy-Denomy, J.; Cheung, V.H.W.; Maxwell, K.L.; Davidson, A.R. A New Group of Phage Anti-CRISPR Genes Inhibits the Type I-E CRISPR-Cas System of Pseudomonas Aeruginosa. mBio 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, E.; Klaenhammer, T.R. Abortive Phage Resistance Mechanism AbiZ Speeds the Lysis Clock To Cause Premature Lysis of Phage-Infected Lactococcus Lactis. J Bacteriol 2007, 189, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Shinedling, S.; Parma, D.; Gold, L. Wild-Type Bacteriophage T4 Is Restricted by the Lambda Rex Genes. J Virol 1987, 61, 3790–3794. [Google Scholar] [CrossRef]
- Bingham, R.; Ekunwe, S.I.N.; Falk, S.; Snyder, L.; Kleanthous, C. The Major Head Protein of Bacteriophage T4 Binds Specifically to Elongation Factor Tu. Journal of Biological Chemistry 2000, 275, 23219–23226. [Google Scholar] [CrossRef] [PubMed]
- Essoh, C.; Blouin, Y.; Loukou, G.; Cablanmian, A.; Lathro, S.; Kutter, E.; Thien, H.V.; Vergnaud, G.; Pourcel, C. The Susceptibility of Pseudomonas Aeruginosa Strains from Cystic Fibrosis Patients to Bacteriophages. PLoS One 2013, 8, e60575. [Google Scholar] [CrossRef]
- Saussereau, E.; Vachier, I.; Chiron, R.; Godbert, B.; Sermet, I.; Dufour, N.; Pirnay, J.-P.; De Vos, D.; Carrié, F.; Molinari, N.; et al. Effectiveness of Bacteriophages in the Sputum of Cystic Fibrosis Patients. Clinical Microbiology and Infection 2014, 20, O983–O990. [Google Scholar] [CrossRef]
- Kutateladze, M.; Adamia, R. Phage Therapy Experience at the Eliava Institute. Med Mal Infect 2008, 38, 426–430. [Google Scholar] [CrossRef]
- Leitner, L.; Ujmajuridze, A.; Chanishvili, N.; Goderdzishvili, M.; Chkonia, I.; Rigvava, S.; Chkhotua, A.; Changashvili, G.; McCallin, S.; Schneider, M.P.; et al. Intravesical Bacteriophages for Treating Urinary Tract Infections in Patients Undergoing Transurethral Resection of the Prostate: A Randomised, Placebo-Controlled, Double-Blind Clinical Trial. Lancet Infect Dis 2021, 21, 427–436. [Google Scholar] [CrossRef]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter Baumannii Infection. Antimicrob Agents Chemother 2017, 61. [Google Scholar] [CrossRef]
- Chan, B.K.; Turner, P.E.; Kim, S.; Mojibian, H.R.; Elefteriades, J.A.; Narayan, D. Phage Treatment of an Aortic Graft Infected with Pseudomonas Aeruginosa. Evol Med Public Health 2018, 2018, 60–66. [Google Scholar] [CrossRef]
- Chan, B.K.; Sistrom, M.; Wertz, J.E.; Kortright, K.E.; Narayan, D.; Turner, P.E. Phage Selection Restores Antibiotic Sensitivity in MDR Pseudomonas Aeruginosa. Sci Rep 2016, 6, 26717. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered Bacteriophages for Treatment of a Patient with a Disseminated Drug-Resistant Mycobacterium Abscessus. Nat Med 2019, 25, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Uyttebroek, S.; Chen, B.; Onsea, J.; Ruythooren, F.; Debaveye, Y.; Devolder, D.; Spriet, I.; Depypere, M.; Wagemans, J.; Lavigne, R.; et al. Safety and Efficacy of Phage Therapy in Difficult-to-Treat Infections: A Systematic Review. Lancet Infect Dis 2022, 22, e208–e220. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, Y.; Boyd, E.F. Mobilization of the Vibrio Pathogenicity Island between Vibrio Cholerae Isolates Mediated by CP-T1 Generalized Transduction. FEMS Microbiol Lett 2002, 214, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Brabban, A.D.; Hite, E.; Callaway, T.R. Evolution of Foodborne Pathogens via Temperate Bacteriophage-Mediated Gene Transfer. Foodborne Pathog Dis 2005, 2, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Maiques, E.; Úbeda, C.; Tormo, M.A.; Ferrer, M.D.; Lasa, I.; Novick, R.P.; Penadés, J.R. Role of Staphylococcal Phage and SaPI Integrase in Intra- and Interspecies SaPI Transfer. J Bacteriol 2007, 189, 5608–5616. [Google Scholar] [CrossRef]
- Parsley, L.C.; Consuegra, E.J.; Kakirde, K.S.; Land, A.M.; Harper, W.F.; Liles, M.R. Identification of Diverse Antimicrobial Resistance Determinants Carried on Bacterial, Plasmid, or Viral Metagenomes from an Activated Sludge Microbial Assemblage. Appl Environ Microbiol 2010, 76, 3753–3757. [Google Scholar] [CrossRef]
- Marti, E.; Variatza, E.; Balcázar, J.L. Bacteriophages as a Reservoir of Extended-Spectrum β -Lactamase and Fluoroquinolone Resistance Genes in the Environment. Clinical Microbiology and Infection 2014, 20, O456–O459. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of Antibiotics and Antibiotic Resistance Genes in Hospital and Urban Wastewaters and Their Impact on the Receiving River. Water Res 2015, 69, 234–242. [Google Scholar] [CrossRef]
- Subirats, J.; Sànchez-Melsió, A.; Borrego, C.M.; Balcázar, J.L.; Simonet, P. Metagenomic Analysis Reveals That Bacteriophages Are Reservoirs of Antibiotic Resistance Genes. Int J Antimicrob Agents 2016, 48, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Marti, R.; Zurfluh, K.; Hagens, S.; Pianezzi, J.; Klumpp, J.; Loessner, M.J. Long Tail Fibres of the Novel Broad-Host-Range T-Even Bacteriophage S16 Specifically Recognize Salmonella OmpC. Mol. Microbiol. 2013, 87, 818–834. [Google Scholar] [CrossRef]
- Marti, E.; Variatza, E.; Balcázar, J.L. Bacteriophages as a Reservoir of Extended-Spectrum β -Lactamase and Fluoroquinolone Resistance Genes in the Environment. Clinical Microbiology and Infection 2014, 20, O456–O459. [Google Scholar] [CrossRef] [PubMed]
- Quirós, P.; Colomer-Lluch, M.; Martínez-Castillo, A.; Miró, E.; Argente, M.; Jofre, J.; Navarro, F.; Muniesa, M. Antibiotic Resistance Genes in the Bacteriophage DNA Fraction of Human Fecal Samples. Antimicrob Agents Chemother 2014, 58, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Torres-Barceló, C. The Disparate Effects of Bacteriophages on Antibiotic-Resistant Bacteria. Emerging Microbes & Infection 2018, 7, 1–12. [Google Scholar] [CrossRef]
- Colomer-Lluch, M.; Jofre, J.; Muniesa, M. Quinolone Resistance Genes (QnrA and QnrS) in Bacteriophage Particles from Wastewater Samples and the Effect of Inducing Agents on Packaged Antibiotic Resistance Genes. Journal of Antimicrobial Chemotherapy 2014, 69, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Fancello, L.; Desnues, C.; Raoult, D.; Rolain, J.M. Bacteriophages and Diffusion of Genes Encoding Antimicrobial Resistance in Cystic Fibrosis Sputum Microbiota. Journal of Antimicrobial Chemotherapy 2011, 66, 2448–2454. [Google Scholar] [CrossRef] [PubMed]
- Enault, F.; Briet, A.; Bouteille, L.; Roux, S.; Sullivan, M.B.; Petit, M.-A. Phages Rarely Encode Antibiotic Resistance Genes: A Cautionary Tale for Virome Analyses. ISME J 2017, 11, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage Treatment of Human Infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef]
- Cislo, M.; Dabrowski, M.; Weber-Dabrowska, B.; Woyton, A. Bacteriophage Treatment of Suppurative Skin Infections. Arch Immunol Ther Exp (Warsz) 1987, 35, 175–183. [Google Scholar]
- Ioseliani, G.D.; Meladze, G.D.; Chkhetiia, N.S.; Mebuke, M.G.; Kiknadze, N.I. [Use of Bacteriophage and Antibiotics for Prevention of Acute Postoperative Empyema in Chronic Suppurative Lung Diseases]. Grudn Khir 1980, 63–67. [Google Scholar]
- Kochetkova, V.A.; Mamontov, A.S.; Moskovtseva, R.L.; Erastova, E.I.; Trofimov, E.I.; Popov, M.I.; Dzhubalieva, S.K. [Phagotherapy of Postoperative Suppurative-Inflammatory Complications in Patients with Neoplasms]. Sov Med 1989, 23–26. [Google Scholar]
- Kwarciński, W.; Lazarkiewicz, B.; Weber-Dabrowska, B.; Rudnicki, J.; Kamiński, K.; Sciebura, M. [Bacteriophage Therapy in the Treatment of Recurrent Subphrenic and Subhepatic Abscess with Jejunal Fistula after Stomach Resection]. Pol Tyg Lek 1994, 49, 535–535. [Google Scholar]
- Perepanova, T.S.; Darbeeva, O.S.; Kotliarova, G.A.; Kondrat’eva, E.M.; Maiskaia, L.M.; Malysheva, V.F.; Baiguzina, F.A.; Grishkova, N. v. [The Efficacy of Bacteriophage Preparations in Treating Inflammatory Urologic Diseases]. Urol Nefrol (Mosk) 1995, 14–17. [Google Scholar]
- Sakandelidze, V.M. [The Combined Use of Specific Phages and Antibiotics in Different Infectious Allergoses]. Vrach Delo 1991, 60–63. [Google Scholar]
- Strój, L.; Weber-Dabrowska, B.; Partyka, K.; Mulczyk, M.; Wójcik, M. [Successful Treatment with Bacteriophage in Purulent Cerebrospinal Meningitis in a Newborn]. Neurol Neurochir Pol 1999, 33, 693–698. [Google Scholar] [PubMed]
- Sarker, S.A.; Sultana, S.; Reuteler, G.; Moine, D.; Descombes, P.; Charton, F.; Bourdin, G.; McCallin, S.; Ngom-Bru, C.; Neville, T.; et al. Oral Phage Therapy of Acute Bacterial Diarrhea With Two Coliphage Preparations: A Randomized Trial in Children From Bangladesh. EBioMedicine 2016, 4, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Leitner, L.; Ujmajuridze, A.; Chanishvili, N.; Goderdzishvili, M.; Chkonia, I.; Rigvava, S.; Chkhotua, A.; Changashvili, G.; McCallin, S.; Schneider, M.P.; et al. Intravesical Bacteriophages for Treating Urinary Tract Infections in Patients Undergoing Transurethral Resection of the Prostate: A Randomised, Placebo-Controlled, Double-Blind Clinical Trial. Lancet Infect Dis 2021, 21, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; le Floch, R.; et al. Efficacy and Tolerability of a Cocktail of Bacteriophages to Treat Burn Wounds Infected by Pseudomonas Aeruginosa (PhagoBurn): A Randomised, Controlled, Double-Blind Phase 1/2 Trial. Lancet Infect Dis 2019, 19, 35–45. [Google Scholar] [CrossRef]
- NIH, U.S. National Library of medicine Bacteriophage Therapy in First Time Chronic Prosthetic Joint Infections. NCT05269121. Available online: https://clinicaltrials.gov/study/NCT05269121 (accessed on 28 October 2023).
- Long Island University, C.D.E.K. Assessing the Efficacy of Anti-Staphylococcal Phages in the Management of Infected Foot Ulcers in Diabetes. NCT04289948. Available online: https://www.cdek.liu.edu/trial/NCT04289948/ (accessed on 28 October 2023).
- National Library of medicine BacterioPHAGE for Gastrointestinal Health 2 Study. NCT04511221. Available online: https://clinicaltrials.gov/study/NCT04511221 (accessed on 28 October 2023).
- National Library of medicine Existence in the Human Digestive Flora of Phages Able to Prevent the Acquisition of Multiresistant Enterobacteria (PHAGO-BMR). NCT03231267. Available online: https://clinicaltrials.gov/study/NCT03231267 (accessed on 28 October 2023).
- Sybesma, W.; Rohde, C.; Bardy, P.; Pirnay, J.-P.; Cooper, I.; Caplin, J.; Chanishvili, N.; Coffey, A.; De Vos, D.; Scholz, A.; et al. Silk Route to the Acceptance and Re-Implementation of Bacteriophage Therapy—Part II. Antibiotics 2018, 7, 35. [Google Scholar] [CrossRef]
- NIH, U.S. National Library of medicine Bacteriophage Effects on Pseudomonas Aeruginosa (MUCOPHAGES). NCT01818206. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01818206 (accessed on 28 October 2023).
- NIH, U.S. National Library of medicine PHAGE Study: Bacteriophages as Novel Prebiotics. NCT03269617. Available online: https://clinicaltrials.gov/study/NCT03269617 (accessed on 28 October 2023).
- NIH, U.S. National Library of medicine A Prospective, Randomized, Double-Blind Controlled Study of WPP-201 for the Safety and Efficacy of Treatment of Venous Leg Ulcers (WPP-201). NCT00663091. Available online: https://clinicaltrials.gov/study/NCT00663091 (accessed on 28 October 2023).
- WCG CenterWatch Safety Tolerability and PK of LBP-EC01 in Patients With Lower Urinary Tract Colonization Caused by E. Coli. Available online: https://www.centerwatch.com/clinical-trials/listings/234324/safety-tolerability-and-pk-of-lbp-ec01-in-patients-with-lower-urinary-tract-colonization-caused-by-e-coli/ (accessed on 28 October 2023).
- Lenneman, B.R.; Fernbach, J.; Loessner, M.J.; Lu, T.K.; Kilcher, S. Enhancing Phage Therapy through Synthetic Biology and Genome Engineering. Curr Opin Biotechnol 2021, 68, 151–159. [Google Scholar] [CrossRef]
- NIH, U.S. National Library of medicine Ascending Dose Study of the Safety of AB-SA01 When Topically Applied to Intact Skin of Healthy Adults. NCT02757755. Available online: https://clinicaltrials.gov/study/NCT02757755 (accessed on 28 October 2023).
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter Baumannii Infection. Antimicrob Agents Chemother 2017, 61. [Google Scholar] [CrossRef]
- Duplessis, C.A.; Stockelman, M.; Hamilton, T.; Merril, G.; Brownstein, M.; Bishop-Lilly, K.; Schooley, R.; Henry, M.; Horne, B.; Sisson, B.M.; et al. A Case Series of Emergency Investigational New Drug Applications for Bacteriophages Treating Recalcitrant Multi-Drug Resistant Bacterial Infections: Confirmed Safety and a Signal of Efficacy. J. Intensive Crit. Care 2019, 5. [Google Scholar]
- Aslam, S.; Courtwright, A.M.; Koval, C.; Lehman, S.M.; Morales, S.; Furr, C.L.L.; Rosas, F.; Brownstein, M.J.; Fackler, J.R.; Sisson, B.M.; et al. Early Clinical Experience of Bacteriophage Therapy in 3 Lung Transplant Recipients. Am J Transplant 2019, 19, 2631–2639. [Google Scholar] [CrossRef]
- Nir-Paz, R.; Gelman, D.; Khouri, A.; Sisson, B.M.; Fackler, J.; Alkalay-Oren, S.; Khalifa, L.; Rimon, A.; Yerushalmy, O.; Bader, R.; et al. Successful Treatment of Antibiotic-Resistant, Poly-Microbial Bone Infection With Bacteriophages and Antibiotics Combination. Clin Infect Dis 2019, 69, 2015–2018. [Google Scholar] [CrossRef]
- Gainey, A.B.; Burch, A.K.; Brownstein, M.J.; Brown, D.E.; Fackler, J.; Horne, B.; Biswas, B.; Bivens, B.N.; Malagon, F.; Daniels, R. Combining Bacteriophages with Cefiderocol and Meropenem/Vaborbactam to Treat a Pan-Drug Resistant Achromobacter Species Infection in a Pediatric Cystic Fibrosis Patient. Pediatr Pulmonol 2020, 55, 2990–2994. [Google Scholar] [CrossRef]
- LeBlanc, L.; Nezami, S.; Yost, D.; Tsourkas, P.; Amy, P.S. Isolation and Characterization of a Novel Phage Lysin Active against Paenibacillus Larvae, a Honeybee Pathogen. 2015, 5, e1080787. [Google Scholar] [CrossRef]
- Bateman, A.; Rawlings, N.D. The CHAP Domain: A Large Family of Amidases Including GSP Amidase and Peptidoglycan Hydrolases. Trends Biochem Sci 2003, 28, 234–237. [Google Scholar] [CrossRef]
- Oliveira, H.; Boas, D.V.; Mesnage, S.; Kluskens, L.D.; Lavigne, R.; Sillankorva, S.; Secundo, F.; Azeredo, J. Structural and Enzymatic Characterization of ABgp46, a Novel Phage Endolysin with Broad Anti-Gram-Negative Bacterial Activity. Front Microbiol 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Lood, R.; Winer, B.Y.; Pelzek, A.J.; Diez-Martinez, R.; Thandar, M.; Euler, C.W.; Schuch, R.; Fischetti, V.A. Novel Phage Lysin Capable of Killing the Multidrug-Resistant Gram-Negative Bacterium Acinetobacter Baumannii in a Mouse Bacteremia Model. Antimicrob Agents Chemother 2015, 59, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.A.; Shin, H.; Heu, S.; Ryu, S. Exogenous Lytic Activity of SPN9CC Endolysin against Gram-Negative Bacteria. J Microbiol Biotechnol 2014, 24, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Djurkovic, N.; McCormack, D.; Casimir, G. The Behavioral Reactions of Victims to Different Types of Workplace Bullying. International Journal of Organization Theory & Behavior 2005, 8, 439–460. [Google Scholar] [CrossRef]
- Fowler, V.G.; Das, A.F.; Lipka-Diamond, J.; Schuch, R.; Pomerantz, R.; Jáuregui-Peredo, L.; Bressler, A.; Evans, D.; Moran, G.J.; Rupp, M.E.; et al. Exebacase for Patients with Staphylococcus Aureus Bloodstream Infection and Endocarditis. J Clin Invest 2020, 130, 3750–3760. [Google Scholar] [CrossRef]
- Davies, G.; Henrissat, B. Structures and Mechanisms of Glycosyl Hydrolases. Structure 1995, 3, 853–859. [Google Scholar] [CrossRef]
- Severi, E.; Hood, D.W.; Thomas, G.H. Sialic Acid Utilization by Bacterial Pathogens. Microbiology (N Y) 2007, 153, 2817–2822. [Google Scholar] [CrossRef]
- Tomlinson, S.; Taylor, P.W. Neuraminidase Associated with Coliphage E That Specifically Depolymerizes the Escherichia Coli K1 Capsular Polysaccharide. J Virol 1985, 55, 374–378. [Google Scholar] [CrossRef]
- Schwarzer, D.; Browning, C.; Stummeyer, K.; Oberbeck, A.; Mühlenhoff, M.; Gerardy-Schahn, R.; Leiman, P.G. Structure and Biochemical Characterization of Bacteriophage Phi92 Endosialidase. Virology 2015, 477, 133–143. [Google Scholar] [CrossRef]
- Murakami, H.; Kuramoto, T.; Mizutani, K.; Nakano, H.; Kitahata, S. Purification and Some Properties of a New Levanase from Bacillus Sp. No. 71. Biosci Biotechnol Biochem 1992, 56, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Jathore, N.R.; Bule, M.V.; Tilay, A.V.; Annapure, U.S. Microbial Levan from Pseudomonas Fluorescens: Characterization and Medium Optimization for Enhanced Production. Food Sci Biotechnol 2012, 21, 1045–1053. [Google Scholar] [CrossRef]
- Dogsa, I.; Brloznik, M.; Stopar, D.; Mandic-Mulec, I. Exopolymer Diversity and the Role of Levan in Bacillus Subtilis Biofilms. PLoS One 2013, 8, e62044. [Google Scholar] [CrossRef]
- Maaroufi, H.; Levesque, R.C. Glycoside Hydrolase Family 32 Is Present in Bacillus Subtilis Phages. Virol J 2015, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, S.; Kanegasaki, S. Smooth Specific Phage Adsorption: Endorhamnosidase Activity of Tail Parts of P22. Biochem Biophys Res Commun 1973, 55, 403–409. [Google Scholar] [CrossRef]
- León, M.; Bastías, R. Virulence Reduction in Bacteriophage Resistant Bacteria. Front Microbiol 2015, 06. [Google Scholar] [CrossRef]
- Thurow, H.; Niemann, H.; Rudolph, C.; Stirm, S. Host Capsule Depolymerase Activity of Bacteriophage Particles Active on Klebsiella K20 and K24 Strains. Virology 1974, 58, 306–309. [Google Scholar] [CrossRef]
- Hanna, A.; Berg, M.; Stout, V.; Razatos, A. Role of Capsular Colanic Acid in Adhesion of Uropathogenic Escherichia Coli. Appl Environ Microbiol 2003, 69, 4474. [Google Scholar] [CrossRef]
- Danese, P.N.; Pratt, L.A.; Kolter, R. Exopolysaccharide Production Is Required for Development of Escherichia Coli K-12 Biofilm Architecture. J Bacteriol 2000, 182, 3593–3596. [Google Scholar] [CrossRef]
- Knirel, Y.A.; Prokhorov, N.S.; Shashkov, A.S.; Ovchinnikova, O.G.; Zdorovenko, E.L.; Liu, B.; Kostryukova, E.S.; Larin, A.K.; Golomidova, A.K.; Letarov, A. v. Variations in O-Antigen Biosynthesis and O-Acetylation Associated with Altered Phage Sensitivity in Escherichia Coli 4s. J Bacteriol 2015, 197, 905–912. [Google Scholar] [CrossRef]
- Thompson, J.E.; Pourhossein, M.; Waterhouse, A.; Hudson, T.; Goldrick, M.; Derrick, J.P.; Roberts, I.S. The K5 Lyase KflA Combines a Viral Tail Spike Structure with a Bacterial Polysaccharide Lyase Mechanism. Journal of Biological Chemistry 2010, 285, 23963–23969. [Google Scholar] [CrossRef] [PubMed]
- Hallenbeck, P.C.; Vimr, E.R.; Yu, F.; Bassler, B.; Troy, F.A. Purification and Properties of a Bacteriophage-Induced Endo-N-Acetylneuraminidase Specific for Poly-Alpha-2,8-Sialosyl Carbohydrate Units. Journal of Biological Chemistry 1987, 262, 3553–3561. [Google Scholar] [CrossRef]
- Freiberg, A.; Morona, R.; Van Den Bosch, L.; Jung, C.; Behlke, J.; Carlin, N.; Seckler, R.; Baxa, U. The Tailspike Protein of Shigella Phage Sf6. Journal of Biological Chemistry 2003, 278, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Knecht, L.E.; Heinrich, N.; Born, Y.; Felder, K.; Pelludat, C.; Loessner, M.J.; Fieseler, L. Bacteriophage S6 Requires Bacterial Cellulose for Erwinia Amylovora Infection. Environ Microbiol 2022. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Itoh, Y. Characterization of Poly-γ-Glutamate Hydrolase Encoded by a Bacteriophage Genome: Possible Role in Phage Infection of Bacillus Subtilis Encapsulated with Poly-γ-Glutamate. Appl Environ Microbiol 2003, 69, 2491–2497. [Google Scholar] [CrossRef]
- Fenton, M.; Keary, R.; McAuliffe, O.; Ross, R.P.; O’Mahony, J.; Coffey, A. Bacteriophage-Derived Peptidase Eliminates and Prevents Staphylococcal Biofilms. Int J Microbiol 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Bharati, A.P.; Singh, N.; Pandey, P.; Joshi, P.; Singh, K.; Mitra, K.; Gayen, J.R.; Sarkar, J.; Akhtar, Md.S. The Prophage-Encoded Hyaluronate Lyase Has Broad Substrate Specificity and Is Regulated by the N-Terminal Domain. Journal of Biological Chemistry 2014, 289, 35225–35236. [Google Scholar] [CrossRef] [PubMed]
- BAKER, J.R.; DONG, S.; PRITCHARD, D.G. The Hyaluronan Lyase of Streptococcus Pyogenes Bacteriophage H4489A. Biochemical Journal 2002, 365, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Briers, Y.; Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; Lavigne, R.; García, P. Role of the Pre-Neck Appendage Protein (Dpo7) from Phage VB_SepiS-PhiIPLA7 as an Anti-Biofilm Agent in Staphylococcal Species. Front Microbiol 2015, 6, 1315. [Google Scholar] [CrossRef]
- Pires, D.P.; Oliveira, H.; Melo, L.D.R.; Sillankorva, S.; Azeredo, J. Bacteriophage-Encoded Depolymerases: Their Diversity and Biotechnological Applications. Appl Microbiol Biotechnol 2016, 100, 2141–2151. [Google Scholar] [CrossRef]
- ALKAWASH, M.A.; SOOTHILL, J.S.; SCHILLER, N.L. Alginate Lyase Enhances Antibiotic Killing of Mucoid Pseudomonas Aeruginosa in Biofilms. APMIS 2006, 114, 131–138. [Google Scholar] [CrossRef]
- Colvin, K.M.; Gordon, V.D.; Murakami, K.; Borlee, B.R.; Wozniak, D.J.; Wong, G.C.L.; Parsek, M.R. The Pel Polysaccharide Can Serve a Structural and Protective Role in the Biofilm Matrix of Pseudomonas Aeruginosa. PLoS Pathog 2011, 7, e1001264. [Google Scholar] [CrossRef]
- Bansal, S.; Harjai, K.; Chhibber, S. Depolymerase Improves Gentamicin Efficacy during Klebsiella Pneumoniae Induced Murine Infection. BMC Infect. Dis 2014, 14, 456. [Google Scholar] [CrossRef]
- Domenech, M.; Ramos-Sevillano, E.; García, E.; Moscoso, M.; Yuste, J. Biofilm Formation Avoids Complement Immunity and Phagocytosis of Streptococcus Pneumoniae. Infect Immun 2013, 81, 2606–2615. [Google Scholar] [CrossRef]
- Eftekhar, F.; Speert, D.P. Alginase Treatment of Mucoid Pseudomonas Aeruginosa Enhances Phagocytosis by Human Monocyte-Derived Macrophages. Infect Immun 1988, 56, 2788–2793. [Google Scholar] [CrossRef]
- Liu, Y.; Leung, S.S.Y.; Huang, Y.; Guo, Y.; Jiang, N.; Li, P.; Chen, J.; Wang, R.; Bai, C.; Mi, Z.; et al. Identification of Two Depolymerases From Phage IME205 and Their Antivirulent Functions on K47 Capsule of Klebsiella Pneumoniae. Front Microbiol 2020, 11, 218. [Google Scholar] [CrossRef]
- Chen, X.; Liu, M.; Zhang, P.; Xu, M.; Yuan, W.; Bian, L.; Liu, Y.; Xia, J.; Leung, S.S.Y. Phage-Derived Depolymerase as an Antibiotic Adjuvant Against Multidrug-Resistant Acinetobacter Baumannii. Front Microbiol 2022, 13. [Google Scholar] [CrossRef]
- Rice, C.J.; Kelly, S.A.; O’Brien, S.C.; Melaugh, E.M.; Ganacias, J.C.B.; Chai, Z.H.; Gilmore, B.F.; Skvortsov, T. Novel Phage-Derived Depolymerase with Activity against Proteus Mirabilis Biofilms. Microorganisms 2021, 9, 2172. [Google Scholar] [CrossRef]
- Melo, L.D.R.; Veiga, P.; Cerca, N.; Kropinski, A.M.; Almeida, C.; Azeredo, J.; Sillankorva, S. Development of a Phage Cocktail to Control Proteus Mirabilis Catheter-Associated Urinary Tract Infections. Front Microbiol 2016, 0, 1024. [Google Scholar] [CrossRef]
- Shahed-Al-Mahmud, Md.; Roy, R.; Sugiokto, F.G.; Islam, Md.N.; Lin, M.-D.; Lin, L.-C.; Lin, N.-T. Phage ΦAB6-Borne Depolymerase Combats Acinetobacter Baumannii Biofilm Formation and Infection. Antibiotics 2021, 10, 279. [Google Scholar] [CrossRef]
- Kaźmierczak, Z.; Piotrowicz, A.; Owczarek, B.; Hodyra, K.; Miernikiewicz, P.; Lecion, D.; Harhala, M.; Górski, A.; Dąbrowska, K. Molecular Imaging of T4 Phage in Mammalian Tissues and Cells. Bacteriophage 2014, 4, e28364. [Google Scholar] [CrossRef]
- Srivastava, A.S.; Kaido, T.; Carrier, E. Immunological Factors That Affect the in Vivo Fate of T7 Phage in the Mouse. J Virol Methods 2004, 115, 99–104. [Google Scholar] [CrossRef]
- Park, K.; Cha, K.E.; Myung, H. Observation of Inflammatory Responses in Mice Orally Fed with Bacteriophage T7. J Appl Microbiol 2014, 117, 627–633. [Google Scholar] [CrossRef]
- Miernikiewicz, P.; Dąbrowska, K.; Piotrowicz, A.; Owczarek, B.; Wojas-Turek, J.; Kicielińska, J.; Rossowska, J.; Pajtasz-Piasecka, E.; Hodyra, K.; Macegoniuk, K.; et al. T4 Phage and Its Head Surface Proteins Do Not Stimulate Inflammatory Mediator Production. PLoS One 2013, 8, e71036. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Kim, J.-E.; Song, Y.-J.; Park, J.-H. Safety of Using Escherichia Coli Bacteriophages as a Sanitizing Agent Based on Inflammatory Responses in Rats. Food Sci Biotechnol 2016, 25, 355–360. [Google Scholar] [CrossRef]
- Carmody, L.A.; Gill, J.J.; Summer, E.J.; Sajjan, U.S.; Gonzalez, C.F.; Young, R.F.; LiPuma, J.J. Efficacy of Bacteriophage Therapy in a Model of Burkholderia Cenocepacia Pulmonary Infection. J Infect Dis 2010, 201, 264–271. [Google Scholar] [CrossRef]
- Dąbrowska, K.; Miernikiewicz, P.; Piotrowicz, A.; Hodyra, K.; Owczarek, B.; Lecion, D.; Kaźmierczak, Z.; Letarov, A.; Górski, A. Immunogenicity Studies of Proteins Forming the T4 Phage Head Surface. J Virol 2014, 88, 12551–12557. [Google Scholar] [CrossRef]
- Edgar, R.; Friedman, N.; Molshanski-Mor, S.; Qimron, U. Reversing Bacterial Resistance to Antibiotics by Phage-Mediated Delivery of Dominant Sensitive Genes. Appl Environ Microbiol 2012, 78, 744–751. [Google Scholar] [CrossRef]
- Międzybrodzki, R.; Fortuna, W.; Weber-Dąbrowska, B.; Górski, A. Phage Therapy of Staphylococcal Infections (Including MRSA) May Be Less Expensive than Antibiotic Treatment Word Count: Coresponding; 2007; Vol. 61.
- Morozova, V.V.; Vlassov, V.V.; Tikunova, N.V. Applications of Bacteriophages in the Treatment of Localized Infections in Humans. Front Microbiol 2018, 9. [Google Scholar] [CrossRef]
- Rose, T.; Verbeken, G.; Vos, D. De; Merabishvili, M.; Vaneechoutte, M.; Lavigne, R.; Jennes, S.; Zizi, M.; Pirnay, J.-P. Experimental Phage Therapy of Burn Wound Infection: Difficult First Steps. Int J Burns Trauma 2014, 4, 66–73. [Google Scholar]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.-A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and Tolerability of a Cocktail of Bacteriophages to Treat Burn Wounds Infected by Pseudomonas Aeruginosa (PhagoBurn): A Randomised, Controlled, Double-Blind Phase 1/2 Trial. Lancet Infect Dis 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Rhoads, D.D.; Wolcott, R.D.; Kuskowski, M.A.; Wolcott, B.M.; Ward, L.S.; Sulakvelidze, A. Bacteriophage Therapy of Venous Leg Ulcers in Humans: Results of a Phase I Safety Trial. J Wound Care 2009, 18, 237–243. [Google Scholar] [CrossRef]
- Markoishvili, K.; Tsitlanadze, G.; Katsarava, R.; Glenn, J.; Morris, M.D., Jr.; Sulakvelidze, A. A Novel Sustained-release Matrix Based on Biodegradable Poly(Ester Amide)s and Impregnated with Bacteriophages and an Antibiotic Shows Promise in Management of Infected Venous Stasis Ulcers and Other Poorly Healing Wounds. Int J Dermatol 2002, 41, 453–458. [Google Scholar] [CrossRef]
- Fish, R.; Kutter, E.; Wheat, G.; Blasdel, B.; Kutateladze, M.; Kuhl, S. Compassionate Use of Bacteriophage Therapy for Foot Ulcer Treatment as an Effective Step for Moving Toward Clinical Trials. In; 2018; pp. 159–170. [CrossRef]
- Abdul-Hassan, H.S.; El-Tahan, E.; Massoud, B.; Gomaa, R. Bacteriophage Therapy of Pseudomonas Burn Wound Sepsis. Ann. Medit. Burn Club 1990, 3, 262–264. [Google Scholar]
- Sarkis, G.J.; Jacobs, W.R.; Hatfulll, G.F. L5 Luciferase Reporter Mycobacteriophages: A Sensitive Tool for the Detection and Assay of Live Mycobacteria. Mol Microbiol 1995, 15, 1055–1067. [Google Scholar] [CrossRef]
- Jacobs, W.R.; Tuckman, M.; Bloom, B.R. Introduction of Foreign DNA into Mycobacteria Using a Shuttle Phasmid. Nature 1987, 327, 532–535. [Google Scholar] [CrossRef]
- Chauthaiwale, V.M.; Therwath, A.; Deshpande, V. V Bacteriophage Lambda as a Cloning Vector. Microbiol Rev 1992, 56, 577–591. [Google Scholar] [CrossRef]
- Marinelli, L.J.; Piuri, M.; Swigoňová, Z.; Balachandran, A.; Oldfield, L.M.; van Kessel, J.C.; Hatfull, G.F. BRED: A Simple and Powerful Tool for Constructing Mutant and Recombinant Bacteriophage Genomes. PLoS One 2008, 3, e3957. [Google Scholar] [CrossRef]
- Hatoum-Aslan, A. Phage Genetic Engineering Using CRISPR–Cas Systems. Viruses 2018, 10, 335. [Google Scholar] [CrossRef]
- Wetzel, K.S.; Guerrero-Bustamante, C.A.; Dedrick, R.M.; Ko, C.-C.; Freeman, K.G.; Aull, H.G.; Divens, A.M.; Rock, J.M.; Zack, K.M.; Hatfull, G.F. CRISPY-BRED and CRISPY-BRIP: Efficient Bacteriophage Engineering. Sci Rep 2021, 11, 6796. [Google Scholar] [CrossRef]
- Pires, D.P.; Cleto, S.; Sillankorva, S.; Azeredo, J.; Lu, T.K. Genetically Engineered Phages: A Review of Advances over the Last Decade. Microbiology and Molecular Biology Reviews 2016, 80, 523–543. [Google Scholar] [CrossRef]
- Summers, W.C. Cholera and Plague in India: The Bacteriophage Inquiry of 1927–1936. J Hist Med Allied Sci 1993, 48, 275–301. [Google Scholar] [CrossRef]
- Sarker, S.A.; Sultana, S.; Reuteler, G.; Moine, D.; Descombes, P.; Charton, F.; Bourdin, G.; McCallin, S.; Ngom-Bru, C.; Neville, T.; et al. Oral Phage Therapy of Acute Bacterial Diarrhea With Two Coliphage Preparations: A Randomized Trial in Children From Bangladesh. EBioMedicine 2016, 4, 124–137. [Google Scholar] [CrossRef]
- Sarker, S.A.; McCallin, S.; Barretto, C.; Berger, B.; Pittet, A.-C.; Sultana, S.; Krause, L.; Huq, S.; Bibiloni, R.; Bruttin, A.; et al. Oral T4-like Phage Cocktail Application to Healthy Adult Volunteers from Bangladesh. Virology 2012, 434, 222–232. [Google Scholar] [CrossRef]
- McCallin, S.; Alam Sarker, S.; Barretto, C.; Sultana, S.; Berger, B.; Huq, S.; Krause, L.; Bibiloni, R.; Schmitt, B.; Reuteler, G.; et al. Safety Analysis of a Russian Phage Cocktail: From MetaGenomic Analysis to Oral Application in Healthy Human Subjects. Virology 2013, 443, 187–196. [Google Scholar] [CrossRef]
- Bourdin, G.; Schmitt, B.; Marvin Guy, L.; Germond, J.-E.; Zuber, S.; Michot, L.; Reuteler, G.; Brüssow, H. Amplification and Purification of T4-Like Escherichia Coli Phages for Phage Therapy: From Laboratory to Pilot Scale. Appl Environ Microbiol 2014, 80, 1469–1476. [Google Scholar] [CrossRef]
- Lu, T.K.; Collins, J.J. Dispersing Biofilms with Engineered Enzymatic Bacteriophage. Proceedings of the National Academy of Sciences 2007, 104, 11197–11202. [Google Scholar] [CrossRef]
- Wu, J.W.; Wang, J.T.; Lin, T.L.; Liu, Y.Z.; Wu, L.T.; Pan, Y.J. Identification of Three Capsule Depolymerases in a Bacteriophage Infecting Klebsiella Pneumoniae Capsular Types K7, K20, and K27 and Therapeutic Application. J Biomed Sci 2023, 30. [Google Scholar] [CrossRef]
- Majkowska-Skrobek, G.; Latka, A.; Berisio, R.; Squeglia, F.; Maciejewska, B.; Briers, Y.; Drulis-Kawa, Z. Phage-Borne Depolymerases Decrease Klebsiella Pneumoniae Resistance to Innate Defense Mechanisms. Front Microbiol 2018, 9. [Google Scholar] [CrossRef]
- Lin, H.; Paff, M.L.; Molineux, I.J.; Bull, J.J. Therapeutic Application of Phage Capsule Depolymerases against K1, K5, and K30 Capsulated E. Coli in Mice. Front Microbiol 2017, 8. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Wang, S.; Guan, L.; Li, X.; Hu, D.; Gao, D.; Song, J.; Chen, H.; Qiana, P. A Novel Tail-Associated O91-Specific Polysaccharide Depolymerase from a Podophage Reveals Lytic Efficacy of Shiga Toxin-Producing Escherichia Coli. Appl Environ Microbiol 2020, 86. [Google Scholar] [CrossRef]
- Mi, L.; Liu, Y.; Wang, C.; He, T.; Gao, S.; Xing, S.; Huang, Y.; Fan, H.; Zhang, X.; Yu, W.; et al. Identification of a Lytic Pseudomonas Aeruginosa Phage Depolymerase and Its Anti-Biofilm Effect and Bactericidal Contribution to Serum. Virus Genes 2019, 55, 394–405. [Google Scholar] [CrossRef]
- Wyres, K.L.; Wick, R.R.; Gorrie, C.; Jenney, A.; Follador, R.; Thomson, N.R.; Holt, K.E. Identification of Klebsiella Capsule Synthesis Loci from Whole Genome Data. Microb Genom 2016, 2, e000102. [Google Scholar] [CrossRef]
- van der Graaf-van Bloois, L.; Chen, H.; Wagenaar, J.A.; Zomer, A.L. Development of Kaptive Databases for Vibrio Parahaemolyticus O- and K-Antigen Genotyping. Microb Genom 2023, 9. [Google Scholar] [CrossRef]
- Pan, Y.-J.; Lin, T.-L.; Chen, C.-T.; Chen, Y.-Y.; Hsieh, P.-F.; Hsu, C.-R.; Wu, M.-C.; Wang, J.-T. Genetic Analysis of Capsular Polysaccharide Synthesis Gene Clusters in 79 Capsular Types of Klebsiella Spp. Sci Rep 2015, 5, 15573. [Google Scholar] [CrossRef]
- Li, J.; Sheng, Y.; Ma, R.; Xu, M.; Liu, F.; Qin, R.; Zhu, M.; Zhu, X.; He, P. Identification of a Depolymerase Specific for K64-Serotype Klebsiella Pneumoniae: Potential Applications in Capsular Typing and Treatment. Antibiotics 2021, 10, 144. [Google Scholar] [CrossRef]
- Park, D.-W.; Park, J.-H. Characterization of a Novel Phage Depolymerase Specific to Escherichia Coli O157:H7 and Biofilm Control on Abiotic Surfaces. J. Microbiol. 2021, 59, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, R.A.; Bamert, R.S.; Belousoff, M.J.; Short, F.L.; Barlow, C.K.; Pickard, D.J.; Wilksch, J.J.; Schittenhelm, R.B.; Strugnell, R.A.; Dougan, G.; et al. Mechanistic Insights into the Capsule-Targeting Depolymerase from a Klebsiella Pneumoniae Bacteriophage. Microbiol. Spectr. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, H.; Luo, S.; Wang, L.; Lu, S.; Fu, Z. Engineering Phage Tail Fiber Protein as a Wide-Spectrum Probe for Acinetobacter Baumannii Strains with a Recognition Rate of 100%. Anal Chem 2022. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





