Submitted:
31 October 2023
Posted:
31 October 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
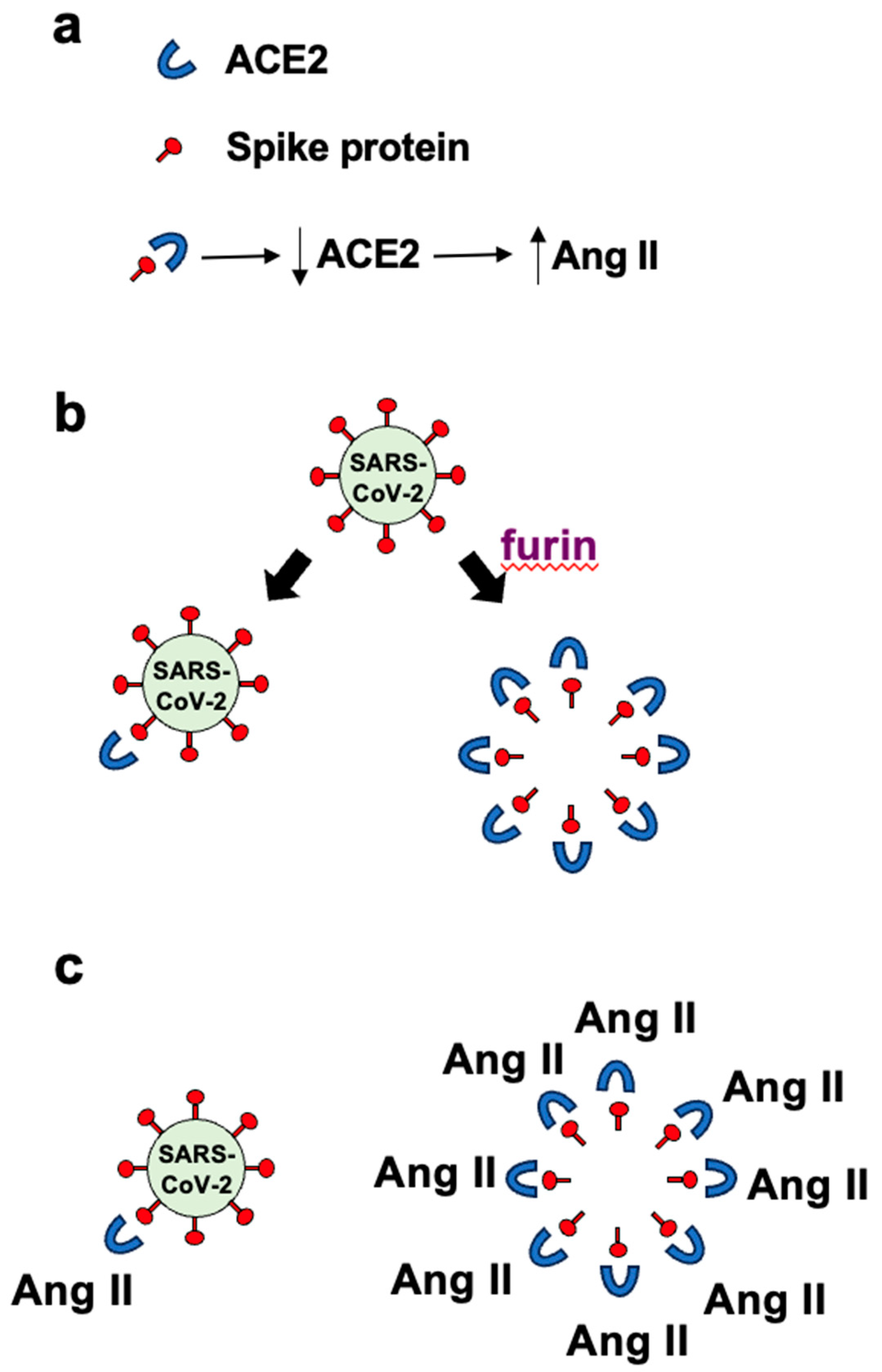
2. The Hypothesis
3. Evaluation of the Hypothesis
- Materials and Methods (Figure 3)
- Results (Figure 3)
4. Consequences of the Hypothesis
- Materials and Methods (Figure 4)
- Results (Figure 4)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19); StatPearls Publishing LLC. 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK554776/.
- Kalra, R.S.; Dhanjal, J.K.; Meena, A.S.; Kalel, V.C.; Dahiya, S.; Singh, B.; Dewanjee, S.; Kandimalla, R. COVID-19, Neuropathology, and Aging: SARS-CoV-2 Neurological Infection, Mechanism, and Associated Complications. Front. Aging Neurosci. 2021, 13, 662786. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Palaiodimou, L.; Zand, R.; Lioutas, V.A.; Krogias, C.; Katsanos, A.H.; Shoamanesh, A.; Sharma, V.K.; Shahjouei, S.; Baracchini, C.; Vlachopoulos, C.; Gournellis, R.; Sfikakis, P.P.; Sandset, E.C.; Alexandrov, A.V.; Tsiodras, S. COVID-19 and cerebrovascular diseases: A comprehensive overview. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420978004. [Google Scholar] [CrossRef] [PubMed]
- Shabani, Z. Demyelination as a result of an immune response in patients with COVID-19. Acta Neurol. Belg. 2021, 121, 859–866. [Google Scholar] [CrossRef]
- Spudich, S.; Nath, A. Nervous system consequences of COVID-19. Science 2022, 375, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Brola, W.; Wilski, M. Neurological consequences of COVID-19. Pharmacol. Rep. 2022, 74, 1208–1222. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-T.; Lu, M.-K.; San, S.; Tsai, C.-H. The Neurologic Manifestations of Coronavirus Disease 2019 Pandemic: A Systemic Review. Front. Neurol. 2020, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; Jbabdi, S.; Taschler, B.; Keating, P.; Winkler, A.M.; Collins, R.; Matthews, P.M.; Allen, N.; Miller, K.L.; Nichols, T.E.; Smith, S.M. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; Miao, X.; Li, Y.; Hu, B. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Chu, H.; Chan, J.F.-W.; Yuen, T.T.-T.; Shuai, H.; Yuan, S.; Wang, Y.; Hu, B.; Yip, C.C.-Y.; Tsang, J.O.-L.; Huang, X.; Chai, Y.; Yang, D.; Hou, Y.; Chik, K.K.-H.; Zhang, X.; Fung, A.Y.-F.; Tsoi, H.-W.; Cai, J.-P.; Chan, W.-M.; Ip, J.D.; Chu, A.W.-H.; Zhou, J.; Lung, D.C.; Kok, K.-H.; To, K.K.-W.; Tsang, O.T.-Y.; Chan, K.-H.; Yuen, K.-Y. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: An observational study. Lancet Microbe 2020, 1, e14–e23. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; Nakao, A.; Takeda, M.; Haro, H.; Inoue, O.; Suzuki-Inoue, K.; Kubokawa, K.; Ogihara, S.; Sasaki, T.; Kinouchi, H.; Kojin, H.; Ito, M.; Onishi, H.; Shimizu, T.; Sasaki, Y.; Enomoto, N.; Ishihara, H.; Furuya, S.; Yamamoto, T.; Shimada, S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; Lehmann, M.; Hassan, O.; Aschman, T.; Schumann, E.; Chua, R.L.; Conrad, C.; Eils, R.; Stenzel, W.; Windgassen, M.; Rößler, L.; Goebel, H.-H.; Gelderblom, H.R.; Martin, H.; Nitsche, A.; Schulz-Schaeffer, W.J.; Hakroush, S.; Winkler, M.S.; Tampe, B.; Scheibe, F.; Körtvélyessy, P.; Reinhold, D.; Siegmund, B.; Kühl, A.A.; Elezkurtaj, S.; Horst, D.; Oesterhelweg, L.; Tsokos, M.; Ingold-Heppner, B.; Stadelmann, C.; Drosten, C.; Corman, V.M.; Radbruch, H.; Heppner, F.L. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Bratosiewicz-Wąsik, J. Neuro-COVID-19: An insidious virus in action. Neurol. Neurochir. Pol. 2022, 56, 48–60. [Google Scholar] [CrossRef]
- Hugon, J. Long-COVID: Cognitive deficits (brain fog) and brain lesions in non-hospitalized patients. Presse Med. 2022, 51, 104090. [Google Scholar] [CrossRef]
- Cosentino, G.; Todisco, M.; Hota, N.; Della Porta, G.; Morbini, P.; Tassorelli, C.; Pisani, A. Neuropathological findings from COVID-19 patients with neurological symptoms argue against a direct brain invasion of SARS-CoV-2: A critical systematic review. Eur. J. Neurol. 2021, 28, 3856–3865. [Google Scholar] [CrossRef] [PubMed]
- Carod-Artal, F.J. Neurological complications of coronavirus and COVID-19. Rev. Neurol. 2020, 70, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Charnley, M.; Islam, S.; Bindra, G.K.; Engwirda, J.; Ratcliffe, J.; Zhou, J.; Mezzenga, R.; Hulett, M.D.; Han, K.; Berryman, J.T.; Reynolds, N.P. Neurotoxic amyloidogenic peptides in the proteome of SARS-COV2: Potential implications for neurological symptoms in COVID-19. Nat. Commun. 2022, 13, 3387. [Google Scholar] [CrossRef]
- Mogi, M.; Horiuchi, M. Effect of angiotensin II type 2 receptor on stroke, cognitive impairment and neurodegenerative diseases. Geriatr. Gerontol. Int. 2013, 13, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Takane, K.; Hasegawa, Y.; Lin, B.; Koibuchi, N.; Cao, C.; Yokoo, T.; Kim-Mitsuyama, S. Detrimental Effects of Centrally Administered Angiotensin II are Enhanced in a Mouse Model of Alzheimer Disease Independently of Blood Pressure. J. Am. Heart Assoc. 2017, 6, e004897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef]
- Patel, V.B.; Zhong, J.-C.; Grant, M.B.; Oudit, G.Y. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ. Res. 2016, 118, 1313–1326. [Google Scholar] [CrossRef]
- Ramos, S.G.; Rattis, B.A.C.; Ottaviani, G.; Celes, M.R.N.; Dias, E.P. ACE2 down-regulation may act as a transient molecular disease causing RAAS dysregulation and tissue damage in the microcirculatory environment among COVID-19 patients. Am. J. Pathol. 2021, 191, 1154–1164. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, Y.; Johnson, A.K. Interactions of the Brain Renin-Angiotensin-System (RAS) and Inflammation in the Sensitization of Hypertension. Front. Neurosci. 2020, 14, 650. [Google Scholar] [CrossRef]
- de Morais, S.D.B.; Shanks, J.; Zucker, I.H. Integrative physiological aspects of brain RAS in hypertension. Curr. Hypertens. Rep. 2018, 20, 10. [Google Scholar] [CrossRef]
- Boily, M.; Li, L.; Vallerand, D.; Girouard, H. Angiotensin II disrupts neurovascular coupling by potentiating calcium increases in astrocytic endfeet. J. Am. Heart Assoc. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, F.; Camins, A.; Ettcheto, M.; Bicker, J.; Falcão, A.; Cruz, M.T.; Fortuna, A. Targeting brain Renin-Angiotensin System for the prevention and treatment of Alzheimer’s disease: Past, present and future. Ageing Res. Rev. 2022, 77, 101612. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Kuba, K.; Ohto-Nakanishi, T.; Penninger, J.M. Angiotensin-Converting Enzyme 2 (ACE2) in Disease Pathogenesis. Circ. J. 2010, 74, 405–410. [Google Scholar] [CrossRef]
- Yang, G.; Chu, P.-L.; Rump, L.C.; Le, T.H.; Stegbauer, J. ACE2 and the Homolog Collectrin in the Modulation of Nitric Oxide and Oxidative Stress in Blood Pressure Homeostasis and Vascular Injury. Antioxid. Redox Signal. 2017, 26, 645–659. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Wang, X.; Yang, L.; Li, H.; Wang, Y.; Liu, M.; Zhao, X.; Xie, Y.; Yang, Y.; Zhang, S.; Fan, Z.; Dong, J.; Yuan, Z.; Ding, Z.; Zhang, Y.; Hu, L. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, P.E.; Chappell, M.C.; Ferrario, C.M.; Tallant, E.A. Distinct roles for ANG II and ANG-(1-7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am. J. Physiol. Cell Physiol. 2006, 290, C420–426. [Google Scholar] [CrossRef]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/Angiotensin-(1–7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1–7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef]
- Suzuki, Y.J. The viral protein fragment theory of COVID-19 pathogenesis. Med. Hypotheses 2020, 144, 110267. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef]
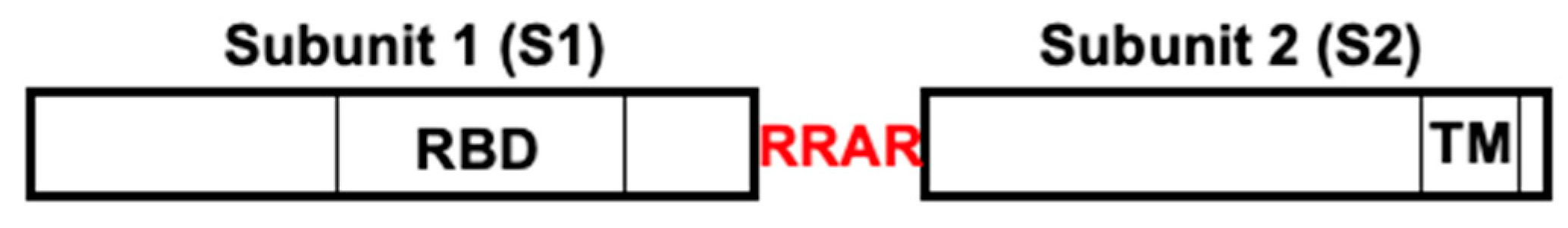
- Takeda, M. Proteolytic activation of SARS-CoV-2 spike protein. Microbiol. Immunol. 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Ogata, A.F.; Maley, A.M.; Wu, C.; Gilboa, T.; Norman, M.; Lazarovits, R.; Mao, C.P.; Newton, G.; Chang, M.; Nguyen, K.; Kamkaew, M.; Zhu, Q.; Gibson, T.E.; Ryan, E.T.; Charles, R.C.; Marasco, W.A.; Walt, D.R. Ultra-sensitive serial profiling of SARS-CoV-2 antigens and antibodies in plasma to understand disease progression in COVID-19 patients with severe disease. Clin. Chem. 2020, 66, 1562–1572. [Google Scholar] [CrossRef]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated With Post-acute Coronavirus Disease 2019 Sequelae. Clin. Infect. Dis. 2023, 76, e487–e490. [Google Scholar] [CrossRef]
- Ogata, A.F.; Cheng, C.A.; Desjardins, M.; Senussi, Y.; Sherman, A.C.; Powell, M.; Novack, L.; Von, S.; Li, X.; Baden, L.R.; Walt, D.R. Circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine antigen detected in the plasma of mRNA-1273 vaccine recipients. Clin. Infect. Dis. 2022, 74, 715–718. [Google Scholar] [CrossRef]
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Burns, M.D.; Kane, A.; Boribong, B.P.; Davis, J.P.; Loiselle, M.; Novak, T.; Senussi, Y.; et al. Circulating spike protein detected in post-COVID-19 mRNA vaccine myocarditis. Circulation 2023, 147, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, M.; Marino, F. The spike hypothesis in vaccine-induced adverse effects: questions and answers. Trends Mol. Med. 2022, 28, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Rhea, E.M.; Logsdon, A.F.; Hansen, K.M.; Williams, L.M.; Reed, M.J.; Baumann, K.K.; Holden, S.J.; Raber, J.; Banks, W.A.; Erickson, M.A. The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nat. Neurosci. 2021, 24, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Braun, E.; Sauter, D. Furin-mediated protein processing in infectious diseases and cancer. Clin. Transl. Immunology 2019, 8, e1073. [Google Scholar] [CrossRef]
- Vidricaire, G.; Denault, J.B.; Leduc, R. Characterization of a secreted form of human furin endoprotease. Biochem. Biophys. Res. Commun. 1993, 195, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; Bao, L.; Zhang, B.; Liu, G.; Wang, Z.; Chappell, M.; Liu, Y.; Zheng, D.; Leibbrandt, A.; Wada, T.; Slutsky, A.S.; Liu, D.; Qin, C.; Jiang, C.; Penninger, J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, F.; Liu, K.; Wang, H.; Rao, S.; Yang, P.; Jiang, C. Endocytosis of the receptor-binding domain of SARS-CoV spike protein together with virus receptor ACE2. Virus Res. 2008, 136, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bayati, A.; Kumar, R.; Francis, V.; McPherson, P.S. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J. Biol. Chem. 2021, 296, 100306. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhu, Q.; Fox, D.M.; Gao, C.; Stanley, S.A.; Luo, K. SARS-CoV-2 down-regulates ACE2 through lysosomal degradation. Mol. Biol. Cell. 2022, 33, ar147. [Google Scholar] [CrossRef] [PubMed]
- Portales, A.E.; Mustafá, E.R.; McCarthy, C.I.; Cornejo, M.P.; Couto, P.M.; Gironacci, M.M.; Caramelo, J.J.; Perelló, M.; Raingo, J. ACE2 internalization induced by a SARS-CoV-2 recombinant protein is modulated by angiotensin II type 1 and bradykinin 2 receptors. Life Sci. 2022, 293, 120284. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Yan, C.; Chen, Y.; Zhou, W.; Zhou, X.; Qiao, Q.; Xu, Z. SIM imaging resolves endocytosis of SARS-CoV-2 spike RBD in living cells. Cell Chem. Biol. 2023, 30, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; Shadel, G.S.; Hepokoski, M.; Lei, T.; Wang, H.; Zhang, J.; Yuan, J.X.; Malhotra, A.; Manor, U.; Wang, S.; Yuan, Z.Y.; Shyy, J.Y. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circ. Res. 2021, 128, 1323–1326. [Google Scholar] [CrossRef]
- Sui, Y.; Li, J.; Venzon, D.J.; Berzofsky, J.A. SARS-CoV-2 spike protein suppresses ACE2 and type I interferon expression in primary cells from macaque lung bronchoalveolar lavage. Front. Immunol. 2021, 12, 658428. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, S.; Gou, J.; Wen, Y.; Fan, L.; Zhou, J.; Zhou, G.; Xu, G.; Zhang, Z. Spike-mediated ACE2 down-regulation was involved in the pathogenesis of SARS-CoV-2 infection. J. Infect. 2022, 85, 418–427. [Google Scholar] [CrossRef]
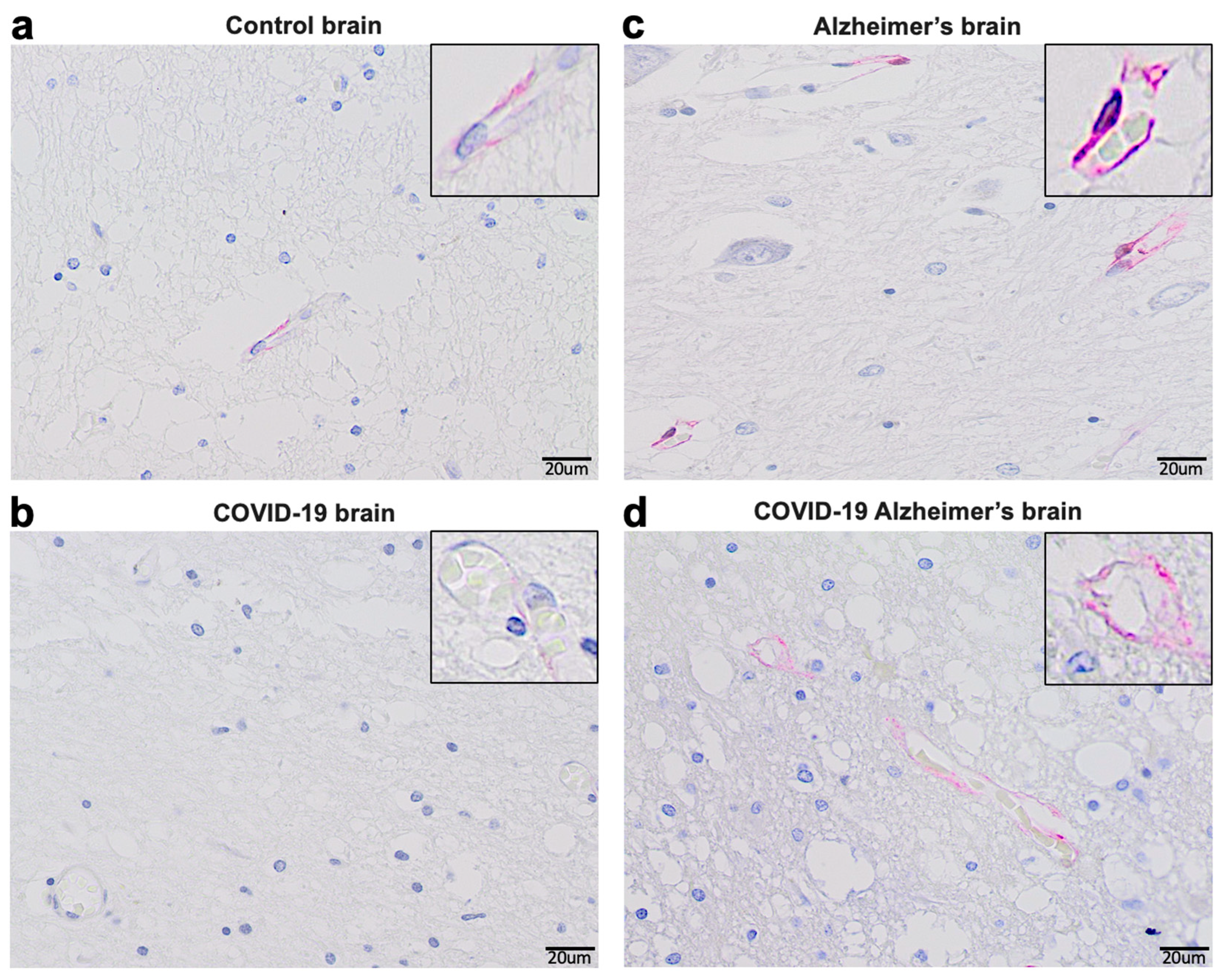
- Ding, Q.; Shults, N.V.; Gychka, S.G.; Harris, B.T.; Suzuki, Y.J. Protein expression of angiotensin-converting enzyme 2 (ACE2) is upregulated in brains with Alzheimer's disease. Int. J. Mol. Sci. 2021, 22, 1687. [Google Scholar] [CrossRef] [PubMed]
- Reveret, L.; Leclerc, M.; Emond, V.; Loiselle, A.; Bourassa, P.; Tremblay, C.; Bennett, D.A.; Hébert, S.; Calon, F. Higher ACE2 expression in the brains of individuals with Alzheimer’s disease. Alzheimers Dement. 2021, 17, e055278. [Google Scholar] [CrossRef]
- Louise, R.; Manon, L.; Vincent, E.; Cyntia, T.; Andréanne, L.; Philippe, B.; David, A.B.; Hébert, S.S.; Frédéric, C. Higher angiotensin-converting enzyme 2 (ACE2) levels in the brain of individuals with Alzheimer's disease. Acta Neuropathol. Commun. 2023, 11, 159. [Google Scholar] [CrossRef]
- Lim, K.H.; Yang, S.; Kim, S.H.; Joo, J.Y. Elevation of ACE2 as a SARS-CoV-2 entry receptor gene expression in Alzheimer’s disease. J. Infect. 2020, 81, e33–e34. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Yang, J.; Zhang, Y.; Dong, M.; Wang, S.; Zhang, Q.; Liu, F.F.; Zhang, K.; Zhang, C. Angiotensin-converting enzyme 2 and angiotensin 1-7: novel therapeutic targets. Nat. Rev. Cardiol. 2014, 11, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lazartigues, E. Expression of ACE2 in human neurons supports the neuro-invasive potential of COVID-19 virus. Cell. Mol. Neurobiol. 2022, 42, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Li, J.; Feng, C.; Zhang, Z.; Wang, L.; Peng, L.; Chen, L.; Qin, Y.; Zhao, D.; Tan, S.; Yin, L.; Xu, J.; Zhou, C.; Jiang, C.; Liu, L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, R.; Zhang, C.; Ren, W.; Yu, A.; Zhou, X. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients. Crit. Care 2020, 24, 290. [Google Scholar] [CrossRef]
- Camargo, R.L.; Bombassaro, B.; Monfort-Pires, M.; Mansour, E.; Palma, A.C.; Ribeiro, L.C.; Ulaf, R.G.; Bernardes, A.F.; Nunes, T.A.; Agrela, M.V.; Dertkigil, R.P.; Dertkigil, S.S.; Araujo, E.P.; Nadruz, W.; Moretti, M.L.; Velloso, L.A.; Sposito, A.C. Plasma angiotensin II is increased in critical coronavirus disease 2019. Front. Cardiovasc. Med. 2022, 9, 847809. [Google Scholar] [CrossRef]
- Ipekci, A.; Biberoglu, S.; Ikizceli, I.; Cakmak, F.; Akdeniz, Y.S.; Kanbakan, A.; Konukoglu, D.; Bolayirli, I.M.; Borekci, S.; Urkmez, S.; Ozkan, S. ACE2 and ANGII levels in patients with COVID-19 based on thoracic tomography findings and PCR test results. J. Infect. Dev. Ctries. 2022, 16, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Satarker, S.; Nampoothiri, M. Structural proteins in severe acute respiratory syndrome coronavirus-2. Arch. Med. Res. 2020, 51, 482–491. [Google Scholar] [CrossRef]
- Neuman, B.W.; Adair, B.D.; Yoshioka, C.; Quispe, J.D.; Orca, G.; Kuhn, P.; Milligan, R.A.; Yeager, M.; Buchmeier, M.J. Supramolecular architecture of severe acute respiratory syndrome coronavirus revealed by electron cryomicroscopy. J. Virol. 2006, 80, 7918–7928. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.J.; Gychka, S.G. SARS-CoV-2 spike protein elicits cell signaling in human host cells: Implications for possible consequences of COVID-19 vaccines. Vaccines 2021, 9, 36. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




