Submitted:
31 October 2023
Posted:
01 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Role of MSCs in myelodysplastic syndromes
2.2. Role of MSCs in acute leukemia
2.3. Role of MSCs in Myeloproliferative Neoplasms
2.4. Role of MSCs in in Chronic lymphocytic leukemia
2.5. Role of MSCs in in Multiple myeloma
3.1. Concluding Remarks and future perspectives
Author Contributions
Conflicts of Interest
References
- Wei, Q. and P.S. Frenette, Niches for Hematopoietic Stem Cells and Their Progeny. Immunity, 2018. 48(4): p. 632-648. [CrossRef]
- Ding, L. and S.J. Morrison, Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature, 2013. 495(7440): p. 231-5. [CrossRef]
- Cordeiro Gomes, A., et al., Hematopoietic Stem Cell Niches Produce Lineage-Instructive Signals to Control Multipotent Progenitor Differentiation. Immunity, 2016. 45(6): p. 1219-1231. [CrossRef]
- Mendes, S.C., C. Robin, and E. Dzierzak, Mesenchymal progenitor cells localize within hematopoietic sites throughout ontogeny. Development, 2005. 132(5): p. 1127-36. [CrossRef]
- Fallati, A., et al., Mesenchymal Stromal Cells (MSCs): An Ally of B-Cell Acute Lymphoblastic Leukemia (B-ALL) Cells in Disease Maintenance and Progression within the Bone Marrow Hematopoietic Niche. Cancers (Basel), 2022. 14(14). [CrossRef]
- Kim, J.A., et al., Identification of a stroma-mediated Wnt/beta-catenin signal promoting self-renewal of hematopoietic stem cells in the stem cell niche. Stem Cells, 2009. 27(6): p. 1318-29. [CrossRef]
- Stier, S., et al., Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med, 2005. 201(11): p. 1781-91. [CrossRef]
- Nakamura, Y., et al., Isolation and characterization of endosteal niche cell populations that regulate hematopoietic stem cells. Blood, 2010. 116(9): p. 1422-32. [CrossRef]
- Crippa, S. and M.E. Bernardo, Mesenchymal Stromal Cells: Role in the BM Niche and in the Support of Hematopoietic Stem Cell Transplantation. Hemasphere, 2018. 2(6): p. e151. [CrossRef]
- Tormin, A., et al., CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood, 2011. 117(19): p. 5067-77. [CrossRef]
- Sacchetti, B., et al., Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell, 2007. 131(2): p. 324-36. [CrossRef]
- Sugiyama, T., et al., Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity, 2006. 25(6): p. 977-88. [CrossRef]
- Jung, Y., et al., Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone, 2006. 38(4): p. 497-508. [CrossRef]
- Krevvata, M., et al., Inhibition of leukemia cell engraftment and disease progression in mice by osteoblasts. Blood, 2014. 124(18): p. 2834-46. [CrossRef]
- Bowers, M., et al., Osteoblast ablation reduces normal long-term hematopoietic stem cell self-renewal but accelerates leukemia development. Blood, 2015. 125(17): p. 2678-88. [CrossRef]
- Kode, A., et al., Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature, 2014. 506(7487): p. 240-4. [CrossRef]
- Zhao, Y., et al., Down-regulation of Dicer1 promotes cellular senescence and decreases the differentiation and stem cell-supporting capacities of mesenchymal stromal cells in patients with myelodysplastic syndrome. Haematologica, 2015. 100(2): p. 194-204. [CrossRef]
- Novoseletskaya, E., et al., Mesenchymal Stromal Cell-Produced Components of Extracellular Matrix Potentiate Multipotent Stem Cell Response to Differentiation Stimuli. Front Cell Dev Biol, 2020. 8: p. 555378. [CrossRef]
- Aithal, A.P., L.K. Bairy, and R.N. Seetharam, Safety and therapeutic potential of human bone marrow-derived mesenchymal stromal cells in regenerative medicine. Stem Cell Investig, 2021. 8: p. 10. [CrossRef]
- Bernardo, M.E. and W.E. Fibbe, Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell, 2013. 13(4): p. 392-402. [CrossRef]
- Nauta, A.J., et al., Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol, 2006. 177(4): p. 2080-7. [CrossRef]
- Tyndall, A. and A. Gratwohl, Adult stem cell transplantation in autoimmune disease. Curr Opin Hematol, 2009. 16(4): p. 285-91. [CrossRef]
- Plakhova, N., et al., Mesenchymal stromal cell senescence in haematological malignancies. Cancer Metastasis Rev, 2023. 42(1): p. 277-296. [CrossRef]
- Ghobrial, I.M., et al., The bone-marrow niche in MDS and MGUS: implications for AML and MM. Nat Rev Clin Oncol, 2018. 15(4): p. 219-233. [CrossRef]
- Weng, Z., et al., Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies. Stem Cells Transl Med, 2022. 11(4): p. 356-371. [CrossRef]
- Hu, D., et al., Cellular senescence and hematological malignancies: From pathogenesis to therapeutics. Pharmacol Ther, 2021. 223: p. 107817. [CrossRef]
- Chen, X., et al., Senescent Mesenchymal Stem Cells in Myelodysplastic Syndrome: Functional Alterations, Molecular Mechanisms, and Therapeutic Strategies. Front Cell Dev Biol, 2020. 8: p. 617466. [CrossRef]
- Vanegas, N.P., et al., Leukemia-Induced Cellular Senescence and Stemness Alterations in Mesenchymal Stem Cells Are Reversible upon Withdrawal of B-Cell Acute Lymphoblastic Leukemia Cells. Int J Mol Sci, 2021. 22(15). [CrossRef]
- O’Hagan-Wong, K., et al., Increased IL-6 secretion by aged human mesenchymal stromal cells disrupts hematopoietic stem and progenitor cells’ homeostasis. Oncotarget, 2016. 7(12): p. 13285-96. [CrossRef]
- Adams, P.D., H. Jasper, and K.L. Rudolph, Aging-Induced Stem Cell Mutations as Drivers for Disease and Cancer. Cell Stem Cell, 2015. 16(6): p. 601-12. [CrossRef]
- Blau, O., et al., Chromosomal aberrations in bone marrow mesenchymal stroma cells from patients with myelodysplastic syndrome and acute myeloblastic leukemia. Exp Hematol, 2007. 35(2): p. 221-9. [CrossRef]
- Huang, J.C., et al., Mesenchymal stromal cells derived from acute myeloid leukemia bone marrow exhibit aberrant cytogenetics and cytokine elaboration. Blood Cancer J, 2015. 5(4): p. e302. [CrossRef]
- Blau, O., et al., Mesenchymal stromal cells of myelodysplastic syndrome and acute myeloid leukemia patients have distinct genetic abnormalities compared with leukemic blasts. Blood, 2011. 118(20): p. 5583-92. [CrossRef]
- von der Heide, E.K., et al., Molecular alterations in bone marrow mesenchymal stromal cells derived from acute myeloid leukemia patients. Leukemia, 2017. 31(5): p. 1069-1078. [CrossRef]
- Medyouf, H., et al., Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell, 2014. 14(6): p. 824-37. [CrossRef]
- Geyh, S., et al., Insufficient stromal support in MDS results from molecular and functional deficits of mesenchymal stromal cells. Leukemia, 2013. 27(9): p. 1841-51. [CrossRef]
- Sui, B.D., et al., Epigenetic Regulation of Mesenchymal Stem Cell Homeostasis. Trends Cell Biol, 2020. 30(2): p. 97-116. [CrossRef]
- Bhagat, T.D., et al., Epigenetically Aberrant Stroma in MDS Propagates Disease via Wnt/beta-Catenin Activation. Cancer Res, 2017. 77(18): p. 4846-4857. [CrossRef]
- Garcia-Gomez, A., et al., Targeting aberrant DNA methylation in mesenchymal stromal cells as a treatment for myeloma bone disease. Nat Commun, 2021. 12(1): p. 421. [CrossRef]
- Poon, Z., et al., Correction: Bone marrow MSCs in MDS: contribution towards dysfunctional hematopoiesis and potential targets for disease response to hypomethylating therapy. Leukemia, 2019. 33(6): p. 1542. [CrossRef]
- Huang, J., et al., Use of methylation profiling to identify significant differentially methylated genes in bone marrow mesenchymal stromal cells from acute myeloid leukemia. Int J Mol Med, 2018. 41(2): p. 679-686. [CrossRef]
- Frassanito, M.A., et al., Halting pro-survival autophagy by TGFbeta inhibition in bone marrow fibroblasts overcomes bortezomib resistance in multiple myeloma patients. Leukemia, 2016. 30(3): p. 640-8. [CrossRef]
- Zhai, Y., et al., Growth differentiation factor 15 contributes to cancer-associated fibroblasts-mediated chemo-protection of AML cells. J Exp Clin Cancer Res, 2016. 35(1): p. 147. [CrossRef]
- Burt, R., et al., Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood, 2019. 134(17): p. 1415-1429. [CrossRef]
- Zi, F.M., et al., Fibroblast activation protein protects bortezomib-induced apoptosis in multiple myeloma cells through beta-catenin signaling pathway. Cancer Biol Ther, 2014. 15(10): p. 1413-22. [CrossRef]
- Duan, C.W., et al., Leukemia propagating cells rebuild an evolving niche in response to therapy. Cancer Cell, 2014. 25(6): p. 778-93. [CrossRef]
- Pan, C., et al., Mesenchymal Stem Cells With Cancer-Associated Fibroblast-Like Phenotype Stimulate SDF-1/CXCR4 Axis to Enhance the Growth and Invasion of B-Cell Acute Lymphoblastic Leukemia Cells Through Cell-to-Cell Communication. Front Cell Dev Biol, 2021. 9: p. 708513. [CrossRef]
- Miyazaki, Y., et al., Adipose-derived mesenchymal stem cells differentiate into pancreatic cancer-associated fibroblasts in vitro. FEBS Open Bio, 2020. 10(11): p. 2268-2281. [CrossRef]
- Rubinstein-Achiasaf, L., et al., Persistent Inflammatory Stimulation Drives the Conversion of MSCs to Inflammatory CAFs That Promote Pro-Metastatic Characteristics in Breast Cancer Cells. Cancers (Basel), 2021. 13(6). [CrossRef]
- Paunescu, V., et al., Tumour-associated fibroblasts and mesenchymal stem cells: more similarities than differences. J Cell Mol Med, 2011. 15(3): p. 635-46. [CrossRef]
- Longhitano, L., et al., The Role of Inflammation and Inflammasome in Myeloproliferative Disease. J Clin Med, 2020. 9(8). [CrossRef]
- Zhu, Y., et al., Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia, 2009. 23(5): p. 925-33. [CrossRef]
- Sun, Z., S. Wang, and R.C. Zhao, The roles of mesenchymal stem cells in tumor inflammatory microenvironment. J Hematol Oncol, 2014. 7: p. 14. [CrossRef]
- Zhang, L., et al., Bone marrow-derived mesenchymal stem/stromal cells in patients with acute myeloid leukemia reveal transcriptome alterations and deficiency in cellular vitality. Stem Cell Res Ther, 2021. 12(1): p. 365. [CrossRef]
- Garcia-Gomez, A., et al., Transcriptomic profile induced in bone marrow mesenchymal stromal cells after interaction with multiple myeloma cells: implications in myeloma progression and myeloma bone disease. Oncotarget, 2014. 5(18): p. 8284-305. [CrossRef]
- Giallongo, C., et al., Mesenchymal Stem Cells (MSC) Regulate Activation of Granulocyte-Like Myeloid Derived Suppressor Cells (G-MDSC) in Chronic Myeloid Leukemia Patients. PLoS One, 2016. 11(7): p. e0158392. [CrossRef]
- Poggi, A. and M. Giuliani, Mesenchymal Stromal Cells Can Regulate the Immune Response in the Tumor Microenvironment. Vaccines (Basel), 2016. 4(4). [CrossRef]
- Azevedo, R.I., et al., Mesenchymal stromal cells induce regulatory T cells via epigenetic conversion of human conventional CD4 T cells in vitro. Stem Cells, 2020. 38(8): p. 1007-1019. [CrossRef]
- Zheng, L., et al., The immunological role of mesenchymal stromal cells in patients with myelodysplastic syndrome. Front Immunol, 2022. 13: p. 1078421. [CrossRef]
- Holthof, L.C., et al., Bone Marrow Mesenchymal Stromal Cells Can Render Multiple Myeloma Cells Resistant to Cytotoxic Machinery of CAR T Cells through Inhibition of Apoptosis. Clin Cancer Res, 2021. 27(13): p. 3793-3803. [CrossRef]
- Giallongo, C., et al., TLR4 signaling drives mesenchymal stromal cells commitment to promote tumor microenvironment transformation in multiple myeloma. Cell Death Dis, 2019. 10(10): p. 704. [CrossRef]
- Li, W., et al., Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ, 2012. 19(9): p. 1505-13. [CrossRef]
- Cao, Y.J., et al., MSC Senescence-Related Genes Are Associated with Myeloma Prognosis and Lipid Metabolism-Mediated Resistance to Proteasome Inhibitors. J Oncol, 2022. 2022: p. 4705654. [CrossRef]
- Waterman, R.S., et al., A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One, 2010. 5(4): p. e10088. [CrossRef]
- Jackson, M.V., et al., Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells, 2016. 34(8): p. 2210-23. [CrossRef]
- Wang, L., et al., Regulation of Inflammatory Cytokine Storms by Mesenchymal Stem Cells. Front Immunol, 2021. 12: p. 726909. [CrossRef]
- Loussouarn, C., et al., Mesenchymal Stromal Cell-Derived Extracellular Vesicles Regulate the Mitochondrial Metabolism via Transfer of miRNAs. Front Immunol, 2021. 12: p. 623973. [CrossRef]
- Thomas, M.A., et al., Human mesenchymal stromal cells release functional mitochondria in extracellular vesicles. Front Bioeng Biotechnol, 2022. 10: p. 870193. [CrossRef]
- Phetfong, J., et al., Bone marrow-mesenchymal stem cell-derived extracellular vesicles affect proliferation and apoptosis of leukemia cells in vitro. FEBS Open Bio, 2022. 12(2): p. 470-479. [CrossRef]
- Giallongo, C., et al., CXCL12/CXCR4 axis supports mitochondrial trafficking in tumor myeloma microenvironment. Oncogenesis, 2022. 11(1): p. 6. [CrossRef]
- Vilaplana-Lopera, N., et al., Crosstalk between AML and stromal cells triggers acetate secretion through the metabolic rewiring of stromal cells. Elife, 2022. 11. [CrossRef]
- Matamala Montoya, M., et al., Metabolic changes underlying drug resistance in the multiple myeloma tumor microenvironment. Front Oncol, 2023. 13: p. 1155621. [CrossRef]
- Chiu, M., et al., Myeloma Cells Deplete Bone Marrow Glutamine and Inhibit Osteoblast Differentiation Limiting Asparagine Availability. Cancers (Basel), 2020. 12(11). [CrossRef]
- Giallongo, C., et al., Inhibition of TLR4 Signaling Affects Mitochondrial Fitness and Overcomes Bortezomib Resistance in Myeloma Plasma Cells. Cancers (Basel), 2020. 12(8). [CrossRef]
- Nemkov, T., A. D’Alessandro, and J.A. Reisz, Metabolic underpinnings of leukemia pathology and treatment. Cancer Rep (Hoboken), 2019. 2(2): p. e1139. [CrossRef]
- Kim, J. and R.J. DeBerardinis, Mechanisms and Implications of Metabolic Heterogeneity in Cancer. Cell Metab, 2019. 30(3): p. 434-446. [CrossRef]
- Kumar, B., et al., Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia, 2018. 32(3): p. 575-587. [CrossRef]
- Dabbah, M., et al., Multiple myeloma BM-MSCs increase the tumorigenicity of MM cells via transfer of VLA4-enriched microvesicles. Carcinogenesis, 2020. 41(1): p. 100-110. [CrossRef]
- Dabbah, M., et al., Microvesicles derived from normal and multiple myeloma bone marrow mesenchymal stem cells differentially modulate myeloma cells’ phenotype and translation initiation. Carcinogenesis, 2017. 38(7): p. 708-716. [CrossRef]
- Dotson, J.L. and Y. Lebowicz, Myelodysplastic Syndrome, in StatPearls. 2023: Treasure Island (FL) ineligible companies. Disclosure: Yehuda Lebowicz declares no relevant financial relationships with ineligible companies.
- Alaggio, R., et al., The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia, 2022. 36(7): p. 1720-1748. [CrossRef]
- Calvi, L.M., A.J. Li, and M.W. Becker, What is the role of the microenvironment in MDS? Best Pract Res Clin Haematol, 2019. 32(4): p. 101113. [CrossRef]
- Lopez-Villar, O., et al., Both expanded and uncultured mesenchymal stem cells from MDS patients are genomically abnormal, showing a specific genetic profile for the 5q- syndrome. Leukemia, 2009. 23(4): p. 664-72. [CrossRef]
- Aanei, C.M., et al., Intrinsic growth deficiencies of mesenchymal stromal cells in myelodysplastic syndromes. Stem Cells Dev, 2012. 21(10): p. 1604-15. [CrossRef]
- Farr, J.N., et al., Identification of Senescent Cells in the Bone Microenvironment. J Bone Miner Res, 2016. 31(11): p. 1920-1929. [CrossRef]
- Fei, C., et al., Senescence of bone marrow mesenchymal stromal cells is accompanied by activation of p53/p21 pathway in myelodysplastic syndromes. Eur J Haematol, 2014. 93(6): p. 476-86. [CrossRef]
- Jann, J.C., et al., Bone marrow derived stromal cells from myelodysplastic syndromes are altered but not clonally mutated in vivo. Nat Commun, 2021. 12(1): p. 6170. [CrossRef]
- Maurizi, G., et al., DNA demethylating therapy reverts mesenchymal stromal cells derived from high risk myelodysplastic patients to a normal phenotype. Br J Haematol, 2017. 177(5): p. 818-822. [CrossRef]
- Wenk, C., et al., Direct modulation of the bone marrow mesenchymal stromal cell compartment by azacitidine enhances healthy hematopoiesis. Blood Adv, 2018. 2(23): p. 3447-3461. [CrossRef]
- Raaijmakers, M.H., et al., Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature, 2010. 464(7290): p. 852-7. [CrossRef]
- Zambetti, N.A., et al., Mesenchymal Inflammation Drives Genotoxic Stress in Hematopoietic Stem Cells and Predicts Disease Evolution in Human Pre-leukemia. Cell Stem Cell, 2016. 19(5): p. 613-627. [CrossRef]
- Lambert, C., Y. Wu, and C. Aanei, Bone Marrow Immunity and Myelodysplasia. Front Oncol, 2016. 6: p. 172. [CrossRef]
- Kobune, M., et al., Stromal cells expressing hedgehog-interacting protein regulate the proliferation of myeloid neoplasms. Blood Cancer J, 2012. 2(9): p. e87. [CrossRef]
- Giallongo, C., et al., MacroH2A1.1 as a crossroad between epigenetics, inflammation and metabolism of mesenchymal stromal cells in myelodysplastic syndromes. Cell Death Dis, 2023. 14(10): p. 686. [CrossRef]
- Chen, S., et al., Massive parallel RNA sequencing of highly purified mesenchymal elements in low-risk MDS reveals tissue-context-dependent activation of inflammatory programs. Leukemia, 2016. 30(9): p. 1938-42. [CrossRef]
- Ping, Z., et al., Activation of NF-kappaB driven inflammatory programs in mesenchymal elements attenuates hematopoiesis in low-risk myelodysplastic syndromes. Leukemia, 2019. 33(2): p. 536-541. [CrossRef]
- Pleyer, L., P. Valent, and R. Greil, Mesenchymal Stem and Progenitor Cells in Normal and Dysplastic Hematopoiesis-Masters of Survival and Clonality? Int J Mol Sci, 2016. 17(7). [CrossRef]
- Calkoen, F.G., et al., Despite differential gene expression profiles pediatric MDS derived mesenchymal stromal cells display functionality in vitro. Stem Cell Res, 2015. 14(2): p. 198-210. [CrossRef]
- Kim, M., et al., Increased expression of interferon signaling genes in the bone marrow microenvironment of myelodysplastic syndromes. PLoS One, 2015. 10(3): p. e0120602. [CrossRef]
- Hayashi, Y., et al., MDS cells impair osteolineage differentiation of MSCs via extracellular vesicles to suppress normal hematopoiesis. Cell Rep, 2022. 39(6): p. 110805. [CrossRef]
- Muntion, S., et al., Microvesicles from Mesenchymal Stromal Cells Are Involved in HPC-Microenvironment Crosstalk in Myelodysplastic Patients. PLoS One, 2016. 11(2): p. e0146722. [CrossRef]
- Jager, P., et al., Acute myeloid leukemia-induced functional inhibition of healthy CD34+ hematopoietic stem and progenitor cells. Stem Cells, 2021. 39(9): p. 1270-1284. [CrossRef]
- Pan, C., et al., Bone marrow mesenchymal stem cells in microenvironment transform into cancer-associated fibroblasts to promote the progression of B-cell acute lymphoblastic leukemia. Biomed Pharmacother, 2020. 130: p. 110610. [CrossRef]
- Leotta, S., et al., Prevention and Treatment of Acute Myeloid Leukemia Relapse after Hematopoietic Stem Cell Transplantation: The State of the Art and Future Perspectives. J Clin Med, 2022. 11(1). [CrossRef]
- Colmone, A., et al., Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science, 2008. 322(5909): p. 1861-5. [CrossRef]
- Kim, J.A., et al., Microenvironmental remodeling as a parameter and prognostic factor of heterogeneous leukemogenesis in acute myelogenous leukemia. Cancer Res, 2015. 75(11): p. 2222-31. [CrossRef]
- Feng, X., et al., Cell circuits between leukemic cells and mesenchymal stem cells block lymphopoiesis by activating lymphotoxin beta receptor signaling. Elife, 2023. 12. [CrossRef]
- Mazur, G., et al., Increased monocyte chemoattractant protein 1 (MCP-1/CCL-2) serum level in acute myeloid leukemia. Neoplasma, 2007. 54(4): p. 285-9.
- Binato, R., et al., The molecular signature of AML mesenchymal stromal cells reveals candidate genes related to the leukemogenic process. Cancer Lett, 2015. 369(1): p. 134-43. [CrossRef]
- Lim, M., et al., Altered mesenchymal niche cells impede generation of normal hematopoietic progenitor cells in leukemic bone marrow. Leukemia, 2016. 30(1): p. 154-62. [CrossRef]
- Falconi, G., et al., Impairment of FOXM1 expression in mesenchymal cells from patients with myeloid neoplasms, de novo and therapy-related, may compromise their ability to support hematopoiesis. Sci Rep, 2022. 12(1): p. 21231. [CrossRef]
- Geyh, S., et al., Functional inhibition of mesenchymal stromal cells in acute myeloid leukemia. Leukemia, 2016. 30(3): p. 683-91. [CrossRef]
- Borella, G., et al., Targeting the plasticity of mesenchymal stromal cells to reroute the course of acute myeloid leukemia. Blood, 2021. 138(7): p. 557-570. [CrossRef]
- Glait-Santar, C., et al., Functional Niche Competition Between Normal Hematopoietic Stem and Progenitor Cells and Myeloid Leukemia Cells. Stem Cells, 2015. 33(12): p. 3635-42. [CrossRef]
- Huang, X., S. Cho, and G.J. Spangrude, Hematopoietic stem cells: generation and self-renewal. Cell Death Differ, 2007. 14(11): p. 1851-9. [CrossRef]
- Beerman, I., et al., The evolving view of the hematopoietic stem cell niche. Exp Hematol, 2017. 50: p. 22-26. [CrossRef]
- Le, Y., et al., Adipogenic Mesenchymal Stromal Cells from Bone Marrow and Their Hematopoietic Supportive Role: Towards Understanding the Permissive Marrow Microenvironment in Acute Myeloid Leukemia. Stem Cell Rev Rep, 2016. 12(2): p. 235-44. [CrossRef]
- Azadniv, M., et al., Bone marrow mesenchymal stromal cells from acute myelogenous leukemia patients demonstrate adipogenic differentiation propensity with implications for leukemia cell support. Leukemia, 2020. 34(2): p. 391-403. [CrossRef]
- Battula, V.L., et al., AML-induced osteogenic differentiation in mesenchymal stromal cells supports leukemia growth. JCI Insight, 2017. 2(13). [CrossRef]
- Hanoun, M., et al., Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell, 2014. 15(3): p. 365-375. [CrossRef]
- Zhang, L., et al., Acute Myeloid Leukemia Cells Educate Mesenchymal Stromal Cells toward an Adipogenic Differentiation Propensity with Leukemia Promotion Capabilities. Adv Sci (Weinh), 2022. 9(16): p. 2105811. [CrossRef]
- Ruiz-Aparicio, P.F. and J.P. Vernot, Bone Marrow Aging and the Leukaemia-Induced Senescence of Mesenchymal Stem/Stromal Cells: Exploring Similarities. J Pers Med, 2022. 12(5). [CrossRef]
- Abdul-Aziz, A.M., et al., Acute myeloid leukemia induces protumoral p16INK4a-driven senescence in the bone marrow microenvironment. Blood, 2019. 133(5): p. 446-456. [CrossRef]
- Collado, M., M.A. Blasco, and M. Serrano, Cellular senescence in cancer and aging. Cell, 2007. 130(2): p. 223-33. [CrossRef]
- Lee, H.R., et al., The Chromatin Remodeling Complex CHD1 Regulates the Primitive State of Mesenchymal Stromal Cells to Control Their Stem Cell Supporting Activity. Stem Cells Dev, 2021. 30(7): p. 363-373. [CrossRef]
- Samudio, I., et al., The warburg effect in leukemia-stroma cocultures is mediated by mitochondrial uncoupling associated with uncoupling protein 2 activation. Cancer Res, 2008. 68(13): p. 5198-205. [CrossRef]
- Wilde, L., et al., Metabolic coupling and the Reverse Warburg Effect in cancer: Implications for novel biomarker and anticancer agent development. Semin Oncol, 2017. 44(3): p. 198-203. [CrossRef]
- Baccelli, I., et al., Mubritinib Targets the Electron Transport Chain Complex I and Reveals the Landscape of OXPHOS Dependency in Acute Myeloid Leukemia. Cancer Cell, 2019. 36(1): p. 84-99 e8. [CrossRef]
- Farge, T., et al., Chemotherapy-Resistant Human Acute Myeloid Leukemia Cells Are Not Enriched for Leukemic Stem Cells but Require Oxidative Metabolism. Cancer Discov, 2017. 7(7): p. 716-735. [CrossRef]
- Forte, D., et al., Bone Marrow Mesenchymal Stem Cells Support Acute Myeloid Leukemia Bioenergetics and Enhance Antioxidant Defense and Escape from Chemotherapy. Cell Metab, 2020. 32(5): p. 829-843 e9. [CrossRef]
- Marlein, C.R., et al., NADPH oxidase-2 derived superoxide drives mitochondrial transfer from bone marrow stromal cells to leukemic blasts. Blood, 2017. 130(14): p. 1649-1660. [CrossRef]
- Moschoi, R., et al., Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood, 2016. 128(2): p. 253-64. [CrossRef]
- Mistry, J.J., et al., Daratumumab inhibits acute myeloid leukaemia metabolic capacity by blocking mitochondrial transfer from mesenchymal stromal cells. Haematologica, 2021. 106(2): p. 589-592. [CrossRef]
- Wang, J., et al., Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on T cell acute lymphoblastic leukemia cells. J Hematol Oncol, 2018. 11(1): p. 11. [CrossRef]
- Shafat, M.S., et al., Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood, 2017. 129(10): p. 1320-1332. [CrossRef]
- Lee, K., J. Kerner, and C.L. Hoppel, Mitochondrial carnitine palmitoyltransferase 1a (CPT1a) is part of an outer membrane fatty acid transfer complex. J Biol Chem, 2011. 286(29): p. 25655-62. [CrossRef]
- Tucci, J., et al., Adipocytes Provide Fatty Acids to Acute Lymphoblastic Leukemia Cells. Front Oncol, 2021. 11: p. 665763. [CrossRef]
- Johnson, S.M., et al., Metabolic reprogramming of bone marrow stromal cells by leukemic extracellular vesicles in acute lymphoblastic leukemia. Blood, 2016. 128(3): p. 453-6. [CrossRef]
- Barbui, T., et al., The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J, 2018. 8(2): p. 15. [CrossRef]
- Nasillo, V., et al., Inflammatory Microenvironment and Specific T Cells in Myeloproliferative Neoplasms: Immunopathogenesis and Novel Immunotherapies. Int J Mol Sci, 2021. 22(4). [CrossRef]
- Rambaldi, B., et al., Heterogeneity of the bone marrow niche in patients with myeloproliferative neoplasms: ActivinA secretion by mesenchymal stromal cells correlates with the degree of marrow fibrosis. Ann Hematol, 2021. 100(1): p. 105-116. [CrossRef]
- Xie, J., et al., Bone mesenchymal stromal cells exhibit functional inhibition but no chromosomal aberrations in chronic myelogenous leukemia. Oncol Lett, 2019. 17(1): p. 999-1007. [CrossRef]
- Avanzini, M.A., et al., Functional and genetic aberrations of in vitro-cultured marrow-derived mesenchymal stromal cells of patients with classical Philadelphia-negative myeloproliferative neoplasms. Leukemia, 2014. 28(8): p. 1742-5. [CrossRef]
- Zhao, Z.G., et al., The characteristics and immunoregulatory functions of regulatory dendritic cells induced by mesenchymal stem cells derived from bone marrow of patient with chronic myeloid leukaemia. Eur J Cancer, 2012. 48(12): p. 1884-95. [CrossRef]
- Jafarzadeh, N., et al., Alteration of cellular and immune-related properties of bone marrow mesenchymal stem cells and macrophages by K562 chronic myeloid leukemia cell derived exosomes. J Cell Physiol, 2019. 234(4): p. 3697-3710. [CrossRef]
- Chai, C., et al., BCR-ABL1-driven exosome-miR130b-3p-mediated gap-junction Cx43 MSC intercellular communications imply therapies of leukemic subclonal evolution. Theranostics, 2023. 13(12): p. 3943-3963. [CrossRef]
- Agarwal, P., et al., Mesenchymal Niche-Specific Expression of Cxcl12 Controls Quiescence of Treatment-Resistant Leukemia Stem Cells. Cell Stem Cell, 2019. 24(5): p. 769-784 e6. [CrossRef]
- Aggoune, D., et al., Bone marrow mesenchymal stromal cell (MSC) gene profiling in chronic myeloid leukemia (CML) patients at diagnosis and in deep molecular response induced by tyrosine kinase inhibitors (TKIs). Leuk Res, 2017. 60: p. 94-102. [CrossRef]
- Schepers, K., et al., Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell, 2013. 13(3): p. 285-99. [CrossRef]
- La Spina, E., et al., Mesenchymal stromal cells in tumor microenvironment remodeling of BCR-ABL negative myeloproliferative diseases. Front Oncol, 2023. 13: p. 1141610. [CrossRef]
- Abbonante, V., et al., Altered fibronectin expression and deposition by myeloproliferative neoplasm-derived mesenchymal stromal cells. Br J Haematol, 2016. 172(1): p. 140-4. [CrossRef]
- Ponce, C.C., et al., The relationship of the active and latent forms of TGF-beta1 with marrow fibrosis in essential thrombocythemia and primary myelofibrosis. Med Oncol, 2012. 29(4): p. 2337-44. [CrossRef]
- Badalucco, S., et al., Involvement of TGFbeta1 in autocrine regulation of proplatelet formation in healthy subjects and patients with primary myelofibrosis. Haematologica, 2013. 98(4): p. 514-7. [CrossRef]
- Schneider, R.K., et al., Activated fibronectin-secretory phenotype of mesenchymal stromal cells in pre-fibrotic myeloproliferative neoplasms. J Hematol Oncol, 2014. 7: p. 92. [CrossRef]
- Ciurea, S.O., et al., Pivotal contributions of megakaryocytes to the biology of idiopathic myelofibrosis. Blood, 2007. 110(3): p. 986-93. [CrossRef]
- Avanzini, M.A., et al., The spleen of patients with myelofibrosis harbors defective mesenchymal stromal cells. Am J Hematol, 2018. 93(5): p. 615-622. [CrossRef]
- Lu, M., et al., Lipocalin produced by myelofibrosis cells affects the fate of both hematopoietic and marrow microenvironmental cells. Blood, 2015. 126(8): p. 972-82. [CrossRef]
- Bedekovics, J., et al., Platelet derived growth factor receptor-beta (PDGFRbeta) expression is limited to activated stromal cells in the bone marrow and shows a strong correlation with the grade of myelofibrosis. Virchows Arch, 2013. 463(1): p. 57-65. [CrossRef]
- Longhitano, L., et al., IGFBP-6/sonic hedgehog/TLR4 signalling axis drives bone marrow fibrotic transformation in primary myelofibrosis. Aging (Albany NY), 2021. 13(23): p. 25055-25071. [CrossRef]
- Schneider, R.K., et al., Gli1(+) Mesenchymal Stromal Cells Are a Key Driver of Bone Marrow Fibrosis and an Important Cellular Therapeutic Target. Cell Stem Cell, 2017. 20(6): p. 785-800 e8. [CrossRef]
- Lataillade, J.J., et al., Does primary myelofibrosis involve a defective stem cell niche? From concept to evidence. Blood, 2008. 112(8): p. 3026-35. [CrossRef]
- Martinaud, C., et al., Osteogenic Potential of Mesenchymal Stromal Cells Contributes to Primary Myelofibrosis. Cancer Res, 2015. 75(22): p. 4753-65. [CrossRef]
- Leimkuhler, N.B., et al., Heterogeneous bone-marrow stromal progenitors drive myelofibrosis via a druggable alarmin axis. Cell Stem Cell, 2021. 28(4): p. 637-652 e8. [CrossRef]
- Nasnas, P., et al., How I Manage Chronic Lymphocytic Leukemia. Hematol Rep, 2023. 15(3): p. 454-464. [CrossRef]
- Gattei, V., et al., Relevance of CD49d protein expression as overall survival and progressive disease prognosticator in chronic lymphocytic leukemia. Blood, 2008. 111(2): p. 865-73. [CrossRef]
- Hartmann, T.N., et al., Circulating B-cell chronic lymphocytic leukemia cells display impaired migration to lymph nodes and bone marrow. Cancer Res, 2009. 69(7): p. 3121-30. [CrossRef]
- Ding, W., et al., Bi-directional activation between mesenchymal stem cells and CLL B-cells: implication for CLL disease progression. Br J Haematol, 2009. 147(4): p. 471-83. [CrossRef]
- Lagneaux, L., et al., Adhesion to bone marrow stroma inhibits apoptosis of chronic lymphocytic leukemia cells. Leuk Lymphoma, 1999. 35(5-6): p. 445-53. [CrossRef]
- Lee, S., et al., Adhesion of B-Cell Chronic Lymphocytic Leukemia Cells to Marrow Stromal Cells is Mediated by alpha4beta1 but not beta2alphaL Integrin: MSC also Prevent Apoptosis of B-CLL Cells. Hematology, 2001. 5(6): p. 463-73. [CrossRef]
- Crompot, E., et al., Extracellular vesicles of bone marrow stromal cells rescue chronic lymphocytic leukemia B cells from apoptosis, enhance their migration and induce gene expression modifications. Haematologica, 2017. 102(9): p. 1594-1604. [CrossRef]
- Paggetti, J., et al., Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood, 2015. 126(9): p. 1106-17. [CrossRef]
- Vangapandu, H.V., et al., B-cell Receptor Signaling Regulates Metabolism in Chronic Lymphocytic Leukemia. Mol Cancer Res, 2017. 15(12): p. 1692-1703. [CrossRef]
- Jitschin, R., et al., Stromal cell-mediated glycolytic switch in CLL cells involves Notch-c-Myc signaling. Blood, 2015. 125(22): p. 3432-6. [CrossRef]
- von Heydebrand, F., et al., Protein kinase C-beta-dependent changes in the glucose metabolism of bone marrow stromal cells of chronic lymphocytic leukemia. Stem Cells, 2021. 39(6): p. 819-830. [CrossRef]
- Vangapandu, H.V., et al., The Stromal Microenvironment Modulates Mitochondrial Oxidative Phosphorylation in Chronic Lymphocytic Leukemia Cells. Neoplasia, 2017. 19(10): p. 762-771. [CrossRef]
- Rassenti, L.Z., et al., ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med, 2004. 351(9): p. 893-901. [CrossRef]
- Ding, W., et al., Platelet-derived growth factor (PDGF)-PDGF receptor interaction activates bone marrow-derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: implications for an angiogenic switch. Blood, 2010. 116(16): p. 2984-93. [CrossRef]
- Kay, N.E., D.F. Jelinek, and L. Peterson, Angiogenesis in B-chronic lymphocytic leukemia. Leuk Res, 2001. 25(8): p. 709-10. [CrossRef]
- Kini, A.R., N.E. Kay, and L.C. Peterson, Increased bone marrow angiogenesis in B cell chronic lymphocytic leukemia. Leukemia, 2000. 14(8): p. 1414-8. [CrossRef]
- Zhang, W., et al., Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat Cell Biol, 2012. 14(3): p. 276-86. [CrossRef]
- Korde, N., S.Y. Kristinsson, and O. Landgren, Monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM): novel biological insights and development of early treatment strategies. Blood, 2011. 117(21): p. 5573-81. [CrossRef]
- Kawano, Y., et al., Targeting the bone marrow microenvironment in multiple myeloma. Immunol Rev, 2015. 263(1): p. 160-72. [CrossRef]
- Brigle, K. and B. Rogers, Pathobiology and Diagnosis of Multiple Myeloma. Semin Oncol Nurs, 2017. 33(3): p. 225-236. [CrossRef]
- Rasch, S., et al., Multiple Myeloma Associated Bone Disease. Cancers (Basel), 2020. 12(8). [CrossRef]
- Gunn, W.G., et al., A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells, 2006. 24(4): p. 986-91. [CrossRef]
- Fowler, J.A., et al., Bone marrow stromal cells create a permissive microenvironment for myeloma development: a new stromal role for Wnt inhibitor Dkk1. Cancer Res, 2012. 72(9): p. 2183-9. [CrossRef]
- Garcia-Gomez, A., et al., Multiple myeloma mesenchymal stromal cells: Contribution to myeloma bone disease and therapeutics. World J Stem Cells, 2014. 6(3): p. 322-43. [CrossRef]
- Todoerti, K., et al., Distinct transcriptional profiles characterize bone microenvironment mesenchymal cells rather than osteoblasts in relationship with multiple myeloma bone disease. Exp Hematol, 2010. 38(2): p. 141-53. [CrossRef]
- Schinke, C., et al., The Pattern of Mesenchymal Stem Cell Expression Is an Independent Marker of Outcome in Multiple Myeloma. Clin Cancer Res, 2018. 24(12): p. 2913-2919. [CrossRef]
- Fernando, R.C., et al., Transcriptome Analysis of Mesenchymal Stem Cells from Multiple Myeloma Patients Reveals Downregulation of Genes Involved in Cell Cycle Progression, Immune Response, and Bone Metabolism. Sci Rep, 2019. 9(1): p. 1056. [CrossRef]
- Lemaitre, L., et al., Imprinting of Mesenchymal Stromal Cell Transcriptome Persists even after Treatment in Patients with Multiple Myeloma. Int J Mol Sci, 2020. 21(11). [CrossRef]
- de Jong, M.M.E., et al., The multiple myeloma microenvironment is defined by an inflammatory stromal cell landscape. Nat Immunol, 2021. 22(6): p. 769-780. [CrossRef]
- Andre, T., et al., Evidences of early senescence in multiple myeloma bone marrow mesenchymal stromal cells. PLoS One, 2013. 8(3): p. e59756. [CrossRef]
- Guo, J., et al., Dicer1 downregulation by multiple myeloma cells promotes the senescence and tumor-supporting capacity and decreases the differentiation potential of mesenchymal stem cells. Cell Death Dis, 2018. 9(5): p. 512. [CrossRef]
- Yaccoby, S., et al., Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica, 2006. 91(2): p. 192-9.
- Romano, A., et al., Immunological dysregulation in multiple myeloma microenvironment. Biomed Res Int, 2014. 2014: p. 198539. [CrossRef]
- Giallongo, C., et al., Granulocyte-like myeloid derived suppressor cells (G-MDSC) are increased in multiple myeloma and are driven by dysfunctional mesenchymal stem cells (MSC). Oncotarget, 2016. 7(52): p. 85764-85775. [CrossRef]
- Chen, D., et al., Bone marrow-derived mesenchymal stem cells promote cell proliferation of multiple myeloma through inhibiting T cell immune responses via PD-1/PD-L1 pathway. Cell Cycle, 2018. 17(7): p. 858-867. [CrossRef]
- Liu, Z., et al., Bone marrow-derived mesenchymal stem cells inhibit CD8(+) T cell immune responses via PD-1/PD-L1 pathway in multiple myeloma. Clin Exp Immunol, 2021. 205(1): p. 53-62. [CrossRef]
- Liu, Z.Y., et al., CD155/TIGIT signalling plays a vital role in the regulation of bone marrow mesenchymal stem cell-induced natural killer-cell exhaustion in multiple myeloma. Clin Transl Med, 2022. 12(7): p. e861. [CrossRef]
- Giuliani, N., et al., Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood, 2005. 106(7): p. 2472-83. [CrossRef]
- van Nieuwenhuijzen, N., et al., From MGUS to Multiple Myeloma, a Paradigm for Clonal Evolution of Premalignant Cells. Cancer Res, 2018. 78(10): p. 2449-2456. [CrossRef]
- Liu, Y., et al., Commitment to Aerobic Glycolysis Sustains Immunosuppression of Human Mesenchymal Stem Cells. Stem Cells Transl Med, 2019. 8(1): p. 93-106. [CrossRef]
- Marlein, C.R., et al., CD38-Driven Mitochondrial Trafficking Promotes Bioenergetic Plasticity in Multiple Myeloma. Cancer Res, 2019. 79(9): p. 2285-2297. [CrossRef]
- Matula, Z., et al., Stromal Cells Serve Drug Resistance for Multiple Myeloma via Mitochondrial Transfer: A Study on Primary Myeloma and Stromal Cells. Cancers (Basel), 2021. 13(14). [CrossRef]
- Matula, Z., et al., The Effect of Belantamab Mafodotin on Primary Myeloma-Stroma Co-Cultures: Asymmetrical Mitochondrial Transfer between Myeloma Cells and Autologous Bone Marrow Stromal Cells. Int J Mol Sci, 2023. 24(6). [CrossRef]
- Barbato, A., et al., Lactate trafficking inhibition restores sensitivity to proteasome inhibitors and orchestrates immuno-microenvironment in multiple myeloma. Cell Prolif, 2023. 56(4): p. e13388. [CrossRef]
- Barbato, A., et al., Mitochondrial Bioenergetics at the Onset of Drug Resistance in Hematological Malignancies: An Overview. Front Oncol, 2020. 10: p. 604143. [CrossRef]
- Desterke, C., et al., Inflammation as a Keystone of Bone Marrow Stroma Alterations in Primary Myelofibrosis. Mediators Inflamm, 2015. 2015: p. 415024. [CrossRef]
- Azab, A.K., et al., CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood, 2009. 113(18): p. 4341-51. [CrossRef]
- Cancilla, D., M.P. Rettig, and J.F. DiPersio, Targeting CXCR4 in AML and ALL. Front Oncol, 2020. 10: p. 1672. [CrossRef]
- Giannoni, P., et al., An interaction between hepatocyte growth factor and its receptor (c-MET) prolongs the survival of chronic lymphocytic leukemic cells through STAT3 phosphorylation: a potential role of mesenchymal cells in the disease. Haematologica, 2011. 96(7): p. 1015-23. [CrossRef]
- Pan, J., et al., Inhibition of Bcl-2/xl With ABT-263 Selectively Kills Senescent Type II Pneumocytes and Reverses Persistent Pulmonary Fibrosis Induced by Ionizing Radiation in Mice. Int J Radiat Oncol Biol Phys, 2017. 99(2): p. 353-361. [CrossRef]
- Hellmich, C., et al., p16INK4A-dependent senescence in the bone marrow niche drives age-related metabolic changes of hematopoietic progenitors. Blood Adv, 2023. 7(2): p. 256-268. [CrossRef]
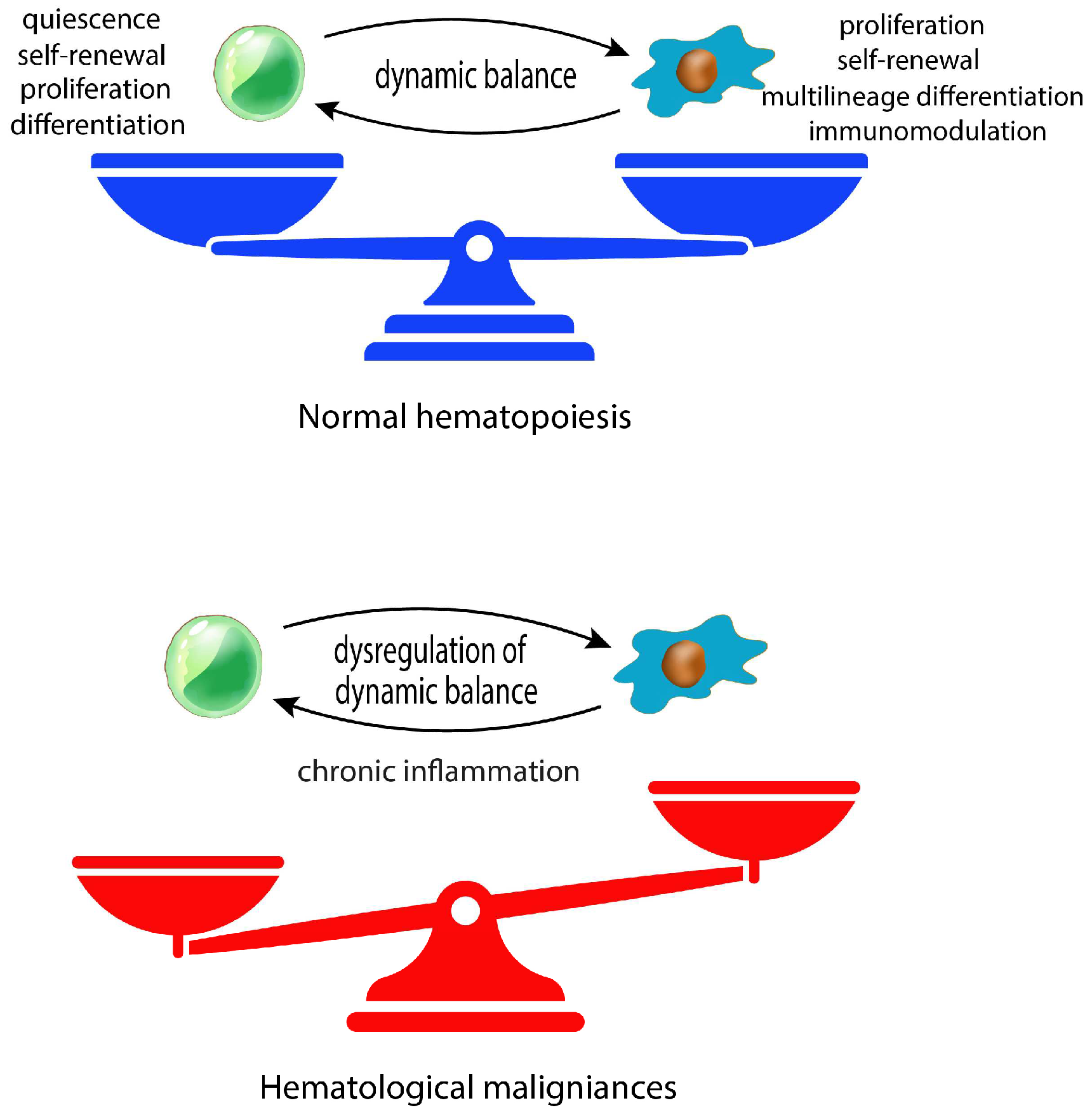
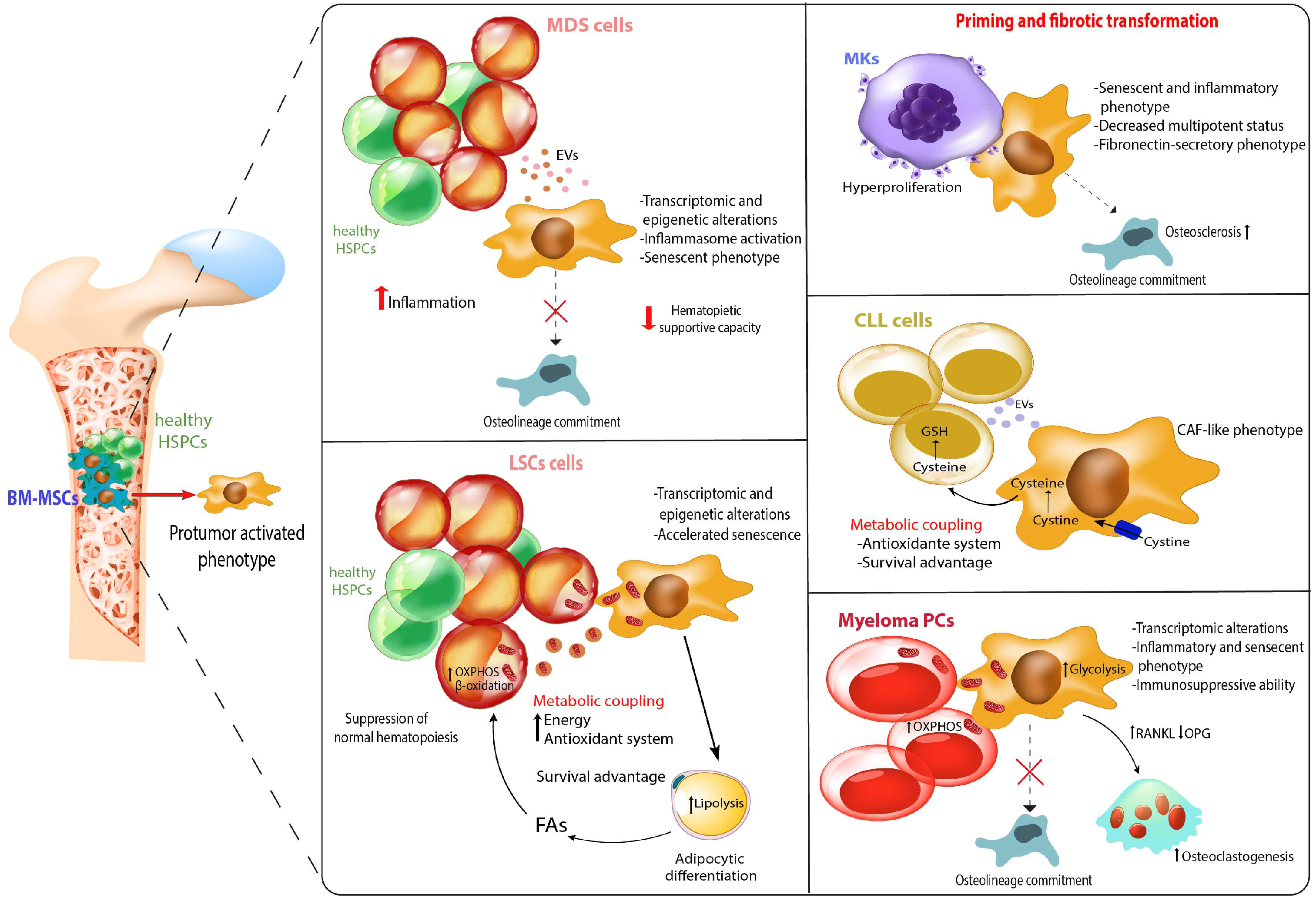
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




