Submitted:
31 October 2023
Posted:
01 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
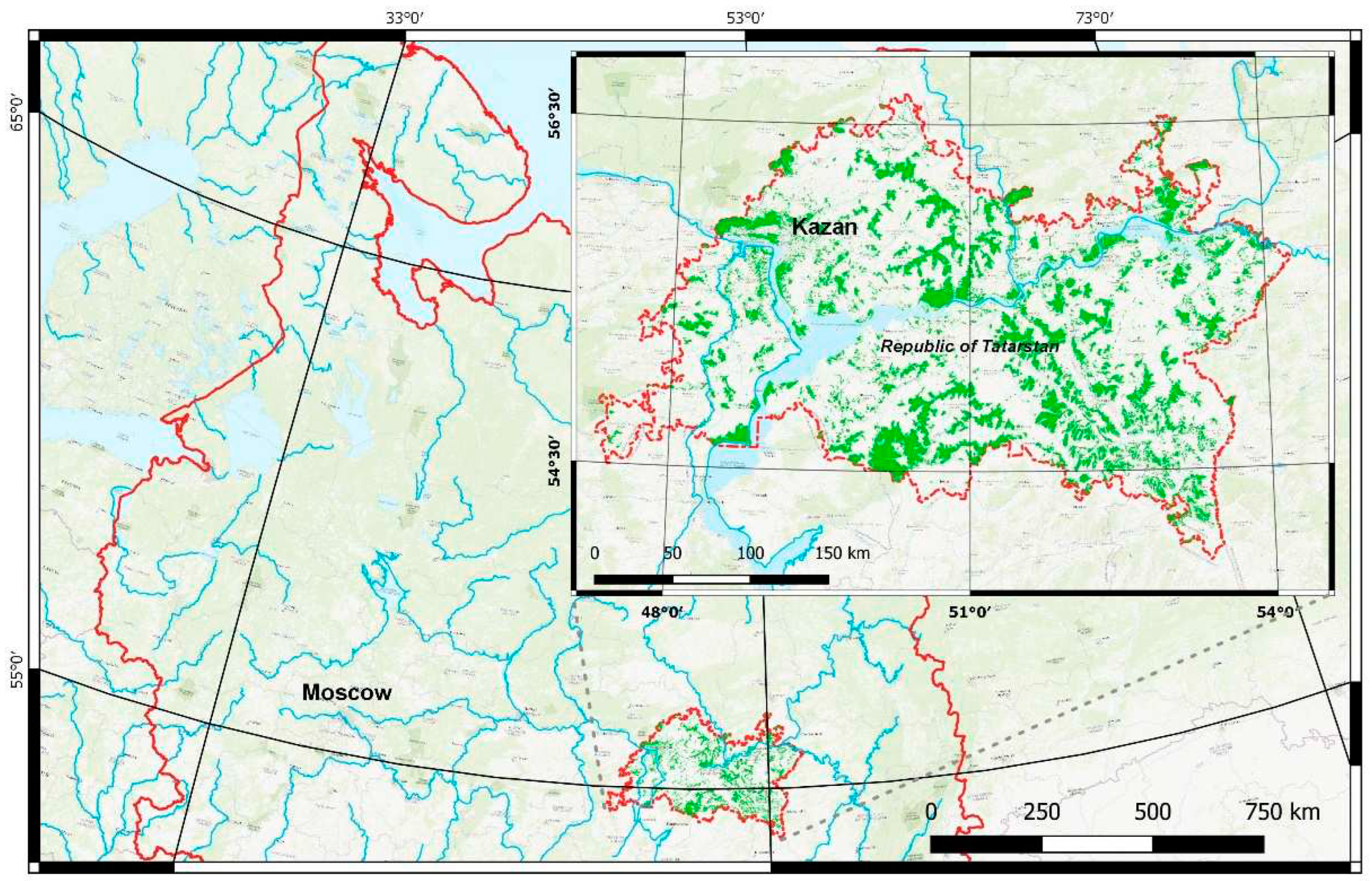
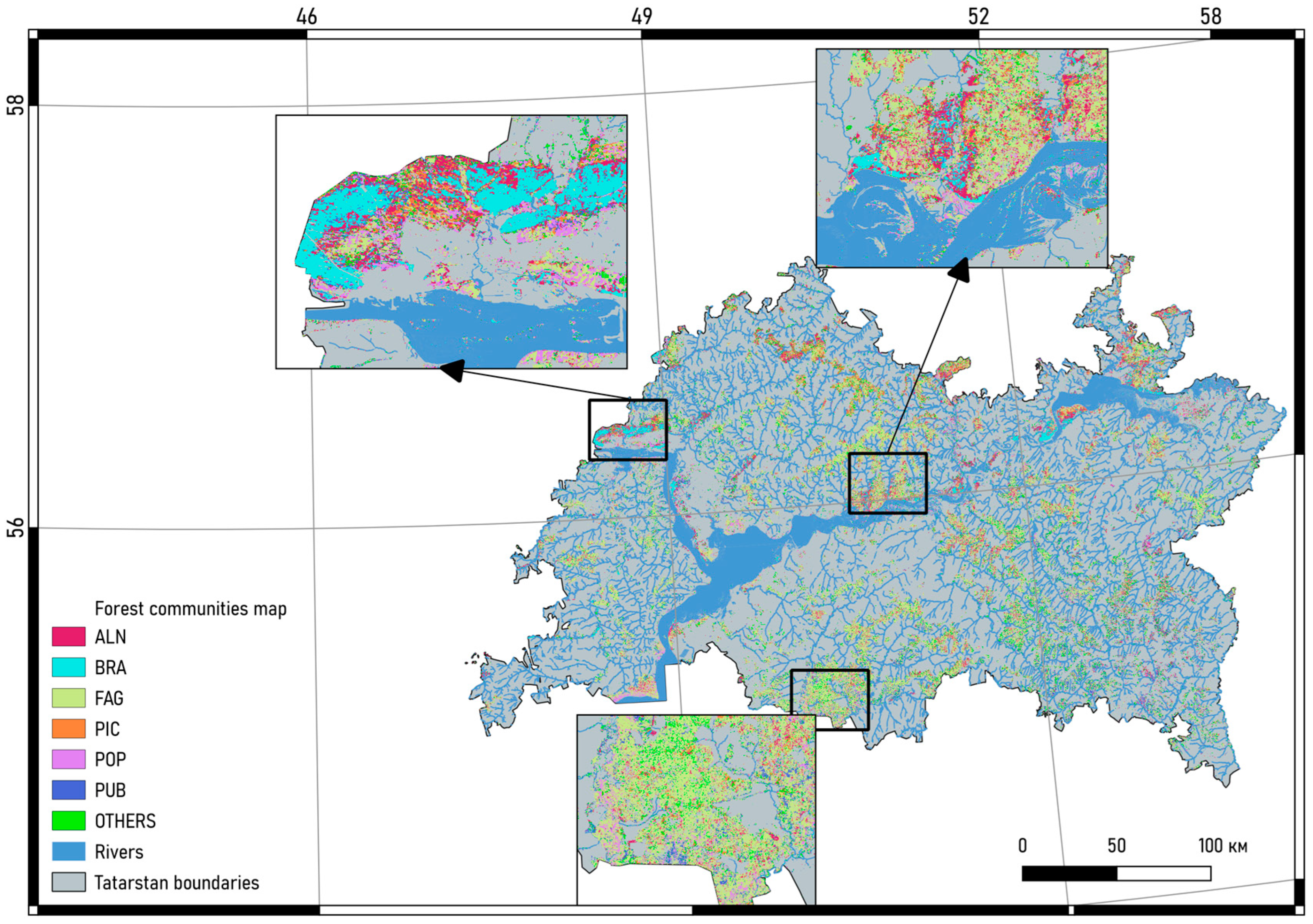
2.1. Study Area
2.2. Data Acquisition
2.3. Google Earth Engine Platform
2.4. Vegetation Indices Computation
2.5. Unsupervised Classification
2.5.1. Data Pre-Processing
2.5.2. Training Dataset Preparation
2.5.3. Clustering Process
2.5.4. Mosaic Creation and Export
2.6. Field Data Collection and Processing
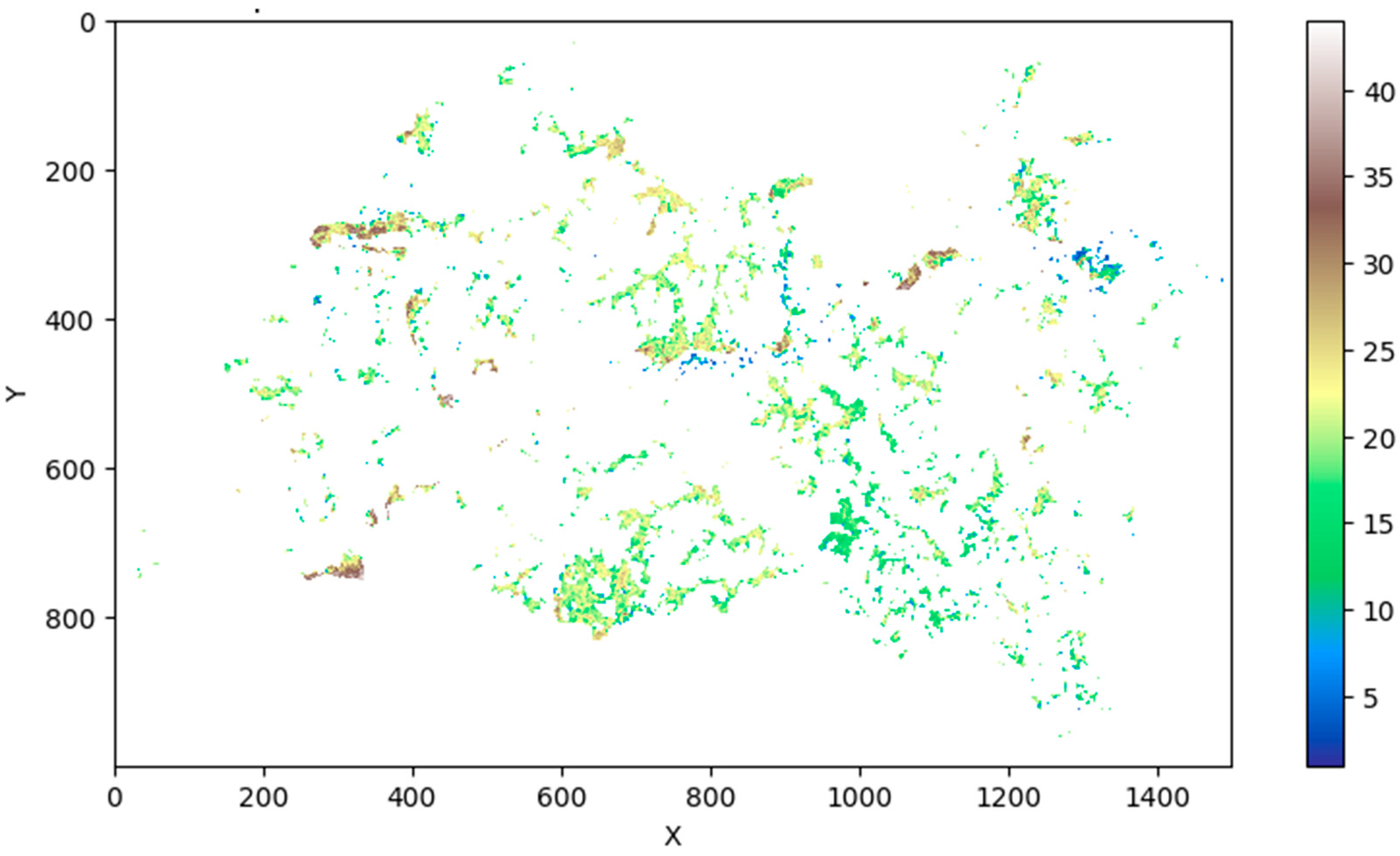
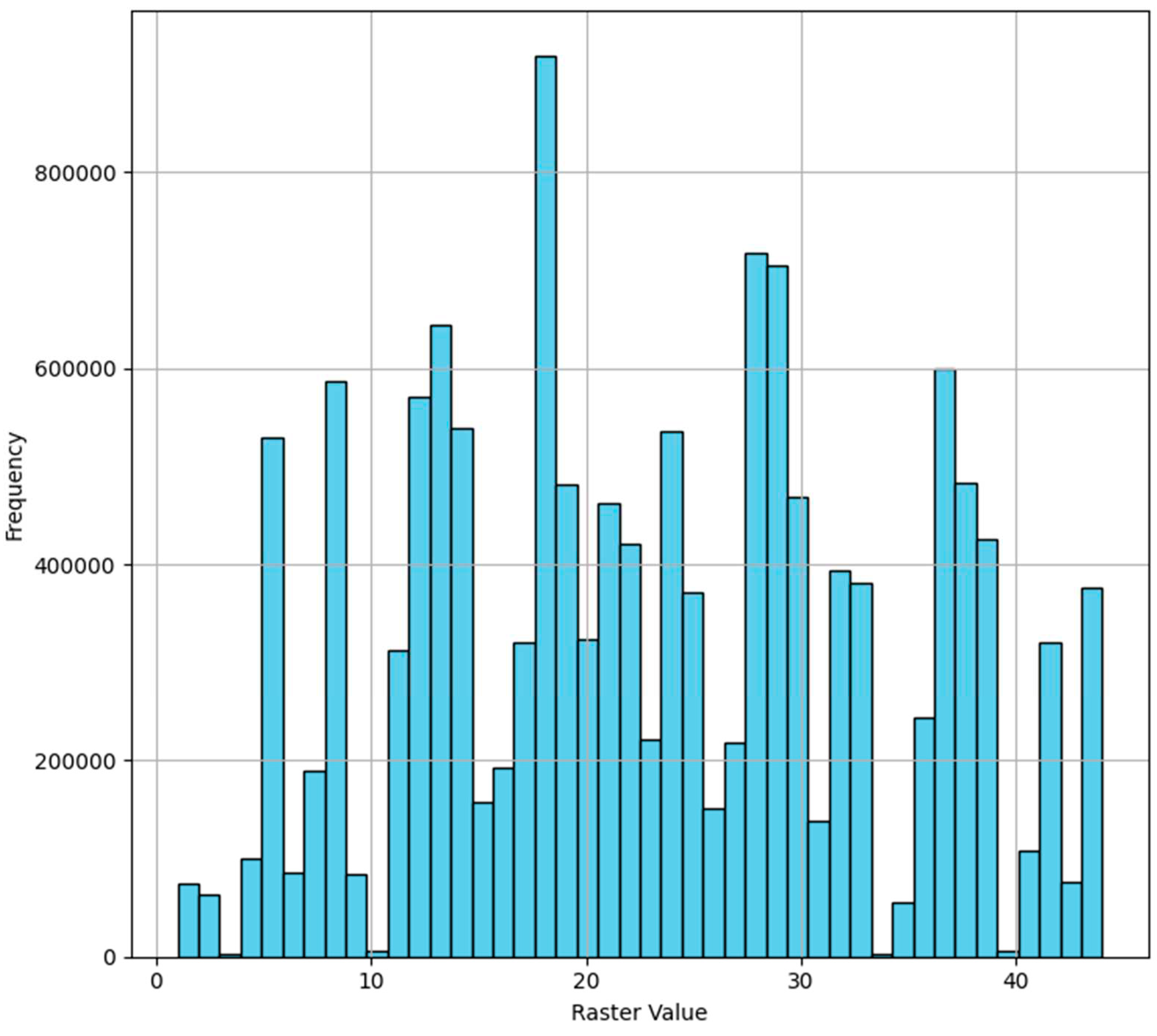
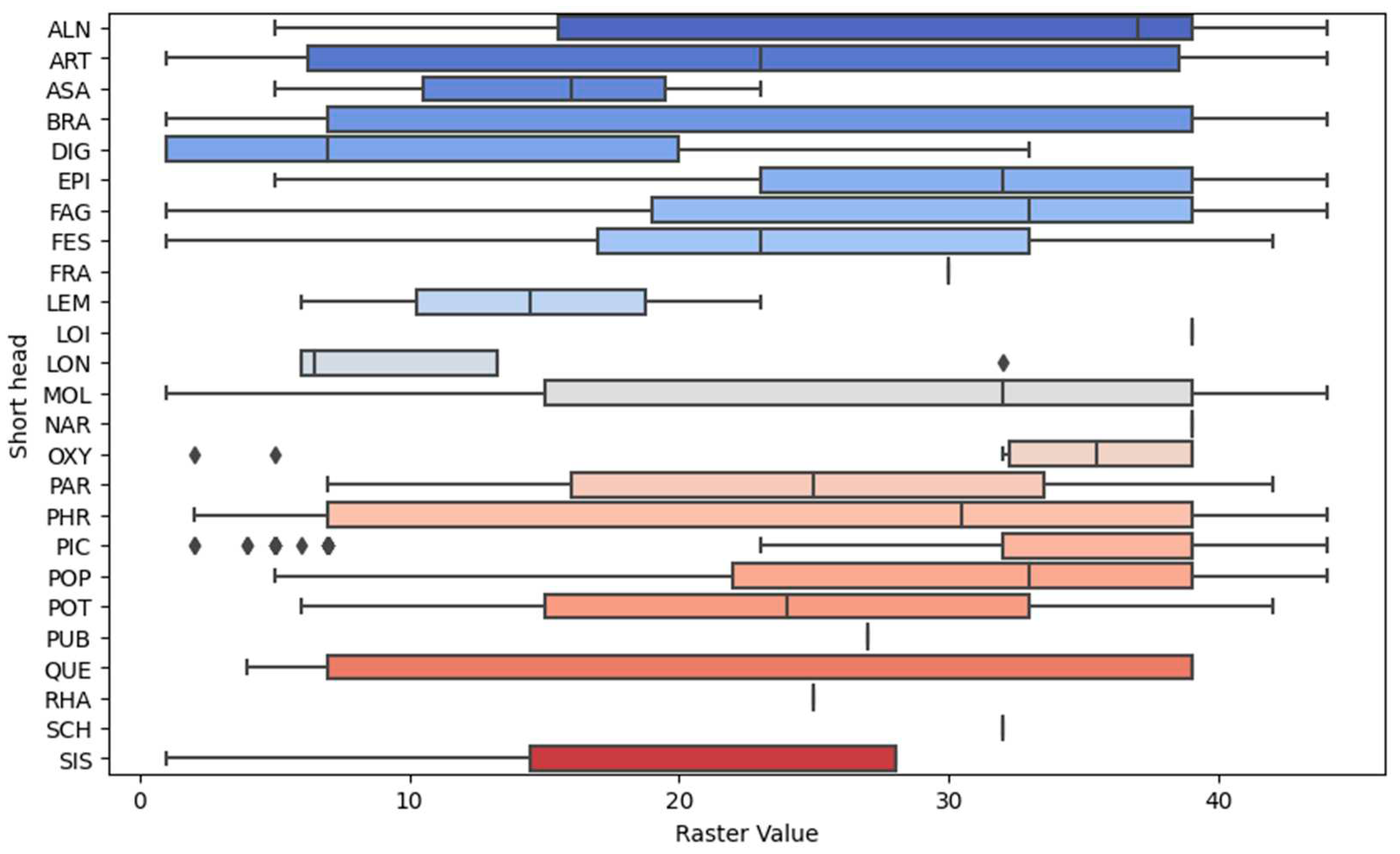
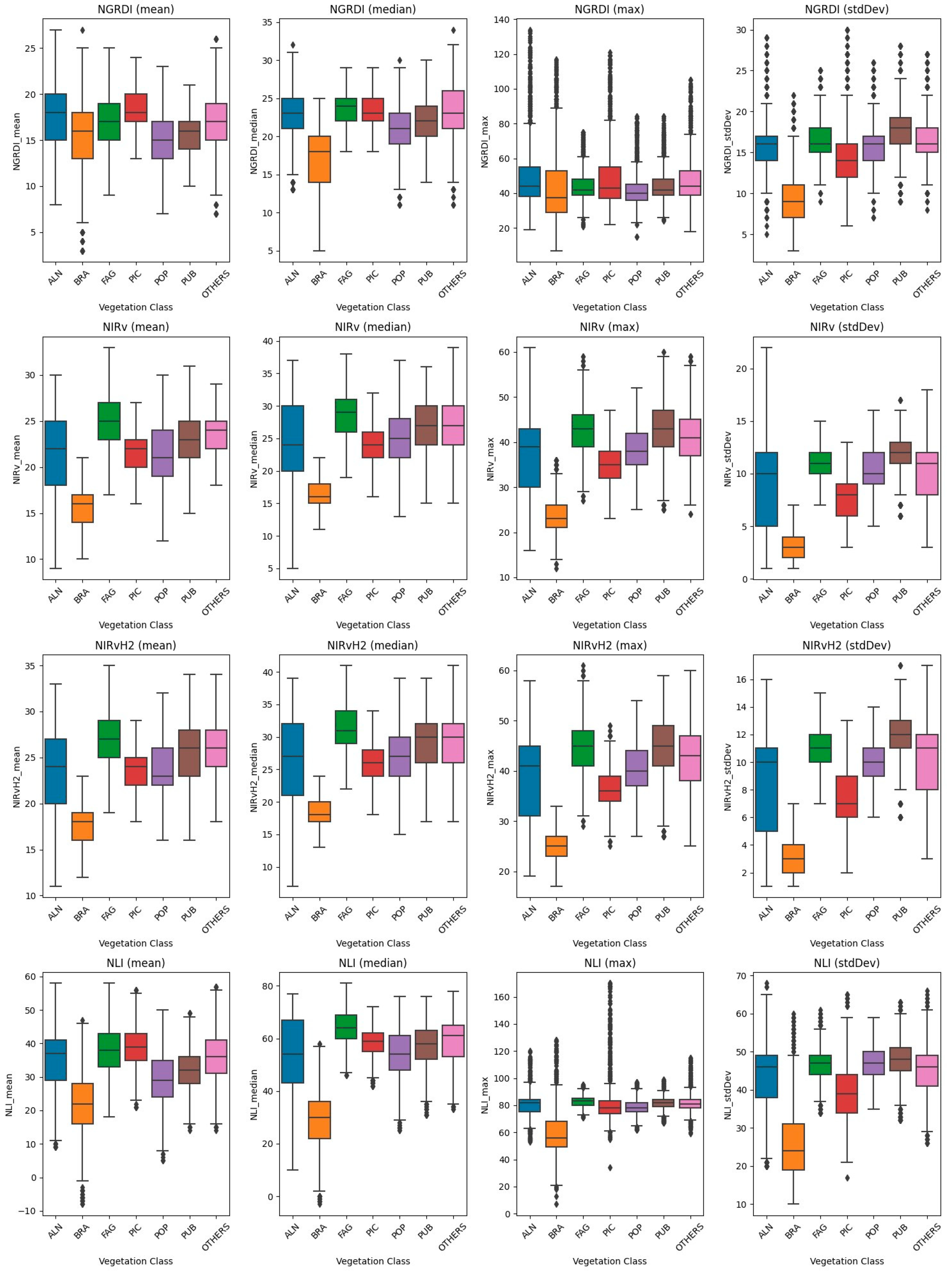
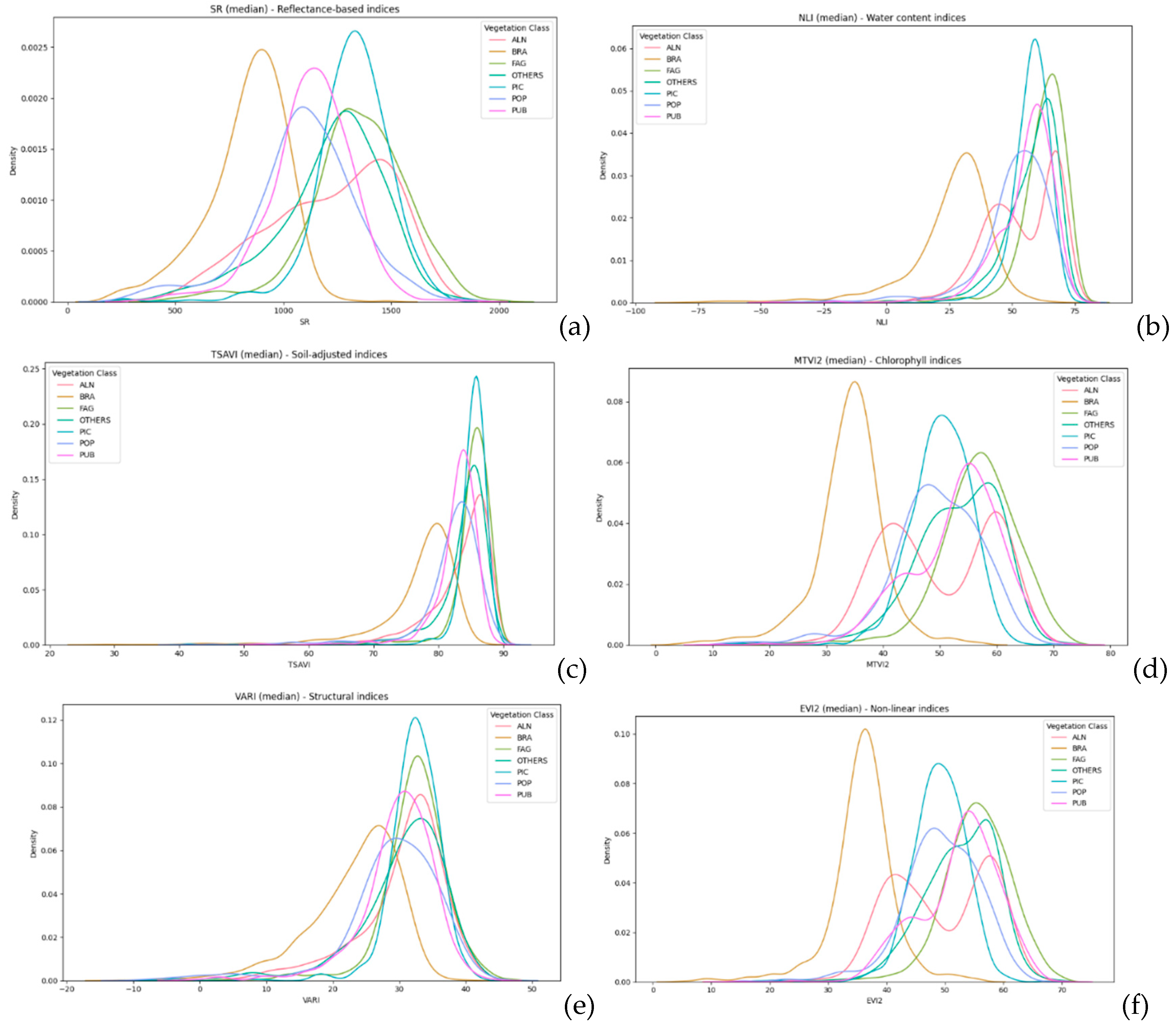
3. Results and discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kurbatova, A.I. Analytical Review of Modern Studies of Changes in the Biotic Components of the Carbon Cycle. Vestn. Ross. univ. družby nar., Ser. Èkol. bezop. žiznedeât. 2020, 28, 428–438. [Google Scholar] [CrossRef]
- Mukharamova, S.; Saveliev, A.; Ivanov, M.; Gafurov, A.; Yermolaev, O. Estimating the Soil Erosion Cover-Management Factor at the European Part of Russia. IJGI 2021, 10, 645. [Google Scholar] [CrossRef]
- Gafurov, A. Mapping of Rill Erosion of the Middle Volga (Russia) Region Using Deep Neural Network. ISPRS International Journal of Geo-Information 2022, 11, 197. [Google Scholar] [CrossRef]
- Fardeeva, M.; Lukyanova, Y.; Eskina, A.; Usmanov, B. Distribution and Habitat Features of Rare Orchid Species ( Orchidaceae Juss) in the National Park “Nizhnyaya Kama”. E3S Web Conf. 2023, 420, 01023. [Google Scholar] [CrossRef]
- Executive Steering Committee for Australian Vegetation Information Australian Vegetation Attribute Manual: National Vegetation Information System, Version 6.0; Department of Environment and Heritage: Canberra, 2003.
- Faber-Langendoen, D.; Keeler-Wolf, T.; Meidinger, D.; Tart, D.; Hoagland, B.; Josse, C.; Navarro, G.; Ponomarenko, S.; Saucier, J.-P.; Weakley, A.; et al. EcoVeg: A New Approach to Vegetation Description and Classification. Ecological Monographs 2014, 84, 533–561. [Google Scholar] [CrossRef]
- De Cáceres, M.; Chytrý, M.; Agrillo, E.; Attorre, F.; Botta-Dukát, Z.; Capelo, J.; Czúcz, B.; Dengler, J.; Ewald, J.; Faber-Langendoen, D.; et al. A Comparative Framework for Broad-scale Plot-based Vegetation Classification. Applied Vegetation Science 2015, 18, 543–560. [Google Scholar] [CrossRef]
- Kozhevnikova, M.; Prokhorov, V. Syntaxonomy of the Xero-Mesophytic Oak Forests in the Republic of Tatarstan (Eastern Europe). VCS 2021, 2, 47–58. [Google Scholar] [CrossRef]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G.; et al. Vegetation of Europe: Hierarchical Floristic Classification System of Vascular Plant, Bryophyte, Lichen, and Algal Communities. Applied Vegetation Science 2016, 19, 3–264. [Google Scholar] [CrossRef]
- De Cáceres, M.; Font, X.; Oliva, F. The Management of Vegetation Classifications with Fuzzy Clustering: Fuzzy Clustering in Vegetation Classifications. Journal of Vegetation Science 2010, 21, 1138–1151. [Google Scholar] [CrossRef]
- Gafurov, A. The Methodological Aspects of Constructing a High-Resolution DEM of Large Territories Using Low-Cost UAVs on the Example of the Sarycum Aeolian Complex, Dagestan, Russia. Drones 2021, 5, 7. [Google Scholar] [CrossRef]
- Duda, T.; Canty, M. Unsupervised Classification of Satellite Imagery: Choosing a Good Algorithm. International Journal of Remote Sensing 2002, 23, 2193–2212. [Google Scholar] [CrossRef]
- Liu, X. Supervised Classification and Unsupervised Classification.; ATS, 2005.
- Kozak, M.; Scaman, C.H. Unsupervised Classification Methods in Food Sciences: Discussion and Outlook. J Sci Food Agric 2008, 88, 1115–1127. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Saha, S. Unsupervised Classification: Similarity Measures, Classical and Metaheuristic Approaches, and Applications; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013; ISBN 978-3-642-32450-5. [Google Scholar]
- Olaode, A.; Naghdy, G.; Todd, C. Unsupervised Classification of Images: A Review. International Journal of Image Processing 2014, 8, 325–342. [Google Scholar]
- Xie, Y.; Sha, Z.; Yu, M. Remote Sensing Imagery in Vegetation Mapping: A Review. Journal of Plant Ecology 2008, 1, 9–23. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, Z.; Zhao, Y.; Zhang, L.; Liu, D.; Ren, T.; Zhang, X.; Li, S. An Unsupervised Crop Classification Method Based on Principal Components Isometric Binning. ISPRS International Journal of Geo-Information 2020, 9, 648. [Google Scholar] [CrossRef]
- Anchang, J.Y.; Ananga, E.O.; Pu, R. An Efficient Unsupervised Index Based Approach for Mapping Urban Vegetation from IKONOS Imagery. International Journal of Applied Earth Observation and Geoinformation 2016, 50, 211–220. [Google Scholar] [CrossRef]
- Ragettli, S.; Herberz, T.; Siegfried, T. An Unsupervised Classification Algorithm for Multi-Temporal Irrigated Area Mapping in Central Asia. Remote Sensing 2018, 10, 1823. [Google Scholar] [CrossRef]
- Yermolaev, O.; Usmanov, B.; Gafurov, A.; Poesen, J.; Vedeneeva, E.; Lisetskii, F.; Nicu, I.C. Assessment of Shoreline Transformation Rates and Landslide Monitoring on the Bank of Kuibyshev Reservoir (Russia) Using Multi-Source Data. Remote Sensing 2021, 13, 4214. [Google Scholar] [CrossRef]
- Perevedentsev, Y.P.; Shantalinskii, K.M.; Guryanov, V.V.; Aukhadeev, T.R. Climatic Changes on the Territory of the Volga Federal District. IOP Conf. Ser.: Earth Environ. Sci. 2020, 606, 012045. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth. BioScience 2001, 51, 933. [Google Scholar] [CrossRef]
- Yermolaev, O.; Usmanov, B. The Basin Approach to the Anthropogenic Impact Assessment in Oil-Producing Region. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM; Bulgaria, June 17 2014; Vol. 2, pp. 681–688. [Google Scholar]
- Prokhorov, V.; Rogova, T.; Kozhevnikova, M. Vegetation Database of Tatarstan. phyto 2017, 47, 309–313. [Google Scholar] [CrossRef]
- Gafurov, A.; Gainullin, I.; Usmanov, B.; Khomyakov, P.; Kasimov, A. Impacts of Fluvial Processes on Medieval Settlement Lukovskoe (Tatarstan, Russia). Proc. IAHS 2019, 381, 31–35. [Google Scholar] [CrossRef]
- Usmanov, B.; Gainullin, I.; Gafurov, A.; Ivanov, M.; Khomyakov, P.; Gubaidullin, A.; Nicu, I.C. Web-Portal “Country of Cities” - Comprehensive Study of the Volga Bulgarian Fortified Settlements. International Journal of Conservation Science 2022, 13, 1143–1158. [Google Scholar]
- Landsat Science. Available online: https://landsat.gsfc.nasa.gov/ (accessed on 7 March 2020).
- Montero, D. Eemont: A Python Package That Extends Google Earth Engine. JOSS 2021, 6, 3168. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-Scale Geospatial Analysis for Everyone. Remote Sensing of Environment 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Google Earth Engine. Available online: https://earthengine.google.com (accessed on 26 May 2021).
- Roujean, J.-L.; Breon, F.-M. Estimating PAR Absorbed by Vegetation from Bidirectional Reflectance Measurements. Remote Sensing of Environment 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Yang, W.; Kobayashi, H.; Wang, C.; Shen, M.; Chen, J.; Matsushita, B.; Tang, Y.; Kim, Y.; Bret-Harte, M.S.; Zona, D.; et al. A Semi-Analytical Snow-Free Vegetation Index for Improving Estimation of Plant Phenology in Tundra and Grassland Ecosystems. Remote Sensing of Environment 2019, 228, 31–44. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a Green Channel in Remote Sensing of Global Vegetation from EOS-MODIS. Remote Sensing of Environment 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Wang, F.; Huang, J.; Tang, Y.; Wang, X. New Vegetation Index and Its Application in Estimating Leaf Area Index of Rice. Rice Science 2007, 14, 195–203. [Google Scholar] [CrossRef]
- Jurgens, C. The Modified Normalized Difference Vegetation Index (mNDVI) a New Index to Determine Frost Damages in Agriculture Based on Landsat TM Data. International Journal of Remote Sensing 1997, 18, 3583–3594. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. 1 January 1974. [Google Scholar]
- Bannari, A.; Asalhi, H.; Teillet, P.M. Transformed Difference Vegetation Index (TDVI) for Vegetation Cover Mapping. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium; IEEE: Toronto, Ont., Canada, 2002; Vol. 5, pp. 3053–3055. [Google Scholar]
- Gitelson, A.A. Wide Dynamic Range Vegetation Index for Remote Quantification of Biophysical Characteristics of Vegetation. Journal of Plant Physiology 2004, 161, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Clevers, J.G.P.W. Application of a Weighted Infrared-Red Vegetation Index for Estimating Leaf Area Index by Correcting for Soil Moisture. Remote Sensing of Environment 1989, 29, 25–37. [Google Scholar] [CrossRef]
- Apan, A.; Held, A.; Phinn, S.; Markley, J. Detecting Sugarcane ‘Orange Rust’ Disease Using EO-1 Hyperion Hyperspectral Imagery. International Journal of Remote Sensing 2004, 25, 489–498. [Google Scholar] [CrossRef]
- Sripada, R.P.; Heiniger, R.W.; White, J.G.; Weisz, R. Aerial Color Infrared Photography for Determining Late-Season Nitrogen Requirements in Corn. Agronomy Journal 2005, 97, 1443–1451. [Google Scholar] [CrossRef]
- Badgley, G.; Field, C.B.; Berry, J.A. Canopy Near-Infrared Reflectance and Terrestrial Photosynthesis. Sci. Adv. 2017, 3, e1602244. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Hao, D.; Badgley, G.; Damm, A.; Rascher, U.; Ryu, Y.; Johnson, J.; Krieger, V.; Wu, S.; Qiu, H.; et al. Estimating Near-Infrared Reflectance of Vegetation from Hyperspectral Data. Remote Sensing of Environment 2021, 267, 112723. [Google Scholar] [CrossRef]
- Klemas, V.; Smart, R. The Influence of Soil Salinity, Growth Form, and Leaf Moisture on-the Spectral Radiance Of. Photogramm. Eng. Remote Sens 1983, 49, 77–83. [Google Scholar]
- Wilson, E.H.; Sader, S.A. Detection of Forest Harvest Type Using Multiple Dates of Landsat TM Imagery. Remote Sensing of Environment 2002, 80, 385–396. [Google Scholar] [CrossRef]
- Wang, L.; Qu, J.J. NMDI: A Normalized Multi-Band Drought Index for Monitoring Soil and Vegetation Moisture with Satellite Remote Sensing. Geophys. Res. Lett. 2007, 34, L20405. [Google Scholar] [CrossRef]
- Huntjr, E.; Rock, B. Detection of Changes in Leaf Water Content Using Near- and Middle-Infrared Reflectances☆. Remote Sensing of Environment 1989, 30, 43–54. [Google Scholar] [CrossRef]
- Wu, W. The Generalized Difference Vegetation Index (GDVI) for Dryland Characterization. Remote Sensing 2014, 6, 1211–1233. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A Modified Soil Adjusted Vegetation Index. Remote Sensing of Environment 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of Soil-Adjusted Vegetation Indices. Remote Sensing of Environment 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Kaufman, Y.J.; Tanre, D. Atmospherically Resistant Vegetation Index (ARVI) for EOS-MODIS. IEEE Trans. Geosci. Remote Sensing 1992, 30, 261–270. [Google Scholar] [CrossRef]
- Huete, A.R. A Soil-Adjusted Vegetation Index (SAVI). Remote Sensing of Environment 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Baret, F.; Guyot, G.; Major, D.J. TSAVI: A Vegetation Index Which Minimizes Soil Brightness Effects On LAI And APAR Estimation. In Proceedings of the 12th Canadian Symposium on Remote Sensing Geoscience and Remote Sensing Symposium; IEEE: Vancouver, Canada, 1989; Vol. 3, pp. 1355–1358. [Google Scholar]
- Gillespie, A.R.; Kahle, A.B.; Walker, R.E. Color Enhancement of Highly Correlated Images. II. Channel Ratio and “Chromaticity” Transformation Techniques. Remote Sensing of Environment 1987, 22, 343–365. [Google Scholar] [CrossRef]
- Bendig, J.; Yu, K.; Aasen, H.; Bolten, A.; Bennertz, S.; Broscheit, J.; Gnyp, M.L.; Bareth, G. Combining UAV-Based Plant Height from Crop Surface Models, Visible, and near Infrared Vegetation Indices for Biomass Monitoring in Barley. International Journal of Applied Earth Observation and Geoinformation 2015, 39, 79–87. [Google Scholar] [CrossRef]
- Haboudane, D. Hyperspectral Vegetation Indices and Novel Algorithms for Predicting Green LAI of Crop Canopies: Modeling and Validation in the Context of Precision Agriculture. Remote Sensing of Environment 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Chen, J.M. Evaluation of Vegetation Indices and a Modified Simple Ratio for Boreal Applications. Canadian Journal of Remote Sensing 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Vincini, M.; Frazzi, E.; D’Alessio, P. A Broad-Band Leaf Chlorophyll Vegetation Index at the Canopy Scale. Precision Agric 2008, 9, 303–319. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel Algorithms for Remote Estimation of Vegetation Fraction. Remote Sensing of Environment 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Vincini, M.; Frazzi, E. Comparing Narrow and Broad-Band Vegetation Indices to Estimate Leaf Chlorophyll Content in Planophile Crop Canopies. Precision Agric 2011, 12, 334–344. [Google Scholar] [CrossRef]
- John E. Plnder, Ill; McLeod, K.W. Indications of Relative Drought Stress in Longleaf Pine from Thematic Mapper Data. Photogrammetric Engineering & Remote Sensing 1999, 65, 495–501. [Google Scholar]
- Jordan, C.F. Derivation of Leaf-Area Index from Quality of Light on the Forest Floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.; Didan, K.; Miura, T. Development of a Two-Band Enhanced Vegetation Index without a Blue Band. Remote Sensing of Environment 2008, 112, 3833–3845. [Google Scholar] [CrossRef]
- Louhaichi, M.; Borman, M.M.; Johnson, D.E. Spatially Located Platform and Aerial Photography for Documentation of Grazing Impacts on Wheat. Geocarto International 2001, 16, 65–70. [Google Scholar] [CrossRef]
- Pinty, B.; Verstraete, M.M. GEMI: A Non-Linear Index to Monitor Global Vegetation from Satellites. Vegetatio 1992, 101, 15–20. [Google Scholar] [CrossRef]
- Escadafal, R.; Huete, A. Etude Des Propriétés Spectrales Des Sols Arides Appliquée à l’amélioration Des Indices de Végétation Obtenus Par Télédétection. Comptes rendus de l’Académie des sciences. Série 2, Mécanique, Physique, Chimie, Sciences de l’univers, Sciences de la Terre 1991, 312, 1385–1391. [Google Scholar]
- Rikimaru, A.; Roy, P.S.; Miyatake, S. Tropical Forest Cover Density Mapping. Tropical ecology 2002, 43, 39–47. [Google Scholar]
- Jiang, H.; Wang, S.; Cao, X.; Yang, C.; Zhang, Z.; Wang, X. A Shadow- Eliminated Vegetation Index (SEVI) for Removal of Self and Cast Shadow Effects on Vegetation in Rugged Terrains. International Journal of Digital Earth 2019, 12, 1013–1029. [Google Scholar] [CrossRef]
- Hancock, D.W.; Dougherty, C.T. Relationships between Blue- and Red-based Vegetation Indices and Leaf Area and Yield of Alfalfa. Crop Science 2007, 47, 2547–2556. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz †, Y.; Merzlyak, M.N. Relationships between Leaf Chlorophyll Content and Spectral Reflectance and Algorithms for Non-Destructive Chlorophyll Assessment in Higher Plant Leaves. Journal of Plant Physiology 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Van Der Tol, C.; Campbell, P.K.E.; Middleton, E.M. Fluorescence Correction Vegetation Index (FCVI): A Physically Based Reflectance Index to Separate Physiological and Non-Physiological Information in Far-Red Sun-Induced Chlorophyll Fluorescence. Remote Sensing of Environment 2020, 240, 111676. [Google Scholar] [CrossRef]
- Ren-hua, Z.; N, R.N.X. and L.K. Approach for a Vegetation Index Resistant to Atmospheric Effect. Journal of Integrative Plant Biology 1996, 38. [Google Scholar]
- Kawashima, S. An Algorithm for Estimating Chlorophyll Content in Leaves Using a Video Camera. Annals of Botany 1998, 81, 49–54. [Google Scholar] [CrossRef]
- Crippen, R. Calculating the Vegetation Index Faster. Remote Sensing of Environment 1990, 34, 71–73. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Wu, Z.; Wang, S.; Sun, H.; Senthilnath, J.; Wang, J.; Robin Bryant, C.; Fu, Y. Modified Red Blue Vegetation Index for Chlorophyll Estimation and Yield Prediction of Maize from Visible Images Captured by UAV. Sensors 2020, 20, 5055. [Google Scholar] [CrossRef] [PubMed]
- Peng Gong; Ruiliang Pu; Biging, G.S.; Larrieu, M.R. Estimation of Forest Leaf Area Index Using Vegetation Indices Derived from Hyperion Hyperspectral Data. IEEE Trans. Geosci. Remote Sensing 2003, 41, 1355–1362. [CrossRef]
- Gu, Y.; Brown, J.F.; Verdin, J.P.; Wardlow, B. A Five-Year Analysis of MODIS NDVI and NDWI for Grassland Drought Assessment over the Central Great Plains of the United States. Geophys. Res. Lett. 2007, 34, L06407. [Google Scholar] [CrossRef]
- Wang, C.; Chen, J.; Wu, J.; Tang, Y.; Shi, P.; Black, T.A.; Zhu, K. A Snow-Free Vegetation Index for Improved Monitoring of Vegetation Spring Green-up Date in Deciduous Ecosystems. Remote Sensing of Environment 2017, 196, 1–12. [Google Scholar] [CrossRef]
- Sulik, J.J.; Long, D.S. Spectral Considerations for Modeling Yield of Canola. Remote Sensing of Environment 2016, 184, 161–174. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and Photographic Infrared Linear Combinations for Monitoring Vegetation. Remote Sensing of Environment 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Z.; Cao, J. Developing a New Method to Identify Flowering Dynamics of Rapeseed Using Landsat 8 and Sentinel-1/2. Remote Sensing 2020, 13, 105. [Google Scholar] [CrossRef]
- Saberioon, M.M.; Amin, M.S.M.; Anuar, A.R.; Gholizadeh, A.; Wayayok, A.; Khairunniza-Bejo, S. Assessment of Rice Leaf Chlorophyll Content Using Visible Bands at Different Growth Stages at Both the Leaf and Canopy Scale. International Journal of Applied Earth Observation and Geoinformation 2014, 32, 35–45. [Google Scholar] [CrossRef]
- Hunt, E.R.; Doraiswamy, P.C.; McMurtrey, J.E.; Daughtry, C.S.T.; Perry, E.M.; Akhmedov, B. A Visible Band Index for Remote Sensing Leaf Chlorophyll Content at the Canopy Scale. International Journal of Applied Earth Observation and Geoinformation 2013, 21, 103–112. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing Prediction Power and Stability of Broadband and Hyperspectral Vegetation Indices for Estimation of Green Leaf Area Index and Canopy Chlorophyll Density. Remote Sensing of Environment 2001, 76, 156–172. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Pickens, A.H.; Tyukavina, A.; Hernandez-Serna, A.; Zalles, V.; Turubanova, S.; Kommareddy, I.; Stehman, S.V.; Song, X.-P.; et al. Global Land Use Extent and Dispersion within Natural Land Cover Using Landsat Data. Environ. Res. Lett. 2022, 17, 034050. [Google Scholar] [CrossRef]
- Kozhevnikova, M.V.; Prokhorov, V.E.; Rogova, T.V. Xeromesophytic Broad-Leaved Forest Communities of the Republic of Tatarstan in the Hierarchy of Syntaxa within the Braun-Blanquet System. Uchenye Zapiski Kazanskogo Universiteta. Seriya Estestvennye Nauki 2018, 160, 445–458. [Google Scholar]
- Kazan Federal University (Kazan, Russian Federation); Kozhevnikova, M.V.; Prokhorov, V.E.; Kazan Federal University (Kazan, Russian Federation); Saveliev, A.A.; Kazan Federal University (Kazan, Russian Federation) Predictive Modeling for the Distribution of Plant Communities of the Order Quercetalia Pubescenti-Petraeae Klika 1933. Tomsk State University Journal of Biology, 2019; 59–73. [CrossRef]
- Tichý, L. JUICE, Software for Vegetation Classification. J Vegetation Science 2002, 13, 451–453. [Google Scholar] [CrossRef]
| Index name | Description |
|---|---|
| REFLECTANCE-BASED INDICES | |
| DVI (Difference Vegetation Index) [32] | Captures the difference in reflectance between the near-infrared (NIR) and red bands. |
| DVIplus [33] | An extension of DVI, considering additional spectral bands or factors. |
| GNDVI (Green Normalized Difference Vegetation Index) [34] | Utilizes the green and NIR bands to emphasize chlorophyll content. |
| GRNDVI (Green-Red Normalized Difference Vegetation Index) [35] | Incorporates green and red bands for enhanced vegetation monitoring. |
| MNDVI (Modified NDVI) [36] | An adjusted form of NDVI to account for atmospheric and canopy background effects. |
| NDVI (Normalized Difference Vegetation Index) [37] | One of the most widely used indices, highlighting areas of active vegetation. |
| RDVI (Renormalized Difference Vegetation Index) [32] | Aims to minimize soil background influences while emphasizing vegetation. |
| TDVI (Transformed Difference Vegetation Index) [38] | A modified version of NDVI to enhance sensitivity to vegetation changes. |
| WDRVI (Wide Dynamic Range Vegetation Index) [39] | Offers a wider dynamic range than NDVI, improving sensitivity. |
| WDVI (Weighted Difference Vegetation Index) [40] | Focuses on minimizing soil background effects by applying a specific weight. |
| WATER CONTENT INDICES | |
| DSWI1 (Drought Stress Water Index 1) [41] | Highlights areas undergoing drought stress, indicating lower water content. |
| GSAVI (Green Soil Adjusted Vegetation Index) [42] | A soil-adjusted index using the green band to minimize soil background influences. |
| NIRv (Near-Infrared Reflectance of Vegetation) [43] | Captures the NIR reflectance associated with vegetation’s structural characteristics. |
| NIRvH2 [44] | A variation of NIRv, considering additional factors for enhanced accuracy. |
| NDII (Normalized Difference Infrared Index) [45] | Helps in assessing vegetation water content. |
| NDMI (Normalized Difference Moisture Index) [46] | Another index for evaluating water content in vegetation. |
| NMDI (Normalized Multi-band Drought Index) [47] | Focuses on monitoring drought conditions across various bands. |
| MSI (Moisture Stress Index) [48] | Highlights moisture stress in vegetation, crucial for drought monitoring. |
| SOIL-ADJUSTED INDICES | |
| GDVI (Green Difference Vegetation Index) [49] | Minimizes soil background influences using the green band. |
| GOSAVI (Green Optimized Soil Adjusted Vegetation Index) [42] | A soil-adjusted index designed for optimal vegetation monitoring. |
| GRVI (Green Red Vegetation Index) [42] | Utilizes green and red bands for vegetation analysis, adjusting for soil effects. |
| MSAVI (Modified Soil Adjusted Vegetation Index) [50] | A soil-adjusted index with modifications for better accuracy. |
| OSAVI (Optimized Soil Adjusted Vegetation Index) [51] | Optimizes the soil adjustment to improve vegetation monitoring in low cover areas. |
| SARVI (Soil Adjusted and Atmospherically Resistant Vegetation Index) [52] | A soil-adjusted and atmospherically resistant index. |
| SAVI (Soil Adjusted Vegetation Index) [53] | Adjusts for the influence of soil brightness when vegetation cover is low. |
| TSAVI (Transformed Soil Adjusted Vegetation Index) [54] | A transformed index for better vegetation representation in diverse conditions. |
| CHLOROPHYLL INDICES | |
| GARI (Green Atmospherically Resistant Vegetation Index) [34] | Designed to monitor chlorophyll content while minimizing atmospheric effects. |
| GCC (Green Chlorophyll Content) [55] | Directly related to chlorophyll content, crucial for assessing vegetation health. |
| MGRVI (Modified Green Red Vegetation Index) [56] | A modified index to enhance sensitivity to chlorophyll content. |
| MCARI2 (Modified Chlorophyll Absorption in Reflectance Index 2) [57] | Targets chlorophyll absorption features for accurate monitoring. |
| MSR (Modified Simple Ratio) [58] | A modified vegetation index to improve sensitivity to chlorophyll content. |
| MTVI2 (Modified Triangular Vegetation Index 2) [57] | Focuses on enhancing the representation of chlorophyll content. |
| OCVI (Optimized Chlorophyll Vegetation Index) [59] | Optimizes chlorophyll representation in vegetation monitoring. |
| VIG (Vegetation Index Green) [60] | Utilizes the green band for chlorophyll monitoring, essential for assessing plant health. |
| STRUCTURAL INDICES | |
| CVI (Chlorophyll Vegetation Index) [61] | Represents vegetation structure and chlorophyll content. |
| DSI (Difference Structure Index) [62] | Highlights structural variations in vegetation. |
| SR (Simple Ratio) [63] | A basic ratio of NIR to red reflectance, indicating vegetation structure. |
| NON-LINEAR INDICES | |
| EVI2 (Enhanced Vegetation Index 2) [64] | An improved version of NDVI, incorporating non-linear enhancements. |
| GBNDVI (Green Blue Normalized Difference Vegetation Index) [35] | A non-linear index utilizing green and blue bands. |
| GLI (Green Leaf Index) [65] | Represents vegetation greenness in a non-linear manner. |
| GEMI (Global Environment Monitoring Index) [66] | A global index for vegetation monitoring with non-linear properties. |
| RI (RapidEye Vegetation Index) [67] | A specific index for RapidEye satellite data. |
| SI (Shadow Index) [68] | Represents vegetation shape characteristics. |
| SEVI (Soil and Atmospherically Resistant Vegetation Index) [69] | Minimizes soil and atmospheric effects using a non-linear approach. |
| VARI (Visible Atmospherically Resistant Index) [60] | Visible spectrum for vegetation monitoring, applying non-linear corrections. |
| OTHER INDICES | |
| BWDRVI (Broadband Width Difference Vegetation Index) [70] | A unique index capturing broadband width differences. |
| CIG (Canopy Index Green) [71] | Represents canopy structure using the green band. |
| FCVI (Floating Canopy Vegetation Index) [72] | A special index for floating canopy vegetation. |
| IAVI (Inverted Attributed Vegetation Index) [73] | An inverted index for enhanced vegetation attribute representation. |
| IKAW (Kawashima Vegetation Index) [74] | A specific vegetation index developed by Kawashima. |
| IPVI (Infrared Percentage Vegetation Index) [75] | Utilizes infrared reflectance for vegetation percentage estimation. |
| MRBVI (Modified Ratio Vegetation Index) [76] | A modified ratio index for improved vegetation monitoring. |
| MNLI (Modified Non-Linear Vegetation Index) [77] | Incorporates non-linear adjustments for enhanced vegetation representation. |
| NDDI (Normalized Difference Drought Index) [78] | Focuses on drought monitoring and water stress assessment. |
| NDGI (Normalized Difference Greenness Index) [33] | Highlights vegetation greenness. |
| NDPI (Normalized Difference Phenology Index) [79] | Utilized for monitoring vegetation phenology. |
| NDYI (Normalized Difference Yellowness Index) [80] | Highlights vegetation yellowness, crucial for certain phenological stages. |
| NGRDI (Normalized Green Red Difference Index) [81] | Utilizes green and red bands for vegetation monitoring. |
| NRFIg (Normalized Red/Far-Red Index Green) [82] | Focuses on the red to far-red ratio using the green band. |
| NRFIr (Normalized Red/Far-Red Index Red) [82] | Utilizes the red to far-red ratio in the red band. |
| NormG (Normalized Green) [42] | Represents normalized green reflectance. |
| NormNIR (Normalized NIR) [42] | Represents normalized near-infrared reflectance. |
| NormR (Normalized Red) [42] | Represents normalized red reflectance. |
| RCC (Red Chromatic Coordinate) [55] | Directly related to chlorophyll content using the red band. |
| RGBVI (Red Green Blue Vegetation Index) [56] | Utilizes the RGB bands for vegetation analysis. |
| RGRI (Red Green Ratio Index) [83] | A ratio index using red and green bands. |
| TGI (Triangular Greenness Index) [84] | Represents vegetation greenness using a triangular approach. |
| TriVI (Triangular Vegetation Index) [85] | A triangular index for enhanced vegetation representation. |
| FC BB* | Main statistics | |||||
|---|---|---|---|---|---|---|
| Mean | Median | Std | Min | Max | Count | |
| ALN | 28.90 | 37.00 | 14.49 | 5 | 44 | 39 |
| ART | 22.23 | 23.00 | 14.13 | 1 | 44 | 126 |
| ASA | 14.67 | 16.00 | 9.07 | 5 | 23 | 3 |
| BRA | 27.91 | 39.00 | 14.66 | 1 | 44 | 464 |
| DIG | 12.40 | 7.00 | 13.89 | 1 | 33 | 5 |
| EPI | 29.47 | 32.00 | 11.86 | 5 | 44 | 15 |
| FAG | 28.48 | 33.00 | 13.22 | 1 | 44 | 1637 |
| FES | 23.75 | 23.00 | 12.13 | 1 | 42 | 61 |
| FRA | 30.00 | 30.00 | NaN | 30 | 30 | 1 |
| LEM | 14.50 | 14.50 | 12.02 | 6 | 23 | 2 |
| LOI | 39.00 | 39.00 | NaN | 39 | 39 | 1 |
| LON | 12.75 | 6.50 | 12.84 | 6 | 32 | 4 |
| MOL | 27.23 | 32.00 | 12.81 | 1 | 44 | 152 |
| NAR | 39.00 | 39.00 | 0.00 | 39 | 39 | 3 |
| OXY | 29.90 | 35.50 | 14.22 | 2 | 39 | 10 |
| PAR | 24.67 | 25.00 | 17.50 | 7 | 42 | 3 |
| PHR | 25.52 | 30.50 | 14.83 | 2 | 44 | 64 |
| PIC | 32.05 | 39.00 | 12.86 | 2 | 44 | 178 |
| POP | 30.18 | 33.00 | 11.30 | 5 | 44 | 121 |
| POT | 24.00 | 24.00 | 25.46 | 6 | 42 | 2 |
| PUB | 27.00 | 27.00 | NaN | 27 | 27 | 1 |
| QUE | 26.44 | 39.00 | 15.30 | 4 | 39 | 25 |
| RHA | 25.00 | 25.00 | NaN | 25 | 25 | 1 |
| SCH | 32.00 | 32.00 | NaN | 32 | 32 | 1 |
| SIS | 19.00 | 28.00 | 15.59 | 1 | 28 | 3 |
| Class | # of pixels | Area (km²) | % of Forested area |
|---|---|---|---|
| ALN | 2082534 | 1874 | 16 |
| BRA | 440568 | 397 | 3 |
| FAG | 6370003 | 5733 | 48 |
| PIC | 564690 | 508 | 4 |
| POP | 876324 | 789 | 7 |
| PUB | 1320184 | 1188 | 10 |
| OTHERS | 1742692 | 1568 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





